Published online Aug 14, 2018. doi: 10.3748/wjg.v24.i30.3414
Peer-review started: May 10, 2018
First decision: June 11, 2018
Revised: June 27, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: August 14, 2018
To explore the role and mechanism of total flavone of Abelmoschus manihot (TFA) on epithelial-mesenchymal transition (EMT) progress of Crohn’s disease (CD) intestinal fibrosis.
First, CCK-8 assay was performed to assess TFA on the viability of intestinal epithelial (IEC-6) cells and select the optimal concentrations of TFA for our further studies. Then cell morphology, wound healing and transwell assays were performed to examine the effect of TFA on morphology, migration and invasion of IEC-6 cells treated with TGF-β1. In addition, immunofluorescence, real-time PCR analysis (qRT-PCR) and western blotting assays were carried out to detect the impact of TFA on EMT progress. Moreover, western blotting assay was performed to evaluate the function of TFA on the Smad and MAPK signaling pathways. Further, the role of co-treatment of TFA and si-Smad or MAPK inhibitors has been examined by qRT-PCR, western blotting, morphology, wound healing and transwell assays.
In this study, TFA promoted transforming growth factor-β1 (TGF-β1)-induced (IEC-6) morphological change, migration and invasion, and increased the expression of epithelial markers and reduced the levels of mesenchymal markers, along with the inactivation of Smad and MAPK signaling pathways. Moreover, we revealed that si-Smad and MAPK inhibitors effectively attenuated TGF-β1-induced EMT in IEC-6 cells. Importantly, co-treatment of TFA and si-Smad or MAPK inhibitors had better inhibitory effects on TGF-β1-induced EMT in IEC-6 cells than either one of them.
These findings could provide new insight into the molecular mechanisms of TFA on TGF-β1-induced EMT in IEC-6 cells and TFA is expected to advance as a new therapy to treat CD intestinal fibrosis.
Core tip: Regulating transforming growth factor-β (TGF-β) and its downstream signaling pathways, mediating the epithelial-mesenchymal transition (EMT) process and restoring the biological function of abnormally activated intestinal fibroblasts, may be an important way to seek drug therapy for Crohn’s disease (CD) intestinal fibrosis. Total flavone of Abelmoschus manihot (TFA) can inhibit TGF-β1-induced morphological change, migration, invasion of rat intestinal epithelial cells, and promote induction of EMT partially by inhibiting TGF-β1-activated Smad and non-Smad signaling pathways. Therefore, TFA is expected to advance as a new therapy to treat CD intestinal fibrosis, and its continued advancement may open the door to a new class of treatment for CD intestinal fibrosis.
- Citation: Yang BL, Zhu P, Li YR, Xu MM, Wang H, Qiao LC, Xu HX, Chen HJ. Total flavone of Abelmoschus manihot suppresses epithelial-mesenchymal transition via interfering transforming growth factor-β1 signaling in Crohn’s disease intestinal fibrosis. World J Gastroenterol 2018; 24(30): 3414-3425
- URL: https://www.wjgnet.com/1007-9327/full/v24/i30/3414.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i30.3414
Crohn’s disease (CD) is a chronic relapsing inflammation of the gut, which causes significant impairment of quality of life with a rising incidence and prevalence during recent decades[1,2]. Although the clinical manifestations and pathologic progress of CD are different, fibrosis of intestinal organization and strictures induced by transmural inflammation will eventually cause intestinal obstruction, which is the characteristic clinical manifestation[3-5]. In addition, more than 1/3 of CD patients need at least one intestinal operation in their lives, while 70% of the CD patients with fibrosis strictures need partial resection of the intestinal tract within 10 years of disease progression, and 70%-90% patients will have a recurrence of anastomotic strictures and over 50% patients will form new strictures[6,7]. Moreover, a large number of clinical and experimental results have confirmed that the main drugs for treatment of CD, such as glucocorticoids, immune agents and biological agents, can effectively inhibit intestinal inflammation, but do not have positive activity in preventing the further progress of intestinal fibrosis[8,9]. Thus, there is still a lack of drugs that can effectively inhibit or reverse CD intestinal fibrosis.
The process of intestinal fibrosis in CD patients involves a variety of cells and multiple molecular signaling pathways[10,11]. Due to the continuous role of chronic intestinal inflammation, activated T and B cells will produce large amounts of pro-inflammatory cytokines and pro-fibrogenic factors, and induce fibroblast, epithelial cells, endothelial cells and stellate cells to migrate, proliferate, activate and differentiate into myofibroblasts, which finally results in excessive proliferation of myofibroblasts and excessive deposition of extracellular matrix (ECM), leading to the formation of intestinal fibrosis[12-14]. Studies have shown that even if inflammation of the intestinal tract is effectively controlled, the process of fibrosis will continue and eventually lead to intestinal stenosis[15]. Epithelial to mesenchymal transition (EMT) plays an important role in the activation of fibroblasts[16]. Epithelial cells will lose epithelial polarity and epithelial phenotype contacted with basement membrane and produce fibroblasts to repair tissue injury caused by trauma and inflammatory reactions through the EMT progress[17]. In physiological states, when the inflammatory reaction is relieved, the transformation process stops spontaneously. However, in the case of continuous activation of the inflammatory reaction, the EMT process will also continue to exist, and eventually cause organ fibrosis. Under pathophysiologic conditions, when the inflammatory reaction is relieved, the transformation process will stop spontaneously. However, in the case of continuous activation of inflammatory response, the EMT process will also exist continuously, and eventually cause organ fibrosis[18,19]. Nowadays, although the role and regulation mechanism of EMT in CD intestinal fibrosis has not been fully understood, the transforming growth factor-β (TGF-β)/Smad/MAPK signaling pathway has been confirmed to play an important role in regulating EMT in organs such as lung, liver, kidney and so on[20-22]. Therefore, studying the role of EMT in the formation of intestinal fibrosis based on the TGF-β/Smad/MAPK signaling pathway, may provide a new target for the treatment of CD intestinal fibrosis.
Total flavone of Abelmoschus manihot L. Medic (TFA), as the main components of the water extract of traditional Chinese medicine Abelmoschus manihot (L.), has been reported to play an important role in the improvement of renal inflammation, nephrotic syndrome, purpura nephritis, IgA nephropathy, membranous nephropathy and diabetic nephropathy (DN) effectively in the clinical trial[23-26]. Further, our previous research has shown that TFA could significantly inhibit the release of the intestinal inflammatory cytokines tumor necrosis factor (TNF)-α and interferon (IFN)-γ in a CD rat model induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS), improve the model animal survival rate, and effectively improve intestinal inflammation. Moreover, immunohistochemical staining showed that the expression of TGF-β, alpha-smooth muscle actin (α-SMA) and matrix metalloproteinase-2 (MMP-2) were obviously reduced after intervention of TFA. Further, Masson staining confirmed that collagen fibrils of the mucosa and lamina propria of the colon were markedly decreased, indicating that TFA had positive inhibitory effects on CD intestinal fibrosis, which may be related to the EMT mechanism mediated by TGF-β. Thus, we hypothesized that TFA could inhibit or reverse the CD intestinal fibrosis via regulation of EMT based on TGF-β and its downstream Smad and MAPK signaling pathways. Therefore, this study was designed to focus on the influence of EMT on CD intestinal fibrosis and to further explore the role and mechanism of TFA on the progress of CD intestinal fibrosis.
Abelmoschus Manihot L. Medic was collected from Jiangyan of Jiangsu province, China. TFA was extracted from the flowers of Abelmoschus Manihot by the Jiangsu Province Hospital of TCM, Nanjing, China. A total of 500 g of Abelmoschus Manihot flowers was immersed in 8000 mL 75% ethanol for 1 h. The mixture was refluxed for 1 h at 90 °C and filtered by analytical filter paper. The extracts were evaporated by rotary evaporation under vacuum at 60 °C[27]. For cell experiments, TFA was dissolved in dimethyl sulfoxide.
Rat intestinal epithelial (IEC-6) cells were obtained from the American Type Culture Collection (Manassas, VA, United States). Cells were cultured in Dulbecco’s modified eagle’s medium (DMEM, Gibco, United States) supplemented with 10% fetal bovine serum (FBS, Invitrogen, CA, United States), 2 mmol/L GlutaMAX-I (Invitrogen, CA, United States), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) in 5% CO2 atmosphere at 37 °C.
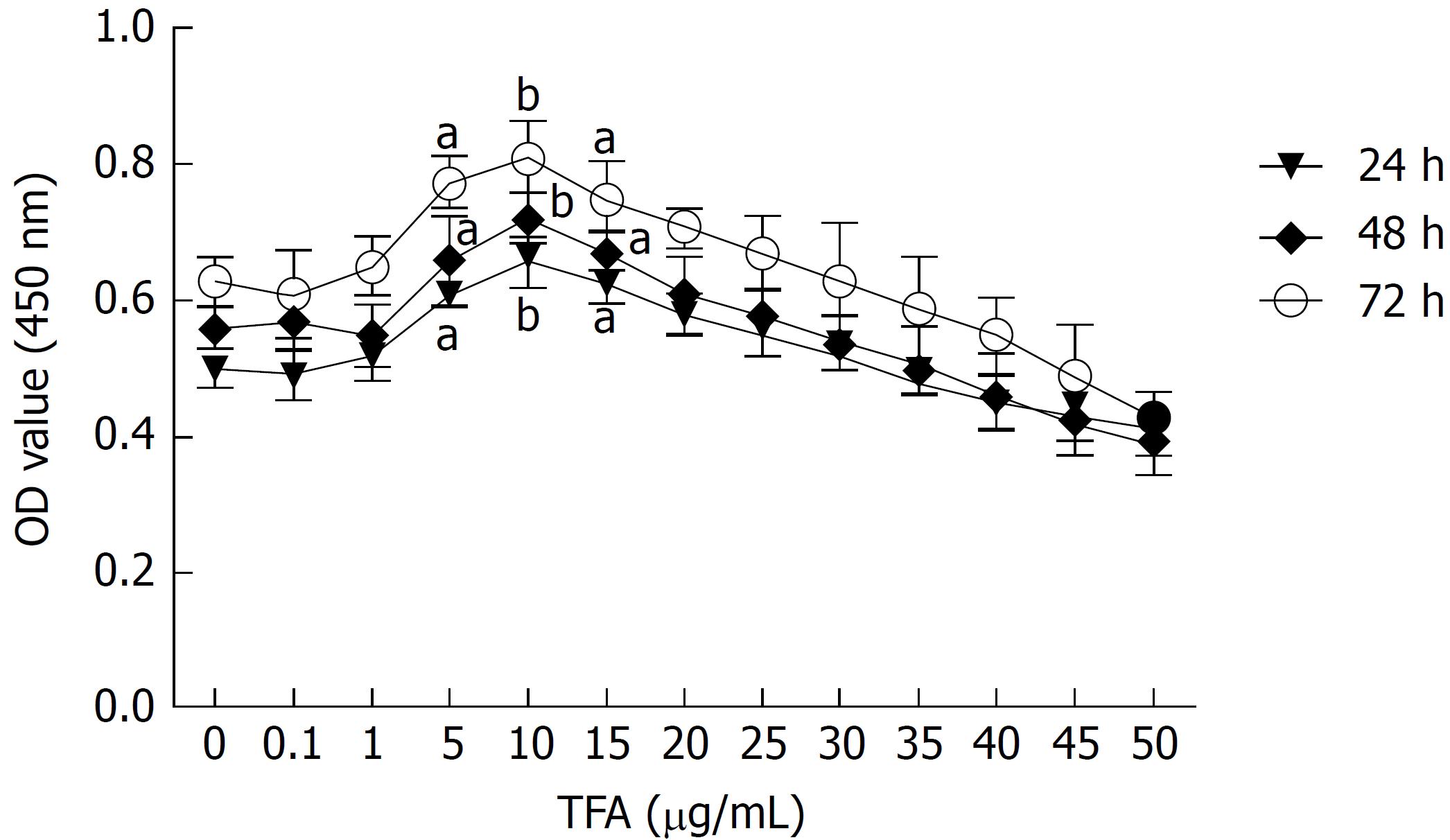
The concentration of TFA for further study was evaluated based on cell viability. Cell viability was determined by Cell Counting Kit-8 assay (CCK-8, Sigma Chemical Co, St Louis, MO, United States). Briefly, IEC-6 cells with a density of 1 × 104 cells/well in 100 μL of complete culture medium were seeded in 96-well plates. After culturing for 24 h, the medium was replaced with serum-free media or serum-free media containing TFA at concentrations ranging from 0.1 μg/mL to 50 μg/mL, and incubated in a humidified incubator at 37 °C for 24, 48 or 72 h. After incubation, 10 μL CCK-8 was added to each well for 2 h at 37 °C. The optical density (OD) was recorded at 450 nm using a microplate reader (Dojindo Molecular Technology, Rockville, MD, United States).
Based on the results in Figure 1A, 5, 10, and 15 μg/mL TFA represented the optimal working concentrations and were used in our subsequent experiments.
Cells were transfected with non-targeting negative control siRNA (Dharmacon, Lafayette, CO, United States) or Smad2/3 (Dharmacon, Lafayette, CO, United States) using LipoRNAiMax according to the manufacturer’s protocol. The cells were maintained for 72 h and then subjected to protein extraction. The antisense and sense oligo template sequences for these siRNAs were: Smad2 siRNA antisense: AAGAGGAGTGCGCTTATATTACCTGTCTC, sense: AATAATATAAGCGCACTCCTCCCTGTCTC; Smad3 siRNA antisense: AATATTCCAGAAACCCCACCCCCTGTCTC, sense: AAGGGTGGGGTT TCTGGAATACCTGTCTC.
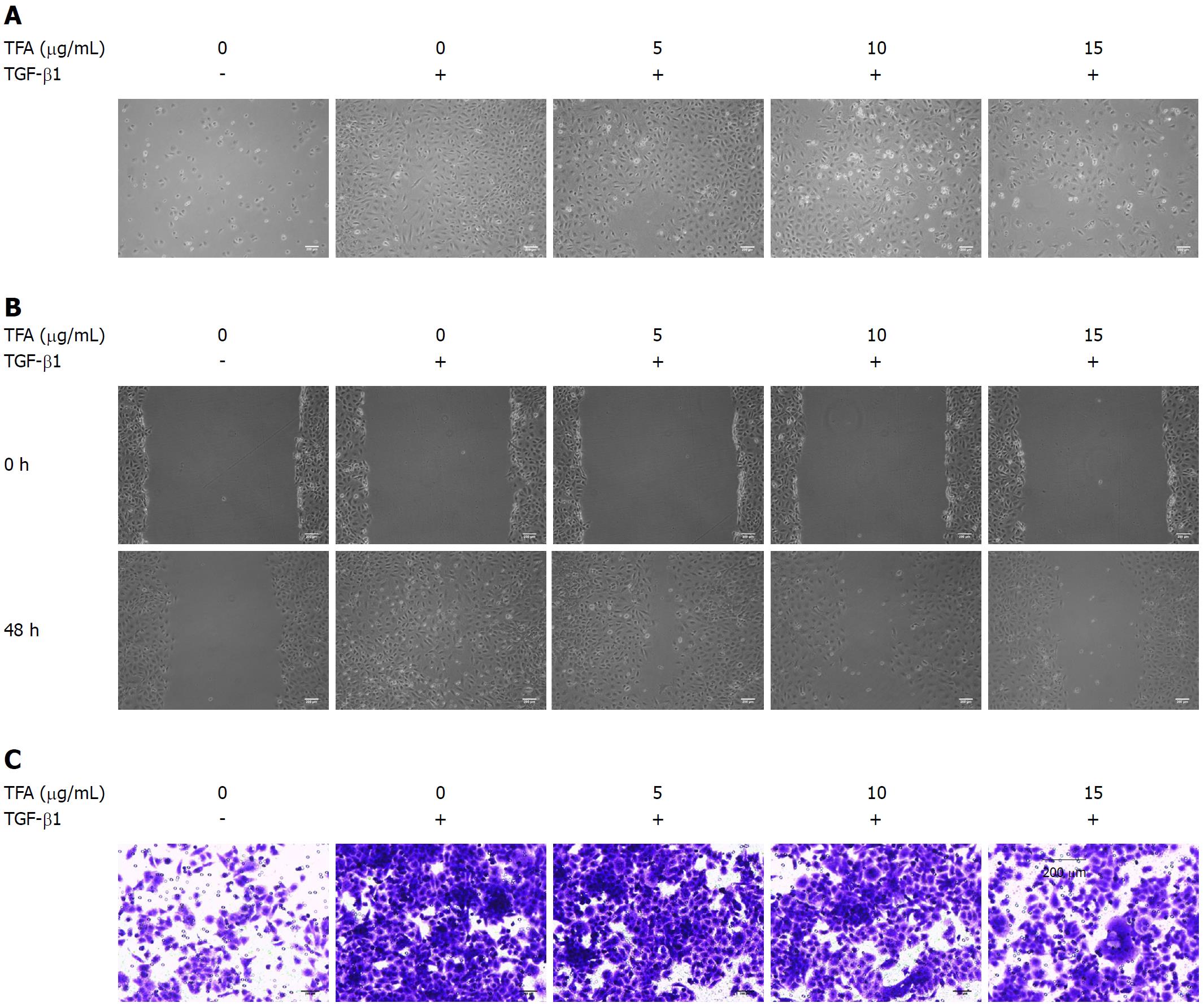
IEC-6 cells were seeded into 6-well plates at a density of 6 × 105 cells per well, and were cultured in fresh culture media to full confluence. After that, we created a wound using a plastic scraper. After being washed with PBS, the medium was replaced with 10 ng/mL TGF-β1[14] and TFA, and incubated at 37 °C for 48 h. The cell migration images were photographed at 0 and 48 h following scraping. Three to four different fields were visualized and photographed under a microscope (Nikon, Tokyo, Japan).
The cell invasion assay was performed using Trans-well chambers (8 μm pore-size, Corning, United States). Matrigel was purchased from BD Biosciences and stored at -20 °C. After thawing at 4 °C overnight, the matrigel was diluted in serum-free medium, and 30 μL of the diluted matrigel were evenly inoculated into the upper chamber to form a gel at 37 °C. Cells (1 × 105) suspended in 300 μL of serum-free medium were seeded into the upper compartments and treated with 10 ng/mL TGF-β1 and TFA for 48 h, and the lower compartments were filled with 600 μL of medium with 20% FBS. After incubation, the non-invasive cells were removed from the upper surface of the membrane by scrubbing. The cells that invaded to the lower surface of the membrane were fixed with 4% paraformaldehyde and stained in 10% crystal violet. Cells were counted under a microscope (Olympus, Tokyo, Japan).
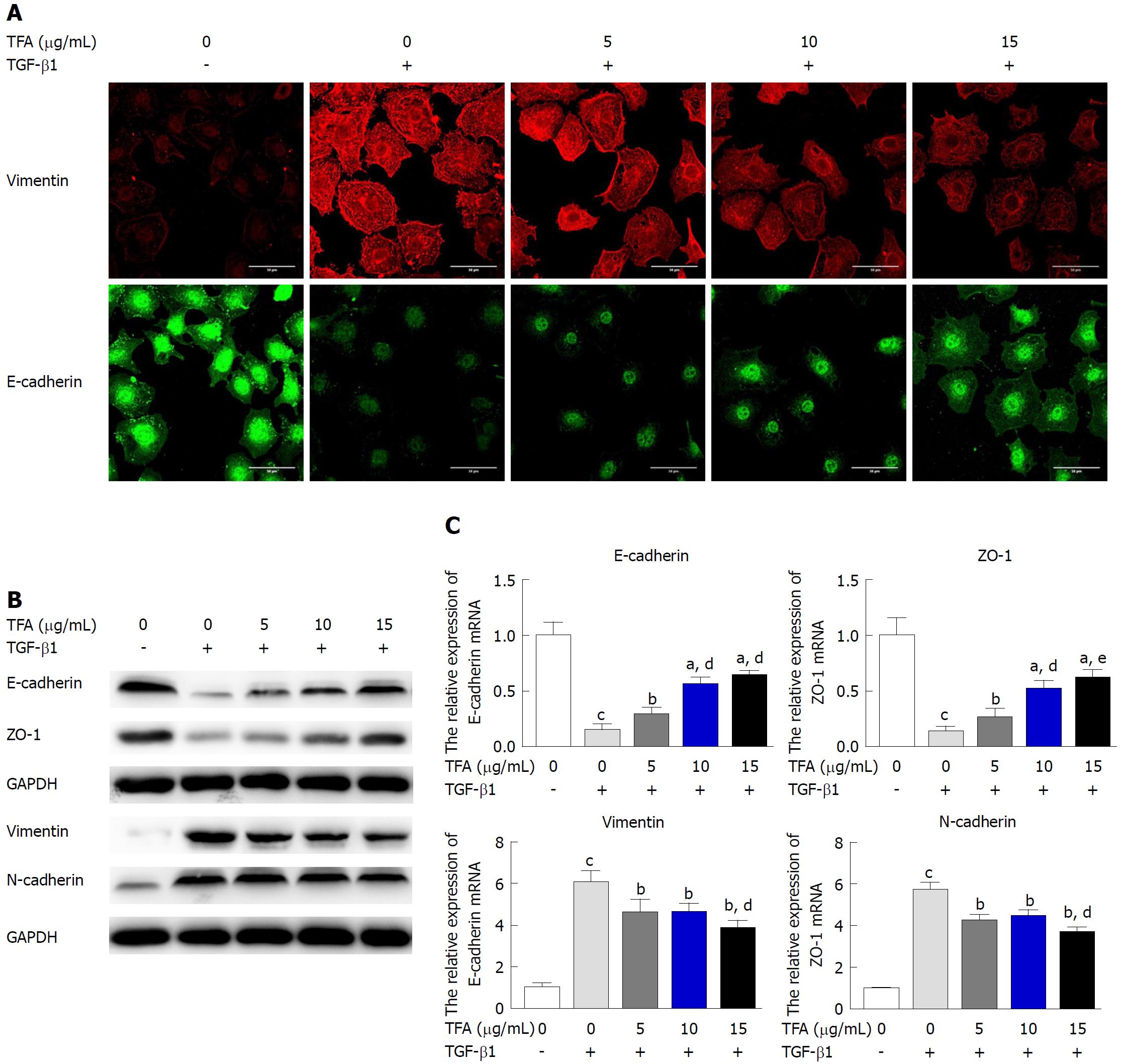
IEC-6 cells were incubated in a 6-well plate with a density of 6 × 105 cell per well. When cells reached 60% confluence, 10 ng/mL TGF-β1 and TFA (0, 5, 10 and 15 μg/mL) were added to the plates and incubated for 48 h. Total RNA was extracted from the cultured cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions and reversed transcribed into cDNA using the TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, United States). The qRT-PCR reactions were performed using the Fast Start Universal SYBR Green Master (Rox) (Roche Applied Science) on a 7500 Real-time system (Applied Biosystems, United States) according to the manufacturer’s protocols. Primer sequences used were designed as follows: E-cadherin forward, 5’-GAGGTCTACATTCCTGGTG-3’, E-cadherin reverse, 5’-TCTGTAGACATTTGAATCGG-3’, ZO-1 forward, 5’-CCATCTTGGACCGATTGCTG-3’, ZO-1 reverse, TAATGCCCGAGCTCCGATG-3’, Vimentin forward, 5’-CCGACACTCCT ACAAGATTTAGA-3’, Vimentin reverse, 5’-CAAAGATTTATTGAAGCAGAACC-3’, N-cadherin forward, 5’-ATCCTACTGGACGGTTCG-3’, N-cadherin reverse, 5’-TTGGCTAATGGCACTTGA-3’, GAPDH forward, 5’ -GGACCTGACCTGCCGTCTAG-3’, GAPDH reverse, 5’-GTAGCCCAGGATG CCCTTGA-3’. GAPDH was used as an internal standard.
IEC-6 cells were plated into 6-well plates at a density of 3 × 105 cells/mL. When cells reached 60% confluence, 10 ng/mL TGF-β1 and TFA (0, 5, 10 and 15 μg/mL) were added to the plates and incubated for 48 h. Each concentration group was performed in triplicate. Protein lysates were prepared using RIPA lysis buffer. Lysates were then subjected to SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes. After blocking with non-fat milk, blots were incubated overnight at 4 °C with the indicated antibodies. After incubation for 24 h, membranes were washed and incubated for 2 h at room temperature with corresponding secondary antibodies. For protein detection, membranes were developed with SuperSignal west femto maximum sensitivity substrate (Pierce, Rockford, IL, United States) and the Gel-Pro Analyzer 6.0 software was applied for image analysis. E-cadherin (1:500), ZO-1 (1:500), Vimentin (1:500), N-cadherin (1:500), p-ERK (1:500), ERK (1:500), p-p38 (1:500), p38 (1:500), p-JNK (1:500), JNK (1:500) and GAPDH (1:1000) antibodies were purchased from Sigma-Aldrich, and anti-mouse secondary antibodies were obtained from Proteintech (Chicago, IL, United States).
IEC-6 cells were plated into 6-well plates at a density of 3 × 105 cells/mL. When cells reached 60% confluence, 10 ng/mL TGF-β1 and TFA (0, 5, 10 and 15 μg/mL) were added to the plates and incubated for 48 h. After that, IEC-6 cells were fixed with 4% paraformaldehyde. After blocking cells with 3% BSA for 2 h at room temperature, the cells were incubated with the anti-E-cadherin (1:50) antibody or anti-Vimentin (1:50) antibody at room temperature for 2 h. After washing three times with PBS, the second antibody conjugated with FITC was incubated on these cells for 1 h at room temperature. The nuclei were counterstained with DAPI for 5 min. Cells were imaged using a Nikon Eclipse TE2000-U fluorescence microscope.
Graph Pad Prism 5.0 statistical software was utilized to analyze the above experimental data. Measurement data were represented as X ± SD (n = 3); One-way analysis of variance was applied to compare differences between multiple groups. When only two groups were compared, Student’s t-test was conducted. A value of P < 0.05 indicated that the difference was statistically significant.
In order to observe the effect of TFA on the EMT progress of IEC-6 cells mediated by TGF-β1, a CCK-8 assay was performed to assess the effect of TFA on the viability of IEC-6 cells and to select the optimal concentrations of TFA for our further studies. IEC-6 cells were treated with increasing concentrations of TFA ranging from 0.1 to 50 μg/mL at different time points (24, 48 and 72 h). From the results of Figure 1, we found that TFA had the positive activities on viability of IEC-6 cells with the concentrations of 5, 10 and 15 μg/mL compared to the control group (TFA 0 μg/mL). Further, the TFA-treated group at 10 μg/mL displayed the maximum proliferative rate at 24, 48 and 72 h. Therefore, 5, 10 and 15 μg/mL TFA were chosen to be the optimal concentrations for our further studies.
TFA inhibited TGF-β1 induced migration and invasion of IEC-6 cells
The EMT process is characterized by alteration of cell morphology, migration and invasion capacity, as well as epithelial and mesenchymal markers expression. In the morphology assay, 10 ng/mL TGF-β1 in the medium for 48 h led to significant morphological changes from cuboidal epithelial cells to fibroblast-like spindle-shaped cells when compared with the control group. Interestingly, TFA effectively suppressed TGF-β1-induced morphological changes of IEC-6 cells in a dose dependent manner (Figure 2A). Moreover, for wound healing assays, 10 ng/ml TGF-β1 could promote the migration of IEC-6 cells compared with the control group, while TFA obviously reduced the TGF-β1-induced migration of IEC-6 cells in a dose dependent manner (Figure 2B). In addition, we examined the effects of TFA on TGF-β1-induced invasion, and we found that 10 ng/mL TGF-β1 in the medium for 48 h induced IEC-6 cells across the membrane, and addition of 5, 10 and 15 μg/mL TFA decreased the number of IEC-6 cells that passed though the Matrigel (Figure 2C). The results indicated that TFA could significantly suppress the morphological change, migration and invasion of IEC-6 cells induced by TGF-β1.
TFA inhibited TGF-β1-induced EMT
To examine the alterations of EMT markers, as shown in Figure 3A, the expression of epithelial marker E-cadherin was decreased along with the increasing expression of the mesenchymal marker Vimentin after treatment with 10 ng/mL TGF-β1. However, TFA effectively inhibited this induction, especially at higher concentrations. Besides, western blotting and qRT-PCR were carried out to examine the alterations of EMT markers, and we found that the protein levels of epithelial markers, including E-cadherin and ZO-1, were markedly increased by TFA treatment. On the contrary, TFA treatment obviously decreased the expression levels of mesenchymal proteins, including Vimentin and N-cadherin (Figure 3B). Likewise, similar changes were observed for mRNA expression of epithelial and mesenchymal markers in TFA-treated TGF-β1-induced IEC-6 cells (Figure 3C). Together, these results demonstrated that TFA could attenuate TGF-β1-induced EMT of IEC-6 cells.
TFA inhibited TGF-β1-induced activation of the Smad signaling pathway
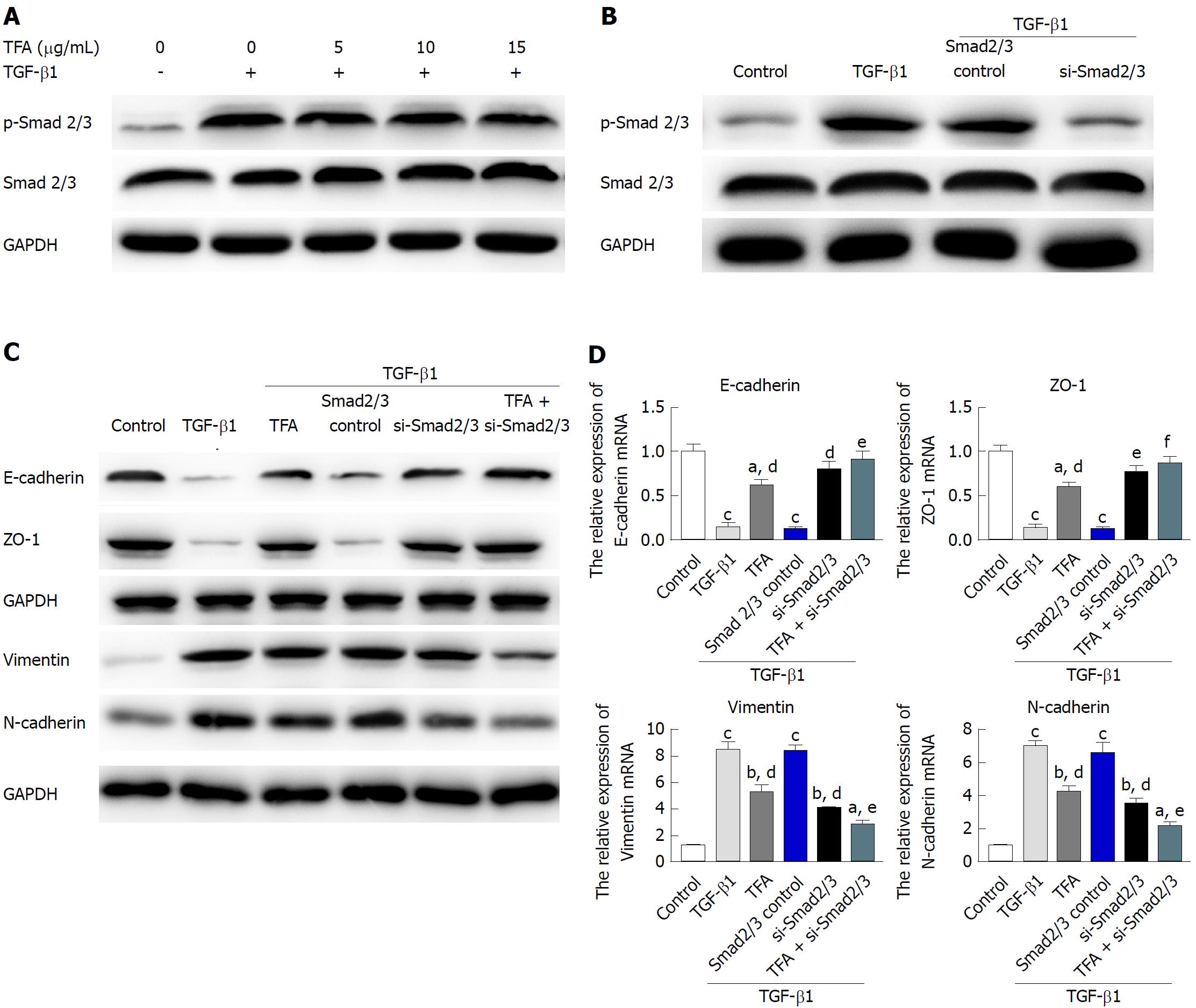
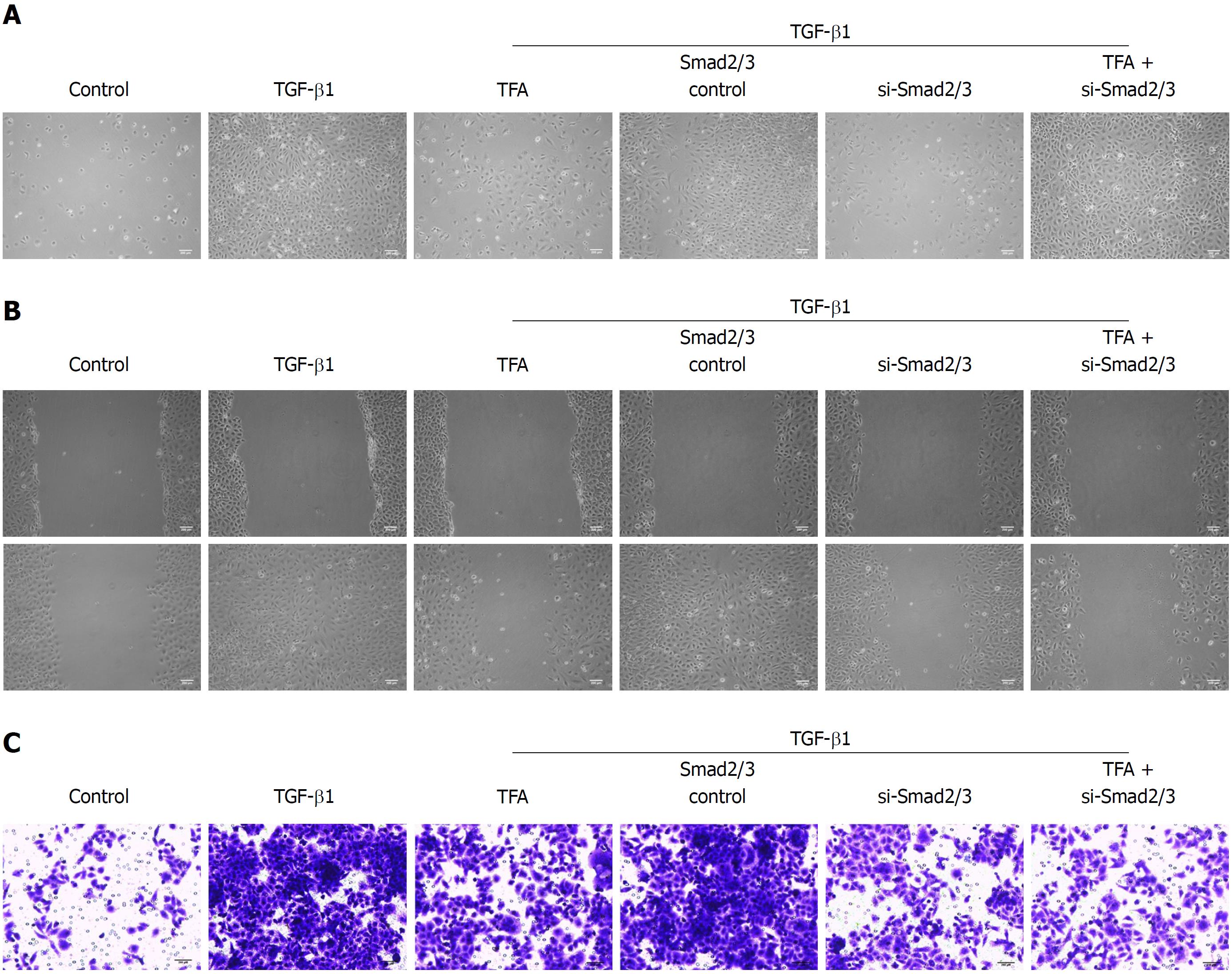
The activation of phosphorylated Smad2/3 plays an important role in the TGF-β1-mediated EMT progress[28]. The status of the Smad pathways was determined in TGF-β1-treated IEC-6 cells following TFA treatment with the concentrations of 5, 10 and 15 μg/mL for 48 h, and the results have shown that TFA remarkably inhibited the level of phosphorylated Smad 2/3 in a dose dependent manner (Figure 4A). Next, we examined the role of Smad in TGF-β1-mediated IEC-6 cells. In IEC-6 cells, western blotting analysis demonstrated that Smad2/3 expression was inhibited after transfection of si-Smad2/3 (Figure 4B). Moreover, our results revealed that si-Smad2/3 could diminish TGF-β1-triggered EMT in IEC-6 cells and inhibit the TGF-β1-elicited changes in the expression of EMT markers, as measured by western blotting and qRT-PCR assays (Figure 4B and C). Besides, si-Smad2/3 suppressed TGF-β1-mediated mensenchymal-like morphological changes in IEC-6 cells as shown in Figure 5A. Wound healing and trans-well assays also showed that si-Smad2/3 suppressed the migration and invasion capacities of IEC-6 cells triggered by TGF-β1 (Figure 5B and C). Furthermore, 15 μg/mL TFA combined with si-Smad2/3 could further restrain TGF-β1-mediated EMT progress in IEC-6 cells. These results suggested that TFA might reverse EMT induced by TGF-β1 via Smad inactivation in IEC-6 cells.
TFA inhibited TGF-β1-induced activation of the non-Smad signaling pathway
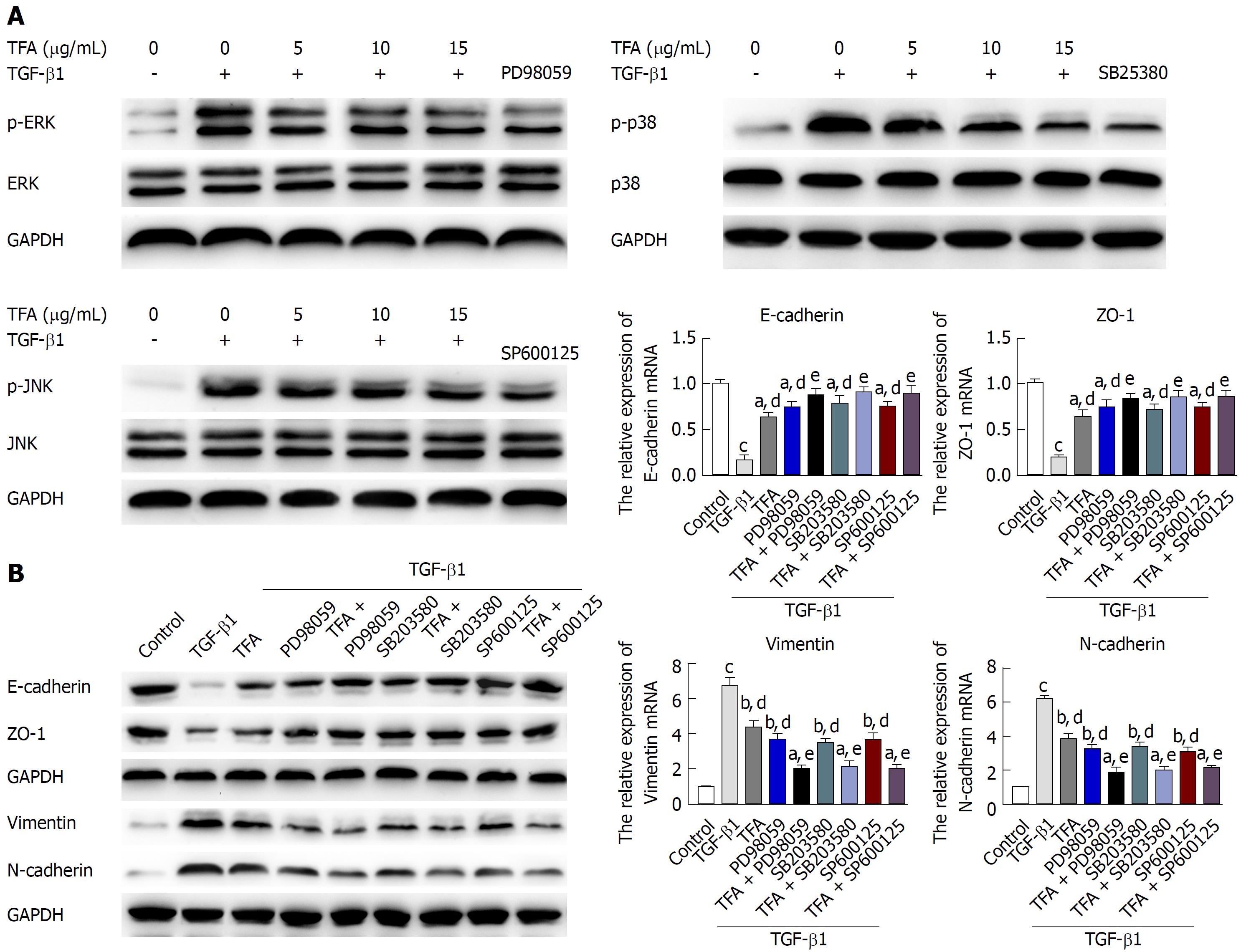
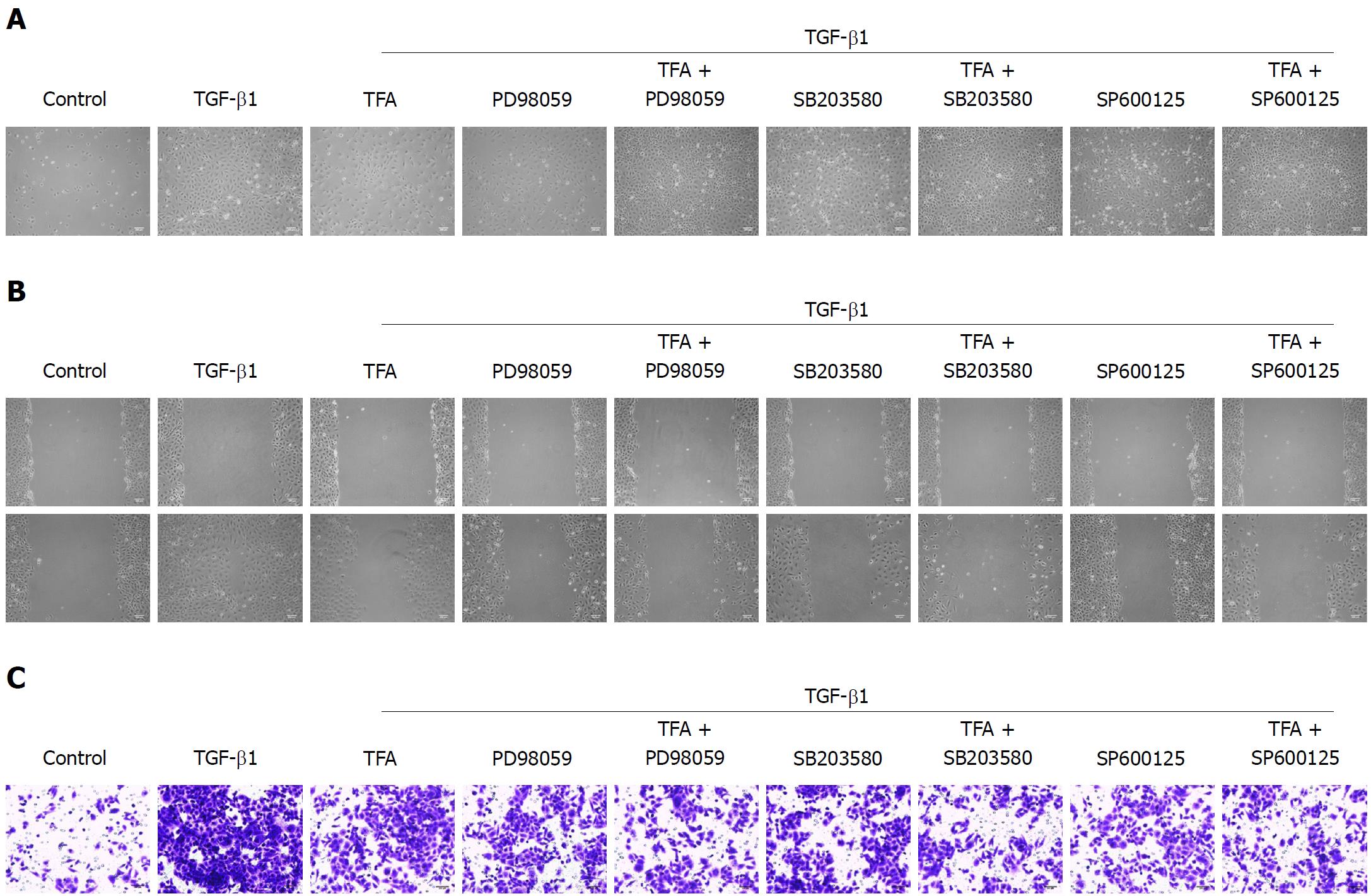
In addition to the Smad2/3 signaling pathway, TGF-β has been reported to activate other signaling molecules, such as MAPKs, which include extracellular signal-regulated kinase (ERK) 1/2, c-Jun NH2-terminal kinase (JNK), and p38 mitogen-activated protein kinase (p38 MAPK)[29]. To explore the involvement of non-Smad signaling in the anti TGF-β1-induced EMT activity of TFA, we tested whether TFA could inhibit the TGF-β1-induced activation of p38, JNK and ERK1/2 by western blotting, and we found that TGF-β1 could significantly activate the expressions of p-p38, p-JNK and p-ERK1/2, while TFA at the concentrations of 5, 10 and 15 μg/mL obviously suppressed the TGF-β1-induced increase in p-p38, p-JNK and p-ERK1/2 levels in IEC-6 cells in a dose dependent manner (Figure 6A). Thus, in order to further explore the role of MAPKs in TGF-β1-treated IEC-6 cells, ERK1/2 inhibitor (PD98059, 5 μmol/L), p38 inhibitor (SB203580, 5 μmol/L) and JNK inhibitor (SP600125, 2 μmol/L) were used to treat IEC-6 cells mediated by TGF-β1. From the results in Figure 6A, we found that PD98059, SB203580 and SP600125 could significantly inhibit the activation of p-p38, p-JNK and p-ERK, respectively. Besides, PD98059, SB203580 and SP600125 could promote the changes in EMT markers by western blotting and qRT-PCR assays (Figure 6B and C). In addition, PD98059, SB203580 and SP600125 treatment inhibited TGF-β1-induced mensenchymal-like morphological changes in IEC-6 cells, as shown in Figure 7A. Moreover, PD98059, SB203580 and SP600125 inhibited the migration and invasion of IEC-6 cells mediated by TGF-β1 via wound healing and trans-well assays (Figure 7B and C). Importantly, co-treatment of TFA with PD98059, SB203580 or SP600125 had better inhibitory effects on TGF-β1-induced EMT in IEC-6 cells than either one of them. Taken together, these results indicated that TFA might exert its role in reversing TGF-β1-mediated EMT via non-Smad inactivation in IEC-6 cells.
CD intestinal fibrosis is characterized by activation of myofibroblasts, resulting in abnormal ECM deposition, which eventually leads to tissue stiffness and progressive intestinal dysfunction[30]. In addition to interstitial cells such as fibroblasts and smooth muscle cells, the differentiation of epithelial cells through EMT is a major source of intestinal fibrotic cells[18]. EMT refers to the transformation of epithelial cells with polar cells to the interstitial cells under a specific physiological and pathological condition. The most important features of EMT are the loss of epithelial cell phenotype and the acquisition of interstitial properties, as reflected in the down-regulation of E-cadherin and ZO-1, resulting in loss of adhesion between cells or between cells and matrix, over-expression of N-cadherin and vimentin, leading to the acquisition of migration and invasion, and over-expression of EMT transcription factors[31,32]. Moreover, EMT plays a key role in tissue formation, organ fibrosis and so on. Through EMT, epithelial cells lose cell polarity and epithelial phenotypes, such as connection to the basement membrane, while acquiring interstitial cell phenotypes with the functions of fibroblasts and myofibroblasts[33-35]. Moreover, in the case of continuous activation of inflammatory response, the EMT process will also continuously exist and eventually cause organ fibrosis. Therefore, it will have a positive significance to search for a method for the treatment of CD intestinal fibrosis.
Our previous studies have found that TFA, a main component of the water extract of traditional Chinese medicine, could reduce the expression of TGF-β, α-SMA and MMP-2 and decrease collagen fibrils, which indicated that TFA may inhibit or reverse EMT during CD intestinal fibrosis. Firstly, we used TGF-β1 to induce EMT of IEC-6 cells and chose the optimal concentrations of TFA to disrupt TGF-β1 activity in IEC-6 cells. From the results, we knew that TGF-β1 could obviously promote the progression of EMT, such as changing morphology from cuboidal epithelial cells to fibroblast-like spindle-shaped cells, and enhancing the abilities of migration and invasion. Despite this, TFA treatment could restrain the changes caused by TGF-β1 in a dose dependent manner.
Effective inhibition of CD intestinal inflammation does not play a positive role in preventing intestinal fibrosis and inhibiting the pro-fibrosis signaling pathway, resulting in persistence of the process of tissue fibrosis[36,37]. Therefore, it is necessary to further study how to block the fibrotic signaling pathway and promote ECM decomposition to inhibit or reverse the process of fibrosis. TGF-β is the strongest inducer, playing an important role in EMT[38]. The Smad signaling pathway induced by TGF-β can promote the expression of ECM and inhibit the transcriptional activity of matrix degradation genes, resulting in ECM deposition in interstitial cells and leading to fibrosis[39]. Actually, in our experiments, we found that 10 ng/mL TGF-β1 could significantly inhibit the expression of p-Smad2/3, while TFA remarkably reversed the phenomenon in a dose dependent manner in TGF-β1-treated IEC-6 cells. Blocking TGF-β/Smad signaling has become a hot spot to inhibit or even reverse fibrosis. It has been reported that silencing Smad 2/3 could block Smad signaling and reduce collagen synthesis and proliferation of fibroblasts[40,41]. We silenced the expression of p-Smad2/3 and explored the role of Smad2/3 in TGF-β1-treated IEC-6 cells. We found that silencing Smad2/3 could effectively improve the EMT progression of IEC-6 cells induced by TGF-β1 no matter morphology, migration, invasion or EMT markers. Interestingly, when TFA was combined with si-Smad2/3, it had better inhibitory activities on EMT progression than either one of them alone, which suggested that TFA might exert its effects in reversing TGF-β1-mediated EMT via Smad inactivation in IEC-6 cells.
Recent researches have shown that the MAPK pathway, including p38, JNK and ERK1/2, is closely related to the regulation of TGF-β signaling pathway and fibrosis[42]. TGF-β can not only carry out signal transduction through TGF-β/Smad, but can also activate p38, JNK and ERK signaling pathways, which indicates that regulation of MAPK signaling pathways can affect the TGF-β/Smad signal transduction pathway and play a role in inhibiting fibrosis[43,44]. In this experiment, we found that 10 ng/mL TGF-β1 could significantly inhibit the expression of p-p38, p-JNK and p-ERK1/2, while TFA obviously suppressed the levels in a dose dependent manner in TGF-β1-treated IEC-6 cells. In addition, we also investigated the role of MAPK in TGF-β1-mediated EMT of IEC-6 cells. We used an ERK1/2 inhibitor (PD98059), p38 inhibitor (SB203580) and JNK inhibitor (SP600125) to inhibit the expression of p-p38, p-JNK and p-ERK1/2 in IEC-6 cells, respectively. We found that PD98059, SB203580 and SP600125 treatment could have positive inhibitory effects on EMT progress, similar to the si-Smad. Moreover, co-treatment of TFA combined with PD98059, SB203580 or SP600125 had better positive activities on inhibiting EMT progress in TGF-β1-mediated IEC-6 cells than either one of them alone, indicating that TFA might exert its role in reversing EMT induced by TGF-β1 via MAPK inactivation in IEC-6 cells.
Stated thus, regulating TGF-β and its downstream Smad and MAPK signaling pathways, mediating the EMT process and restoring the biological function of abnormally activated intestinal fibroblasts, may be an important way to seek drug therapy for CD intestinal fibrosis. TFA is able to inhibit TGF-β1-induced morphological change, migration, invasion of IEC-6 cells. TFA promoted the induction of EMT partly by inhibiting TGF-β1-activated Smad signaling pathway and non-Smad signaling pathway. To conclude, TFA is expected to advance as a new therapy to treat CD intestinal fibrosis, and its continued advancement may open the door to a new class of treatment for CD intestinal fibrosis.
Epithelial-mesenchymal transition (EMT) is a crucial process in Crohn’s disease (CD) intestinal fibrosis. Total flavone of Abelmoschus manihot (TFA) has been found as an effective component to reduce CD intestinal fibrosis in vivo. However, the role and mechanism of TFA on EMT progress of CD intestinal fibrosis have not been understood yet.
EMT is a crucial process in CD intestinal fibrosis. TFA has been found as an effective component to reduce CD intestinal fibrosis in vivo. However, the role and mechanism of TFA on EMT progress of CD intestinal fibrosis have not been understood yet. In the present study, we performed CCK-8, morphology, wound healing, transwell, qRT-PCR, western blotting and immunofluorescence assays to explore the role and the underlying mechanisms of TFA on CD intestinal fibrosis, and the results indicated that TFA was expected to advance as a new therapy to treat CD intestinal fibrosis.
To explore the role and mechanism of TFA on EMT progress of CD intestinal fibrosis.
First, a CCK-8 assay was performed to assess the effect of TFA on the viability of IEC-6 cells and to select the optimal concentrations of TFA for our further studies. Then cell morphology, wound healing and transwell assays were performed to examine the effect of TFA on morphology, migration and invasion of IEC-6 cells treated with transforming growth factor-β1 (TGF-β1). In addition, immunofluorescence, qRT-PCR and western blotting assays were carried out to detect the impact of TFA on EMT progress. Moreover, western blotting assay was performed to evaluate the function of TFA on the Smad and MAPK signaling pathways. Further, the role of co-treatment of TFA and si-Smad or MAPK inhibitors was examined by qRT-PCR, western blotting, morphology, wound healing and transwell assays.
In this study, TFA promoted TGF-β1-induced IEC-6 cell morphological change, migration and invasion, and increased the expression of epithelial markers and reduced the levels of mesenchymal markers, along with the inactivation of Smad and MAPK signaling pathways. Moreover, we revealed that si-Smad and MAPK inhibitors effectively attenuated TGF-β1-induced EMT in TEC-6 cells. Importantly, co-treatment of TFA and si-Smad or MAPK inhibitors had better inhibitory effects on TGF-β1-induced EMT in IEC-6 cells than either one of them alone.
These findings could provide new insight into the molecular mechanisms of TFA on TGF-β1-induced EMT in IEC-6 cells, and TFA is expected to advance as a new therapy to treat CD intestinal fibrosis.
TFA promoted the induction of EMT partly by inhibiting TGF-β1-activated Smad signaling pathway and non-Smad signaling pathway. TFA is expected to advance as a new therapy to treat CD intestinal fibrosis, and its continued advancement may open the door to a new class of treatment for CD intestinal fibrosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sipahi AM, Sultan K, Smith SM S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Hirten RP, Shah S, Sachar DB, Colombel JF. The Management of Intestinal Penetrating Crohn’s Disease. Inflamm Bowel Dis. 2018;24:752-765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Stidham RW, Wu J, Shi J, Lubman DM, Higgins PD. Serum Glycoproteome Profiles for Distinguishing Intestinal Fibrosis from Inflammation in Crohn’s Disease. PLoS One. 2017;12:e0170506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Wilkens R, Hagemann-Madsen RH, Peters DA, Nielsen AH, Nørager CB, Glerup H, Krogh K. Validity of Contrast-enhanced Ultrasonography and Dynamic Contrast-enhanced MR Enterography in the Assessment of Transmural Activity and Fibrosis in Crohn’s Disease. J Crohns Colitis. 2018;12:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 4. | Rogler G, Hausmann M. Factors Promoting Development of Fibrosis in Crohn’s Disease. Front Med (Lausanne). 2017;4:96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Hui CK, Hui NK. Collagenous colitis presenting with skip lesions mimicking Crohn’s disease and complicated by intestinal obstruction. J Dig Dis. 2017;18:487-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 6. | Ma C, Moran GW, Benchimol EI, Targownik LE, Heitman SJ, Hubbard JN, Seow CH, Novak KL, Ghosh S, Panaccione R. Surgical Rates for Crohn’s Disease are Decreasing: A Population-Based Time Trend Analysis and Validation Study. Am J Gastroenterol. 2017;112:1840-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Ha FJ, Thong L, Khalil H. Quality of Life after Intestinal Resection in Patients with Crohn Disease: A Systematic Review. Dig Surg. 2017;34:355-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Sobrado CW, Leal RF, Sobrado LF. THERAPIES FOR CROHN’S DISEASE: a clinical update. Arq Gastroenterol. 2016;53:206-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Deepak P, Loftus EV Jr. Ustekinumab in treatment of Crohn’s disease: design, development, and potential place in therapy. Drug Des Devel Ther. 2016;10:3685-3698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Wang X, Lu Y, Wu L, Zhao C, Song C, Yu S, Zhao B, Zhao T, Liu H, Dou C. Moxibustion Inhibits the ERK Signaling Pathway and Intestinal Fibrosis in Rats with Crohn’s Disease. Evid Based Complement Alternat Med. 2013;2013:198282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Abreu MT, Taylor KD, Lin YC, Hang T, Gaiennie J, Landers CJ, Vasiliauskas EA, Kam LY, Rojany M, Papadakis KA. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn’s disease. Gastroenterology. 2002;123:679-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 324] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Hassan-Zahraee M, Banerjee A, Cheng JB, Zhang W, Ahmad A, Page K, von Schack D, Zhang B, Martin SW, Nayak S. Anti-MAdCAM Antibody Increases ß7+ T Cells and CCR9 Gene Expression in the Peripheral Blood of Patients With Crohn’s Disease. J Crohns Colitis. 2018;12:77-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Zheng Y, Ge W, Ma Y, Xie G, Wang W, Han L, Bian B, Li L, Shen L. miR-155 Regulates IL-10-Producing CD24hiCD27B+ Cells and Impairs Their Function in Patients with Crohn’s Disease. Front Immunol. 2017;8:914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Mortensen JH, Godskesen LE, Jensen MD, Van Haaften WT, Klinge LG, Olinga P, Dijkstra G, Kjeldsen J, Karsdal MA, Bay-Jensen AC. Fragments of Citrullinated and MMP-degraded Vimentin and MMP-degraded Type III Collagen Are Novel Serological Biomarkers to Differentiate Crohn’s Disease from Ulcerative Colitis. J Crohns Colitis. 2015;9:863-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Kurahara LH, Hiraishi K, Sumiyoshi M, Doi M, Hu Y, Aoyagi K, Jian Y, Inoue R. Significant contribution of TRPC6 channel-mediated Ca2+ influx to the pathogenesis of Crohn’s disease fibrotic stenosis. J Smooth Muscle Res. 2016;52:78-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Jiang H, Shen J, Ran Z. Epithelial-mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018;11:294-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Scharl M, Frei P, Frei SM, Biedermann L, Weber A, Rogler G. Epithelial-to-mesenchymal transition in a fistula-associated anal adenocarcinoma in a patient with long-standing Crohn’s disease. Eur J Gastroenterol Hepatol. 2014;26:114-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Scharl M, Huber N, Lang S, Fürst A, Jehle E, Rogler G. Hallmarks of epithelial to mesenchymal transition are detectable in Crohn’s disease associated intestinal fibrosis. Clin Transl Med. 2015;4:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Chen Y, Xiao Y, Ge W, Zhou K, Wen J, Yan W, Wang Y, Wang B, Qu C, Wu J. miR-200b inhibits TGF-β1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis. 2013;4:e541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Liu TJ, Guo JL, Wang HK, Xu X. Semaphorin-7A contributes to growth, migration and invasion of oral tongue squamous cell carcinoma through TGF-β-mediated EMT signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:1035-1043. [PubMed] [Cited in This Article: ] |
| 21. | Sommerova L, Ondrouskova E, Vojtesek B, Hrstka R. Suppression of AGR2 in a TGF-β-induced Smad regulatory pathway mediates epithelial-mesenchymal transition. BMC Cancer. 2017;17:546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Sun S, Xie F, Zhang Q, Cui Z, Cheng X, Zhong F, He K, Zhou J. Advanced oxidation protein products induce hepatocyte epithelial-mesenchymal transition via a ROS-dependent, TGF-β/Smad signaling pathway. Cell Biol Int. 2017;41:842-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Liu Z, Liu S, Zhou L, Gao X, Ju W, Tan H, Yang C. Effects of HuangKui capsules on glibenclamide pharmacokinetics in rats. J Ethnopharmacol. 2012;139:1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Tu Y, Sun W, Wan YG, Che XY, Pu HP, Yin XJ, Chen HL, Meng XJ, Huang YR, Shi XM. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J Ethnopharmacol. 2013;147:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Ge J, Miao JJ, Sun XY, Yu JY. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator-activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J Ethnopharmacol. 2016;189:238-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Liu S, Ye L, Tao J, Ge C, Huang L, Yu J. Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm Biol. 2017;56:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Guo J, Xue C, Duan JA, Qian D, Tang Y, You Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine. 2011;18:1250-1254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Chen Q, Yang W, Wang X, Li X, Qi S, Zhang Y, Gao MQ. TGF-β1 Induces EMT in Bovine Mammary Epithelial Cells Through the TGFβ1/Smad Signaling Pathway. Cell Physiol Biochem. 2017;43:82-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Ling G, Ji Q, Ye W, Ma D, Wang Y. Epithelial-mesenchymal transition regulated by p38/MAPK signaling pathways participates in vasculogenic mimicry formation in SHG44 cells transfected with TGF-β cDNA loaded lentivirus in vitro and in vivo. Int J Oncol. 2016;49:2387-2398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Cayci M, Bostanci EB, Turhan N, Karaman K, Dalgic T, Ozer I, Ercan M, Ulas M, Akoglu M. The analysis of clinico-pathologic characteristics in patients who underwent surgery due to stricturing and non-perineal fistulizing forms of Crohn’s disease: a retrospective cohort study. Int J Surg. 2015;15:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Conway J, Al-Zahrani KN, Pryce BR, Abou-Hamad J, Sabourin LA. Transforming growth factor β-induced epithelial to mesenchymal transition requires the Ste20-like kinase SLK independently of its catalytic activity. Oncotarget. 2017;8:98745-98756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren Y, Li Z, Pang D, Qian C. MiR-23a promotes TGF-β1-induced EMT and tumor metastasis in breast cancer cells by directly targeting CDH1 and activating Wnt/β-catenin signaling. Oncotarget. 2017;8:69538-69550. [PubMed] [Cited in This Article: ] |
| 33. | You J, Li M, Tan Y, Cao L, Gu Q, Yang H, Hu C. Snail1-expressing cancer-associated fibroblasts induce lung cancer cell epithelial-mesenchymal transition through miR-33b. Oncotarget. 2017;8:114769-114786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Ke X, Yang D, Liang J, Wang X, Wu S, Wang X, Hu C. Human Endothelial Progenitor Cell-Derived Exosomes Increase Proliferation and Angiogenesis in Cardiac Fibroblasts by Promoting the Mesenchymal-Endothelial Transition and Reducing High Mobility Group Box 1 Protein B1 Expression. DNA Cell Biol. 2017;36:1018-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Guan R, Wang X, Zhao X, Song N, Zhu J, Wang J, Wang J, Xia C, Chen Y, Zhu D. Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation. Sci Rep. 2016;6:35696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Boland BS, Vermeire S. Janus Kinase Antagonists and Other Novel Small Molecules for the Treatment of Crohn’s Disease. Gastroenterol Clin North Am. 2017;46:627-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Ramos GP, Faubion WA, Papadakis KA. Targeting Specific Immunologic Pathways in Crohn’s Disease. Gastroenterol Clin North Am. 2017;46:577-588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Chen H, Chen Q, Jiang CM, Shi GY, Sui BW, Zhang W, Yang LZ, Li ZY, Liu L, Su YM. Triptolide suppresses paraquat induced idiopathic pulmonary fibrosis by inhibiting TGFB1-dependent epithelial mesenchymal transition. Toxicol Lett. 2018;284:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Perumal N, Perumal M, Halagowder D, Sivasithamparam N. Morin attenuates diethylnitrosamine-induced rat liver fibrosis and hepatic stellate cell activation by co-ordinated regulation of Hippo/Yap and TGF-β1/Smad signaling. Biochimie. 2017;140:10-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | Luong VH, Chino T, Oyama N, Matsushita T, Sasaki Y, Ogura D, Niwa SI, Biswas T, Hamasaki A, Fujita M. Blockade of TGF-β/Smad signaling by the small compound HPH-15 ameliorates experimental skin fibrosis. Arthritis Res Ther. 2018;20:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Ma YL, Chen F, Yang SX, Chen BP, Shi J. MicroRNA-21 promotes the progression of peritoneal fibrosis through the activation of the TGF-β/Smad signaling pathway: An in vitro and in vivo study. Int J Mol Med. 2018;41:1030-1038. [PubMed] [Cited in This Article: ] |
| 42. | Cao Y, Liu Y, Ping F, Yi L, Zeng Z, Li Y. miR-200b/c attenuates lipopolysaccharide-induced early pulmonary fibrosis by targeting ZEB1/2 via p38 MAPK and TGF-β/smad3 signaling pathways. Lab Invest. 2018;98:339-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Bansal T, Chatterjee E, Singh J, Ray A, Kundu B, Thankamani V, Sengupta S, Sarkar S. Arjunolic acid, a peroxisome proliferator-activated receptor α agonist, regresses cardiac fibrosis by inhibiting non-canonical TGF-β signaling. J Biol Chem. 2017;292:16440-16462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Che X, Wang Q, Xie Y, Xu W, Shao X, Mou S, Ni Z. Astragaloside IV suppresses transforming growth factor-β1 induced fibrosis of cultured mouse renal fibroblasts via inhibition of the MAPK and NF-κB signaling pathways. Biochem Biophys Res Commun. 2015;464:1260-1266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |