Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3513
Peer-review started: June 1, 2018
First decision: July 6, 2018
Revised: July 11, 2018
Accepted: July 22, 2018
Article in press: July 22, 2018
Published online: August 21, 2018
Processing time: 77 Days and 15.2 Hours
Primary biliary cholangitis (PBC) is an autoimmune cholestatic liver disease with multiple debilitating complications. Osteoporosis is a common complication of PBC resulting in frequent fractures and leading to significant morbidity in this population, yet evidence for effective therapy is lacking. We sought to summarize our current understanding of the pathophysiology of osteoporosis in PBC, as well as current and emerging therapies in order to guide future research directions. A complete search with a comprehensive literature review was performed with studies from PubMed, EMBASE, Web of Science, Cochrane database, and the Countway Library. Osteoporosis in PBC is driven primarily by decreased bone formation, which differs from the increased bone resorption seen in postmenopausal osteoporosis. Despite this fundamental difference, current treatment recommendations are based primarily on experience with postmenopausal osteoporosis. Trials specific to PBC-related osteoporosis are small and have not consistently demonstrated a benefit in this population. As it stands, prevention of osteoporosis in PBC relies on the mitigation of risk factors such as smoking and alcohol use, as well as encouraging a healthy diet and weight-bearing exercise. The primary medical intervention for the treatment of osteoporosis in PBC remains bisphosphonates though a benefit in terms of fracture reduction has never been shown. This review outlines what is known regarding the pathogenesis of bone disease in PBC and summarizes current and emerging therapies.
Core tip: This article reviews the available literature on the pathophysiology and management of osteoporosis in primary biliary cholangitis (PBC). PBC-related osteoporosis is driven mainly by decreased bone formation as opposed to the increased bone resorption seen in postmenopausal osteoporosis. Despite this and a lack of evidence of efficacy, bisphosphonates remain the cornerstone of treatment. Future attention should be given to the use of anabolic bone agents in the treatment of PBC-related osteoporosis.
- Citation: Danford CJ, Trivedi HD, Papamichael K, Tapper EB, Bonder A. Osteoporosis in primary biliary cholangitis. World J Gastroenterol 2018; 24(31): 3513-3520
- URL: https://www.wjgnet.com/1007-9327/full/v24/i31/3513.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i31.3513
Primary biliary cholangitis (PBC) is a chronic, progressive cholestatic liver disease associated with numerous extrahepatic complications. Osteoporosis, a disease of decreased bone density and strength, is a common complication of PBC[1-3]. The prevalence of osteoporosis in patients with PBC is roughly 30%[1-3] and higher in advanced stages of liver disease, up to 44% in those awaiting liver transplantation[4]. Osteoporosis is a major risk factor for fractures[3,5,6]. The incidence of fractures in PBC ranges from 0-14% over a two-year period[6-9]. The prevalence of fractures is reported to be 10%-20%[3,5,6], increasing to 22% in transplant waitlisted patients[4]. Serious injuries such as fractures are more difficult to manage in patients with advanced liver disease with perioperative morbidity and mortality approaching 80% and 60% respectively in cirrhotics undergoing emergent total hip arthroplasty[10]. Patients would therefore benefit from a timely diagnosis, effective risk factor modification, and treatment of PBC-related osteoporosis.
While effective treatments exist for postmenopausal osteoporosis, management of osteoporosis in PBC is limited by an incomplete understanding of pathophysiology specific to this disease process and a paucity of studies evaluating potential treatment. The current American Association for the Study of Liver Diseases guidelines recommend vitamin D and calcium supplementation based on experience in postmenopausal women as well as alendronate based on a single RCT in PBC[11]. An overreliance on postmenopausal osteoporosis data may lead to relatively ineffective treatment of PBC-related osteoporosis. To this end, we aim to summarize our current understanding of the pathophysiology behind bone disease in PBC as well as the evidence behind current and emerging therapies for osteoporosis in PBC.
The term hepatic osteodystrophy is sometimes used to refer to all metabolic bone disease seen in chronic liver disease. It refers to both osteomalacia, or decreased bone mineralization, and osteoporosis, or decreased bone mass, which can both be seen in advanced liver disease. Osteomalacia was once thought to be common in cholestatic liver disease; theoretically due dietary vitamin D malabsorption in the setting of severe cholestasis and impaired hepatic 25-hydroxylation[12,13]. However, it appears osteomalacia is actually quite rare in PBC and cholestatic liver disease and early studies were plagued by selection bias and a loose definition of osteomalacia[14]. It is now widely accepted that osteoporosis is the primary metabolic bone disease in PBC[15] and this review will focus on the management of osteoporosis in PBC.
The pathogenesis of osteoporosis in PBC appears to be largely driven by decreased bone formation, though increased bone resorption may play a role in certain scenarios. Several studies evaluating bone histomorphometry have shown decreased tetracycline double-labeling, decreased bone formation rates, decreased osteoblast numbers, and decreased serum osteocalcin, a marker of bone formation, all pointing towards osteoblast dysfunction and deficient bone formation as central to the pathogenesis of PBC-related osteoporosis[5,16-18].
Osteoblast dysfunction is a multifactorial process caused both by decreased osteoblast stimulation and increased osteoblast inhibition. Serum levels of insulin-like growth factor-1 (IGF-1), an osteoblast trophic factor, are lower in cirrhotics compared to controls[19]. Supplementation of IGF-1 in cirrhotic rats results in improvement in bone mass and bone density[20]. Vitamin K is also involved in bone metabolism through carboxylation of the non-collagenous bone protein, osteocalcin, and has been shown to stimulate osteoblastogenesis and inhibit osteoclastogenesis[21,22]. Vitamin K levels may be decreased in patients with severe cholestasis and impaired fatty-soluble vitamin absorption resulting in impaired osteoblast function. Indeed a meta-analysis does indicate some reduction in bone loss with vitamin K supplementation, but was not performed exclusively in patients with chronic liver disease[23].
Elevated levels of bilirubin, bile salts, and altered fibronectin production may also play a role in decreased bone formation through osteoblast inhibition in PBC. In one study, plasma mitogenic activity of osteoblasts was significantly lower in patients with cholestatic liver disease compared to healthy controls[24]. In addition, removal of bilirubin by plasma photobleaching resulted in improved plasma mitogenic activity[24]. Similarly, elevated lithocolic acid concentrations have been shown to decrease osteoblast survival through impaired vitamin D stimulation of osteoblast gene transcription[25]. Patients with chronic liver disease also exhibit altered hepatic fibronectin production, resulting in increased production of a fibronectin isoform containing the oncofetal domain, which inhibits osteoblast-mediated mineralization in humans and mice[26].
Increased bone resorption may also play a role in osteoporosis in PBC in certain populations such as post-menopausal women and men with hypogonadism[5,14,16]. One study showed increased osteoblast numbers and increased eroded surface area indicative of increased resorption, but only in female patients with cholestatic liver disease[16]. Estrogen promotes apoptosis of osteoclasts and its absence results in a sharp decline in bone mineral density (BMD) after menopause[27,28]. Men with cholestatic liver disease show no signs of increased bone resorption despite similar degrees of osteopenia[16].
Theoretically, calcium and vitamin D deficiencies may develop in those with cholestatic liver disease leading to secondary hyperparathyroidism and increased bone resorption. Data is conflicting, however. Some studies have found decreased calcium absorption and serum vitamin D levels in PBC patients compared to controls[5,29], but others have found normal vitamin D, calcium, and PTH levels even among osteoporotic patients with PBC[16,17,26]. In addition, vitamin D supplementation to normal levels has not been shown to improve BMD in PBC[30,31]. While vitamin D absorption may be impaired in those taking cholestyramine, this is overcome by increasing the oral dose of vitamin D[32].
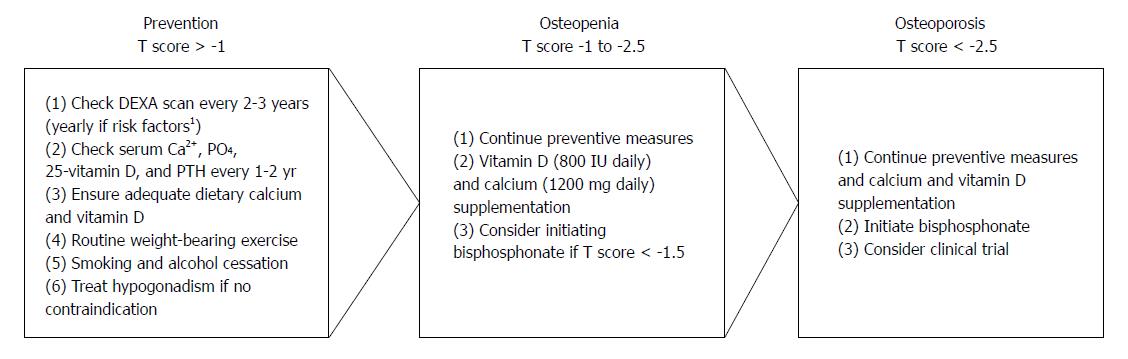
No data exists as to the optimal timing of screening and monitoring for osteoporosis in PBC, however, expert opinion recommends bone densitometry be performed in all PBC patients at diagnosis[11]. The World Health Organization defines osteoporosis as BMD at the spine or proximal femur less than 2.5 standard deviations (SDs) below the mean of a young adult population (expressed as a T score)[33]. Osteopenia refers to those with a T score between -1.0 and -2.5 SDs below the mean. Patients who initially have normal bone densitometry should be reassessed every 2-3 years with repeat bone densitometry, while those with additional risk factors for low bone density and fractures (i.e., severe cholestasis, long-term corticosteroid use, postmenopausal women, BMI < 19, menopause before age 45, alcohol abuse, smoking) should be reassessed annually. Serum calcium, phosphorus, 25-vitamin D, and parathyroid hormone levels should also be checked at diagnosis of PBC and yearly thereafter[14,34,35]. Patients in whom treatment has been initiated should have repeat BMD measured every 1-2 years[36].
General measures to prevent bone loss in PBC are extrapolated from osteoporosis risk factors in the general population. Epidemiologic data suggests lifestyle factors such as tobacco and alcohol use, low dietary calcium intake, and low levels of exercise are associated with decreased bone density and fracture risk[33]. Based on this, tobacco and alcohol cessation are recommended in all PBC patients to reduce risk of bone loss. A balanced diet with adequate levels of calcium and vitamin D should also be encouraged. General recommendations for daily dietary calcium and vitamin D intake in at-risk populations (women older than 50) are 1200 mg and 800 IU daily, respectively[33].
Evidence for routine weight-bearing exercise comes from menopausal women. In a 16-year prospective study of early-postmenopausal women with mild osteopenia, those who exercised regularly had significant improvement in BMD compared to controls. This finding was sustained and even greater at 16 years compared to 4[37].
Timing of treatment is based on recommendations from the postmenopausal osteoporosis literature[36]. Treatment should be initiated in all PBC patients with osteoporosis (T score < -2.5). The ideal time to start treatment in those with osteopenia (T score < -1 to -2.5) is less well established. The National Osteoporosis Foundation recommends treatment in individuals with a prior hip or vertebral fracture or 10-year hip fracture risk ≥ 3% or osteoporosis-related fracture risk ≥ 20% based on the World Health Organization Fracture Risk Assessment Tool in those with osteopenia[36]. In PBC, patients with a T score < 1.5 appear to be at increased risk of fracture[3] and initiation of specific treatment could be considered at this point (Figure 1). A number of different therapeutic approaches have been evaluated, however, most are limited by small sample size and short follow-up (Tables 1 and 2). The duration of treatment is unclear even in the postmenopausal population, though the National Osteoporosis Foundation emphasizes that treatment should not be indefinite and generally is continued for anywhere from 2 to 5 years based on individual risk assessment[36].
| Agent | Treatment | No. of patients (n) | BMD changes at 1 yr (%) | BMD changes at 2 yr (%) | Fractures (n) |
| Etidronate | 400 mg/d (3 mo cycles) | 6 (etidronate) | +1.0 (L), +0.2 (F) | N/A | 0 |
| Wolfhagen et al[43], 1997 | 6 (no treatment)3 | -1.7 (L), +0.4 (F) | 0 | ||
| Lindor et al[6], 2000 | 400 mg/d (3 mo cycles) | 29 (etidronate) | +0.7 (L), +1.3 (F) | +1.0 (L), +0.5 (F) | 4 (V) |
| 31 (placebo) | -0.6 (L), +0.9 (F) | +2.6 (L), +0.8 (F) | 4 (V) | ||
| Alendronate | 70 mg/wk | 15 (alendronate) | +10.4 (L)12, +1.4 (F)1 | N/A | 1 (V), 0 (P) |
| Zein et al[44], 2005 | 13 (placebo) | -0.1 (L)1, -2.1 (F)1 | 0 (V), 1 (P) | ||
| HRT | 50 mcg twice weekly TD estradiol + 2.5 mg/d progestin | 8 (HRT) | +3.1 (L)2, +1.7 (F)12 | N/A | 0 |
| Ormarsdottir et al[51], 2004 | 9 (no treatment)3 | +1.0 (L), -0.6 (F)1 | 0 | ||
| Boone et al[9], 2006 | 0.05 mg/d TD estradiol + 0.25 mg/d TD progestin | 8 (HRT) | N/A | -0.6 (L), +0.2 (F) | 0 (V) |
| 14 (placebo) | -0.8 (L), -3.7 (F)2 | 2 (V) | |||
| Sodium fluoride | 50 mg/d sodium fluoride | 8 (fluoride) | N/A | +2.9 (L)1 | 0 |
| Guañabens et al[7], 1992 | 8 (placebo) | -6.6 (L)12 | 0 | ||
| Calcitriol | 0.5 mcg/d BID calcitriol | 17 (calcitriol) | +0.1 (L)1 | N/A | N/A |
| Shiomi et al[38], 1999 | 17 (no treatment)3 | -3.1 (L)1 | |||
| Vitamin K | 45 mg/d vitamin K2 | 15 (vitamin K) | +0.3 (L)1 | -0.8 (L)1 | N/A |
| Nishiguchi et al[57], 2001 | 15 (no treatment)3 | -3.5 (L)1 | -6.9 (L)1 |
| Agent | Treatment | No. of patients (n) | BMD changes at 1 yr (%) | BMD changes at 2 yr (%) | Fractures (n) |
| Guañabens et al[39], 1997 | |||||
| Etidronate | 400 mg/d (3 mo cycles) | 13 (etidronate) | -0.1 (L), -0.4 (F) | +0.5 (L)2, -0.2 (F) | 0 (V), 3 (P) |
| Sodium fluoride | 50 mg/d sodium fluoride | 10 (fluoride) | -1.7 (L), -0.6 (F) | -2.1 (L), -1.5 (F) | 2 (V), 2 (P) |
| Guañabens et al[45], 2003 | |||||
| Alendronate | 10 mg/d | 13 (alendronate) | +5.8 (L)12, +3.9 (F)12 | 0 (V), 2 (P) | |
| Etidronate | 400 mg/d (3 mo cycles) | 13 (etidronate) | +1.9 (L)1, +0.4 (F)1 | 0 (V), 1 (P) | |
| Guañabens et al[2], 2005 | |||||
| Alendronate | 10 mg/d | 16 (alendronate) | +3.3 (L)2, +1.2 (F)2 | ||
| Alendronate | 70 mg/wk | 10 (alendronate) | +1.2 (L), -0.3 (F) | ||
| Guañabens et al[46], 2013 | |||||
| Ibandronate | 150 mg/mo | 14 (ibandronate) | +3.8 (L), +1.0 (F) | +5.7 (L)2, +1.1 (F) | 0 (V), 0 (P) |
| Alendronate | 70 mg/wk | 19 (alendronate) | +4.6 (L), +1.4 (F) | +4.5 (L)2, +2.5 (F) | 1 (V), 0 (P) |
In PBC patients with osteopenia or osteoporosis, vitamin D and calcium supplementation has not been shown to improve BMD or reduce fracture risk, though given few side effects and potential for deficiency, supplementation is generally recommended[14,34,35]. The only randomized controlled trial (RCT) looking at calcitriol supplementation did not find a significant improvement in BMD from baseline at one year, though BMD did significantly worsen in those who received no treatment[38]. Most studies use some combination of calcium and vitamin D supplementation as standard of care in both the intervention and control groups, though notably, BMD continues to worsen in the control group despite supplementation[9,39,40].
Bisphosphonates reduce bone resorption and are effective in increasing BMD while reducing fractures in postmenopausal osteoporosis[41]. Their effectiveness in PBC is not as clear due to the small number of studies with small study size and short follow-up. A 2011 Cochrane review of bisphosphonates in PBC concluded there was insufficient data supporting improved BMD and fracture risk[42]. It appears etidronate is not effective in improving BMD and preventing fractures in PBC. In two RCTs comparing etidronate to calcium alone or placebo, neither demonstrated an improvement in BMD from baseline with etidronate[6,43], though one did show significantly less bone loss compared to calcium alone[43]. Neither showed a difference in fracture risk over 1-2 years of follow-up[6,43].
Alendronate, a nitrogen-containing bisphosphonate as opposed to etidronate, has had more promising results in PBC. Only one RCT has been performed comparing alendronate to placebo in PBC[44]. In this study, 34 patients were randomized to receive oral alendronate or placebo. After 1 year, patients in the alendronate arm experienced a 10.4% increase in lumbar spine BMD compared to -0.12% for placebo[44]. However, there was no difference in fracture incidence over a year and the study included only one male and two post-menopausal females[44].
Several studies have also compared bisphosphonates to one another. Alendronate was found to be superior to etidronate in terms of BMD improvement in a small trial of 32 women with PBC and osteopenia in which patients were randomized to receive cyclical etidronate or weekly alendronate[45]. The alendronate group had significantly higher lumbar BMD (5.8%) at the end of 2 years compared to the etidronate group (1.9%). There was no significant improvement in either lumbar or femoral BMD in the etidronate group from baseline. However, 2 patients in the alendronate group experienced new non-vertebral fractures during the study period compared to 1 in the etidronate group[45].
Ibandronate was also compared to alendronate in a more recent study of 42 post-menopausal women with PBC and osteoporosis[46]. In this study, women were randomized to receive IV ibandronate monthly or weekly oral alendronate. There was no significant difference in BMD between the 2 groups at the end of 2 years, however, compliance was significantly better in the ibandronate group[46]. One patient in the alendronate group developed a new vertebral fracture. Another advantage of IV administration is the avoidance of the theoretical concern of esophagitis and variceal bleeding with oral bisphosphonates in cirrhotics. However, none of the studies with alendronate and etidronate noted any esophagitis or variceal bleeds[6,40,43-46].
Evidence for the use of bisphosphonates in PBC is limited with only one small trial of alendronate showing improvement in BMD compared to placebo, though this occurred almost exclusively in postmenopausal women, and none demonstrating fracture risk reduction[6,43,44]. Similarly powered trials in postmenopausal osteoporosis have shown improvement benefit in terms of both fracture reduction and BMD improvement[47], and the PBC-specific benefit of bisphosphonates remains unclear.
Estrogens also have strong anti-resorptive effect on bones and for a period of time were widely used in postmenopausal osteoporosis[48]. Concerns over worsening of cholestasis initially limited their use in PBC. However, several observational studies did not show any significant worsening in liver disease with hormone replacement therapy (HRT)[49,50] and subsequently two RCTs similarly found no worsening in liver disease[9,51]. In these RCTs, those randomized to transdermal estrogen and progesterone had femoral and vertebral BMD compared to controls after 1-2 years of treatment[9,51], though only one showed an improvement in BMD from baseline[51] and neither showed a reduction in fracture risk[9,51]. In addition, both studies noted an increase in noncholestatic adverse events, such as vaginal bleeding and headaches, in the HRT arm leading to increased dropout[9,51]. Hormone replacement is also associated with increased risk of venous thromboembolism, stroke, ischemic heart disease, and breast cancer and is not recommended in women older than age 60 or greater than 10 years after menopause[52]. Hormone replacement may be effective in improving BMD in PBC, though no improvement in fracture risk has been shown and side effects limit their use.
Calcitonin has been shown to have some effect in postmenopausal osteoporosis, however, data in PBC is lacking. A crossover study of IV calcitonin for 6 mo compared to oral calcium supplementation did not find a significant difference between the two and BMD ultimately fell in both groups[53]. A 3-year study of vitamin D, calcium, and IM calcitonin found significantly less BMD loss in treatment patients compared to controls who received no treatment[54,55]. However, there was no significant improvement in the calcitonin group and, since the control group received no therapy, the stabilization of BMD may have been from calcium and vitamin D supplementation[55].
Raloxifene is a selective estrogen receptor modulator that maintains the antiresorptive effects of estrogen in bone with anti-estrogen effects in the uterus and breast. A single pilot study has examined the efficacy of raloxifene in PBC. In this study, 9 women with PBC treated with raloxifene were compared to 3 age-matched controls[56]. After 1 year, there was a small (0.02 g/cm2vs 0.00 g/cm2) but significant improvement in lumbar BMD compared to controls, though there was no difference in femoral BMD and no data on fracture risk[56].
Sodium fluoride increases bone formation and has been shown to be effective in postmenopausal osteoporosis, but is not as effective as anti-resorptive agents. One randomized, controlled trial examined sodium fluoride in PBC and found a significant increase in BMD (+2.9%) compared to placebo (-6.6%) in 22 women with PBC followed for 2 years[7]. No fractures occurred in either group[7]. A subsequent trial comparing sodium fluoride to etidronate, however, found etidronate to be superior and actually observed a decrease in femoral BMD in the sodium fluoride group after 2 years[39]. In addition, 2 patients in the sodium fluoride group experienced new vertebral fractures compared to none in the etidronate group[39].
Patients with severe cholestasis may have decreased absorption of fat-soluble vitamins, such as vitamin K. Vitamin K is involved in bone formation through stimulation of osteoblastogenesis[21,22]. One meta-analysis did indicate some reduction in bone loss with vitamin K supplementation, but did not focus on patients with cholestatic liver disease[23]. One small RCT in PBC found significantly less bone loss in vitamin K patients compared to controls, but was ultimately not effective in improving BMD[57].
Human parathyroid hormone (PTH) improves BMD and reduces fractures in postmenopausal osteoporosis through stimulating bone formation, the major driver of osteoporosis in PBC[58,59]. The recombinant form consisting of the bioactive portion of the hormone (teriparatide) is approved for treatment of postmenopausal osteoporosis, but has not been studied in PBC. Recombinant human PTH 1-34 (rhPTH 1-34) has been studied in rats that have undergone biliary ductal ligation[60]. In this study, rats that underwent biliary ductal ligation had significant worsening in BMD compared to rats that underwent a sham operation. Biliary ductal ligation rats were then administered rhPTH 1-34 at 40 and 80 μg/kg/d. Those rats who received 40 μg/kg/d experienced significant improvement in femoral and tibial BMD compared to untreated rats and those who received 80 μg/kg/d (who did not experience significant improvement compared to untreated rats)[60]. No trials have been undertaken in humans with PBC.
Therapies directed at PBC itself have not been shown to improve bone disease. Patients enrolled in a randomized, controlled trial of ursodeoxycholic acid (UDCA) were followed over a 3-year period with dual-photon densitometry annually. After 3 years, there was no significant difference in lumbar BMD between UDCA and placebo[61].
In the initial phase 3 trial of obeticholic acid, BMD was measured at baseline and 12 mo[62]. BMD continued to decline in the obeticholic acid groups (both 5-10 mg and 10 mg groups), however, this decline was significantly less at the femoral neck compared to placebo. BMD decreased in both groups at the lumbar spine as well and, while there was a trend toward less decline in the obeticholic acid groups, there was no significant difference[62]. There was no difference in fracture rates[62].
No data currently exists evaluating the effects of fibrates on BMD when used to treat PBC.
Concerns over long-term efficacy and safety of bisphosphonate use in postmenopausal osteoporosis have led to ongoing development of new medications. Abaloparatide, a parathyroid hormone-related peptide analog, was recently approved by the Food and Drug Administration for treatment of postmenopausal osteoporosis and may avoid the pro-resorptive and hypercalcemic effects of teriparatide[63]. Along with teriparatide, these recombinant human PTH agents differ in that they promote bone formation, the primary deficit in PBC, as opposed to decrease resorption, the driving issue in postmenopausal osteoporosis and the current target of most treatments. Based on our understanding of the pathogenesis of osteoporosis in PBC, future studies should include therapies that promote bone formation and ample male and premenopausal female PBC patients to decrease the likelihood that results reflect only treatment of postmenopausal osteoporosis.
Osteoporosis is a common complication of PBC resulting in a significantly increased risk of fractures especially in those with a T score < -1.5. Severity of bone disease is related to the severity and duration of the underlying liver disease as well as increasing age. The mechanism of decreased bone density primarily involves decreased bone formation resulting from decreased osteoblast function. Increased bone resorption may also play a role in postmenopausal women.
For prevention of osteoporosis, mitigation of risk factors is recommended through smoking and alcohol cessation as well as a balanced diet and regular weight-bearing exercise. Treatment is recommended in patients who have experienced fractures or have osteoporosis (T score < -2.5), though may also be considered in those with a T score < -1.5 in PBC. No treatment has been adequately shown to reduce fractures in PBC, though the bisphosphonates ibandronate and alendronate may be effective in increasing BMD. HRT may also be effective in improving BMD in PBC, though with more side effects. Treatment of the underlying liver disease with UDCA or OCA does not appear to effectively treat the bone disease. Ultimately further research is required, with special attention to anabolic bone agents, to identify effective treatment in PBC-related osteoporosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Takahashi T S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Newton J, Francis R, Prince M, James O, Bassendine M, Rawlings D, Jones D. Osteoporosis in primary biliary cirrhosis revisited. Gut. 2001;49:282-287. [PubMed] [DOI] [Full Text] |
| 2. | Guañabens N, Parés A, Ros I, Caballería L, Pons F, Vidal S, Monegal A, Peris P, Rodés J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573-577. [PubMed] [DOI] [Full Text] |
| 3. | Guañabens N, Cerdá D, Monegal A, Pons F, Caballería L, Peris P, Parés A. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138:2348-2356. [PubMed] [DOI] [Full Text] |
| 4. | Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12:1390-1402. [PubMed] [DOI] [Full Text] |
| 5. | Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85:1356-1362. [PubMed] |
| 6. | Lindor KD, Jorgensen RA, Tiegs RD, Khosla S, Dickson ER. Etidronate for osteoporosis in primary biliary cirrhosis: a randomized trial. J Hepatol. 2000;33:878-882. [PubMed] [DOI] [Full Text] |
| 7. | Guañabens N, Parés A, del Rio L, Roca M, Gómez R, Muñoz J, Rodés J. Sodium fluoride prevents bone loss in primary biliary cirrhosis. J Hepatol. 1992;15:345-349. [PubMed] |
| 8. | Guañabens N, Parés A, Navasa M, Martínez de Osaba MJ, Hernández ME, Muñoz J, Rodés J. Cyclosporin A increases the biochemical markers of bone remodeling in primary biliary cirrhosis. J Hepatol. 1994;21:24-28. [PubMed] |
| 9. | Boone RH, Cheung AM, Girlan LM, Heathcote EJ. Osteoporosis in primary biliary cirrhosis: a randomized trial of the efficacy and feasibility of estrogen/progestin. Dig Dis Sci. 2006;51:1103-1112. [PubMed] [DOI] [Full Text] |
| 10. | Cohen SM, Te HS, Levitsky J. Operative risk of total hip and knee arthroplasty in cirrhotic patients. J Arthroplasty. 2005;20:460-466. [PubMed] [DOI] [Full Text] |
| 11. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [PubMed] [DOI] [Full Text] |
| 12. | Long RG, Varghese Z, Meinhard EA, Skinner RK, Wills MR, Sherlock S. Parenteral 1,25-dihydroxycholecalciferol in hepatic osteomalacia. Br Med J. 1978;1:75-77. [PubMed] |
| 13. | Compston JE, Crowe JP, Horton LW. Treatment of osteomalacia associated with primary biliary cirrhosis with oral 1-alpha-hydroxy vitamin D3. Br Med J. 1979;2:309. [PubMed] |
| 14. | Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124:795-841. [PubMed] [DOI] [Full Text] |
| 15. | Hay JE. Bone disease in cholestatic liver disease. Gastroenterology. 1995;108:276-283. [PubMed] |
| 16. | Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Bone metabolism in advanced cholestatic liver disease: analysis by bone histomorphometry. Hepatology. 2002;36:895-903. [PubMed] [DOI] [Full Text] |
| 17. | Hodgson SF, Dickson ER, Wahner HW, Johnson KA, Mann KG, Riggs BL. Bone loss and reduced osteoblast function in primary biliary cirrhosis. Ann Intern Med. 1985;103:855-860. [PubMed] |
| 18. | Ackerman Z, Weinreb M, Amir G, Pollak RD. Bone mineral metabolism and histomorphometry in rats with cholestatic liver disease. Liver. 2002;22:166-172. [PubMed] |
| 19. | Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695-699. [PubMed] [DOI] [Full Text] |
| 20. | Cemborain A, Castilla-Cortázar I, García M, Quiroga J, Muguerza B, Picardi A, Santidrián S, Prieto J. Osteopenia in rats with liver cirrhosis: beneficial effects of IGF-I treatment. J Hepatol. 1998;28:122-131. [PubMed] [DOI] [Full Text] |
| 21. | Houben RJ, Rijkers DT, Stanley TB, Acher F, Azerad R, Käkönen SM, Vermeer C, Soute BA. Characteristics and composition of the vitamin K-dependent gamma-glutamyl carboxylase-binding domain on osteocalcin. Biochem J. 2002;364:323-328. [PubMed] |
| 22. | Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol. 2003;176:339-348. [PubMed] [DOI] [Full Text] |
| 23. | Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. [PubMed] [DOI] [Full Text] |
| 24. | Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95:2581-2586. [PubMed] [DOI] [Full Text] |
| 25. | Ruiz-Gaspà S, Guañabens N, Enjuanes A, Peris P, Martinez-Ferrer A, de Osaba MJ, Gonzalez B, Alvarez L, Monegal A, Combalia A. Lithocholic acid downregulates vitamin D effects in human osteoblasts. Eur J Clin Invest. 2010;40:25-34. [PubMed] [DOI] [Full Text] |
| 26. | Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A, Singer MV, Nakchbandi IA. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res. 2008;23:1278-1286. [PubMed] [DOI] [Full Text] |
| 27. | Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2:1132-1136. [PubMed] [DOI] [Full Text] |
| 28. | Armas LA, Recker RR. Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol Metab Clin North Am. 2012;41:475-486. [PubMed] [DOI] [Full Text] |
| 29. | Bengoa JM, Sitrin MD, Meredith S, Kelly SE, Shah N, Baker AL, Rosenberg IH. Intestinal calcium absorption and vitamin D status in chronic cholestatic liver disease. Hepatology. 1984;4:261-265. [PubMed] |
| 30. | Herlong HF, Recker RR, Maddrey WC. Bone disease in primary biliary cirrhosis: histologic features and response to 25-hydroxyvitamin D. Gastroenterology. 1982;83:103-108. [PubMed] |
| 31. | Matloff DS, Kaplan MM, Neer RM, Goldberg MJ, Bitman W, Wolfe HJ. Osteoporosis in primary biliary cirrhosis: effects of 25-hydroxyvitamin D3 treatment. Gastroenterology. 1982;83:97-102. [PubMed] |
| 32. | Compston JE, Thompson RP. Intestinal absorption of 25-hydroxyvitamin D and osteomalacia in primary biliary cirrhosis. Lancet. 1977;1:721-724. [PubMed] |
| 33. | Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General. US Heal Hum Serv (Internet). 2004;437. [PubMed] [DOI] [Full Text] |
| 34. | Parés A, Guañabens N. Osteoporosis in primary biliary cirrhosis: pathogenesis and treatment. Clin Liver Dis. 2008;12:407-24. [PubMed] [DOI] [Full Text] |
| 35. | Glass LM, Su GL. Metabolic Bone Disease in Primary Biliary Cirrhosis. Gastroenterol Clin North Am. 2016;45:333-343. [PubMed] [DOI] [Full Text] |
| 36. | Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R; National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359-2381. [PubMed] [DOI] [Full Text] |
| 37. | Kemmler W, Engelke K, von Stengel S. Long-Term Exercise and Bone Mineral Density Changes in Postmenopausal Women--Are There Periods of Reduced Effectiveness? J Bone Miner Res. 2016;31:215-222. [PubMed] [DOI] [Full Text] |
| 38. | Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, Ochi H. Calcitriol for bone loss in patients with primary biliary cirrhosis. J Gastroenterol. 1999;34:241-245. [PubMed] |
| 39. | Guañabens N, Parés A, Monegal A, Peris P, Pons F, Alvarez L, de Osaba MJ, Roca M, Torra M, Rodés J. Etidronate versus fluoride for treatment of osteopenia in primary biliary cirrhosis: preliminary results after 2 years. Gastroenterology. 1997;113:219-224. [PubMed] [DOI] [Full Text] |
| 40. | Musialik J, Petelenz M, Gonciarz Z. Effects of alendronate on bone mass in patients with primary biliary cirrhosis and osteoporosis: preliminary results after one year. Scand J Gastroenterol. 2005;40:873-874. [PubMed] [DOI] [Full Text] |
| 41. | Sanderson J, Martyn-St James M, Stevens J, Goka E, Wong R, Campbell F, Selby P, Gittoes N, Davis S. Clinical effectiveness of bisphosphonates for the prevention of fragility fractures: A systematic review and network meta-analysis. Bone. 2016;89:52-58. [PubMed] [DOI] [Full Text] |
| 42. | Rudic JS, Giljaca V, Krstic MN, Bjelakovic G, Gluud C. Bisphosphonates for osteoporosis in primary biliary cirrhosis. Cochrane Database Syst Rev. 2011;CD009144. [PubMed] [DOI] [Full Text] |
| 43. | Wolfhagen FH, van Buuren HR, den Ouden JW, Hop WC, van Leeuwen JP, Schalm SW, Pols HA. Cyclical etidronate in the prevention of bone loss in corticosteroid-treated primary biliary cirrhosis. A prospective, controlled pilot study. J Hepatol. 1997;26:325-330. [PubMed] |
| 44. | Zein CO, Jorgensen RA, Clarke B, Wenger DE, Keach JC, Angulo P, Lindor KD. Alendronate improves bone mineral density in primary biliary cirrhosis: a randomized placebo-controlled trial. Hepatology. 2005;42:762-771. [PubMed] [DOI] [Full Text] |
| 45. | Guañabens N, Parés A, Ros I, Alvarez L, Pons F, Caballería L, Monegal A, Martínez de Osaba MJ, Roca M, Peris P. Alendronate is more effective than etidronate for increasing bone mass in osteopenic patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98:2268-2274. [PubMed] [DOI] [Full Text] |
| 46. | Guañabens N, Monegal A, Cerdá D, Muxí Á, Gifre L, Peris P, Parés A. Randomized trial comparing monthly ibandronate and weekly alendronate for osteoporosis in patients with primary biliary cirrhosis. Hepatology. 2013;58:2070-2078. [PubMed] [DOI] [Full Text] |
| 47. | El Dareer SM, Tillery KF, Hill DL. Disposition of 5-methyltetrahydrohomofolate in mice, dogs, and monkeys. Cancer Treat Rep. 1979;63:201-207. [PubMed] [DOI] [Full Text] |
| 48. | Hillard TC, Whitcroft SJ, Marsh MS, Ellerington MC, Lees B, Whitehead MI, Stevenson JC. Long-term effects of transdermal and oral hormone replacement therapy on postmenopausal bone loss. Osteoporos Int. 1994;4:341-348. [PubMed] |
| 49. | Olsson R, Mattsson LA, Obrant K, Mellström D. Estrogen-progestogen therapy for low bone mineral density in primary biliary cirrhosis. Liver. 1999;19:188-192. [PubMed] [DOI] [Full Text] |
| 50. | Menon KV, Angulo P, Boe GM, Lindor KD. Safety and efficacy of estrogen therapy in preventing bone loss in primary biliary cirrhosis. Am J Gastroenterol. 2003;98:889-892. [PubMed] [DOI] [Full Text] |
| 51. | Ormarsdóttir S, Mallmin H, Naessén T, Petrén-Mallmin M, Broomé U, Hultcrantz R, Lööf L. An open, randomized, controlled study of transdermal hormone replacement therapy on the rate of bone loss in primary biliary cirrhosis. J Intern Med. 2004;256:63-69. [PubMed] [DOI] [Full Text] |
| 52. | de Villiers TJ, Gass ML, Haines CJ, Hall JE, Lobo RA, Pierroz DD, Rees M. Global Consensus Statement on menopausal hormone therapy. Maturitas. 2013;74:391-392. [PubMed] [DOI] [Full Text] |
| 53. | Camisasca M, Crosignani A, Battezzati PM, Albisetti W, Grandinetti G, Pietrogrande L, Biffi A, Zuin M, Podda M. Parenteral calcitonin for metabolic bone disease associated with primary biliary cirrhosis. Hepatology. 1994;20:633-637. [PubMed] |
| 54. | Floreani A, Chiaramonte M, Giannini S, Malvasi L, Lodetti MG, Castrignano R, Giacomini A, D’Angelo A, Naccarato R. Longitudinal study on osteodystrophy in primary biliary cirrhosis (PBC) and a pilot study on calcitonin treatment. J Hepatol. 1991;12:217-223. [PubMed] [DOI] [Full Text] |
| 55. | Floreani A, Zappala F, Fries W, Naccarato R, Plebani M, D’Angelo A, Chiaramonte M. A 3-year pilot study with 1,25-dihydroxyvitamin D, calcium, and calcitonin for severe osteodystrophy in primary biliary cirrhosis. J Clin Gastroenterol. 1997;24:239-244. [PubMed] [DOI] [Full Text] |
| 56. | Levy C, Harnois DM, Angulo P, Jorgensen R, Lindor KD. Raloxifene improves bone mass in osteopenic women with primary biliary cirrhosis: results of a pilot study. Liver Int. 2005;25:117-121. [PubMed] [DOI] [Full Text] |
| 57. | Nishiguchi S, Shimoi S, Kurooka H, Tamori A, Habu D, Takeda T, Kubo S. Randomized pilot trial of vitamin K2 for bone loss in patients with primary biliary cirrhosis. J Hepatol. 2001;35:543-545. [PubMed] [DOI] [Full Text] |
| 58. | Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434-1441. [PubMed] [DOI] [Full Text] |
| 59. | Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016;316:722-733. [PubMed] [DOI] [Full Text] |
| 60. | Dresner-Pollak R, Gabet Y, Steimatzky A, Hamdani G, Bab I, Ackerman Z, Weinreb M. Human parathyroid hormone 1-34 prevents bone loss in experimental biliary cirrhosis in rats. Gastroenterology. 2008;134:259-267. [PubMed] [DOI] [Full Text] |
| 61. | Lindor KD, Janes CH, Crippin JS, Jorgensen RA, Dickson ER. Bone disease in primary biliary cirrhosis: does ursodeoxycholic acid make a difference? Hepatology. 1995;21:389-392. [PubMed] |
| 62. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [PubMed] [DOI] [Full Text] |
| 63. | Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898-907. [PubMed] [DOI] [Full Text] |