Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3556
Peer-review started: June 9, 2018
First decision: July 4, 2018
Revised: July 18, 2018
Accepted: July 21, 2018
Article in press: July 21, 2018
Published online: August 21, 2018
Processing time: 69 Days and 21.5 Hours
To evaluate the ability of PillCamColon2 to visualize colonic segments missed by incomplete optical colonoscopy (OC) and to assess the diagnostic yield.
This prospective multicentre study included 81 patients from nine centres who underwent second-generation colon capsule endoscopy (CCE) following incomplete OC performed by an experienced gastroenterologist (> 1000 colonoscopies). Patients with stenosis were excluded. According to patient preferences, CCE was performed the following day (protocol A) after staying on clear liquids and 0.75 L Moviprep in the morning or within 30 d after new split-dose Moviprep (protocol B). Boosts consisted of 0.75 L and 0.25 L Moviprep, and phospho-soda was given as a rescue if the capsule was not excreted after seven hours.
Seventy-four patients were analysed (51% of them in group A; 49% in group B). Bowel cleansing was adequate in 67% of cases, and CCE could visualize colonic segments missed by incomplete colonoscopy in 90% of patients under protocol A and 97% of patients under protocol B (P = 0.35, n.s.). Significant polyps including adenocarcinoma were detected in 24% of cases. Detection rates for all polyps and significant polyps per patient were similar in both protocols. Polyps were found predominantly in the right colon (86%) in segments that were not reached by OC. Extracolonic findings - such as reflux esophagitis, suspected Barrett esophagus, upper GI-bleeding, gastric polyps, gastric erosions and angiectasia - were detected in eight patients. PillCamColon2 capsule was retained in the ileum of one patient (1.4%) without symptoms and removed during an uneventful resection for unknown Crohn’s disease that was diagnosed as the cause of anemia, which was the indication for colonoscopy. CCE was well tolerated. One patient suffered from self-limiting vomiting after consuming the phospho-soda.
Second-generation CCE using a low-volume preparation is useful after incomplete OC, and it allows for the detection of additional relevant findings, but cleansing efficiency could be improved.
Core tip: Colonoscopy is the gold standard for visualization of the colon, but it may be incomplete, not reaching the cecum. Second-generation colon capsule endoscopy (CCE) with low-volume preparations could complement incomplete colonoscopies in 90% of cases, and it could help to detect additional relevant colonic and extracolonic findings. Protocols with either CCE the day following an incomplete colonoscopy or within 30 d after a new low-volume preparation were both feasible and well tolerated; however, the protocols could be improved with respect to bowel cleanliness and complete colon visualization.
- Citation: Baltes P, Bota M, Albert J, Philipper M, Hörster HG, Hagenmüller F, Steinbrück I, Jakobs R, Bechtler M, Hartmann D, Neuhaus H, Charton JP, Mayershofer R, Hohn H, Rösch T, Groth S, Nowak T, Wohlmuth P, Keuchel M. PillCamColon2 after incomplete colonoscopy - A prospective multicenter study. World J Gastroenterol 2018; 24(31): 3556-3566
- URL: https://www.wjgnet.com/1007-9327/full/v24/i31/3556.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i31.3556
Flexible optical colonoscopy (OC) is the gold standard to detect and treat colorectal diseases and proved to be safe and effective in colorectal cancer screening (CRC)[1]. However, OC may be incomplete for various reasons, with completion rates between 90% and 98%[2-5] for screening colonoscopy and 81%-94% in mixed series[6-9]. Risk factors for incomplete colonoscopy are female gender, elder patients, previous pelvic or abdominal surgery, diverticulosis, redundant colon, stenosing tumors, poor preparation, inflammatory bowel disease; in contrast sedation, male gender and higher body mass index were reported as protective factors[4,6,7,10,11]. Of the patients with incomplete OC, 4.3% had advanced neoplasia in the right colon[5]. A five-year follow-up study showed a higher risk for colorectal cancer in patients with failed colonoscopy[12], demonstrating the need for additional investigations in these patients. PillCamColon has been cleared by the US Food and Drug Administration (FDA) for detection of colon polyps in patients after an incomplete optical colonoscopy with adequate preparation. Later the indication had been expanded to the detection of colon polyps in patients with evidence of lower gastrointestinal origin and major risks for colonoscopy or moderate sedation.
First-generation PillCamColon1 with a constant rate of two frames per second (fps) had limited sensitivity compared to a colonoscopy[13-15]. Technical improvements of the second-generation PillCamColon2 that was used in the present study included adaptive frame rates (4 fps and 35 fps) and an increased viewing angle. It also included automatic small bowel detection with consecutive timing of boosters to shorten transit times and to improve cleansing levels[16], which was shown to have a significantly higher diagnostic yield for polyps[17]. A decisive factor for CCE accuracy is bowel cleansing; recent data have shown that low-volume Polyethylene glycol (PEG)-based protocols may be feasible instead of high-volume preparations[18,19].
Our study aimed to assess the impact of second-generation CCE after incomplete colonoscopies by analyzing the complementation rates and incremental diagnostic yields with two different low-volume cleansing protocols. Patients were offered to either stay on clear liquids with a CCE the next day or to start a new bowel preparation procedure within 30 d.
This prospective, multicentre study including nine centres (tertiary care hospitals or private endoscopy offices) in Germany. It was approved by the ethics committee of Hamburg Chamber of Physicians (PV3467, 20.05.2010). The http://www.clinicaltrials.gov identifier is NCT01480635.
All patients included in the study were 18 years or older who had incomplete colonoscopies that were performed by an experienced endoscopist (> 1000 colonoscopies performed). Incomplete OC was defined as a failure to reach the cecum or ileo-cecal anastomosis due to looping, bowel angulation, adhesions, and intolerance of sedation or inflammation. The presumed area of the colon reached by OC was documented, as were reasons for termination, detection of polyps and tumours, other findings and adverse events. Patients with stenosis, inadequate preparation, or exchange of endoscope were excluded. Other exclusion criteria for CCE have been described previously[18]. Written informed consent was obtained from all patients.
After an incomplete colonoscopy, patients were rescheduled for the following day (protocol A) or they were given a separate appointment within 30 d (protocol B), according to their preference. CCE was performed with PillCamColon2 (Given Imaging, Yoqneam, Israel) after a low-volume cleansing regimen with PEG and ascorbic acid (Moviprep, Norgine, Marburg/Lahn, Germany). Following capsule ingestion, the boosts consisted of 0.75 L after small bowel detection and 0.25 L Moviprep if the capsule had not been excreted five hours after ingestion. 30 mL sodium picosulfate (NaP; Fleet, Recordati, Ulm, Germany) was used as an additional boost if the capsule was not excreted seven hours after ingestion. In protocol A, patients stayed on clear liquids after the colonoscopy, and they received an additional cleansing of 0.75 L Moviprep the next morning (at the latest, one hour before CCE). In protocol B, patients were allowed to eat after the OC. A new bowel cleansing procedure was performed within 30 d: split-dose 1 L of Moviprep was consumed in the evening and 1 L was consumed in the morning, each followed by 1 L of water. Boosts with Moviprep or NaP were identical in both protocols (Table 1).
| Time | Procedure | |
| Evening and morning before colonoscopy | Standard bowel prep for colonoscopy | |
| Incomplete colonoscopy (OC) | ||
| After incomplete colonoscopy | Patient´s choice of protocol A or B for colon capsule endoscopy (CCE) | |
| Protocol A (CCE next day) | Protocol B (CCE within 30 d) | |
| After incomplete colonoscopy | Patient stays on clear liquids | Patient can eat |
| 2 d before CCE | Low residue diet | |
| Day before CCE | NA | Clear liquids only |
| Evening before CCE | 1 L Moviprep + 1 L water | |
| Morning of CCE | 0.75 L Moviprep + water | 1 l Moviprep + 1 L water |
| Colon capsule ingestion | ||
| Small bowel detection | 0.75 L Moviprep + water (1st boost) | |
| 5 h after ingestion | 0.5 L Moviprep + water (2nd boost)1 | |
| 7 h after ingestion | 30 mL NaP + water (´Rescue boost)1 | |
| Bisacodyl supp1 | ||
| 11 h after ingestion | Removal of equipment1 | |
CCE studies were read with Rapid7 or Rapid8 software in each centre by an experienced endoscopist with additional experience in capsule endoscopy (> 1000 colonoscopies, > 100 small bowel capsule endoscopies (SBCE) and > 25 CCEs performed). All readers had completed a dedicated two-day CCE evaluation course. Polyp size was estimated with the integrated software tool.
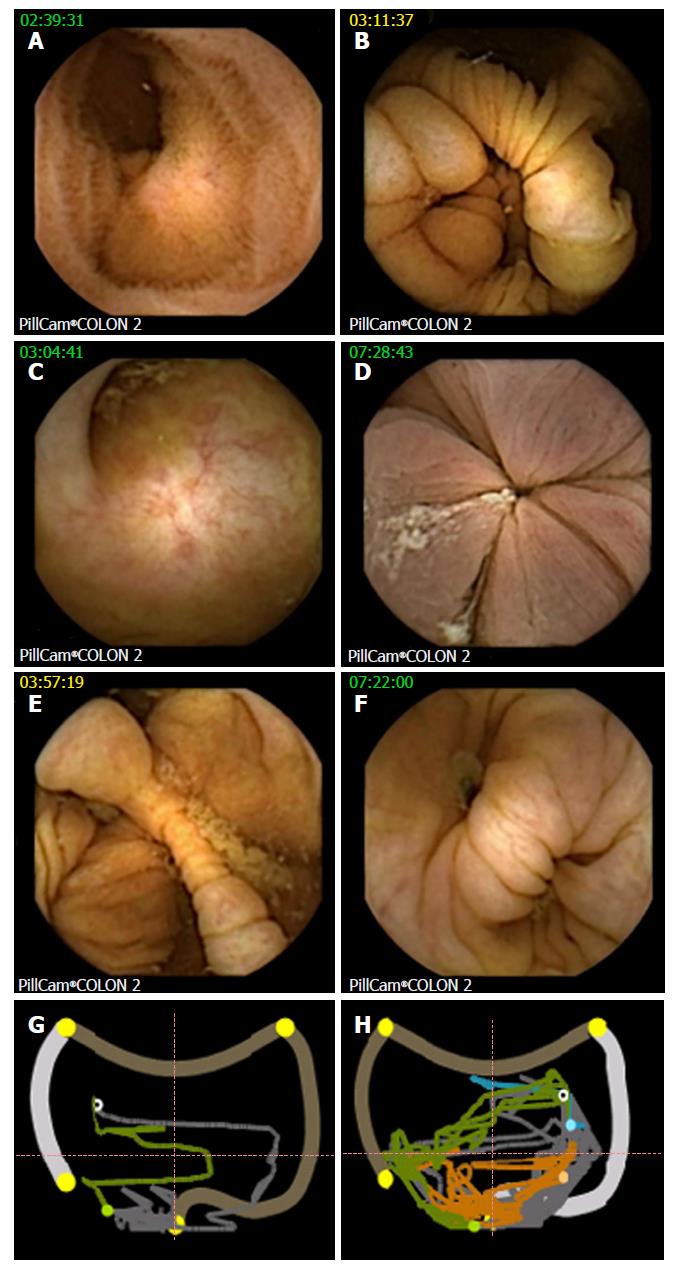
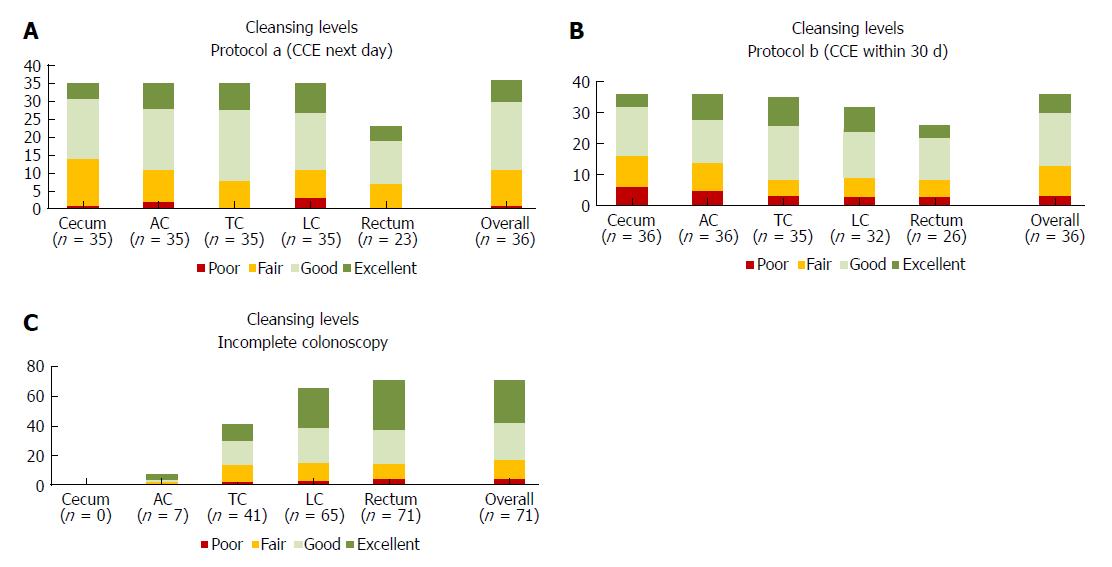
For both examination modalities, cleansing levels were documented, as described previously[15], using four grades: excellent, good (adequate), fair and poor (inadequate). Each colon segment [that is, cecum, ascending colon (AC), transverse colon (TC), left colon (LC) and rectum, see Figure 1] and overall cleansing status were evaluated.
For CCE, visualization of colonic segments, complementation of previous colonoscopy, completeness of CCE, and adverse events were recorded. Complete CCE was defined as excretion of capsule during recording time or by identification of the haemorrhoidal plexus. Detection of polyps, significant polyps, tumours or other relevant findings were documented for segments reached and not reached by the previous standard colonoscopy. According to previous studies, significant polyps were defined by size (≥ 6 mm) or number (≥ 3)[19-21]. Other findings were considered important if they explained the indication for the colonoscopy or if they had further diagnostic or therapeutic implications. Follow-up took place via a telephone call one week after the procedure. During the call, adverse events were documented, and capsule excretion was confirmed. Any adverse events were recorded, as well as the time of capsule excretion.
The primary outcome parameter was complementation rate of CCE in patients with incomplete OC, which was defined by visualization of colonic segments not reached by OC. The secondary outcome parameters were as follows: additional (incremental) diagnostic yield of CCE compared to incomplete OC (all polyps, significant polyps defined by size or number and other significant findings) in segments not reached by OC; CCE findings in the upper gastrointestinal (GI) tract and small bowel; rate of complete CCE, as defined above; cleansing level of the colon (overall and segments) following the low-volume protocol for CCE; visualization of the Z-Line; and adverse events (number, type and severity).
Continuous variables were reported as mean and standard deviation, and categorical variables were reported as percentage. The null hypothesis (H0) for the primary endpoint (complementation of incomplete colonoscopy) was constructed using data from a former study that used PillCamColon1 and that showed a complementation rate of 50%[22]. Accordingly, H0 was set to μ = 0.5 with an expectation for complementation of OC by PillCamColon2 of 0.67. Using the Fisher exact test with a power of 80% (which is equal to 1-β), 74 patients had to be recruited. Categorical values were compared using a chi-squared test (χ2), while continuous values with a normal distribution were compared by a Student´s t-test. P values < 0.05 were considered significant. An intention-to-treat (ITT) analysis was performed for complementation rates, cleansing levels, detection of significant polyps and safety. Statistical review was performed by one of the authors (Peter Wohlmuth).
Eighty-one consecutive patients were enrolled from nine participating centres between 2010 and 2013. Seven patients (four in the protocol A group and three in the protocol B group) had to be excluded due to technical failure (n = 1), protocol noncompliance as a result of incorrect timing of CCE (n = 4), exchange of colonoscope (n = 1) or early removal of recorder by the patient (n = 1) (Figure 2). In total, data of 74 patients were analysed per protocol. Demographics are shown in Table 2.
| Protocol A (CCE next day) | Protocol B ( CCE within 30 d) | Significance | |
| Demographics | |||
| Patients | 38 (51.4) | 36 (48.6) | |
| Female | 20/38 (52.6) | 24/36 (66.7) | |
| Age, mean ± SD | 68.0 ± 12.8 yr | 63.9 ± 13.0 yr | |
| Body mass index | 26.0 ± 3.9 | 26.5 ± 4.9 | |
| Reasons for termination of colonoscopy | |||
| Looping of colon | 23/38 (60.5) | 23/36 (63.9) | |
| Angulation of colon | 6/38 (15.8) | 5/36 (13.9) | |
| Susp. adhesions | 5/38 (13.2) | 6/36 (16.7) | |
| Risk of perforation | 2/38 (5.3) | 0 | P = 0.710 (NS); χ2 test |
| Sedation problems | 2/38 (5.3) | 2/36 (5.6) | |
| Results of CCE | |||
| Complete CCE | 24/38 (63.3) | 24/36 (66.7) | P = 0.560 (NS); χ2 test |
| Complementation of colonoscopy | 34/38 (89.5) | 35/36 (97.2) | P = 0.350 (NS); χ2 test |
| Adequate cleansing | 25/36 (69.4) | 23/36 (63.9) | P = 0.820 (NS); χ2 test |
| Patients with significant colon polyps | 10/38 (26.3) | 11/36 (30.6) | P = 0.500 (NS); χ2 test |
| Patients with other colon findings | 0 | Angiectasia (n = 3) | P = 0.045; χ2 test |
| Diverticulitis (n = 1) | |||
| Patients with small bowel findings | Angiectasia (n = 1) | 0 | P = 0.174 (NS); χ2 test |
| Crohn’s disease (n = 1) | |||
| Patients with upper GI findings | Reflux esophagitis (n = 1) | Reflux-esophagitis (n = 1) | P = 0.949 (NS); χ2 test |
| Upper GI-bleeding (n = 1) | Susp. Barrett esophagus (n = 1) | ||
| Gastric polyps (n = 1) | Gastric erosions (n = 1) | ||
Reasons for referral to colonoscopy were CRC screening (22%), anemia (15%), hematochezia (15%), irregular stool (12%), abdominal pain (12%), B symptoms (7%), colitis (5%) and other reasons (12%). Thirty-six patients (48.6%) had prior abdominal surgery, while 14 patients (19%) had more than one surgical intervention. Most common surgeries were appendectomies (23%) and hysterectomies (19%). Only three patients had colonic surgery (4%), one patient had an ileocecal and one patient had a Billroth IIanastomosis.
An experienced endoscopist (> 1000 colonoscopies performed) performed OC with a standard colonoscope. The mean duration of the procedure was 45 ± 17 min (range: 15-101 min). Unfavourable anatomy was the reason for termination in 92% of procedures. OC reached the sigmoid colon in 27% of cases, the descending colon (DC) in 4% of cases, the splenic flexure in 12% of cases, the TC in 14% of cases, the hepatic flexure in 35% of cases and the AC in 8% of cases. Adequate cleansing was achieved in 76% of procedures. In 12 of 74 patients (16%), polyps were detected, with a mean size of 6 ± 4.2 mm. Six patients had more than one polyp (range: 2-5 polyps). Diverticula were found in 35 patients. Other findings were diverticulitis, erosions, angiectasias and erythema.
Primary endpoint: complementation of incomplete colonoscopy: Incomplete colonoscopy could be complemented by CCE in 69 of 74 patients (93%; see Table 2). Complete CCE was achieved in 48 of 74 patients (65%). In four additional patients, the capsule reached the rectum but did not visualize the haemorrhoidal plexus (5%).
Complementation of OC could be achieved by CCE with protocol A in 89.5% of procedures and with protocol B in 97.2% of procedures (P = 0.35, not significant; Table 2). There were no differences between the rates of complementation using CCE for protocol A or B and the diagnostic yield. In the patients with incomplete visualization of the missing segments (n = 5), the capsule was able to display the hepatic flexure in two patients. One patient had a capsule retention in the small bowel because of unknown stenosing Crohn’s disease. In one patient, the capsule did not reach the colon during recording time, and in one patient, visualization of the colon was incomplete due to recording gaps. The capsule was excreted within seven hours of ingestion and before the need for an additional NaP booster in 17 of 38 patients (44.7%) following protocol A, and in 12 of 36 patients (33.3%; P = 0.25, not significant) in protocol B.
Secondary endpoints: CCE was performed on the day after colonoscopy in 38 patients (protocol A; 51%) or within 30 d in 36 patients (protocol B; 49%). Overall, cleansing was adequate in 48 of 72 (67%), and cleansing was adequate in the cecum in 58% of cases, in the AC in 65% of cases, in the TC in 77% of cases, in the LC in 70% of cases and in the rectum in 63% of cases (Figure 3); there were no differences between the protocols. Two capsules did not allow for visualization of the colon. Poor cleansing was rare (4 of 72 patients; 5.6%).
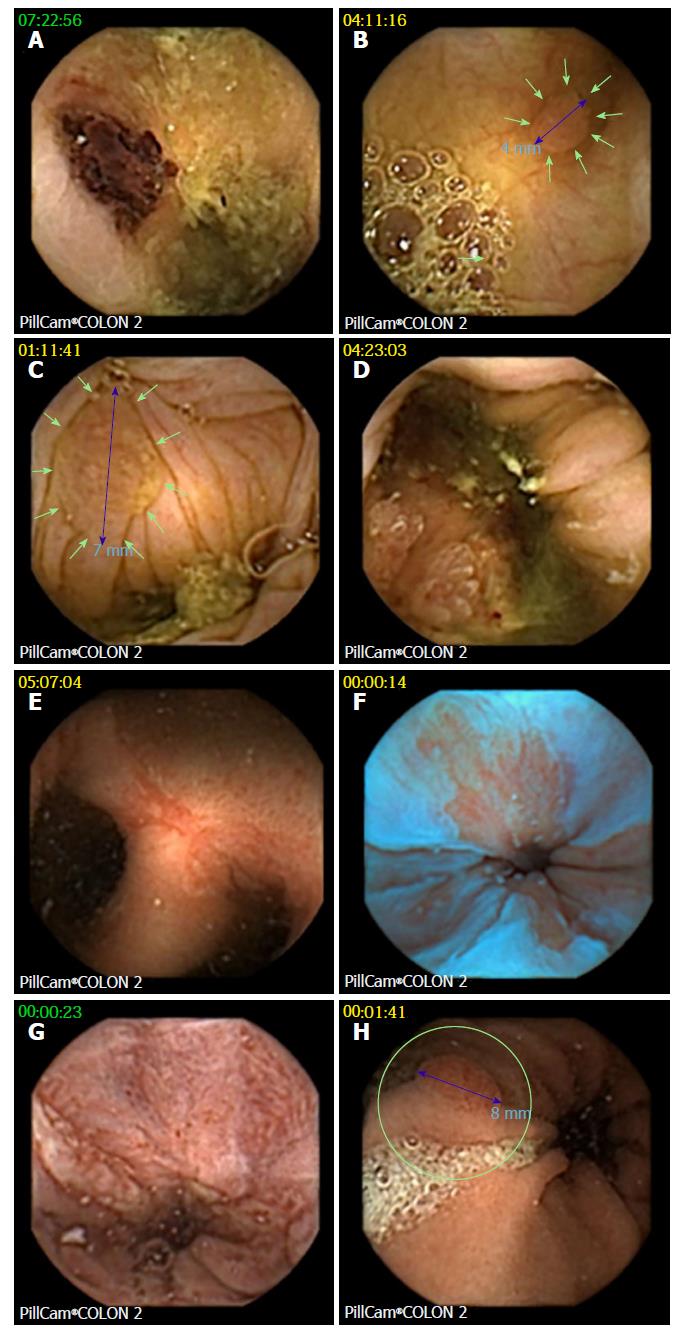
CCE detected 76 polyps in 35 of 74 patients (47%; Figure 4). Twenty-one patients (28%) had significant polyps (Table 3), and 14 patients had an insignificant number of polyps. In 9 of 21 patients, polyp size was ≥ 6 mm; in 3 patients, the number of polyps was ≥ 3; and in 9 patients, both parameters were positive. A total of 59 polyps (mean size 8 ± 4.5 mm) were detected in the 21 patients with significant polyps. Significant polyps were predominantly found in the ascending colon in segments that had not been reached by OC (86%). Incremental diagnostic yield of CCE compared to previous incomplete OC per patient for detecting significant polyps was 24%, and there were no differences between the two protocols. A cecal polyp of 26 mm turned out to be an adenocarcinoma, and hemicolectomy was performed in this patient.
| No. | Significance based on | Size of largest polyp (mm) | Number of polyps | Localization of polyps | Farest point reached in OC | All segments with polyps in CCE reached by colonoscopy |
| 1 | Size/number | 6 | 5 | Rectum | HF | Yes |
| 2 | Size | 8 | 2 | Sigma | SF | Yes |
| 3 | Size/number | 8 | 3 | Sigma | AC | Yes |
| 4 | Size/number | 8 | 3 | 2 × Cecum, 1 × AC | TC | No |
| 5 | Size | 14 | 1 | AC | HF | No |
| 6 | Size | 26 | 1 | AC | SF | No |
| 7 | Size | 6 | 2 | AC | Sigma | No |
| 8 | Size/number | 10 | 3 | 2 × AC, 1 × TC | SF | No |
| 9 | Size/number | 10 | 3 | 2 × LC, 1 × AC | Sigma | No |
| 10 | Size | 10 | 1 | AC | SF | No |
| 11 | Size/number | 9 | 3 | 2 ×AC, 1 × LC | HF | No |
| 12 | Size | 6 | 1 | AC | Sigma | No |
| 13 | Size | 8 | 2 | 1 × AC/1 × LC | HF | No |
| 14 | Size | 7 | 1 | HF | Sigma | No |
| 15 | Number | 5 | 4 | AC, TC, 2 × sigma | Sigma | No |
| 16 | Size/number | 12 | 4 | 1 × Cecum, 1 × AC, 1 × LC, 1 × rectum | Sigma | No |
| 17 | Number | 4 | 3 | 1 × Cecum, 2 × rectum | TC | No |
| 18 | Number | 5 | 5 | 2 × AC, 1 × TC, 2 × LC | HF | No |
| 20 | Size/number | 10 | 7 | 4 × AC, 3 × LC | TC | No |
| 20 | Size | 9 | 2 | 2 × Cecum | LC | No |
| 21 | Size/number | 12 | 3 | AC | LC | No |
Z-line was visualized in 45 of 74 patients (60.8%). CCE findings in the upper GI tract were reflux esophagitis (n = 2), suspected Barrett´s esophagus (n = 1), hemorrhagic gastropathy (n = 1), gastric polyps (n = 1; consecutive gastroscopy revealed foveolar hyperplasia in previously undiagnosed atrophic gastritis with vitamin B12 deficiency) and upper GI bleeding (n = 1).
In the small bowel, angiectasia and previously unknown Crohn’s disease was detected in one patient each. In colon segments missed by incomplete colonoscopy, angiectasias were detected in three patients and diverticulitis was detected in one patient (all patients were part of protocol B).
ITT analysis of all 81 patients found a complementation rate of 89%, adequate cleansing in 65%, significant polyps in 26% of patients, and no additional adverse events. The low complementation rate that was observed in this cohort is consistent with the exclusion criteria [technical problems, n = 1, and noncompliance of study protocol, n = 6 (for example, early removal of recorder and incorrect CCE timing)].
In one patient (protocol A), the capsule was retained in the small bowel without symptoms. Surgery was indicated following a new diagnosis of stenosing and fistulating Crohn’s disease with iron deficiency anemia (indication for colonoscopy). During an uneventful surgery, the capsule was retrieved. Another patient (protocol B) complained of self-limiting nausea and vomiting after the NaP boost.
In this prospective, multicentre study, second-generation CCE allowed for the visualization of colon segments that were not reached by a previously incomplete colonoscopy in 93% of 74 patients. Fifty-one percent of patients opted for protocol A, and 49% for protocol B. The complementation rate following incomplete OC was higher in group B (97%) compared with group A (89%), but the results did not reach statistical significance. There were no differences between the rate of complete CCE, the cleansing level, the diagnostic yield or the number of adverse events.
The complementation rate identified in the present study corresponded well with results from recent trials using a first-generation colon capsule. A Spanish study found a complementation rate of incomplete colonoscopies of 85.3% and a diagnostic yield of 45%[23]. A French trial including patients as well with contraindicated as incomplete OC, complete CCE was achieved in 83% of patients. An Italian trial reported a complete PillcamColon2 CCE after incomplete OC in 98% of patients using a separate preparation with Senna tablets, 4 L of PEG and two boosters with NaP and gastrografin[24]. This rate was higher than in our low-volume protocol, but it was accompanied more often by preparation-related complaints (28.0% vs 1.4%). Nevertheless, our primary aim to complement colon visualization was achieved in 93% of patients, which was similar to a Greek study that achieved results using PillCamColon1 in 91% of patients[14]. In the present study, unfavourable anatomy was the reason for terminating the colonoscopy, which was usually performed under propofol sedation, in 92% of patients. In contrast, pain was reported by 45% of patients as the reason for incomplete colonoscopy in the Italian trial. This selection bias toward unfavourable anatomy might have also influenced the rate of complete consecutive CCEs (65%) in the present study. It may also be relevant when comparing our results to series with unselected patients without previously incomplete colonoscopies. In these trials, complete CCE was achieved in 76% of patients using a similar low-volume preparation[18], in 88% of patients using PEG[25], in 76% of patients using NaP[14] and in 98% of patients using a PEG-, NaP- or gastrografin-based regimen[24].
In the present study, colon cleansing using a low-volume preparation was adequate in 67% of patients, and the results were similar in both protocols. These results are comparable to other studies that used PillCamColon1 for incomplete colonoscopies with PEG preparation and NaP boosts (65%)[23], and 60% vs 63% in the right and left colon, respectively[14]. Adequate cleansing following use of the PillcamColon1 was observed in 76% of patients following consumption of 1-2 L Moviprep and two NaP boosts in a mixed cohort, including 28% of patients with contraindicated colonoscopy without anatomical problems[19]. A recent Spanish multicentre trial found adequate cleansing levels in 75% of patients using a standard 4 L PEG preparation with a NaP booster for PillCamColon2 after an incomplete colonoscopy[26]. Even in a large trial in a screening population without negative selection towards unfavorable anatomy, CCE was technically insufficient in 9% of patients due to inadequate cleansing or rapid transit of the capsule[27].
Twenty-four percent of our patients (ITT 22%) had additional relevant finding following the CCE, which led to a recommendation for further diagnostics or treatment. Similarly, PillCamColon1 was useful in guiding management after failed OC in a Spanish trial[23].
Most of the significant polyps (86%), including a carcinoma, were found in the AC. Our data confirmed previous observations[5] that CCE can identify relevant lesions in segments not reached by incomplete OC (Table 3).
Device-assisted colonoscopy with either spiral endoscopy and double or single balloons have identical rates, similar to CCE, of complementing incomplete OC of about 90%[28-31]. These flexible endoscopic techniques additionally provide the possibility to directly treat detected lesions, but they require equipment and expertise, and they are restricted to specialized centres. A cap-assisted colonoscopy was also successful in 93% of patients, and one perforation occurred with this technique after failed OC[32].
Computed tomographic colonography (CTC) after incomplete OC has a good diagnostic yield and may be advantageous if extra-colonic findings are considered[33], for example, in the case of tumour. However, CTC detected fewer small polyps than CCE[24]. Furthermore, radiation-free CCE could additionally be used to visualize the Z-line in 60.8% of patients, as previously reported for PillCamColon1 (60%)[34]. In the present trial, eight patients had relevant extra-colonic findings, seven of which were presumably not detectable with CTC.
Capsule retention occurred in one patient, corresponding to a retention rate of 1.4%, which was similar to the rate reported for SBCE[35]. This capsule retention resulted in a new diagnosis of Crohn´s disease and was considered to be diagnostic rather than a complication. However, possible retention must also be considered when applying CCE to complement incomplete OC and should be included as part of the informed consent discussion. Although adhesions were supposed to be the cause of incomplete OC in some patients, no related clinical problems or capsule retention following CCE were observed.
In conclusion, second-generation CCE using low-volume bowel preparations is useful, well tolerated and is able to detect additional relevant lesions. Risk of retention is as low as in SBCE, but must be considered. Similar results were found in the present study between the two protocols for the complementation rate and presence of significant polyps.
Patients could choose between preparation protocols for CCE without randomization. The area reached by the colonoscopy was described, but tattooing was only optional. Long-term follow-up was not part of the present study.
Optical colonoscopy (OC) is the gold standard for visualization of the colon. However, it may be incomplete e.g. due to unfavorable anatomy. Colon capsule endoscopy (CCE) is cleared by the US Food and Drug Administration for patients with previously incomplete OC. Second generation CCE has been shown to have a higher sensitivity for detection of colon polyps than first generation. Low volume bowel prep with Moviprep has been shown to be feasible for CCE.
Bowel preparation for CCE is more extensive than for OC. Thus, we aimed to evaluate, if second generation CCE is feasible using either repeated low volume bowel prep or staying on clear liquids following an incomplete OC.
Main research objective was the ability of CCE to visualize those colon segments not reached by incomplete OC. Secondary objectives were additional diagnostic yield of CCE, rate of complete colon visualization by CCE, cleansing levels, and safety.
In this prospective multicenter study 81 patients underwent second generation colon capsule endoscopy with PillCamColon2 after incomplete OC. CCE was performed either the following day (protocol A) after staying on clear liquids and 0.75 L Moviprep in the morning or within 30 d after new split-dose Moviprep (protocol B). Boosts consisted of 0.75 L and 0.25 L Moviprep, and phospho-soda as rescue if the capsule was not excreted after 7 hours.
Seventy-four patients were finally analyzed per protocol. Of those, cleansing was adequate in 67% of cases and CCE could visualize the colonic segments missed by incomplete colonoscopy in 90% (protocol A) and 97% (protocol B, P = 0.35, n.s.) of the patients. Detection rates were similar with both protocols: Significant polyps and one adenocarcinoma were detected in 24% of cases. Polyps were found predominantly in the right colon (86%) in segments not reached by OC. Extra-colonic findings as reflux esophagitis, suspected Barrett esophagus, upper GI-bleeding, gastric polyps, gastric erosions, and angiectasia were detected in 8 patients. One capsule (1.4%) was retained in the ileum without symptoms and removed during uneventful resection for unknown Crohn`s disease diagnosed as cause of unclear anemia. CCE was well tolerated. One patient suffered from self-limiting vomiting after phospho-soda.
Second generation CCE using low volume prep is useful to complement incomplete OC, detects additional relevant findings including extra-colonic lesions, and is well tolerated. CCE is feasible the following day after staying on clear liquids or after new prep within 30 d. Potential risk of capsule retention must be considered.
Future studies should address improvement of colon cleansing levels and completeness of CCE after incomplete OC. Cost-effectiveness of CCE after incomplete OC should be addressed by future research in comparison with other methods as CT colonoscopy, MR colonoscopy, and device assisted colonoscopy.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Dutta AK, Paduani G, Teramoto-Matsubara OT S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Pox CP, Altenhofen L, Brenner H, Theilmeier A, Von Stillfried D, Schmiegel W. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460-1467.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Brenner H, Altenhofen L, Katalinic A, Lansdorp-Vogelaar I, Hoffmeister M. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol. 2011;174:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Sarwar S, Anwar MM, Ryan B, O’Morain C. Colonoscopy completion rates - are we prepared for a national screening programme? Ir Med J. 2007;100:585-587. [PubMed] |
| 4. | Koido S, Ohkusa T, Nakae K, Yokoyama T, Shibuya T, Sakamoto N, Uchiyama K, Arakawa H, Osada T, Nagahara A. Factors associated with incomplete colonoscopy at a Japanese academic hospital. World J Gastroenterol. 2014;20:6961-6967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Neerincx M, Terhaar sive Droste JS, Mulder CJ, Räkers M, Bartelsman JF, Loffeld RJ, Tuynman HA, Brohet RM, van der Hulst RW. Colonic work-up after incomplete colonoscopy: significant new findings during follow-up. Endoscopy. 2010;42:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Dafnis G, Granath F, Påhlman L, Ekbom A, Blomqvist P. Patient factors influencing the completion rate in colonoscopy. Dig Liver Dis. 2005;37:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Loffeld RJ, van der Putten AB. The completion rate of colonoscopy in normal daily practice: factors associated with failure. Digestion. 2009;80:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Hansel SL, Prechel JA, Horn B, Crowell MD, DiBaise JK. Observational study of the frequency of use and perceived usefulness of ancillary manoeuvres to facilitate colonoscopy completion. Dig Liver Dis. 2009;41:812-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Tsai MS, Su YH, Liang JT, Lai HS, Lee PH. Patient factors predicting the completion of sedation-free colonoscopy. Hepatogastroenterology. 2008;55:1606-1608. [PubMed] |
| 11. | Crispin A, Birkner B, Munte A, Nusko G, Mansmann U. Process quality and incidence of acute complications in a series of more than 230,000 outpatient colonoscopies. Endoscopy. 2009;41:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Britton EJ, Sidhu S, Geraghty J, Psarelli E, Sarkar S. The 5-year outcome of patients having incomplete colonoscopy. Colorectal Dis. 2015;17:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Triantafyllou K, Viazis N, Tsibouris P, Zacharakis G, Kalantzis C, Karamanolis DG, Ladas SD. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc. 2014;79:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 16. | Adler S, Hassan C, Metzger Y, Sompolinsky Y, Spada C. Accuracy of automatic detection of small-bowel mucosa by second-generation colon capsule endoscopy. Gastrointest Endosc. 2012;76:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Spada C, Pasha SF, Gross SA, Leighton JA, Schnoll-Sussman F, Correale L, González Suárez B, Costamagna G, Hassan C. Accuracy of First- and Second-Generation Colon Capsules in Endoscopic Detection of Colorectal Polyps: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1533-1543.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Hartmann D, Keuchel M, Philipper M, Gralnek IM, Jakobs R, Hagenmüller F, Neuhaus H, Riemann JF. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy. 2012;44:482-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Pioche M, de Leusse A, Filoche B, Dalbiès PA, Adenis Lamarre P, Jacob P, Gaudin JL, Coulom P, Letard JC, Borotto E. Prospective multicenter evaluation of colon capsule examination indicated by colonoscopy failure or anesthesia contraindication. Endoscopy. 2012;44:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Spada C, Hassan C, Marmo R, Petruzziello L, Riccioni ME, Zullo A, Cesaro P, Pilz J, Costamagna G. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin Gastroenterol Hepatol. 2010;8:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Triantafyllou K, Tsibouris P, Kalantzis C, Papaxoinis K, Kalli T, Kalantzis N, Ladas SD. PillCam Colon capsule endoscopy does not always complement incomplete colonoscopy. Gastrointest Endosc. 2009;69:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, Gimeno-García AZ, Nicolás-Pérez D, Jiménez A, Quintero E. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol. 2013;11:534-540.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Spada C, Hassan C, Barbaro B, Iafrate F, Cesaro P, Petruzziello L, Minelli Grazioli L, Senore C, Brizi G, Costamagna I. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2015;64:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581-589.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 26. | Nogales Ó, García-Lledó J, Luján M, Nicolás D, Juanmartiñena JF, González-Suárez B, Sánchez Ceballos F, Couto I, Olmedo J, Garfia C, Carretero C, Fernández Urién I, Rodríguez S, Asteinza M, Olivencia P, Masedo Á, Muñoz-Navas M, Merino B, González Asanza C. Therapeutic impact of colon capsule endoscopy with PillCam™ COLON 2 after incomplete standard colonoscopy: a Spanish multicenter study. Rev Esp Enferm Dig. 2017;109:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Rex DK, Adler SN, Aisenberg J, Burch WC Jr, Carretero C, Chowers Y, Fein SA, Fern SE, Fernandez-Urien Sainz I, Fich A, Gal E, Horlander JC Sr, Isaacs KL, Kariv R, Lahat A, Leung WK, Malik PR, Morgan D, Papageorgiou N, Romeo DP, Shah SS, Waterman M. Accuracy of capsule colonoscopy in detecting colorectal polyps in a screening population. Gastroenterology. 2015;148:948-957.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Becx MC, Al-Toma A. Double-balloon endoscopy: an effective rescue procedure after incomplete conventional colonoscopy. Eur J Gastroenterol Hepatol. 2014;26:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Dzeletovic I, Harrison ME, Pasha SF, Crowell MD, Decker GA, Gurudu SR, Leighton JA. Comparison of single- versus double-balloon assisted-colonoscopy for colon examination after previous incomplete standard colonoscopy. Dig Dis Sci. 2012;57:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Schembre DB, Ross AS, Gluck MN, Brandabur JJ, McCormick SE, Lin OS. Spiral overtube-assisted colonoscopy after incomplete colonoscopy in the redundant colon. Gastrointest Endosc. 2011;73:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Sulz MC, Frei R, Semadeni GM, Sawatzki M, Borovicka J, Meyenberger C. The role of single-balloon colonoscopy for patients with previous incomplete standard colonoscopy: Is it worth doing it? Surg Endosc. 2016;30:1876-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Lee YT, Hui AJ, Wong VW, Hung LC, Sung JJ. Improved colonoscopy success rate with a distally attached mucosectomy cap. Endoscopy. 2006;38:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Pullens HJ, van Leeuwen MS, Laheij RJ, Vleggaar FP, Siersema PD. CT-colonography after incomplete colonoscopy: what is the diagnostic yield? Dis Colon Rectum. 2013;56:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |