Published online Oct 14, 2018. doi: 10.3748/wjg.v24.i38.4412
Peer-review started: May 21, 2018
First decision: June 21, 2018
Revised: August 2, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: October 14, 2018
Processing time: 145 Days and 20 Hours
Gastric polyposis is a rare disease. Not all polyps progress to cancer. Monoallelic mutation in Fanconi anemia (FA) genes, unlike biallelic gene mutations that causes typical FA phenotype, can increase risks of cancers in a sporadic manner. Aberrations in the FA pathway were reported in all molecular subtypes of gastric cancer. We studied a patient with synchronous gastric cancer from gastric polyposis by conducting a 13-year long-term follow up. Via pathway-driven massive parallel genomic sequencing, a germline mutation at FANCA D1359Y was identified. We identified several recurrent mutations in DNA methylation (TET1, V873I), the β-catenin pathway (CTNNB1, S45F) and RHO signaling pathway (PLEKHG5, R203C) by comparing the genetic events between benign and malignant gastric polyps. Furthermore, we revealed gastric polyposis susceptible genes and genetic events promoting malignant transformation using pathway-driven targeted gene sequencing.
Core tip: The genetic events that predispose benign polyps to carcinoma are rarely explored in gastric cancer. We studied a rare case that progressed from benign gastric polyposis to gastric cancer and disclosed a monoallelic germline mutation (D1359Y) at Fanconi’s anemia gene, which may contribute to the phenotype. Comparing the genetic events between benign and malignant gastric polyps, several recurrent mutations in DNA methylation (TET1, V873I), the β-catenin pathway (CTNNB1, S45F) and RHO signaling pathway (PLEKHG5, R203C) were identified.
- Citation: Huang JP, Lin J, Tzen CY, Huang WY, Tsai CC, Chen CJ, Lu YJ, Chou KF, Su YW. FANCA D1359Y mutation in a patient with gastric polyposis and cancer susceptibility: A case report and review of literature. World J Gastroenterol 2018; 24(38): 4412-4418
- URL: https://www.wjgnet.com/1007-9327/full/v24/i38/4412.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i38.4412
Gastric cancer is one of the most common cancers worldwide. Most gastric cancers are associated with infectious agents such as Helicobacter pylori (H. pylori) and Epstein-Barr virus (EBV) and a minority (1%-3%) of gastric cancer cases are associated with germline mutations in E-cadherin (CDH1) or mismatch repair genes[1]. H. pylori infection and certain genetic conditions such as adenomatous polyposis coli (APC) gene mutation are also associated with benign gastric polyps either in sporadic or inherited form[2,3]. Although benign gastric polyps result from the accumulation of multiple genetic and epigenetic alterations, only a minority of cases progress to gastric cancer. Given that gastric polyposis is an exceedingly rare disease, the genetic cause of adenoma-adenocarcinoma consequence is unclear.
A recent large-scale investigation in gastric cancer genomics found that there are four major subtypes of gastric cancer[4]. Although the molecular processes driving tumorigenesis vary among patients, aberrations in the Fanconi anemia (FA) pathway were observed in all molecular subtypes of gastric cancer[5]. Germline mutations of genes encoding FA proteins are associated with FA, a rare hereditary autosomal recessive syndrome, characterized by progressive bone marrow failure, variable congenital malformations, and increased risk of cancers. Monoallelic mutation in FA genes, unlike biallelic or homozygous gene mutation that causes typical FA phenotype, can increase the susceptibility to a variety of solid tumors in a sporadic manner[6]. The major cellular function of the FA pathway is to maintain stability of genome during the process of DNA replication and damage repair.
In this report, we explore the genetic profiles in a patient with sporadic gastric polyposis and carcinoma. We hypothesize that multiple biopsies from recurrent gastric polyps will serve as an important model to study gastric homeostasis and gastric tumorigenesis. We have also sought to demonstrate the use of a pathway-driven massive parallel genetic sequencing approach for elucidating important tumoral characteristics.
A 65-year-old man, without significant medical history, presented with recurrent passage of black stool and iron-deficiency anemia (IDA) from the age of 48 (2002). Multiple polypoid tumors at the stomach with easy touch bleeding were noted by esophagogastroduodenoscopy (EGD) since 2002. Systemic survey showed that the polyposis was limited to the stomach and H. pylori infection was absent. There was only one sporadic polyp in the colon as shown by colonfibroscopy. The biopsy of the gastric polyps showed hyperplastic polyps, in which dilated, complex, tortuous, gastric, foveolar type glands were seen.
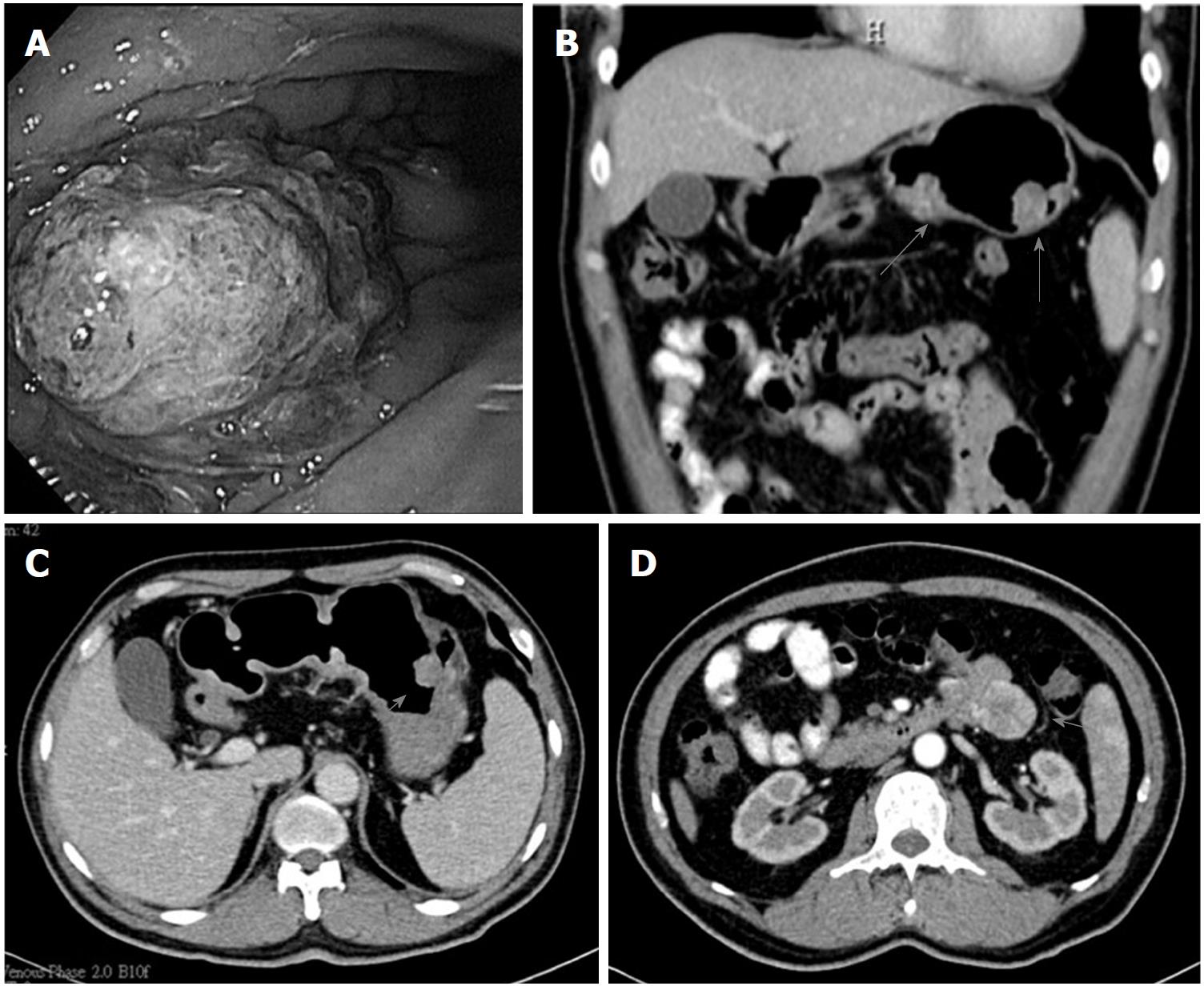
After medical treatment with iron supplement, he was essentially free of gastrointestinal symptoms and the levels of hemoglobin were kept in subnormal ranges (11.5-12 g/dL) until 2011 when recurrent passage of tarry stool with IDA developed. EGD was performed several times during 2011-2015 (between the ages of 57 and 61). The serial EGD showed the maximal size of gastric polypoid tumors gradually increased from 1.5 cm in 2011, to 2.5 cm in 2012, and to 5 cm in 2015. Microscopically, surface chronic ulceration and inflammation on the hyperplastic polyps were noted between 2011 and 2012. In later follow-ups in 2015, low-grade to high-grade intraepithelial neoplasia and intramucosal carcinoma in the background of tubular adenoma was found. CT of the abdomen in 2015 showed multiple intraluminal polypoid tumors and an exophytic tumor at the upper jejunum (Figure 1). Therefore, the patient underwent subtotal gastrectomy and resection of the jejunal tumor. In addition to benign gastric polyposis, adenocarcinoma at the stomach and gastrointestinal stromal tumor (GIST) at the jejunum were diagnosed after surgery. After the procedure, there was no further drop in the level of hemoglobin and no evidence of recurrence of cancer was noted in the subsequent follow-ups until the end of 2017.
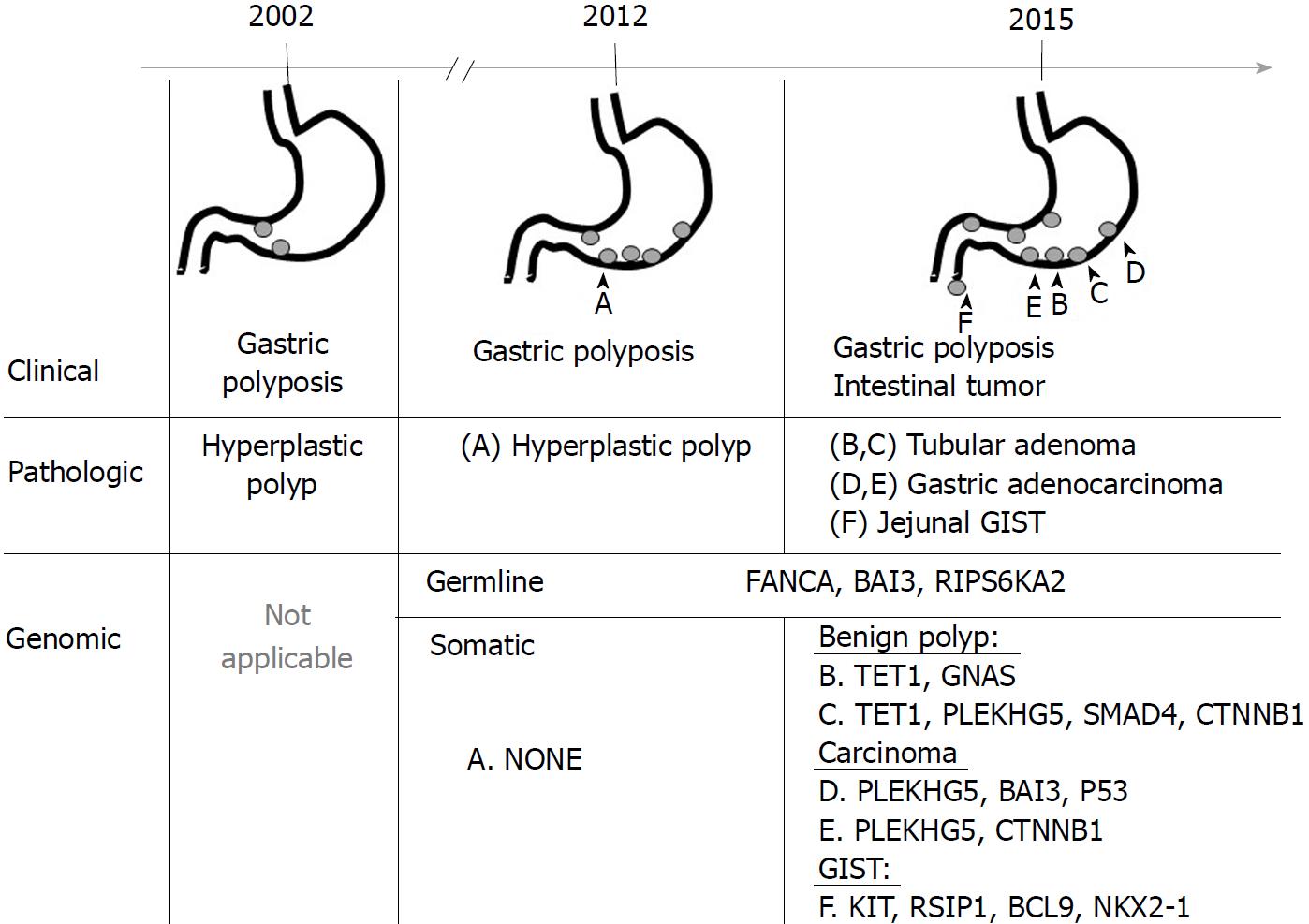
The clinical course of gastric polyposis in this patient is summarized in Figure 2. To study the genetic association between benign gastric polyp, gastric adenocarcinoma, and jejunal GIST, we used a commercially available multiplexed gene sequencing panel (ACTOnco®, ACT Genomics Co., Ltd. Taipei, Taiwan) that detects mutations in 409 genes pertinent to cancer treatment, prognosis, and diagnosis.
Ethic approval: The approval for conducting this study was granted by the Ethic Committee of Mackay Memorial Hospital, No. 15MMHIS190e. Informed consent was obtained from the patient.
Sample preparation: DNA from formalin-fixed paraffin-embedded tumor specimens (benign gastric polyp, gastric adenocarcinoma, and jejunal GIST tumor) were extracted using QIAamp DNA FFPE Tissue Kit (Qiagen Inc., Valencia, CA, United States) according to the manufacturer’s protocol. DNA was quantified using Quant-iTTM dsDNA HS Assay (Invitrogen) and tested for the integrity of genomic DNA using Fragment Analyzer™ (Advanced Analytical Technologies, Inc.) according to the manufacturer’s protocol.
ACTOnco comprehensive cancer panel sequencing: Eighty nanograms of genomic DNA from each sample were amplified using four pools of 15992 primer pairs (Ion AmpliSeq Comprehensive Cancer Panel, Thermo Fisher Scientific) to target all coding exons of 409 cancer-related genes (1688650 target bases). Amplicons were ligated with barcoded adaptors using the Ion Ampliseq Library Kit (Thermo Fisher Scientific). They were subsequently conjugated with sequencing beads by emulsion polymerase chain reaction and enriched using IonChef (Thermo Fisher Scientific) according to the manufacturer’s protocol. Sequencing was performed on the Ion Proton Sequencer with the Ion PI chip (Thermo Fisher Scientific).
Raw data generated by the sequencer were mapped to the hg19 reference genome using Ion Torrent Suite (v. 4.4). Single nucleotide variants (SNVs) and short insertion/deletions (INDELs) were identified using the Torrent Variant Caller plug-in. Variant Effect Predictor (VEP) was used to annotate every variant. Genetic alterations of all coding exons and all exon-intron boundaries covered by the gene panel were analyzed. Identified variants were included both in protein-coding sequences and splice-site variants. For variants in protein-coding sequences, only non-synonymous mutations were analyzed. Any variant with less than 25 reads or lower than 5% variant frequency was filtered out. With the threshold for filtering, 99% of the captured region was covered in each case. Total numbers of mapped reads in the studied samples were between 20907271 and 37358579. The mean base coverage depth ranged from 1355 to 2397. Variants that were included in 1000 Genomes project Phase 1 (Asian populations) with > 1% minor allele frequency (MAF) were considered as polymorphisms and excluded from further analysis.
Mutational analysis of benign and malignant gastric polyps, and jejunal gastrointestinal stromal tumor: Massively parallel sequencing for a panel of 409 cancer-related genes in these tumors identified three germline mutations (BAI3 p.S311W, FANCA pD1359Y, RPS6KA2 p.T595I) and twelve somatic mutations in three benign and three malignant tumors (Table 1). Among the benign tumors, there was no identifiable somatic mutation in the hyperplastic polyp collected in 2011. However, several newly developed somatic mutations were found four years later: TET1 p.V873I and GNAS p.R844C in one adenomatous polyp, and TET1 p.V873I, SMAD4 p.R361C, CTNNB1 p.S45F, and PLEKHG5 p.R203C in another adenomatous polyp with low-to-high-grade intraepithelial neoplasia. In the malignant gastric polyps, two new mutations (BAI3 pL617P, and P53 p.K164fs) were found, but no mutations in TET1 or SMAD4 were identified. The jejunal GIST tumor harbored unique somatic mutations in KIT p. S501_A502insAY, RSIP1 p.S514N, BCL9 p.A1191G, BCL9 p.P1195A, and NKX2-1 p.F209L, which were not observed in either benign or malignant gastric polyps.
| Gene | DNA change | Amino acid change | A | B | C | D | E | F |
| Benign polyp | Malignant polyp | |||||||
| Hyperplastic polyp | Tubular adenoma | Tubular adenoma with low-to-high-grade intraepithelial neoplasia | Tubular adenoma with signet ring cell gastric adenocarcinoma | Tubular adenoma with focal intramucosal carcinoma | Jejunal GIST | |||
| 1.5 cm | 2.5 cm | N/A | 2.5 cm | 5 cm | N/A | |||
| 1FANCA | c. 4075G>T | p. D1359Y | 48.40% | 54.00% | 47.50% | 52.00% | 37.80% | 45.70% |
| 1BAI3 | c. 932C>G | p. S311W | 50.30% | 46.10% | 40.90% | 50.40% | 51.30% | 49.30% |
| 1RPS6KA2 | c. 1784C>T | p. T595I | 48.00% | 49.40% | 47.70% | 45.70% | 51.50% | 48.40% |
| TET1 | c. 2617G>A | p. V873I | 10.10% | 7.40% | ||||
| GNAS | c. 2530C>T | p. R844C | 6.30% | |||||
| SMAD4 | c. 1081C>T | p. R361C | 6.40% | |||||
| CTNNB1 | c. 134C>T | p. S45F | 23.60% | 4.20% | ||||
| PLEKHG5 | c. 607C>T | p. R203C | 40.70% | 15.30% | 35.40% | |||
| BAI3 | c. 1850T>C | p. L617P | 37.70% | |||||
| P53 | c. 489dupC | p. K164fs | 49.90% | |||||
| KIT | C1502_1503insTGCCTA | p. S501_A502insAY | 37.30% | |||||
| PSIP1 | c. 1541G>A | p. S514N | 14.00% | |||||
| BCL9 | c. 3572C>G | p. A1191G | 13.10% | |||||
| BCL9 | c. 3583C>G | p. P1195A | 13.00% | |||||
| NKX2-1 | C627C>A | p. F209L | 7.50% | |||||
We reported a patient with H. pylori-negative gastric polyposis presenting with bleeding and anemia from the age of 48. The polyp progressed from hyperplastic polyp to adenomatous polyp, then led to malignant transformation after 13 years of follow-up, and a jejunal GIST was identified incidentally. Because the polyps spread throughout the whole stomach and no other family members were affected by similar disease, the patient did not fulfill the diagnostic criteria of Gastric Adenocarcinoma and Proximal Polyposis of the Stomach (GAPPS), a recently described autosomal-dominant heritable syndrome characterized by isolated proximal gastric polyposis and risk of gastric cancer[7,8]. Therefore, we studied the genomic profiles from benign to malignant polyp by massive parallel sequencing and unexpectedly identified that the patient was a germline carrier of FANCA, BAI3, and RPS6KA2 mutations. Comparing the genomic profiling from benign to malignant gastric polyp and jejunal GIST revealed clonal evolution between gastric polyposis and not jejunal GIST.
The adenoma-carcinoma sequence, first proposed in the 1990’s, is now a widely accepted theory of step-wise carcinogenesis from normal epithelium to adenoma, carcinoma in situ, and invasive and metastatic tumor in colorectal cancer[9]. Genomic instability plays a critical role to initiate and facilitate the adenoma-carcinoma sequence[10]. In this case, the monoallelic germline mutation (D1359Y) in a DNA damage repair (DDR) gene, FANCA, might account for the predisposition to chromosomal instability and the development of tumors.
FA is a rare, genetically heterogeneous syndrome with an increased predisposition to cancers and bone marrow failure[11]. To date, mutations in 16 genes have been identified in FA, including FANCA, B, C, D1/BRCA2, FANCD2, E, F, G, I, J, L, M, N/PALPB2, FANCO/RAD51C, FANCP/SLX4, and FANCQ[12]. The above proteins work together in the FA pathway in response to DNA damage to coordinate DNA repair and maintain genomic stability. Therefore, mutation in any of the 16 FA genes may lead to defects in DDR and chromosomal instability.
Germline FANCA mutation is the most frequent in patients with FA but less reported in other solid tumors[13]. In the TCGA database, somatic mutations in FANC genes were approximately 3% in stomach cancer[5,14]. Hierarchical clustering analysis showed that the DDR pathways, especially the FA pathway, were deranged across all subtypes of gastric cancer[5]. FANCA D1359Y, the amino acid substitution found in our case, results in a change from a charged to a hydrophobic amino acid[15]. It not only has a weaker interaction with FANCC and FANCG but also affects the ubiquitination of FANCD2 and subsequent cellular response to DNA damage[16].
The association with clinical development of cancers is different in homozygous and heterozygous germline FA gene mutation carriers. In patients with inherited homozygous (biallelic) mutations of FA genes, the relative risk of nonhematologic malignancies is increased especially for squamous cell carcinomas (700-fold more affected than the normal population) and the median onset age of cancers is much earlier (16 years old in affected compared to 68 years old in non-FA population)[17,18]. In contrast, heterozygous (monoallelic) FA gene mutations somatically do not cause the FA phenotype but significantly increase cancer susceptibility in a sporadic manner, e.g. later onset without overt family history[6]. Some of the FA genes (FANCD1, FANCD2, FANCJ, FANCN and FANCO) have been described as breast and ovarian cancer susceptibility genes[19-22]. To our knowledge, our case is the first report of a FANCA mutation in gastric polyposis and cancer.
In addition to genetic susceptibility, environmental factors play critical roles in the development of gastric cancer, such as H. pylori, chronic use of a proton pump inhibitor, high salt diet, and smoking[23,24]. In response to tissue damage from the environmental stimuli, the stomach can initiate a regenerative program to accelerate stem cell division and lineage differentiation to replenish damaged cells[25]. In this study, two somatic mutations frequently found in gastric cancer were identified in malignant polyps: tumor suppressor genes TP53 and oncogene CTNNB1. Interestingly, PLEKHG5, a less described mutation in gastric cancer, was found in three out of five examined samples, and TET1 mutation was only identified in two benign polyps.
PLEKHG5, a member of pleckstrin homology and Rho guanine nucleotide exchange factor domain-containing G protein family, is currently considered to play a role in activating RhoA exchange factors and the nuclear factor kappa B (NFκB) signaling pathway[26]. The cellular function of PLEKHG5 is to promote cell polarity, migration, adhesion, and degradation through the interaction with RHO downstream effectors[27,28]. In the molecular subtypes proposed by TCGA[5], RHOA mutation was found almost exclusively in genomically stable tumors. Although PLEKHG5 mutation found in this study may theoretically activate the RHOA-driven signaling pathway and contribute to the invasive phenotype, its actual cellular function remains to be experimentally elucidated.
TET1, tet methylcytosine dioxygenase 1, belongs to the TET protein family and plays a role in DNA methylation and gene activation. One recent study reported that TET1 can inhibit gastric cancer growth and AKT signaling through demethylation and re-expression of PTEN[29]. To our knowledge, only one breast cancer cell line, HCC1143, was reported to have this mutation (TET1 V873I) from dbEM (A database of epigenetic modifier, http://www.crdd.osdd.net). In this study, TET1 mutation was recurrently seen in benign polyps but not in malignant lesions. Whether this mutation can prevent malignant transformation requires more investigation.
To our knowledge, the association between an FA gene mutation and a gastric polyp or an adenocarcinoma has not been reported.
With the advent of new sequencing technologies, we were able to detect germline mutations/variations that may cause susceptibility to the development of gastric polyps and cancer, which might shed light in finding potential genetic events contributing to malignant transformation. Although more research is needed to elucidate the cellular phenotypes of the mutations observed in our study and their role in gastric carcinogenesis, the advent of a pathway-driven genomic profiling approach brings clinicians closer to the central cause of the disease and holds a great potential in providing better care for individuals.
A 65-year-old man had iron deficiency anemia from the age of 48 due to gastric polyposis with recurrent upper gastrointestinal bleeding.
Gastric polyposis
Clinically, differential diagnoses for gastric polyposis include familial adenomatous polyposis syndrome, inherited autosomal-dominant syndrome “Gastric Adenocarcinoma and Proximal Polyposis of the Stomach” (GAPPS), or sporadic gastric polyposis. Histologically, gastric polyps should be differentiated between benign epithelial polyps (such as hyperplastic, fundic-gland, adenomatous, and inflammatory fibrinoid polyps), gastric neuroendocrine tumors (carcinoids), gastrointestinal stromal tumors (GIST), and malignant polyps (such as adenomatous carcinoma).
Iron deficiency anemia
Polyposis at the stomach and upper jejunum by computed tomography scan
He was initially diagnosed with gastric hyperplastic polyp between the ages of 48 years to 58 years, and then gastric adenocarcinoma and jejunal GIST at the age of 61 years. Through comparing the genetic events between benign and malignant gastric polyps, a germline mutation at FANCA D1359Y and several recurrent mutations in DNA methylation (TET1, V873I), the β-catenin pathway (CTNNB1, S45F) and RHO signaling pathway (PLEKHG5, R203C) were identified in the polyps with malignant transformation.
Endoscopic polypectomy for lesions larger than 0.5-1 cm, followed by annual upper endoscopic surveillance; subtotal gastrectomy for gastric adenocarcinoma and resection of the jejunal GIST.
The adenoma-carcinoma sequence in gastric cancer is recapitulated in the case study. Germline mutation at FANCA D1359Y is identified as a potential gastric polyposis susceptible gene.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
P- Reviewer: Ierardi E, Misra V, Pylkas K, Ranzani GN S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Bian YN
| 1. | Boland CR, Yurgelun MB. Historical Perspective on Familial Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2017;3:192-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Li J, Woods SL, Healey S, Beesley J, Chen X, Lee JS, Sivakumaran H, Wayte N, Nones K, Waterfall JJ. Point Mutations in Exon 1B of APC Reveal Gastric Adenocarcinoma and Proximal Polyposis of the Stomach as a Familial Adenomatous Polyposis Variant. Am J Hum Genet. 2016;98:830-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Elhanafi S, Saadi M, Lou W, Mallawaarachchi I, Dwivedi A, Zuckerman M, Othman MO. Gastric polyps: Association with Helicobacter pylori status and the pathology of the surrounding mucosa, a cross sectional study. World J Gastrointest Endosc. 2015;7:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology. 2015;149:1153-1162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 364] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 5. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4848] [Article Influence: 440.7] [Reference Citation Analysis (2)] |
| 6. | Chen H, Zhang S, Wu Z. Fanconi anemia pathway defects in inherited and sporadic cancers. Transl Pediatr. 2014;3:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 7. | Brosens LA, Giardiello FM, Offerhaus GJ, Montgomery EA. Syndromic Gastric Polyps: At the Crossroads of Genetic and Environmental Cancer Predisposition. Adv Exp Med Biol. 2016;908:347-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Worthley DL, Phillips KD, Wayte N, Schrader KA, Healey S, Kaurah P, Shulkes A, Grimpen F, Clouston A, Moore D. Gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS): a new autosomal dominant syndrome. Gut. 2012;61:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8004] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 10. | Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5:19-27. [PubMed] |
| 11. | Brosh RM Jr, Bellani M, Liu Y, Seidman MM. Fanconi Anemia: A DNA repair disorder characterized by accelerated decline of the hematopoietic stem cell compartment and other features of aging. Ageing Res Rev. 2017;33:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Bogdanova N, Helbig S, Dörk T. Hereditary breast cancer: ever more pieces to the polygenic puzzle. Hered Cancer Clin Pract. 2013;11:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Wilkes DC, Sailer V, Xue H, Cheng H, Collins CC, Gleave M, Wang Y, Demichelis F, Beltran H, Rubin MA. A germline FANCA alteration that is associated with increased sensitivity to DNA damaging agents. Cold Spring Harb Mol Case Stud. 2017;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4732] [Cited by in RCA: 7140] [Article Influence: 310.4] [Reference Citation Analysis (0)] |
| 15. | Savino M, Ianzano L, Strippoli P, Ramenghi U, Arslanian A, Bagnara GP, Joenje H, Zelante L, Savoia A. Mutations of the Fanconi anemia group A gene (FAA) in Italian patients. Am J Hum Genet. 1997;61:1246-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Adachi D, Oda T, Yagasaki H, Nakasato K, Taniguchi T, D’Andrea AD, Asano S, Yamashita T. Heterogeneous activation of the Fanconi anemia pathway by patient-derived FANCA mutants. Hum Mol Genet. 2002;11:3125-3134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Alter BP. Cancer in Fanconi anemia, 1927-2001. Cancer. 2003;97:425-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003;101:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 19. | Ghoussaini M, Pharoah PDP, Easton DF. Inherited genetic susceptibility to breast cancer: the beginning of the end or the end of the beginning? Am J Pathol. 2013;183:1038-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi Anemia Registry. Haematologica. 2008;93:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Levy-Lahad E. Fanconi anemia and breast cancer susceptibility meet again. Nat Genet. 2010;42:368-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, Freund M, Lichtner P, Hartmann L, Schaal H. Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet. 2010;42:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 530] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 23. | Isomoto H, Furusu H, Ohnita K, Takehara Y, Wen CY, Kohno S. Effect of Helicobacter pylori eradication on gastric hyperplastic polyposis in Cowden’s disease. World J Gastroenterol. 2005;11:1567-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Freeman HJ. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J Gastroenterol. 2008;14:1318-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Kim TH, Shivdasani RA. Notch signaling in stomach epithelial stem cell homeostasis. J Exp Med. 2011;208:677-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Kim HJ, Hong YB, Park JM, Choi YR, Kim YJ, Yoon BR, Koo H, Yoo JH, Kim SB, Park M. Mutations in the PLEKHG5 gene is relevant with autosomal recessive intermediate Charcot-Marie-Tooth disease. Orphanet J Rare Dis. 2013;8:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Iwatake M, Nishishita K, Okamoto K, Tsukuba T. The Rho-specific guanine nucleotide exchange factor Plekhg5 modulates cell polarity, adhesion, migration, and podosome organization in macrophages and osteoclasts. Exp Cell Res. 2017;359:415-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Dachsel JC, Ngok SP, Lewis-Tuffin LJ, Kourtidis A, Geyer R, Johnston L, Feathers R, Anastasiadis PZ. The Rho guanine nucleotide exchange factor Syx regulates the balance of dia and ROCK activities to promote polarized-cancer-cell migration. Mol Cell Biol. 2013;33:4909-4918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Pei YF, Tao R, Li JF, Su LP, Yu BQ, Wu XY, Yan M, Gu QL, Zhu ZG, Liu BY. TET1 inhibits gastric cancer growth and metastasis by PTEN demethylation and re-expression. Oncotarget. 2016;7:31322-31335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |