Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.95
Peer-review started: September 12, 2018
First decision: October 24, 2018
Revised: December 1, 2018
Accepted: December 13, 2018
Article in press: December 13, 2018
Published online: January 7, 2019
Processing time: 118 Days and 1.2 Hours
Abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs) are the most common cause of recurrent abdominal pain in children. Despite its high prevalence, the underlying pathophysiology of this condition is poorly understood.
To assess the role of gastric dysmotility and autonomic nervous system dysfunction in the pathophysiology of AP-FGIDs.
One hundred children, fulfilling Rome III criteria for AP-FGIDs, and 50 healthy controls, aged 5 to 12 years, were recruited after obtaining parental consent. All patients were investigated for underlying organic disorders. Gastric motility and cardiovascular autonomic functions were assessed using validated non-invasive techniques.
The main gastric motility parameters assessed (gastric emptying rate [45.7 vs 59.6 in controls], amplitude [48.7 vs 58.2], frequency of antral contractions [8.3 vs 9.4], and antral motility index [4.1 vs 6.4]) were significantly lower in children with AP-FGIDs (P < 0.05). The post-prandial antral dilatation at 1 min after the test meal significantly correlated with the severity of abdominal pain (P < 0.05). Assessment of autonomic functions in AP-FGID patients showed neither a significant difference compared to the control group, nor a correlation with gastric motility abnormalities (P > 0.05). The duration of pain episodes negatively correlated with the parasympathetic tone (maladaptive parasympathetic tone) (P < 0.05).
Children with AP-FGIDs have abnormal gastric motility but normal cardiovascular autonomic functions. There is no relationship between abnormal gastric motility and autonomic functions. The pathogenesis of AP-FGIDs is not related to cardiovascular autonomic dysfunction.
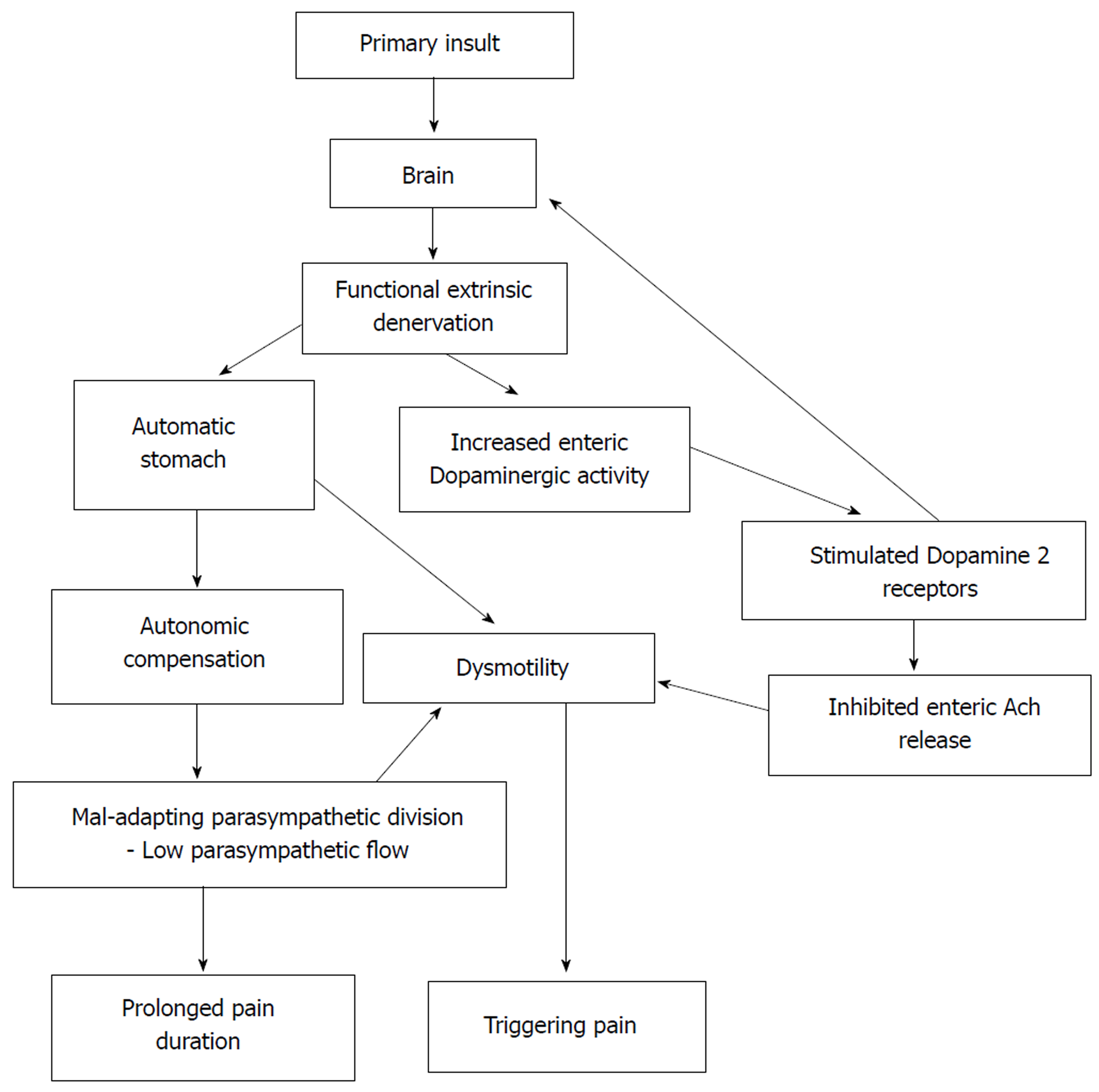
Core tip: In this study, we examined the relationship between cardiovascular autonomic functions and functional abdominal pain disorders in children. We failed to demonstrate a significant difference in autonomic functions and a significant relationship between gastric motor abnormalities and autonomic functions in affected children. In this paper, we propose functional extrinsic denervation and maladaptive parasympathetic division as possible contributing factors for the impaired gastric motility and symptoms in functional abdominal pain disorders, which is demonstrated in the ‘Automatic Stomach’ model.
- Citation: Karunanayake A, Rajindrajith S, de Silva HA, Gunawardena S, Devanarayana NM. Autonomic functions and gastric motility in children with functional abdominal pain disorders. World J Gastroenterol 2019; 25(1): 95-106
- URL: https://www.wjgnet.com/1007-9327/full/v25/i1/95.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i1.95
Abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs) are one of the most recognized groups of gastrointestinal disorders in children across the world. It has an estimated global prevalence of 13.5% in community- and school-based surveys[1]. This group of disorders consists of irritable bowel syndrome (IBS), functional abdominal pain (FAP), functional dyspepsia (FD) and abdominal migraine[2]. Although not directly related to mortality, AP-FGIDs have a considerable effect on health-related quality of life and healthcare expenditure[3,4]. Even with the highly advanced modern technologies, pathophysiological mechanisms of FGIDs are not yet clearly understood. The recognized pathophysiological mechanisms include visceral hypersensitivity, altered gastrointestinal motility, immunological dysfunction, altered gastrointestinal microbiota, altered intestinal permeability, genetic factors and psychosocial disturbances[5].
Abnormalities in gastrointestinal motor function have been suggested as a potential pathophysiological mechanism in AP-FGIDs. They include, dilated gastric antrum at fasting period[6,7], delayed gastric emptying[6,8-11], impaired initial distribution of a meal[12], impaired gastric accommodation to a meal[13] and antral hypomotility[8-11,14].
The autonomic nervous system (ANS) is an integral part of the brain-gut axis that is involved in regulating gastrointestinal motility. Some studies have demonstrated dysfunctions in both sympathetic and parasympathetic divisions of the ANS in children and adults with functional gastrointestinal disorders (FGIDs)[15-17]. Elsenbruch and Orr have noted a significant correlation between vagal response and post-prandial abdominal symptoms in patients with diarrhoea-predominant IBS[18]. Abnormalities of gastric motility and underlying vagal defects have been demonstrated in adult patients with IBS[15,18]. In addition, the ANS is thought to play an important role in modulating visceral sensitivity in FGIDs[19]. However, the relationship between autonomic function and gastric motility has not been studied in affected children.
The main objective of this study was to assess ANS functions in paediatric patients with AP-FGIDs and its relationship with gastric motor functions.
This is a comparative, cross-sectional study to assess cardiovascular autonomic functions and gastric motility in children with AP-FGIDs.
All consecutive patients aged 5-12 years who were eligible according to the inclusion criteria were recruited from the paediatric out-patient clinics of North Colombo Teaching Hospital, Ragama, Sri Lanka and investigated in the Gastroenterology Research Laboratory, Faculty of Medicine, University of Kelaniya, Sri Lanka. A detailed history was taken from each subject and his or her parents after obtaining written informed consent. Details regarding pain characteristics and autonomic symptoms were obtained using an interviewer-administered pre-tested questionnaire. AP-FGIDS were diagnosed using Rome III criteria[2].
(1) Fulfilment of Rome III criteria for at least one AP-FGID AND;
(2) Abdominal pain at least once per week for at least 2 mo prior to diagnosis AND;
(3) Pain severity more than 25 mm on a 100-mm visual analogue scale and severe enough to interrupt the activities of the child (e.g., sleep, play, schooling, etc).
All patients were screened for organic diseases using a detailed history, and complete physical examination, including growth parameters, stool microscopy, urine microscopy and culture, full blood count, C-reactive protein, liver function tests, renal function tests and ultrasound scan abdomen. Special investigations performed, based on clinical judgment of the consultant paediatrician who assessed the patients, included upper and lower gastrointestinal endoscopy, serum amylase and X-ray kidney-ureter-bladder. Patients were not screened for coeliac disease since it is extremely rare in Sri Lanka[20].
(1) Clinical or laboratory evidence suggestive of an organic pathology.
(2) Chronic medical or surgical diseases other than AP-FGIDs.
(3) Long-term medication for any illness other than AP-FGIDs.
(4) Previous abdominal surgery.
(5) Subjects who had received prokinetic drugs or any other drugs that can alter gastrointestinal motility during the 30 d prior to the diagnosis being made.
An age- and sex-compatible group of children were recruited from the community of the same geographical area as controls after obtaining written parental consent. None of the controls had acute or chronic disease or symptoms related to the gastrointestinal tract.
Autonomic functions and gastric motility were assessed on the same day (gastric motility from 8:30 am to 9:30 am and autonomic function test from 9:30 am to 10:30 am) under thermo-neutral conditions (26°C). All girls who had attained menarche underwent laboratory investigations during the proliferative phase of their menstrual cycles. All medications with adrenergic and cholinergic properties were discontinued for a period of at least five times the half-life of the specific medication. All subjects were advised to refrain from ingesting beverages containing caffeine, nicotine or alcohol for at least 8 h prior to testing. They were in a fasting state for at least six hours prior to the study. A standard breakfast was given with water after completion of gastric motility assessment. The autonomic function was assessed in all subjects 30 minutes after completion of breakfast.
Gastric motility was measured in all children with AP-FGIDs and the controls by a previously reported and validated ultrasound method[21] using a high-resolution real-time scanner (Siemens ACUSON X300™) with 1.8 MHz to 6.4 MHz curve linear transducer and with facilities to record and playback. All gastric motility parameters were assessed by the same investigator (NMD) who was blind to the diagnosis and results of the autonomic function tests. The main gastric motility parameters assessed were fasting antral area, gastric emptying rate, frequency and amplitude of antral contractions and antral motility index.
All subjects underwent autonomic cardiovascular tests according to the test battery described by Ewing et al[22], using the standard procedures described. All autonomic functions were assessed by the same investigator (AK) who was blind to the gastric motility status. The test battery consisted of four autonomic function tests conducted in the following order, and the results were recorded in a data sheet.
(1) Blood pressure (BP) response to standing from lying down position.
(2) Heart rate response to standing from lying down position.
(3) Heart rate variation with deep breathing.
(4) Valsalva test.
Before the test, the procedures were explained and mimicked for the benefit of each subject.
After instrumentation, children were subjected to ten minutes of mandatory rest. At the end of the rest period, the electrocardiogram (ECG) recording from lead II was started along with the BP recording. Thereafter, two readings of BP and heart rate were obtained at an interval of two minutes between two consecutive recordings. The average of the two readings was recorded as resting heart rate and BP.
Test 1 - BP response to standing from lying down position: BP readings were recorded one minute after the unaided standing up, maintaining the arm cuff at the level of the heart. The one-minute systolic BP was compared with the resting systolic BP, and the postural change in systolic BP was calculated. An automated BP machine (A&D Medical®) with a paediatric cuff, which was calibrated against a standard mercury sphygmomanometer, was used. The BP response to standing is dependent upon sympathetic adrenergic function[23].
Test 2 - Heart rate response to standing from lying down position: ECG was recorded for a further 60 seconds after standing. The heart rate ratio (30:15 ratio) was calculated as the ratio between the longest R-R interval at around the 30th beat (R-R 30) and the shortest R-R interval at or around the 15th beat after standing (R-R 15). The 30:15 ratio was calculated as R-R 30/R-R 15. An increment in the 30:15 ratio was considered an increased parasympathetic response[24].
Test 3 - Heart rate response to deep breathing: Subjects were instructed to sit quietly and to breathe deeply at six breaths per minute (five seconds in and five seconds out). The investigator guided them through the manoeuvre by counting. Continuous ECG recording (Lead II) was completed for three consecutive artefact-free cycles of deep inspiration and expiration. The difference between maximum and minimum heart rates during each cycle was calculated, and the mean difference of the three cycles was obtained. Impairment in heart rate variability is a sign of parasympathetic dysfunction[23]. Increased parasympathetic response is indicated by widening of the difference[25].
Test 4 - Valsalva test: Subjects were asked to exhale into a mouthpiece connected to a mercury manometer and to maintain the expiratory pressure at 20 mmHg for 15 s in the sitting position. ECG was recorded during this manoeuvre and for 45 seconds afterwards. Pre-testing showed that most of the children were not able to achieve 40 mmHg expiratory pressure proposed by Ewing. Therefore, we set the value at 20 mmHg, which was achieved by the children. The child was allowed to rest for one minute before repeating the Valsalva manoeuvre. The Valsalva ratio was calculated by dividing the maximum R-R interval following Valsalva manoeuvre with the minimum R-R interval during the Valsalva procedure. The mean ratio of the two attempts was calculated. A reduced ratio indicates parasympathetic dysfunction[23].
Autonomic symptoms were assessed by a modified composite autonomic symptom scale (commonly known as COMPASS) which was translated and validated for the local language[26].
Gastrointestinal symptoms were assessed using a translated and validated Rome III questionnaire[27,28]. The severity of abdominal symptoms was recorded on a 100-mm visual analogue scale.
The sample size was calculated using the 30:15 ratio taken from a previous study done on obese children aged 5-10 years in India[29]. The similarity with the race and age group was considered for selecting values from the Indian study. At a power of 90% and significance level of 95%, the minimum sample required was 26 per group.
All statistical analyses were completed using PSPP version 0.8.3-g5f9212 statistics software (Free Software Foundation, Inc. http://fsf.org/). Means and standard deviations were calculated for continuous variables, and frequencies and percentages were taken for categorical variables. For continuous data, nonparametric, a Mann Witney U test was used. For dichotomous data, a chi-square test was used to assess differences between the two groups. A two-tailed level of significance of 0.05 was used for the analysis.
This study protocol was approved by the Ethics Review Committee, Faculty of Medicine, University of Kelaniya, Sri Lanka.
A total of 100 patients with AP-FGIDs [39 (39%) boys, mean age 7.9 years (SD 2.1 years)] and 50 healthy controls [20 (40%) boys, mean age 8.6 years (SD 1.9 years)] were recruited for this study. The AP-FGID group consisted of 54 (54%) children with FAP, 33 (33%) with IBS, and 13 (13%) with FD.
The autonomic symptoms related to the gastrointestinal tract were significantly higher among the AP-FGID group (Table 1). The extra-intestinal symptoms, with the exception of the presence of cold feet, were higher among the AP-FGID group, though the difference was not statistically significant.
The autonomic parameters were not significantly different between AP-FGIDs and control groups (Table 2). All parasympathetic parameters were lower in the AP-FGIDs group, but these were not statistically significant. Resting heart rate, which is under parasympathetic inhibition, was higher among the AP-FGIDs group but with no statistical significance.
| Autonomic test | Measurement | AP-FGIDs, n = 100 | Controls, n = 50 | P value1 |
| Median (range) | Median (range) | |||
| Resting heart rate (beats/min) | Resting heart rate (beats/min) | 89.0 (57-110) | 87.0 (63-114) | 0.18 |
| Heart rate response to deep breathing | Maximum-minimum heart rate (beats/min) | 30.8 (10-60) | 32.0 (10-57) | 0.90 |
| Lying to standing heart rate response | 30:15 ratio | 1.2 (0.9-1.6) | 1.2 (0.9-1.8) | 0.67 |
| Valsalva manoeuvre | Valsalva ratio | 1.5 (1-2.1) | 1.5 (1-2.5) | 0.23 |
When pain characters of children with AP-FGIDs were correlated with autonomic parameters, the duration of pain episode negatively correlated with the 30:15 ratio (r = -0.21, P = 0.024, Pearson correlation coefficient). Other characteristics had no such correlation.
Gastric emptying rate, frequency of antral contractions, the amplitude of antral contractions and antral motility index were significantly lower in AP-FGIDs group (Table 3). Pain severity positively correlated with the antral area at 1 min (r = 0.2, P = 0.02).
| Gastric motility parameter | AP-FGIDs, n = 100 | Controls, n = 50 | P value1 |
| Median (range) | Median (range) | ||
| Fasting antral area, cm2 | 1.6 (0.4-5.9) | 1.3 (0.4-5.1) | 0.19 |
| Antral area at 1 min, cm2 | 9.8 (3.8-14.6) | 9.8 (3.4-19.1) | 0.42 |
| Antral area at 15 min, cm2 | 4.8 (0.9-10.7) | 3.6 (0.6-11.2) | < 0.0001 |
| Gastric emptying rate, % | 46.3 (16.0-77.9) | 61.6 (10.0-88.5) | < 0.0001 |
| Frequency of antral contraction, /3 min | 8.0 (6-11) | 9.0 (8-11) | < 0.0001 |
| Amplitude of antral contraction, % | 47.2 (22.8-83.0) | 59.4 (32.6-85.4) | < 0.0001 |
| Antral motility index | 4.0 (1.7-7.5) | 5.7 (2.9-8.1) | < 0.0001 |
In healthy controls, the gastric emptying rate and the frequency of antral contractions positively correlated with the 30:15 ratio. Furthermore, the Valsalva ratio positively correlated with the frequency of antral contractions in healthy controls (Table 4). There was no such correlation between autonomic functions and gastric motility in patients with AP-FGIDs.
| Gastric motility parameter | Valsalva ratio | Minimum -maximum heart rate | 30:15 ratio | |
| AP-FGIDs, | FAA | -0.01 | 0.04 | -0.09 |
| n = 100 | AA1 | -0.02 | -0.02 | -0.06 |
| AA15 | -0.01 | -0.03 | -0.07 | |
| GER | 0.07 | -0.03 | 0.06 | |
| FAA | -0.02 | -0.07 | 0.04 | |
| AAC | 0.07 | -0.06 | 0.13 | |
| MI | 0.07 | -0.07 | 0.14 | |
| Controls, | FAA | 0.11 | -0.05 | -0.11 |
| n = 50 | AA1 | 0.03 | 0.01 | -0.01 |
| AA15 | 0.07 | 0.05 | 0.17 | |
| GER | 0.2 | 0.1 | 0.39a | |
| FAC | 0.7a | -0.09 | 0.14a | |
| AAC | -0.07 | 0.11 | -0.2 | |
| MI | 0.05 | 0.17 | 0.07 |
The current study assessed the cardiovascular autonomic functions and gastric motility in children with AP-FGIDs. The assessment of autonomic functions in AP-FGID patients showed no significant difference when compared with the control group. The gastric motility parameters were significantly impaired in children with AP-FGIDs. None of the autonomic function tests showed significant correlation with any of the gastric motility parameters in the AP-FGIDs group.
The lack of differences in the autonomic parameters in the two groups indicates the possibility of normal autonomic function in children with AP-FGIDs. Since there are no tests that can directly assess the autonomic function of the gastrointestinal system and its interactions with the brain, the cardiovascular autonomic functions are used as a proxy to assess autonomic function of the gastrointestinal system[30]. Chelimsky et al[16] noted orthostatic intolerance in six out of eight patients (reflected by excessive increase in heart rate or reduction in BP) and low Valsalva ratio in two patients. Heart rate response to deep breathing, which exclusively assesses parasympathetic function, was within the normal limits in all eight patients[16]. However, this was an observational study with no controls. In addition, the authors did not classify recurrent abdominal pain to definitive functional gastrointestinal disorders using the standard Rome criteria. Several studies have assessed heart rate variability (HRV) in adult patients with IBS using different methods[31]. However, no significant difference in vagal activity and sympatho-vagal balance between children with FAP/IBS and healthy controls have been shown in HRV assessments[32]. Furthermore, meta-analysis of studies assessing HRV found that there could be a significantly lower vagal influence in IBS patients compared to controls[33]. The studies included in these meta-analyses used a different method to assess autonomic function, and therefore, we cannot directly compare our findings with the meta-analyses. Similar to our findings, some other studies have failed to demonstrate a significant association between autonomic functions and functional gastrointestinal disorders[34,35].
In addition, the lack of significant difference of extra-intestinal autonomic symptoms between children with AP-FGIDs compared to controls potentially indicates that children with AP-FGIDs do not have generalized autonomic dysfunction. Similarly, Chelimsky et al[16] did not find extra-intestinal autonomic symptoms in children with AP-FGIDs.
Current autonomic tests are only a proxy measure of gastrointestinal autonomic function. Apart from that, contribution of the autonomic input of the local neuronal network within the stomach has not been taken into account during the testing. These factors may at least partially contributed to the lack of differences in autonomic function in children with AP-FGIDs. However, it is essential to understand that with the current knowledge and available tests, this is the closest that we could come to making a reasonable assessment of autonomic functions.
Gastric motility abnormalities have been reported in children and adults with FGIDs[6,8-11,14,36-38]. In the current study, we also noted a similar pattern of abnormalities in children with AP-FGIDs. In addition, we found a positive correlation between abdominal pain and abnormalities in gastric motility, similar to previous studies[6,10,11,39-41]. These findings suggest a potential pathophysiological relationship between gastric motility abnormalities and AP-FGIDs.
When we correlated gastric motility with autonomic parameters, we did not find a clear correlation between them in children with AP-FGIDs. However, the finding of a significant correlation in controls indicates parasympathetic control of gastrointestinal motor function. None of the other studies in children have assessed the association between autonomic function and gastric motility, and therefore, we could not make a clear comparison.
The ANS is a physiological stress system. It is involved in adapting to various stimuli. Available literature has shown that autonomic activity may present as being normal[38], hypo-functioning[42] or hyper-active[43] in functional abdominal pain. Dysfunction of the ANS can cause significant gastrointestinal problems[44]. At the central level, there is a strong connection between autonomic activation and nociception, which is supported by the anatomical and functional overlap of pain processing structures and autonomic regulating structures[45]. The interaction between pain and autonomic response becomes maladaptive in chronic pain[46]. In some chronic pain states, sympathetic hyperactivity contributes to increased sensitivity to pain[47]. In contrast, the pain can result in reduced parasympathetic activity[48]. Association of pain and low parasympathetic flow has been reported in women with IBS[49].
In the current study, pain duration negatively correlated with the 30:15 ratio (parasympathetic), which can be interpreted as increased pain duration when parasympathetic activity is reduced. The 30:15 ratio is a sensitive index to detect autonomic abnormalities in children[50]. Therefore, we suggest that parasympathetic division may adapt to the initial phase of the disease, as shown in Figure 1. However, a decrease in the parameters after 12 mo of disease may be a feature of mal-adapting autonomic flow. Furthermore, progressive autonomic dysfunction over time has been demonstrated in adults with IBS[51]. Therefore, the degree of parasympathetic functional impairment may present as a spectrum extending from normal to severe impairment. In this context, we may not see a similar response in every FGID patient.
The three cardinal findings in this study, such as lack of difference in extra- intestinal autonomic symptoms between AP-FGIDs and controls, lack of differences in autonomic functions between the two groups and lack of correlation between gastric motility and autonomic parameters in those with FGIDs, suggest that the ANS does not play a major role in the pathogenesis of AP-FGIDs in children. In this context, abnormalities in parasympathetic flow are unlikely to be the primary cause for impaired motility in AP-FGIDs. Therefore, the stomach’s unresponsiveness to extrinsic autonomic signals (functional extrinsic denervation) would be a possible underlying primary pathophysiological mechanism for gastric motility abnormalities seen in AP-FGIDs.
Based on these observations, we have developed a hypothetical model to explain the possible mechanism of pathogenesis of AP-FGIDs, which we term ‘’automatic stomach in AP-FGIDs’’. According to the proposed model, functional extrinsic denervation is able to impair motility by three mechanisms (Figure 1). Both impaired motility and maladapted parasympathetic flow have an impact on pain. Possible functional extrinsic denervation demonstrated in the current study would affect gastric motility by increasing dopaminergic inhibition on the stomach, possibly via DAR2 receptors, leading to an “automated stomach” that does not directly respond to the outflow of the autonomic nervous system (Figure 1). Additionally, we have incorporated the modulatory effects of peripheral dopamine receptors on the central dopaminergic system as another possible effect of functional extrinsic denervation[52].
There are several strengths of this study. We have employed well established, non -invasive techniques to assess cardiovascular autonomic functions and gastric motility. The two investigators who assessed gastric motility and autonomic functions were blinded to the diagnosis of the study subjects. In addition, the impact of diurnal variation on motility was minimized by conducting the study from 8:30 am to 10:30 am. The large sample size (100 AP-FGIDs and 50 controls) and detailed evaluation of patients (using history, examination and investigations) to exclude possible underlying organic disorders were the other strengths of the study. Therefore, we believe that our results can be applied to the whole population of children with AP-FGIDs. However, we did not separate children with AP-FGIDs into specific disease entities such as IBS, FD and FAP. In addition, we used cardiovascular autonomic functions to assess the autonomic functions of the gastrointestinal tract, which is only a proxy measure at best.
In conclusion, children with AP-FGIDs showed abnormal gastric motility parameters, while their cardiovascular autonomic functions were normal. There was no correlation between gastric motility parameters and autonomic functions, indicating that abnormalities in the autonomic nervous system do not play a major role in the pathogenesis of AP-FGIDs. However, we believe maladaptive parasympathetic flow and the proposed automated stomach model can shed some light upon the pathophysiology of AP-FGIDs in children.
Abdominal pain-predominant functional gastrointestinal disorders (AP-FGIDs) are a common clinical problem in paediatric practice across the globe, with an estimated prevalence of 13.5%. Although thought to be benign in nature, as a group they are known to associate with poor health-related quality of life and high healthcare burden.
The pathophysiology of AP-FGIDs is not clearly understood. Previous studies have shown abnormalities in gastroduodenal motility, such as delayed gastric emptying, impaired antral motility, and impaired gastric accommodation as potential pathophysiological mechanisms in children. Studies among adults have found autonomic dysfunction in patients with IBS. However, the association between autonomic dysfunction and gastric motility in children with AP-FGIDs had not been previously evaluated.
The main objective of our study was to assess the autonomic functions in children with AP-FGIDs and their relationship to gastric motor functions.
One hundred children fulfilling Rome III criteria for AP-FGIDs and 50 healthy controls aged 5 to 12 years were recruited for the study. All patients were thoroughly investigated to rule out underlying organic disorders. Gastric motility and cardiovascular autonomic functions were assessed using validated, non-invasive techniques.
Gastric emptying rate, amplitude of antral contractions, and antral motility index were significantly lower in children with AP-FGIDs. Autonomic functions, including blood pressure and heart rate responses to standing from lying down position, heart rate response to deep breathing, and Valsalva test, showed no difference between children with AP-FGIDs and controls. These parameters did not show any correlation with gastric motor functions. However, the duration of pain episodes negatively correlated with the parasympathetic tone.
Although children with AP-FGIDs have abnormal gastric motility parameters, their cardiovascular autonomic functions are normal. In addition, there is no correlation between autonomic functions and gastric motility. Our findings indicate that the autonomic nervous system is not chronically abnormal in patients with AP-FGIDs. Based on currently available evidence, we propose maladaptive parasympathetic flow and an automated stomach model as a potential pathophysiological mechanism for AP-FGIDs.
We would like to acknowledge Mrs. Liyanayage JCD and Mrs. Ariyawansa J, Technical Officers, Department of Physiology, Faculty of Medicine, University of Kelaniya, Sri Lanka, for their assistance in gastric motility and autonomic function tests. We also acknowledge Dr. Perera BJC, Joint Editor of Sri Lanka Journal of Child Health for editing the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sri Lanka
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kehagias I, Zhang Z S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Korterink JJ, Diederen K, Benninga MA, Tabbers MM. Epidemiology of pediatric functional abdominal pain disorders: a meta-analysis. PLoS One. 2015;10:e0126982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 309] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 2. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 1077] [Article Influence: 56.7] [Reference Citation Analysis (6)] |
| 3. | Devanarayana NM, Rajindrajith S, Benninga MA. Quality of life and health care consultation in 13 to 18 year olds with abdominal pain predominant functional gastrointestinal diseases. BMC Gastroenterol. 2014;14:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Park R, Mikami S, LeClair J, Bollom A, Lembo C, Sethi S, Lembo A, Jones M, Cheng V, Friedlander E, Nurko S. Inpatient burden of childhood functional GI disorders in the USA: an analysis of national trends in the USA from 1997 to 2009. Neurogastroenterol Motil. 2015;27:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Korterink J, Devanarayana NM, Rajindrajith S, Vlieger A, Benninga MA. Childhood functional abdominal pain: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2015;12:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Devanarayana NM, de Silva DG, de Silva HJ. Gastric myoelectrical and motor abnormalities in children and adolescents with functional recurrent abdominal pain. J Gastroenterol Hepatol. 2008;23:1672-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Hausken T, Li XN, Goldman B, Leotta D, Ødegaard S, Martin RW. Quantification of gastric emptying and duodenogastric reflux stroke volumes using three-dimensional guided digital color Doppler imaging. Eur J Ultrasound. 2001;13:205-213. [PubMed] |
| 8. | Devanarayana NM, Rajindrajith S, Bandara C, Shashiprabha G, Benninga MA. Ultrasonographic assessment of liquid gastric emptying and antral motility according to the subtypes of irritable bowel syndrome in children. J Pediatr Gastroenterol Nutr. 2013;56:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Devanarayana NM, Rajindrajith S, Benninga MA. Abdominal migraine in children: association between gastric motility parameters and clinical characteristics. BMC Gastroenterol. 2016;16:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Devanarayana NM, Rajindrajith S, Perera MS, Nishanthanie SW, Benninga MA. Gastric emptying and antral motility parameters in children with functional dyspepsia: association with symptom severity. J Gastroenterol Hepatol. 2013;28:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Devanarayana NM, Rajindrajith S, Rathnamalala N, Samaraweera S, Benninga MA. Delayed gastric emptying rates and impaired antral motility in children fulfilling Rome III criteria for functional abdominal pain. Neurogastroenterol Motil. 2012;24:420-425, e207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Troncon LE, Bennett RJ, Ahluwalia NK, Thompson DG. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994;35:327-332. [PubMed] |
| 13. | Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346-1352. [PubMed] |
| 14. | Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, Marengo M, Corinaldesi R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036-1042. [PubMed] |
| 15. | Aggarwal A, Cutts TF, Abell TL, Cardoso S, Familoni B, Bremer J, Karas J. Predominant symptoms in irritable bowel syndrome correlate with specific autonomic nervous system abnormalities. Gastroenterology. 1994;106:945-950. [PubMed] |
| 16. | Chelimsky G, Boyle JT, Tusing L, Chelimsky TC. Autonomic abnormalities in children with functional abdominal pain: coincidence or etiology? J Pediatr Gastroenterol Nutr. 2001;33:47-53. [PubMed] |
| 17. | Heitkemper M, Jarrett M, Cain KC, Burr R, Levy RL, Feld A, Hertig V. Autonomic nervous system function in women with irritable bowel syndrome. Dig Dis Sci. 2001;46:1276-1284. [PubMed] |
| 18. | Elsenbruch S, Orr WC. Diarrhea- and constipation-predominant IBS patients differ in postprandial autonomic and cortisol responses. Am J Gastroenterol. 2001;96:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Azpiroz F. Hypersensitivity in functional gastrointestinal disorders. Gut. 2002;51 Suppl 1:i25-i28. [PubMed] |
| 20. | Cummins AG, Roberts-Thomson IC. Prevalence of celiac disease in the Asia-Pacific region. J Gastroenterol Hepatol. 2009;24:1347-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Kusunoki H, Haruma K, Hata J, Tani H, Okamoto E, Sumii K, Kajiyama G. Real-time ultrasonographic assessment of antroduodenal motility after ingestion of solid and liquid meals by patients with functional dyspepsia. J Gastroenterol Hepatol. 2000;15:1022-1027. [PubMed] |
| 22. | Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8:491-498. [PubMed] |
| 23. | Thumshirn M, Camilleri M. Evaluation of gastrointestinal autonomic function. In: Horowitz M, Samsom M, Gastrointestinal function in diabetes mellitus. London: John Wiley & Sons, 2004: 304-330. . |
| 24. | Bellavere F, Ewing DJ. Autonomic control of the immediate heart rate response to lying down. Clin Sci (Lond). 1982;62:57-64. [PubMed] |
| 25. | Clarke BF, Ewing DJ. Cardiovascular reflex tests; in the natural history of diabetic autonomic neuropathy. N Y State J Med. 1982;82:903-908. [PubMed] |
| 26. | Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’Brien PC, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52:523-528. [PubMed] |
| 27. | Devanarayana NM, Mettananda S, Liyanarachchi C, Nanayakkara N, Mendis N, Perera N, Rajindrajith S. Abdominal pain-predominant functional gastrointestinal diseases in children and adolescents: prevalence, symptomatology, and association with emotional stress. J Pediatr Gastroenterol Nutr. 2011;53:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Walker LS, Caplan A, Rasquin A. Rome III diagnostic questionnaire for the pediatric functional GI disorders. In: Drossman DA, Corazziari E, Delvaux M, Talley NJ, Thompson WG, Whitehead WE, Rome III: The Functional Gastrointestinal Disorders. McLean, VA: Degnon Associates, 2006: 961-990. . |
| 29. | Bedi M, Khullar S, Varshney VP. Assessment of autonomic function activity in obese children. Vascular Disease Prevention. 2009;6:139-141. [DOI] [Full Text] |
| 30. | Chelimsky G, Chelimsky TC. Autonomic nervous system testing. In: Faure C, Di Lorenzo C, Thapar N, Pediatric Neurogastroenterology. New York: Springer Science, 2013: 177-186. . |
| 31. | Tak LM, Riese H, de Bock GH, Manoharan A, Kok IC, Rosmalen JG. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol Psychol. 2009;82:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Jarrett M, Heitkemper M, Czyzewski D, Zeltzer L, Shulman RJ. Autonomic nervous system function in young children with functional abdominal pain or irritable bowel syndrome. J Pain. 2012;13:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Liu Q, Wang EM, Yan XJ, Chen SL. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: a meta-analysis. J Dig Dis. 2013;14:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Olafsdottir E, Ellertsen B, Berstad A, Fluge G. Personality profiles and heart rate variability (vagal tone) in children with recurrent abdominal pain. Acta Paediatr. 2001;90:632-637. [PubMed] |
| 35. | Feuerstein M, Barr RG, Francoeur TE, Houle M, Rafman S. Potential biobehavioral mechanisms of recurrent abdominal pain in children. Pain. 1982;13:287-298. [PubMed] |
| 36. | Gilja OH, Hausken T, Wilhelmsen I, Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996;41:689-696. [PubMed] |
| 37. | Hoffman I, Tack J. Assessment of gastric motor function in childhood functional dyspepsia and obesity. Neurogastroenterol Motil. 2012;24:108-112, e81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Olafsdottir E, Gilja OH, Aslaksen A, Berstad A, Fluge G. Impaired accommodation of the proximal stomach in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2000;30:157-163. [PubMed] |
| 39. | Riezzo G, Cucchiara S, Chiloiro M, Minella R, Guerra V, Giorgio I. Gastric emptying and myoelectrical activity in children with nonulcer dyspepsia. Effect of cisapride. Dig Dis Sci. 1995;40:1428-1434. [PubMed] |
| 40. | Duan LP, Zheng ZT, Li YN. A study of gastric emptying in non-ulcer dyspepsia using a new ultrasonographic method. Scand J Gastroenterol. 1993;28:355-360. [PubMed] |
| 41. | Kamino D, Manabe N, Hata J, Haruma K, Tanaka S, Chayama K. Long-term Ultrasonographic Follow-up Study of Gastric Motility in Patients with Functional Dyspepsia. J Clin Biochem Nutr. 2008;42:144-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Battistella PA, Carrà S, Zaninotto M, Ruffilli R, Da Dalt L. Pupillary reactivity in children with recurrent abdominal pain. Headache. 1992;32:105-107. [PubMed] |
| 43. | Jørgensen LS, Christiansen P, Raundahl U, Ostgaard S, Christensen NJ, Fenger M, Flachs H. Autonomic nervous system function in patients with functional abdominal pain. An experimental study. Scand J Gastroenterol. 1993;28:63-68. [PubMed] |
| 44. | McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33-44. [PubMed] |
| 45. | Jänig W. Relationship between pain and autonomic phenomena in headache and other pain conditions. Cephalalgia. 2003;23 Suppl 1:43-48. [PubMed] |
| 46. | Ge HY, Fernández-de-las-Peñas C, Arendt-Nielsen L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin Neurophysiol. 2006;117:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1236] [Cited by in RCA: 1431] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 48. | Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res Ther. 2007;9:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 49. | Burr RL, Heitkemper M, Jarrett M, Cain KC. Comparison of autonomic nervous system indices based on abdominal pain reports in women with irritable bowel syndrome. Biol Res Nurs. 2000;2:97-106. [PubMed] |
| 50. | Verrotti A, Chiarelli F, Blasetti A, Morgese G. Autonomic neuropathy in diabetic children. J Paediatrics Child Health. 1995;31:545-548. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Cheng P, Shih W, Alberto M, Presson AP, Licudine A, Mayer EA, Naliboff BD, Chang L. Autonomic response to a visceral stressor is dysregulated in irritable bowel syndrome and correlates with duration of disease. Neurogastroenterol Motil. 2013;25:e650-e659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Sudakov SK, Bashkatova VG. Effect of peripheral D2 dopamine receptor antagonist domperidone on metabolism, feeding behavior, and locomotor activity of rats. Bull Exp Biol Med. 2013;155:705-707. [PubMed] |