Published online Jan 7, 2019. doi: 10.3748/wjg.v25.i1.85
Peer-review started: May 27, 2018
First decision: July 4, 2018
Revised: August 22, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: January 7, 2019
Processing time: 225 Days and 22.3 Hours
To assess the efficiency of endoscopic trans-esophageal submucosal tunneling surgery (EESTS) technique for diseases located around the aorta ventralis.
Nine pigs were assigned to EESTs. The procedures were as follows: First, a long esophageal submucosal tunnel was established. Second, full-thickness myotomy was created. Third, an endoscope was entered into the abdominal cavity through a muscle incision and the endoscope was around the aorta ventralis. Eventually, celiac trunk ganglion neurolysis, partial hepatectomy and splenectomy, partial tissue resection in the area of the posterior peritoneum, and endoscopic submucosal dissection (ESD) combined with lymph node dissection were performed. The animals were given antibiotics for 5 d and necropsied 7 d after surgery.
In all surgeries, one pig died from intraperitoneal hemorrhage after doing partial splenectomy, while the other pigs were alive after successfully operating other surgeries. For surgery of celiac trunk ganglion damage, at necropsy, there was no exudation in the abdominal cavity. Regarding surgery of partial hepatectomy, the wound with part healing was observed in the left hepatic lobe, and no bleeding or obvious exudation was seen. In surgery of partial splenectomy, massive hemorrhage was observed on the splenic wound surface, and the metal clips could not stop bleeding. After surgery of retroperitoneal tissue resection, mild tissue adhesion was observed in the abdominal cavity of one animal, and another one suffered from severe infection. For surgery of ESD and lymph node dissection, a moderate tissue adhesion was observed.
EESTS is a feasible and safe technique for diseases located around the aorta ventralis.
Core tip: Endoscopic trans-esophageal submucosal tunneling surgery (EESTS) technique is a new branch of endoscopic tunneling technology for diagnosing and treating diseases located around the aorta ventralis. The objective of our study was to simulate surgeries in a porcine model and to assess the efficiency of this new strategy. The surgeries included celiac trunk ganglion neurolysis, partial hepatectomy and splenectomy, partial tissue resection in the area of the posterior peritoneum, and endoscopic submucosal dissection combined with lymph node dissection. And we confirmed that EESTS is a feasible and safe technique.
- Citation: Xiong Y, Chen QQ, Chai NL, Jiao SC, Ling Hu EQ. Endoscopic trans-esophageal submucosal tunneling surgery: A new therapeutic approach for diseases located around the aorta ventralis. World J Gastroenterol 2019; 25(1): 85-94
- URL: https://www.wjgnet.com/1007-9327/full/v25/i1/85.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i1.85
Nowadays, surgical procedures are shifting paradigms in minimally invasive surgery, including natural orifice transluminal endoscopic surgery (NOTES)[1]. NOTES is a technology of utilizing a flexible endoscope through a natural orifice (e.g., the mouth, esophagus, stomach, rectum, vagina and urethra) to diagnose diseases and perform surgeries[2]. The emergence of endoscopic tunnel technique makes the diseases which used to need surgical or laparoscopic surgical treatments enter into the endoscopic therapy[3]. There is a significant question in scholars’ mind why endoscopic tunnel technique has such an appropriate curative effect and has some advantages (e.g., fewer complications). To answer this question, we can explain that the submucosal tunneling technique could well prevent the communication between the intra-luminal and the extra-luminal space by sealing the entry incision of the submucosal tunnel, and that gas or fluid within the lumen is prevented from entering the extra-luminal space, which could ensure the endoscopic therapy free of perforation[4,5]. In 2000, Kalloo et al[6] reported the first NOTES. They performed liver biopsy and examined the peritoneal cavity by using upper gastrointestinal endoscopy in a live swine model. In addition, they successfully confirmed the feasibility and safety of NOTES. In 2006, Rao et al[7] made the first attempt of transgastric NOTES in human. Subsequently, a number of scholars used NOTES to simulate a variety of intraperitoneal surgeries including cholecystectomy, colectomy, liver resection, and thyroidectomy in experimental animals[8-11]. A significant and hybrid model of NOTES was proposed for abdominal surgeries using transcutaneous rigid laparoscopes in combination with a flexible endoscope passing through a visceral incision[12,13]. Moreover, it was confirmed that NOTES had been less invasive, safer, and more cost-effective than either traditional or laparoscopic surgery[14].
However, there are several technical challenges in the development of NOTES[14-16]. First, it is difficult to close the entrance of natural orifices, which lacks an economic and precise suture technique. Second, the infection caused by liquid and air from natural orifices to the body cavity would unavoidably occur. Third, it would be lost in body cavity due to the insufficient localization and navigation. Forth, a flexible endoscope could not create a stable operative platform in order to perform a very precise resection of tissues. Eventually, the loss of triangulation is a stubborn problem in either pure NOTES or laparoscopy. These challenges may puzzle endoscopists.
In this study, we attempted to put forward a new approach using endoscopic tunneling techniques to perform NOTES, which was named endoscopic trans-esophageal submucosal tunneling surgery (EESTS). Additionally, some preliminary studies were performed in a porcine model. The proposed technique can resolve the problem of closing entrance and infection. The aim of this study was to perform some endoscopic surgeries in animals’ body cavity, including celiac trunk ganglion damage, partial hepatectomy, partial splenectomy, resection of the retroperitoneal organs, and endoscopic submucosal dissection (ESD) and lymph node dissection.
Experiments were performed with the contribution of five males and four female porcine corpses at animal laboratory of Pinggu District Hospital (Beijing, China), and approved by the Ethics Committee of the Animal Facility of Chinese PLA General Hospital. All these animals were treated humanely and underwent a 3 d quarantine and acclimation period.
All procedures were performed under general anesthesia, using the combination of chloramine hydrochloride (5 mg/kg) and Su Mian Xin II (0.1 mL/kg), as well as endotracheal intubation. All animals received antibiotic prophylaxis with a single dose of intravenous penicillin (3.2 million units) at 24 h before operating surgery. The pigs were in supine and raised right shoulder positions. Furthermore, the basal body temperature was measured before surgery. All endoscopic instruments were sterile whereas EESTS equipment underwent a high-level disinfection with 25% glutaraldehyde solution (Instrunet, Laboratorios Inibsa, Spain).
The procedures were performed by an experienced endoscopist. A gastroscope (GIF-Q260J, Olympus Medical Systems, Corp., Tokyo, Japan) was inserted through the pig’s mouth which was secured with a self-made overtube. The procedure was maintained with CO2 insufflation through the endoscope. The vital signs of pigs were closely monitored during the operation.
A submucosal tunnel was made as follows: (1) the esophagus and stomach cavity were rinsed repeatedly with saline, and this was followed by injecting 100 mL of metronidazole and retaining the fluid for 15 min before removing it; (2) the site of interest was the right posterior wall of the cardia and located by injecting 0.5 mL of methylene blue (1:2); (3) 10 mL of saline with methylene blue (1:10000) was injected to create a submucosal cushion in the right posterior wall at 5 cm above the esophagogastric junction; (4) inverted T-shaped incision was created in the mucosal layer of the esophagus with an electric knife (KD-V451M, Olympus Medical Systems, Corp., Tokyo, Japan) and an electrosurgical unit (PSD-30, Olympus Medical Systems, Corp., Tokyo, Japan); (5) the endoscope was introduced into the submucosal space and gently separated the submucosa from the muscularis propria, creating a submucosal tunnel. The dark blue localization spot in the cardia was then identified; (6) at the end of the tunnel, a progressive full-thickness incision was created through the muscularis propria with the needle-knife and the endoscope was entered into the abdominal cavity. Once the scope was moved into the lesser omental sac, blunt dissection with an electric knife was used to advance the scope until the aorta ventralis was identified; (7) an artificial pneumoperitoneum was created and a 20 mL needle was used to puncture exhaust to maintain relatively stable intra-abdominal pressure; and (8) after simulating the abdominal operation, the submucosal space was closed by applying clips at the mucosal entry site.
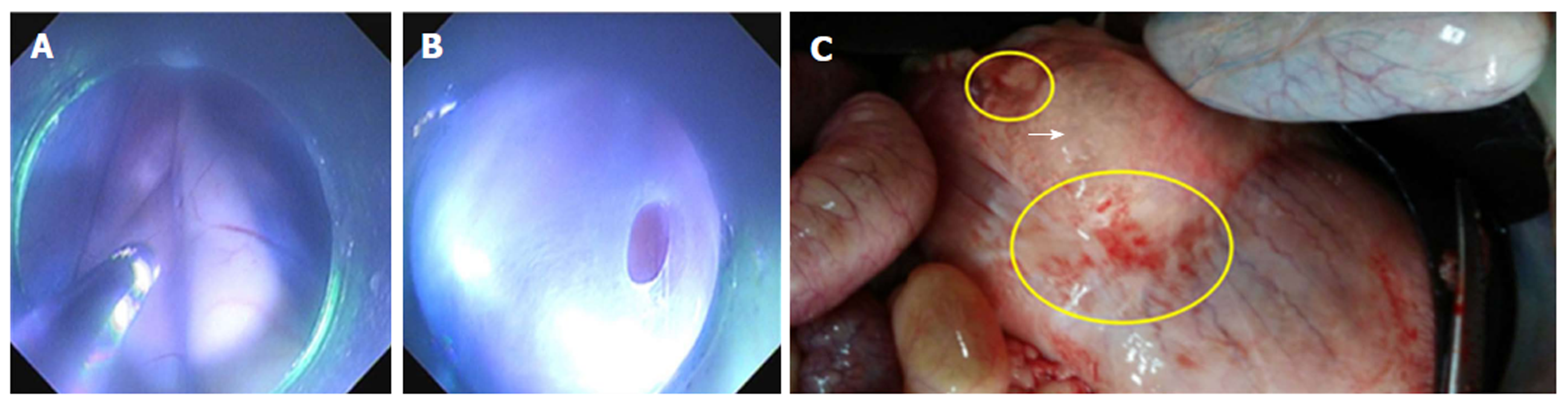
Surgery of celiac trunk ganglion damage: At the end of the esophageal tunnel, the endoscope was inserted into the abdominal cavity from the posterior wall of the gastric fundus near the cardia. The aorta ventralis and its branches were observed in the endoscopic direct status. The abdominal ganglions were invisible in healthy pigs. Thus, the surgery was simulated by partially removing tissues in the angle formed between the aorta ventralis and the coeliac trunk using thermal biopsy forceps and an electric knife (Figure 1A and B)
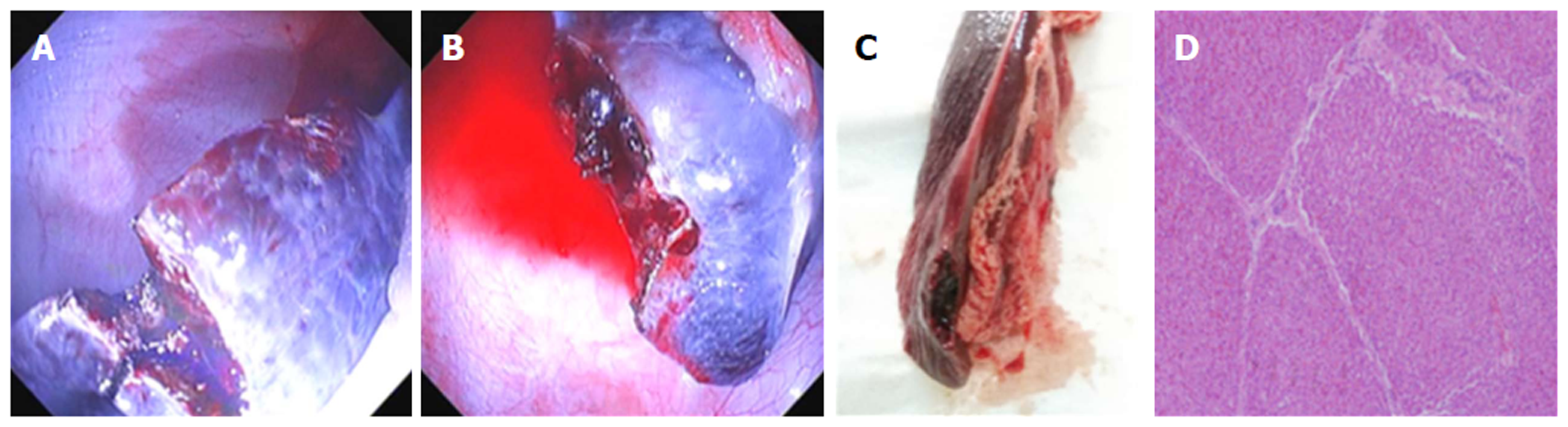
Surgery of partial hepatectomy: The endoscope was inserted into the abdominal cavity. The left hepatic lobe was observed by twisting the body of the endoscope to the right (clockwise) or left (counter-clockwise) direction. Wedge resection of the liver tissues was carried out with an electric knife using a hybrid cutting mode (electro-coagulation was higher than that for cutting). Specimens were collected and stored for diagnostic pathology investigation. The wound was treated with conventional maneuvers (coagulation forceps, Olympus Medical Systems, Corp., Tokyo, Japan) as displayed in Figure 2A and B)
Surgery of partial splenectomy: The endoscope was inserted into the area around the aorta ventralis. It was attempted to find the left hepatic lobe and then, explore the spleen by upward turning the endoscope. Pig’s spleen is in banded shape and the spleen tail was relatively unfixed and in free status in the abdominal cavity. Wedge resection of the spleen tissues was done with an electric knife using a hybrid cutting mode. Specimens were collected and stored for diagnostic pathology investigation. The wound was treated with conventional maneuvers (coagulation forceps), as shown in Figure 3A and B.
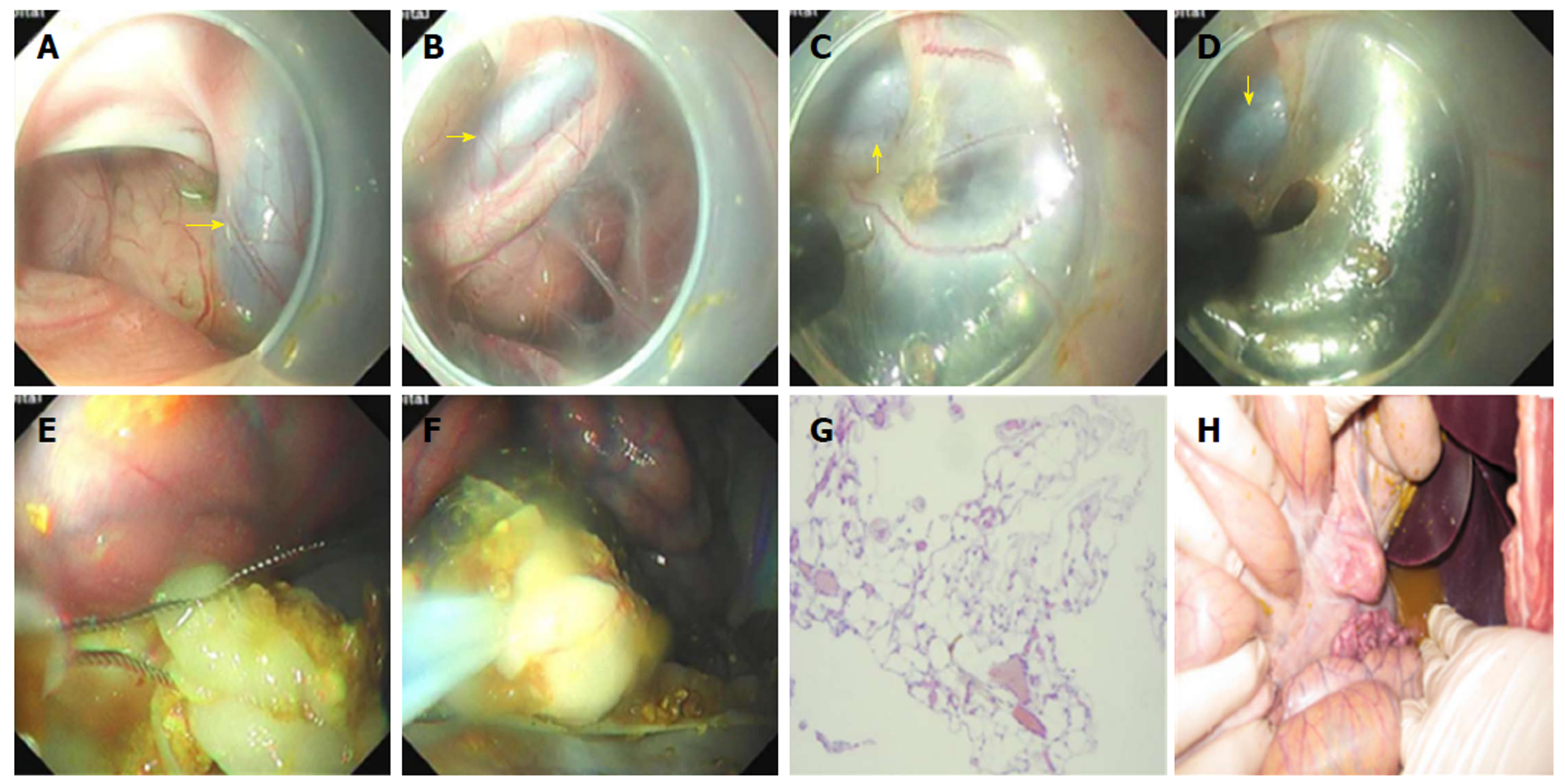
Surgery of retroperitoneal tissue resection: The endoscope was inserted into the area around the aorta ventralis. The retroperitoneum was observed in the endoscopic direct status and the aorta ventralis was the predetermined structure for identification (Figure 4A and B). Tissues in the retroperitoneum were partially removed using an electric knife, avoiding thick blood vessels (Figure 4C and D). This aimed to simulate surgical removal of the retroperitoneal tumors. The omentum majus was observed when the endoscope was reversed continually. Afterwards, some tissues of the omentum majus were removed using endoloops (Olympus Medical Systems, Corp., Tokyo, Japan) (Figure 4E and F).
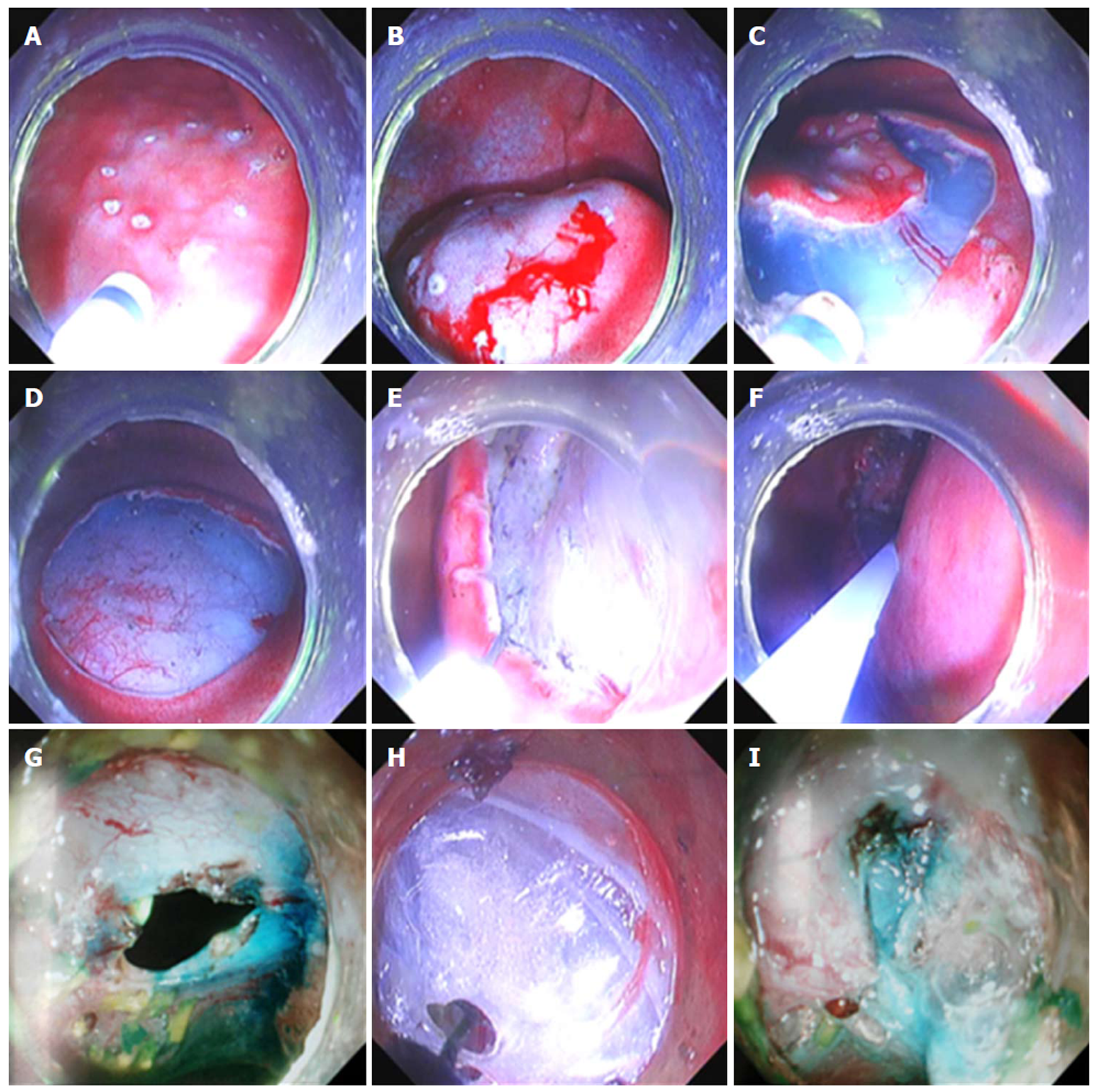
Surgery of ESD and lymph node dissection: The endoscope was inserted into the area around the aorta ventralis. In the endoscopic direct status, the gastric fundus and the posterior wall of the gastric body were observed. Blunt dissection of omental tissue outside the stomach wall was undertaken with an electric knife. The endoscope exited from the esophageal mucosal tunnel and entered into the gastric cavity. The simulation of ESD for early gastric carcinoma in posterior gastric body wall was undertaken. After completing submucosal dissection, 0.5 mL of methylene blue (1:2) and saline mixture was used as a marker and injected to the intrinsic muscle layer surrounding the wound. The endoscope was inserted into the posterior wall of the gastric fundus near the cardia in the abdominal cavity through the end of the esophageal tunnel. Methylene blue marker was identified and lymph nodes outside the gastric wall were then dissected. A full-thickness incision was created in the posterior part of the gastric body and the opening was enlarged to the extent that the metal clip could not close it. Another endoscope was inserted into the abdominal cavity through this esophageal tunnel. Lateral wall of the perforation was pulled by the tongs from outside the gastric wall for alignment and then, this perforation was closed by that endoscope inside the gastric cavity using metal clips (Olympus Medical Systems, Corp., Tokyo, Japan).
Postoperative care and necropsy: All animals were fasted within 24 h after doing surgery. They were orally administered with glucose (1000 g/d) for 2 d and then, were given regular meals. All animals received intravenous penicillin (3.2-million units/d) after surgery and were monitored for signs of abdominal infection and sepsis during the next 6 d. Necropsy was made for the evidence of ascites and signs of infection, injuries in other organs and tissue adhesion in surgery position. The surgical specimens were stained with hematoxylin and eosin for pathological diagnosis.
In this study, nine animals weighing 28.5 ± 5.2 kg were examined. In addition, eight animals, in which the surgery was completed, survived until they were euthanized on the 7th day.
Two pigs survived well after finishing the operation without complications such as delayed air leak, severe infection and injuries of other organs. But both had the complication of mild tissue adhesion. The procedure time was 32 min and 28 min, respectively, and the incision size was 5 mm for both. At necropsy, there was no exudation in the abdominal cavity. In addition, the lesser sac in the posterior gastric wall was adhered to adjacent tissue (Figure 1C). The postoperative pathology showed that one was in part healing and one was in minimal inflammatory reaction.
Two pigs survived well after doing the operation without the complications of delayed air leak and injuries of other organs. But one had the complications of severe infection and medium tissue adhesion. One did not have the complication of severe infection, but had the complication of tissue adhesion. The procedure time was 47 min and 39 min, and the incision size was 10 mm and 15 mm, respectively. At necropsy, the wound with part healing was observed in the left hepatic lobe, and no bleeding or obvious exudation was seen in the abdominal cavity (Figure 2C). Furthermore, the lesser sac in the posterior gastric wall was adhered to adjacent tissue. The liver tissue, which was excised by an electric knife, was further confirmed by a pathological examination as illustrated in Figure 2D. The postoperative pathology showed that both were in part healing.
One pig was dead after completing the operation without the complications of delayed air leak and injuries of other organs. But this pig had the complications of severe infection and severe tissue adhesion. The procedure time was 92 min. The incision size was 15 mm. During the procedure, partial splenectomy was made in the spleen tail and massive hemorrhage was observed on the wound surface. The wound was repeatedly rinsed with norepinephrine saline (1:20000) and was treated with conventional maneuvers (coagulation forceps) without a proper effect. Then, metal clips were applied to clamp the splenic vessels, and bleeding significantly decreased. The splenic vessels could not be fully clamped, and limited by the small size of metal clips. Consequently, a small amount of bleeding was observed in the splenic wound (Figure 3A and B). After rapid recovery from the anesthesia in 3 h after doing the surgery, the animal’s state was extremely poor and it immediately received antibiotics fluid transfusion. On the second day, the pig died. At necropsy, massive bleeding was seen in the abdominal cavity and diffuse blood oozing was observed in the splenic wound, as displayed in Figure 3C. The spleen tissue, which was excised by an electric knife, was further confirmed by a pathological examination (Figure 3D). The postoperative pathology showed that one pig was in intraperitoneal hemorrhage.
Two pigs survived well after completing the operation without the complications of delayed air leak and injuries of other organs. But one had the complications of severe infection and moderate tissue adhesion. One did not have the complication of severe infection, but had the complication of mild tissue adhesion. The procedure time was 42 min and 51 min, and the incision sizes was 15 mm and 20 mm, respectively. At necropsy, mild tissue adhesion was observed between the posterior gastric wall and retroperitoneal space in one of the pigs. Besides, another one suffered from severe infection. Because of amounts of chyme in the stomach and longer operative time, chyme refluxed into the abdominal cavity through the entrance of the esophageal tunnel. During this procedure, plenty of saline was used to flush the abdominal cavity without an appropriate effect. The pig had a fever up to 43.2 °C. At necropsy, a severe tissue adhesion was observed between the posterior gastric wall and retroperitoneal space. The omentum majus tissue, which was excised with an electric knife, was further confirmed by a pathological examination (Figure 4D). The postoperative pathology showed that one was in minimal inflammatory reaction and one is in complete healing.
Two pigs were employed to simulate the ESD of early gastric carcinoma, and dissection of lymph nodes outside the gastric wall survived well (Figure 5). All animals survived well after doing the operation without the complications of delayed air leak and injuries of other organs. But both had the complications of severe infection and medium tissue adhesion. The procedure time was 57 min and 66 min, and the incision size of ESD was 25 mm and 20 mm, respectively. The incision size of lymph node dissection were both 5 mm. At necropsy, a moderate tissue adhesion was observed in the abdominal cavity.
It is noteworthy that the area around the aorta ventralis is difficult to reach in laparoscopic surgery, which makes doing the operation to be difficult. However, the endoscope could easily approach to this area, which makes it simple and convenient.
In a previous study performed by authors, it confirmed some surgical techniques as follows: (1) Supine and raised right shoulder position was chosen as surgical positioning; (2) Methylene blue was injected to preoperative cardiac submucosa in the posterior wall as a marker of piercing site; and (3) An esophageal submucosal tunnel was established to the cardia, a progressive full-thickness incision through the muscularis propria was created, and an endoscope was inserted into the abdominal cavity. In this study, we inserted the endoscope into the area around the aorta ventralis to simulate intraperitoneal surgeries, including celiac trunk ganglion damage, partial hepatectomy, partial splenectomy, resection of regional tissues in the retroperitoneum, and ESD and lymph node dissection.
In the surgery of celiac trunk ganglion damage, it was revealed that the aorta ventralis could be identified as the predetermined structure in retroperitoneal space when the endoscope was in the direct status. The abdominal ganglions were scarcely found in healthy pigs. Thus, a part of tissue around the aorta ventralis was removed using thermal biopsy forceps and an electric knife. All animals received accurate postoperative care and survived after doing surgery as well; consequently, the surgery was successful. However, finding and recognizing ganglions around the aorta ventralis was difficult. Thus, it necessitates that endoscopists should increase their knowledge about the anatomy of the abdomen and predetermined structure should be confirmed as well.
In the surgery of partial hepatectomy, the liver tissues were dissected with an electric knife using the hybrid cutting mode. No obvious bleeding occurred during the operation and all animals survived well after finishing surgery. The liver tissue was confirmed by a pathological examination. A massive liver resection could not be operated because of the restriction to the suitable endoscopic instruments and severe hemorrhage complications of large vessel injuries. Thus, further studies should concentrate on the development of endoscopic instruments and techniques.
In the surgery of partial splenectomy, the surgery was performed in the spleen tail and massive hemorrhage was found on the wound surface, while massive bleeding was seen in the abdominal cavity, and diffuse blood oozing was observed in the splenic wound. Several conventional maneuvers (coagulation forceps) were applied to stop bleeding, however, that failed. Then, metal clips were used to clamp the splenic vessels, while those metal clips could not fully block the splenic blood supply, which was limited to a specification for metal clips (5 mm of arm length and 135° of opening angle). A pig died on the second day after doing the operation. This can be justified as follows: (1) Because of the features of the spleen with fragile tissue and rich blood supply; and (2) A traumatic spleen rupture requires emergency total splenectomy by surgeons. Moreover, it is necessary to suture the splenic artery and vein in order to stop bleeding during the operation. Hence, this surgery could not be properly undertaken by endoscopic technique.
In the surgery of retroperitoneal tissue resection, some tissues were removed in the retroperitoneal space. No obvious complications occurred during the operation, and all animals survived after completing the surgery. However, one of the pigs suffered from severe infection, while that survived. Besides, omentum majus tissue was confirmed by a pathological examination.
Due to the development of endoscopic technology and the improvement of cancer screening systems, the diagnostic rate of early gastric cancer (EGC) has been remarkably increased. This has led to an increase of survival rate of gastric cancer. Recently, ESD has been preferred in order to treat EGC with strict indications within the superficial submucosa (SM1). In addition, the expanded indications within SM1 and the submucosa (SM2), particularly accompanied with lymph node metastasis (LNM), have been a subject of debate among clinicians[17]. EGC invasion into the deep submucosa (SM3) is an absolute indication of surgery[17]. Additionally, patients with EGC and LNM should receive lymph node dissection. However, subtotal gastrectomy is difficult to be accepted by the patients because of greater trauma and poor quality of life after operation. Conversely, endoscopic physicians could not perform lymphadenectomy. Thus, a hybrid NOTES technique enables minimal surgical resection and would be a new alternative in EGC treatment. Hybrid NOTES utilizes the ESD technique for local excision of early carcinoma, which is simultaneously combined with a laparoscopic lymphadenectomy in case of EGC with a risk of LNM[13,17]. Satisfactory results were reported in some studies[13,18]. Although laparoscopic surgery is a minimally invasive treatment, a laparoscope is inserted into the abdominal cavity by cutting the abdominal wall.
In the surgery of ESD and lymph node dissection, two endoscopes were utilized instead of a laparoscope to clear the lymph nodes around the stomach. Moreover, it was confirmed that this surgery is feasible. A new and simple method of perforation closure with two endoscopes was also proposed in the experiment. One endoscope with tongs was used to pull the lateral wall of the perforation from outside the gastric wall for alignment, and another endoscope with metal clips was employed to close this perforation.
However, the indication of EESTS seems to be limited at the present time because it contains several limitations. First, tunnel and penetration site could not be created in the same place because of scar healing. Second, various degrees of tissue adhesion and abdominal infection were inevitable regardless of the application of antibiotics before and after operation. Third, a flexible endoscope easily gets lost in the abdominal cavity. Sufficient localization and navigation of endoscope would be key points. Forth, instruments, which are specially designed for NOTES treatments in the abdominal cavity, have not been developed. Furthermore, in this study, an electric knife was used for ESD, thermal biopsy forceps and snare were utilized as well, which limited the operations. Therefore, only a small amount of tissues could be removed. Moreover, hemostasis was difficult, and the abdominal vessels could not be fully clipped.
In conclusion, EESTS by the submucosal tunneling technique to simulate surgeries around the aorta ventralis is feasible, efficient, and relatively safe in a porcine model. However, the safety of profile has to be improved before adopting in a clinical setting. And developing new endoscopic instruments suitable for the technique will be one of the future directions.
Surgical procedures are shifting paradigms in minimally invasive surgery nowadays, including natural orifice transluminal endoscopic surgery (NOTES), which is a technology of utilizing a flexible endoscope through a natural orifice to diagnose diseases and perform surgeries. The emergence of endoscopic tunnel technique makes the diseases which used to need surgical or laparoscopic surgical treatments enter into the endoscopic therapy.
We attempted to put forward a new approach using endoscopic tunneling techniques to perform NOTES, which was named endoscopic trans-esophageal submucosal tunneling surgery (EESTS).
To assess the efficiency of endoscopic trans-esophageal submucosal tunneling surgery technique for diseases located around the aorta ventralis.
We simulated surgeries in a porcine model and to assess the efficiency of this new strategy.
One pig died from intraperitoneal hemorrhage after doing partial splenectomy, while the other pigs were alive after successfully operating all surgeries.
We confirmed that EESTS is feasible and safe.
EESTS by the submucosal tunneling technique to simulate surgeries for diseases located around the aorta ventralis is feasible, efficient, and relatively safe in a porcine model at least. Developing new endoscopic instruments suitable for the technique will be one of the future directions. However, the safety of profile has to be improved before adopting in a clinical setting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Konishi H, Lo GH, Sun SY, Tantau A S- Editor: Wang XJ L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Lee GC, Sylla P. Shifting Paradigms in Minimally Invasive Surgery: Applications of Transanal Natural Orifice Transluminal Endoscopic Surgery in Colorectal Surgery. Clin Colon Rectal Surg. 2015;28:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Bazzi WM, Raheem OA, Cohen SA, Derweesh IH. Natural orifice transluminal endoscopic surgery in urology: Review of the world literature. Urol Ann. 2012;4:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Bingener J, Ibrahim-zada I. Natural orifice transluminal endoscopic surgery for intra-abdominal emergency conditions. Br J Surg. 2014;101:e80-e89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Ryou M, Thompson CC. Transmural gastric closure and NOTES--how far have we come? Endoscopy. 2009;41:558-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Córdova H, Cubas G, Boada M, Rodríguez de Miguel C, Martínez-Pallí G, Gimferrer JM, Fernández-Esparrach G. Adverse events of NOTES mediastinoscopy compared to conventional video-assisted mediastinoscopy: a randomized survival study in a porcine model. Endosc Int Open. 2015;3:E571-E576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Giday SA, Magno P, Kalloo AN. NOTES: the future. Gastrointest Endosc Clin N Am. 2008;18:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Rao G, Reddy N. Transgastric appendectomy in humans. Oral presentation at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) conference; 26-29 April 2006. Dallas, Tex, USA;. 2006;. |
| 8. | Oliveira ALA, Zorron R, Oliveira FMM, Santos MBD, Scheffer JP, Rios M, Antunes F. Transcolonic Perirectal NOTES Access (PNA): A feasibility study with survival in swine model. An Acad Bras Cienc. 2017;89:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Khereba M, Thiffault V, Goudie E, Tahiri M, Hadjeres R, Razmpoosh M, Ferraro P, Liberman M. Transtracheal thoracic natural orifice transluminal endoscopic surgery (NOTES) in a swine model. Surg Endosc. 2016;30:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Zhu HY, Li F, Li KW, Zhang XW, Wang J, Ji F. Transumbilical endoscopic cholecystectomy in a porcine model. Acta Cir Bras. 2013;28:762-766. [PubMed] |
| 11. | Katagiri T, Otsuka Y, Horgan S, Sandler BJ, Jacobsen GR, Coker AM, Tsuchiya M, Maeda T, Kaneko H. Feasibility and Technique for Transvaginal Natural Orifice Transluminal Endoscopic Surgery Liver Resection: A Porcine Model. Surg Laparosc Endosc Percutan Tech. 2017;27:e6-e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Moloney JM, Gan PS. Hybrid Transvaginal NOTES and Mini-Laparoscopic Colectomy: Benefit Through Synergy. JSLS. 2016;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Park YS, Kim SH, Ryu HY, Cho YK, Jo YJ, Son TI, Hong YO. Hybrid Natural Orifice Transluminal Endoscopic Surgery with Sentinel Lymph Node Navigation for Deep Early Gastric Cancer in the Fundic Region. Clin Endosc. 2016;49:298-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Zuo S, Wang S. Current and emerging robotic assisted intervention for Notes. Expert Rev Med Devices. 2016;13:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/SAGES Working Group on Natural Orifice Translumenal Endoscopic Surgery. October 2005. Surg Endosc. 2006;20:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Saxena P, Khashab MA. New NOTES Clinical Training and Program Development. Gastrointest Endosc Clin N Am. 2016;26:385-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Li H, Lu P, Lu Y, Liu CG, Xu HM, Wang SB, Chen JQ. Predictive factors for lymph node metastasis in poorly differentiated early gastric cancer and their impact on the surgical strategy. World J Gastroenterol. 2008;14:4222-4226. [PubMed] |
| 18. | Cho WY, Kim YJ, Cho JY, Bok GH, Jin SY, Lee TH, Kim HG, Kim JO, Lee JS. Hybrid natural orifice transluminal endoscopic surgery: endoscopic full-thickness resection of early gastric cancer and laparoscopic regional lymph node dissection--14 human cases. Endoscopy. 2011;43:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |