Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3531
Peer-review started: April 13, 2018
First decision: May 17, 2018
Revised: May 26, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: August 21, 2018
Processing time: 127 Days and 3.9 Hours
To image stomach wall blood vessels and tissue, layer-by-layer.
We built up the acoustic resolution photoacoustic microscopy (AR-PAM) system for imaging layered tissues, such as the stomach wall. A tunable dye laser system was coupled to a fiber bundle. The fibers of the bundle were placed in nine directions with an incident angle of 45° around a high-frequency ultrasound transducer attached to the acoustic lens. This structure formed a dark field on the tissue surface under the acoustic lens and the nine light beams from the fibers to be combined near the focal point of the acoustic lens. The sample piece was cut from a part of the porcine stomach into a petri dish. In order to realize photoacoustic depth imaging of tumor, we designed a tumor model based on indocyanine green (ICG) dye. The ICG solution (concentration of 129 μM/mL) was mixed into molten gel, and then a gel mixture of ICG (concentration of 12.9 μM/mL) was injected into the stomach submucosa. The injection quantity was controlled by 0.1 mL to make a small tumor model.
An acoustic resolution photoacoustic microscopy based on fiber illumination was established and an axial resolution of 25 μm and a lateral resolution of 50 μm in its focal zone range of 500 μm has been accomplished. We tuned the laser wavelength to 600 nm. The photoacoustic probe was driven to do B-scan imaging in tissue thickness of 200 μm. The photoacoustic micro-image of mucosa and submucosa of the tissue have been obtained and compared with a pathological photograph of the tissue stained by hematoxylin-eosin staining. We have observed more detailed internal structure of the tissue. We also utilized this photoacoustic microscopy to image blood vessels inside the submucosa. High contrast imaging of the submucosa tumor model was obtained using ICG dye.
This AR-PAM is able to image layer-by-layer construction and some blood vessels under mucosa in the stomach wall without any contrast agents.
Core tip: In order to image layered tissue and blood vessels, acoustic resolution photoacoustic microscopy based on fiber illumination was established and an axial resolution of 25 μm and a lateral resolution of 50 μm in its focal zone range of 500 μm was accomplished. Layer-by-layer imaging of the stomach tissue and stomach mucosa blood vessels were obtained. High contrast imaging of the submucosa tumor model was obtained using ICG dye.
- Citation: Wang C, Lu YF, Cai CM, Xiang HZ, Zheng G. Stomach wall structure and vessels imaging by acoustic resolution photoacoustic microscopy. World J Gastroenterol 2018; 24(31): 3531-3537
- URL: https://www.wjgnet.com/1007-9327/full/v24/i31/3531.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i31.3531
Stomach submucosal tumors (SMT) are any intramural growth underneath the mucosa of the gastrointestinal tract[1]. These tumors are very hard to find because they are usually asymptomatic and therefore most often discovered as accidental findings during surgery, autopsy, and so on. When examining submucosal tumors, standard optical stomach endoscopy, capsule optical endoscopy and push-and-pull enteroscopy together with barium contrast X-ray do not alone provide sufficient information. Endoscopic ultrasound (EUS), computed tomography (CT) and magnetic resonance imaging (MRI) are recommended as supplementary tools[2] because CT and MRI often lack either sufficient spatial resolution or satisfactory contrast (or both) to be effective for early-stage tumor imaging[3]. Optimal EUS imaging of an SMT needs submersion of the tumor under water. However, benign SMTs, for example the submucosal inflammation, cannot be distinguished endosonographically[4]. Therefore, early-phase tumor detection or in situ characterization of diseased tissue is challenging for EUS. Because the mechanism of EUS imaging is ultrasound imaging, this is based on tissue bulk mechanical properties. Tumor tissue boundaries and blood vessel structures are clinically relevant and provide necessary information for assessing disease stage and planning treatment therapies.
Recently, optical endoscopic imaging modalities, like narrow band imaging endoscopy[5], endoscopic optical coherent tomography[6] and confocal laser endomicroscopy[7] have been developed. They can detect tissue or tissue changes with high sensitivity and high spatial resolution. Because of the strong optical scattering properties of tissue, these techniques are unable to image targets beyond a 1-2 mm depth. Photoacoustic imaging has a lot of advantages, such as, endogenous optical chromophore contrast enables label-free imaging of the microvasculature with a high resolution[8], and in vivo blood vessel photoacoustic imaging can cover the length scale from a superficial capillary[9] to an abdominal aorta[10], demonstrating the potential of photoacoustic imaging to bridge the resolution and penetration gaps between microvascular microscopy, clinical angiography and so on[11,12]. This paper’s aim is to demonstrate that photoacoustic imaging is able to image the layered tissue and blood vessels under the surface of the tissue. In the future, we can design and develop an photoacoustic endoscopic imaging system to image deeper tissue with higher resolution for diagnosis and treatment.
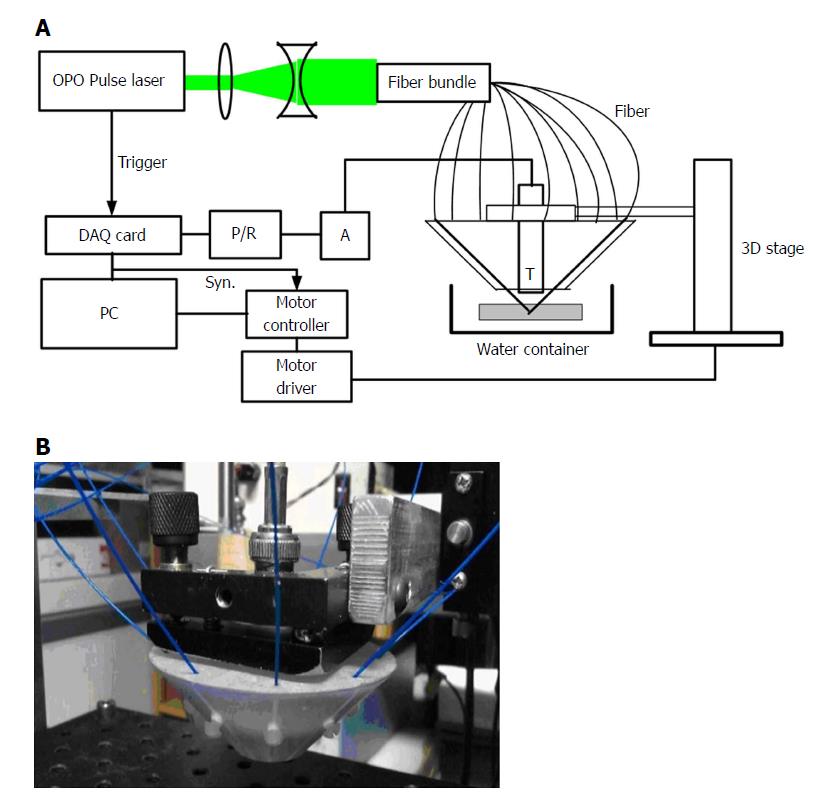
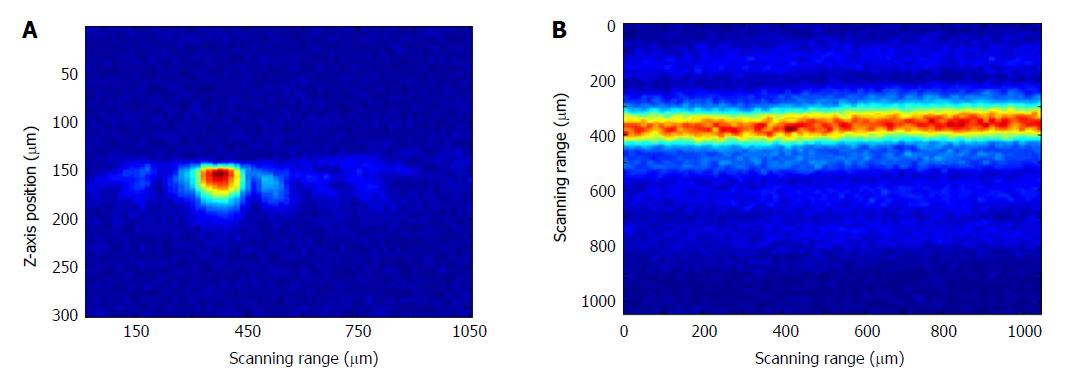
The acoustic resolution photoacoustic microscopy (AR-PAM) system for stomach wall imaging was shown in Figure 1A. A tunable dye laser system pumped by a Q-switch Nd:YAG laser (ND6000, continuum) was used to provide laser pulse with a pulse repetition of 10 Hz and a pulse width of 5.5 ns. The tunable range of the laser light was 415 nm to 940 nm. A pair of concave and convex lenses expanded and collimated the light beam to approximately 5 mm in diameter. Then coupling to a fiber bundle that was composed by nine optical fibers (FT400UMT, Thorlabs) which have a 400 μm core diameter and numerical aperture of 0.39 were placed in nine directions (40° intervals in 360°) around a high-frequency (50-MHz, bandwidth of 30 MHz) ultrasound transducer attached with the acoustic lens (the f/# is 1.3 and focal length is 4 mm). The cross angle between the fibers with the transducer axis direction were set to 45°, so that it formed a dark field on the tissue surface under the acoustic lens and the nine light beams from the fibers were combined near the focal point of the acoustic lens, i.e. it is then weakly focused into the tissue with the focal region coaxially overlapping the ultrasonic focus inside the tissue. In an optically transparent medium, the optical focus is about 2 mm in diameter, which is much wider than the ultrasonic focus. The focal acoustic transducer and output end of the fibers were fastened with a holder made by a 3D printer. We can refer to it as a photoacoustic probe (Figure 1B). According to the parameters of the ultrasonic transducer, an axial resolution of 25 μm and a lateral resolution of 50 μm in its focal zone range of 500 μm can be accomplished. The photoacoustic probe is translated in a water bath. A window at the bottom of the water container is sealed with an optically and ultrasonically transparent disposable polyethylene membrane (thickness: 0.04 mm). After commercial ultrasound gel is applied to the region of interest on the sample for acoustic coupling, the sample is placed between the sample supporter and the water container for imaging. The photoacoustic wave is recorded at each location of the ultrasonic transducer and subsequently converted into a one-dimensional (1D) depth-resolved image (A-scan) based on the sound velocity in soft tissue (1.54 mm/us). Images were generated by one dimension (B-Scan) in the X or Y direction or two-dimensional (C-Scan) raster scanning of the photoacoustic probe in the X-Y plane with a step size of 30 μm. In addition to, we can also scan in the Z direction with a step of 200 μm for producing an image of the deeper tissue by utilizing deeply penetrable diffused light to excite photoacoustic signals. At each scanning position, signals were recorded by an 8 bits digitizer card (DP1400, Agilent Tech, United States) after it was amplified by a preamplifier (AU-2A-0150-BNC, MTEQ, United States) and then amplified and filtered (high pass 1 MHz) by a pulser/receiver (5073PR, Olympus, Japan). No signal averaging is performed. As shown in Figure 2, photoacoustic imaging of a hair with a diameter of 80 μm were obtained with this photoacoustic system for verifying the imaging ability. Figure 2A was a B-scan image and Figure 2B was a C-scan image of the hair.
A porcine stomach was received the day after the animal was sacrificed for an unrelated study. The sample piece was cut from a part of the porcine stomach into a petri dish with a diameter of 140 mm and a thickness of 22 mm. In order to obtain a photoacoustic depth image of the tumor, we designed a tumor model based on indocyanine green (ICG) dye, wherein the ICG solution at a concentration of 129 μM/mL was mixed into molten gel, and then the gel mixture of ICG at a concentration of 12.9 μM/mL was injected into the stomach submucosa at a 2-3 mm position. The injection quantity was controlled to 0.1 mL to make a small tumor model.
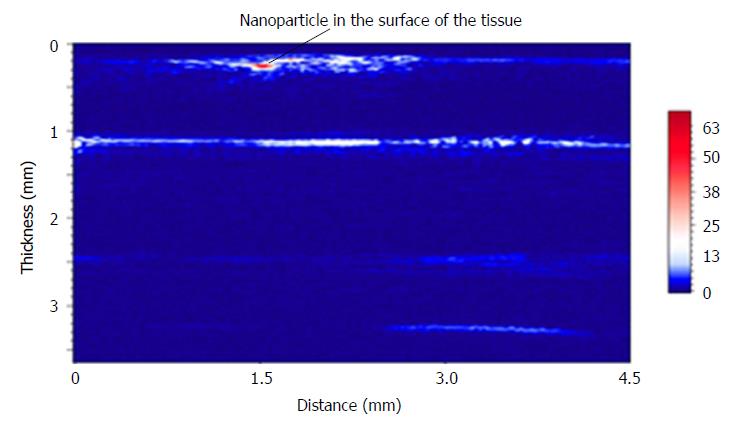
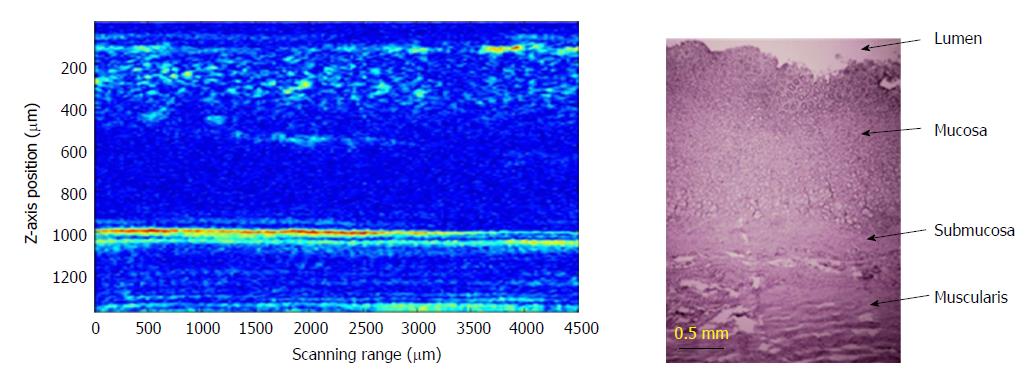
In order to verify photoacoustic imaging can also be used to implement stratified imaging of the stomach wall, we tuned the laser wavelength to 600 nm. The photoacoustic probe obtained many B-scan images at tissue thickness direction with a lateral scanning step of 200 μm. Then, all of the B-scan images were combined to complete an image of the layered structure of the stomach wall (Figure 3). A particle with the diameter of 300 μm was placed on the surface of stomach tissue, in order to indicate the surface position of the tissue. As shown in Figure 4, the photoacoustic microimage of the mucosa and submucosa of the tissue have been obtained and compared with a pathological photograph of the tissue stained by hematoxylin-eosin staining. We have observed more detailed internal structure of the tissue by the photoacoustic imaging.
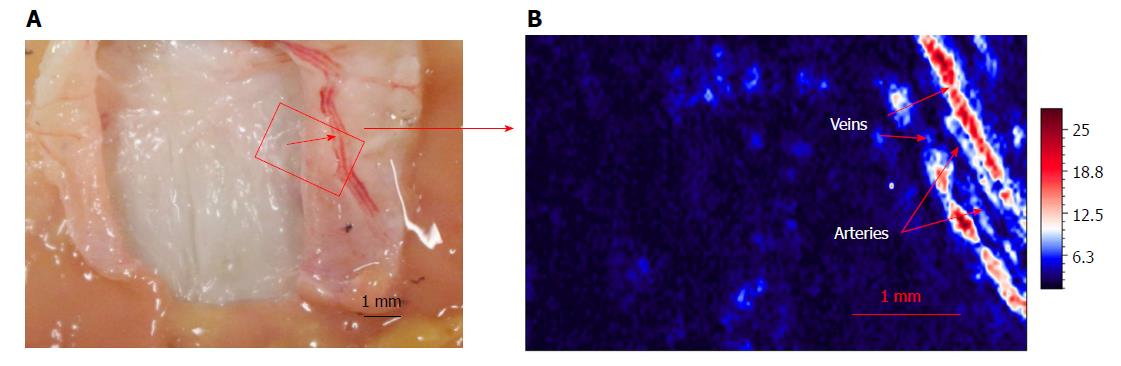
In addition, we also utilized this photoacoustic microscopy to image blood vessels inside the submucosa. The dissected stomach wall after imaging by photoacoustic microscopy is shown in Figure 5A. The vessels under submucosa after imaging by photoacoustic microscopy is shown in Figure 5B. We very clearly saw the artery vessels and vein vessels.
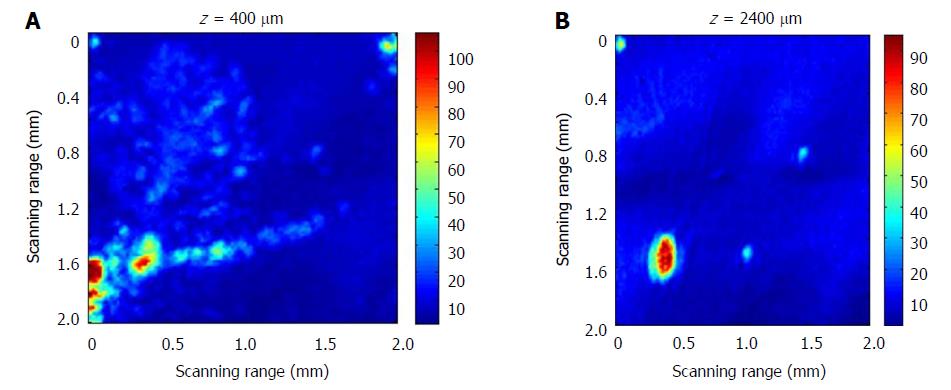
In order to realize the photoacoustic depth imaging of the tumor, we designed a labeled tumor model based on ICG dye, wherein 0.1 mL ICG gelatin solution with a concentration of 129 μM/mL was injected into the stomach submucosa at 2-3 mm deep. After the ICG-gel mixture was injected into the tissue and coagulated for 20 minutes, the C-scan imaging at different depths was completed. When doing the scanning, the output wavelength of the laser was tuned to 700 nm because is the absorption peak of the ICG dye with molar extinction coefficient of 1.1 × 105 cm-1/M in water. This will improve the signal: noise ratio of the image.
Some diffusion phenomena of the ICG resolution at the mucosal site of 400 μm was observed (Figure 6A). The ICG dye may have combined with blood in the vessels, so some tubular structures were observed. At a depth of 2400 μm, the ICG labeled tumor model was still visible (Figure 6B). This indicated that we can achieve deeper detection.
Ultrasound uses high-frequency sound waves to produce images of the organs and structures inside the body such as ovaries, uterus, liver, gallbladder, pancreas, or aorta. EUS combines endoscopy and ultrasound in order to obtain images and information about the digestive tract and the surrounding tissue and organs. EUS can obtain information about the layers of the intestinal wall as well as adjacent areas such as lymph nodes and the blood vessels. EUS provides doctors with more information than other imaging tests by providing detailed images of the digestive tract. Although contrast-enhanced ultrasonic techniques[13,14] such as Doppler ultrasound[15] are able to image blood vessels, the resolution of these imaging techniques are much lower than that of PAM, which has recently achieved lateral resolution up to 15 mm[16]. Additionally, photoacoustic imaging provides functional information with endogenous contrast and with the aid of an exogenous contrast agent. Therefore, photoacoustic endoscopy based on photoacoustic tomography and EUS, which has achieved spatially coincident photoacoustic and ultrasonic imaging, provides unprecedented information and promotes morphologic and functional understanding of the gastrointestinal tract imaging[17]. But the research on tissue structure based on photoacoustic imaging technique has not been published yet. This paper reported that utilizing AR-PAM to image layered tissue in the stomach wall and blood vessels under the mucosa in s without any contrasting agents. In the next step, this acoustic resolution photoacoustic microscopy will combine with endoscopy and minimize the photoacoustic probe to make a micro-photoacoustic endoscopy for obtaining morphologic and functional information with higher resolution and depth in vivo.
In conclusion, we have presented a fiber illumination based acoustic resolution photoacoustic microscopy method. We have realized an axial resolution of 25 μm and a lateral resolution of 50 μm in its focal zone range of 500 μm. This system was utilized to image layered stomach wall tissue and blood vessels under the mucosa without any contrasting agents. Using ICG dye enhance a tumor model, the tumor model was imaged to depths of 2400 μm with very high contrast. The photoacoustic microscopy has the ability to image layered tissue with high resolution and high contrast.
When checking submucosal tumors, traditional methods (such as standard optical stomach endoscopy, capsule optical endoscopy and push-and-pull enteroscopy together with barium contrast X-ray) can’t provide accurate information. Computed tomography and magnetic resonance imaging are often lower in resolution and contrast. Optimal endoscopic ultrasound (EUS) imaging of stomach submucosal tumors (SMT) needs submersion of the tumor under water. However, benign SMTs, for example the submucosal inflammation, cannot be distinguished endosonographically. Therefore, it is still a challenge for EUS to detect early-phase tumor in situ. Our team aimed to demonstrate that photoacoustic imaging is able to image layered tissue and blood vessels under the surface of the tissue. In the future, we can design and develop a photoacoustic endoscopic imaging system to image deeper tissues with higher resolution for diagnosis and treatment.
To image layered tissue and blood vessels in layered tissue.
Our aim is to demonstrate that photoacoustic imaging can detect the structure of layered tissue and blood vessels beneath the surface of the tissue.
Our team established the acoustic resolution photoacoustic microscopy (AR-PAM) system for stomach wall structure imaging. Photoacoustic microimaging of the stomach wall structure was compared to an HE pathology image to verify the structure, vessels, and vessel direction.
As a result, we have established a fiber illumination based acoustic resolution photoacoustic microscopy method. The imaging ability has an axial resolution of 25 μm and a lateral resolution of 50 μm in its focal zone range of 500 μm. We have observed more detailed internal structure of the tissue from AR-PAM imaging. We also utilized this photoacoustic microscopy to image blood vessels inside the submucosa. By using ICG dye enhance a tumor model in the submucosa, the images obtained at a depth of 2400 μm depth had very high contrast.
In this study, we have established a fiber illumination based acoustic resolution photoacoustic microscopy. Layer-by-layer imaging of the stomach tissue and blood vessels under stomach mucosa were obtained. By using ICG dye to enhance the tumor model, high contrast images at a depth of 2400 μm tissue were obtained. This proved that photoacoustic microscopy has the ability to image layered tissue and deep tissue targets with high resolution and high contrast. In the near future, this technique combined with endoscopy will supply a simple tool for physicians to see differences in layered tissues and imaging of tumor angiogenesis in submucosa.
This technique combined with endoscopy will supply a simple tool to visualize layered tissues and tumor angiogenesis in submucosa. Scientific research should aim at solving practical problems in clinical practice. Innovative scientific research is demonstrated in the ability to effectively solve the difficult problems doctors encounter in clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Goral V S- Editor: Wang JL L- Editor: Filipodia E- Editor: Huang Y
| 1. | Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002;56:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Hsu CC, Chen JJ, Changchien CS. Endoscopic features of metastatic tumors in the upper gastrointestinal tract. Endoscopy. 1996;28:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Hu S, Wang LV. Photoacoustic imaging and characterization of the microvasculature. J Biomed Opt. 2010;15:011101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Shim CS, Jung IS. Endoscopic removal of submucosal tumors: preprocedure diagnosis, technical options, and results. Endoscopy. 2005;37:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Han S, Hsu A, Wassef WY. An update in the endoscopic management of gastric cancer. Curr Opin Gastroenterol. 2016;32:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ding Z, Qiu J, Shen Y, Chen Z, Bao W. Lens-free all-fiber probe with an optimized output beam for optical coherence tomography. Opt Lett. 2017;42:2814-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Karia K, Kahaleh M. A Review of Probe-Based Confocal Laser Endomicroscopy for Pancreaticobiliary Disease. Clin Endosc. 2016;49:462-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Zhang HF, Maslov K, Stoica G, Wang LV. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat Biotechnol. 2006;24:848-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1018] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 9. | Maslov K, Zhang HF, Hu S, Wang LV. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt Lett. 2008;33:929-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 394] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 10. | Brecht HP, Su R, Fronheiser M, Ermilov SA, Conjusteau A, Liopo A, Motamedi M, Oraevsky AA. Optoacoustic 3D whole-body tomography: experiments in nude mice. Proc SPIE. 2009;7177:71770E. [DOI] [Full Text] |
| 11. | Wang X, Xie X, Ku G, Wang LV, Stoica G. Noninvasive imaging of hemoglobin concentration and oxygenation in the rat brain using high-resolution photoacoustic tomography. J Biomed Opt. 2006;11:024015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Hu S, Maslov K, Zhang Y, Xia Y, Wang LV. In vivo integrated photoacoustic and confocal microscopy of hemoglobin oxygen saturation and oxygen partial pressure. Opt Lett. 2011;36:1029-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Ignee A, Jenssen C, Hocke M, Dong Y, Wang WP, Cui XW, Woenckhaus M, Iordache S, Saftoiu A, Schuessler G. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc Ultrasound. 2017;6:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Dong Y, Wang WP, Mao F, Ji ZB, Huang BJ. Application of imaging fusion combining contrast-enhanced ultrasound and magnetic resonance imaging in detection of hepatic cellular carcinomas undetectable by conventional ultrasound. J Gastroenterol Hepatol. 2016;31:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Xuan JW, Bygrave M, Jiang H, Valiyeva F, Dunmore-Buyze J, Holdsworth DW, Izawa JI, Bauman G, Moussa M, Winter SF. Functional neoangiogenesis imaging of genetically engineered mouse prostate cancer using three-dimensional power Doppler ultrasound. Cancer Res. 2007;67:2830-2839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Xing W, Wang L, Maslov K, Wang LV. Integrated optical- and acoustic-resolution photoacoustic microscopy based on an optical fiber bundle. Opt Lett. 2013;38:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Yang JM, Favazza C, Chen R, Yao J, Cai X, Maslov K, Zhou Q, Shung KK, Wang LV. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat Med. 2012;18:1297-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |