INTRODUCTION
Fluorescence-guided surgery (FGS) is a modality of intraoperative navigation that provides the surgeon with enhanced visualization of anatomical structures and/or an improved understanding of an organ’s current perfusion in real time[1,2]. To obtain a fluorescence signal, the operative field is exposed to near-infrared light sources, while the target is injected with a fluorescent dye, which can emit a fluorescence signal after being excited by ad hoc laser sources. The fluorescence signal can be visualized either directly on the operative field during open surgical procedures or captured by specific cameras and displayed on a screen in a minimally invasive setting. FGS is currently more adapted to the needs of surgical navigation because it does not require bulky equipment in the operating room and the enhanced information provided to the surgeon is displayed in real time, without interfering with the surgical workflow[3].
In the last few years, fluorescence imaging using the fluorescent properties of indocyanine green (ICG) has become widespread in several medical and surgical specialties[4]. Due to its relatively low cost and high availability, ICG is the most employed fluorophore in the clinical setting of general surgery.
The main goal of this overview is to survey and discuss the most recent and ongoing clinical applications of ICG-based fluorescence in visceral, hepatobiliary and pancreatic (HBP) surgery. Up to date, vascular assessment of anastomotic stump in visceral surgery and extrahepatic biliary anatomy identification during difficult cholecystectomies are the most well established applications of ICG-based fluorescence surgery.
The in-depth analysis presented here relies on three main sources of information. First, the analysis draws on the authors’ significant experience with this technology in their medical institutions. Second, an up-to-date review of the most relevant articles published on this topic between 2014 and 2018 presents the latest developments in this rapidly developing field. Lastly, lengthy discussions of the authors with key opinion leaders in the field during recent conferences and congresses add novel insights on the ongoing applications that will appear in the literature in the near future. In particular, the rationale of applying this technique to visceral and HBP surgery, the relevant physiopathological insights, the available data, and the ongoing clinical trials are thoroughly surveyed and discussed. For each application, the benefits and limitations of this technique are identified, and applicable future directions are described.
REAL-TIME VISCERAL PERFUSION
The estimation of real-time visceral perfusion is a promising intraoperative application of ICG-based fluorescence surgery. ICG-based fluorescence angiography might guide the identification of the optimal resection site and help estimate the blood supply of visceral anastomosis in both upper GI and colorectal surgery. The rationale behind fluorescence angiography is that the fluorescent dye, upon systemic injection, should reach and highlight only vascularized areas.
Focusing on colorectal surgery, it is well known that the rate of anastomotic leaks ranges between 5% and 7%, even in high-volume centers[5]. The introduction into daily clinical practice of a tool capable of delivering optimal stump vascularization could reduce this rate. In turn, this may eliminate dehiscence due to inadequate vascularization despite a macroscopically satisfactory appearance[6-8]. Some phase II trials have confirmed the feasibility, low cost, and high success rate of this procedure[9], reporting a leakage rate (< 3%) lower than the one expected on the basis of historical series in rectal anastomoses. The rate of changes in the transection line varies from 5% to 40%[10]. However, a few reports did not confirm these data; for instance, in a series of 346 colorectal interventions, Kin and colleagues reported a leakage rate of 7.5% and 6.4% with and without ICG intraoperative assessment, respectively[11]. Some randomized prospective controlled trials are currently ongoing[12].
Similar use of ICG for intraoperative assessments has been investigated for esophagectomy and gastrectomy. When making the gastric tube during esophagectomy, the most cranial portion represents a critical point because the gastroepiploic vascularization reaches a macroscopically unclear cut-off at that level[13]. A few retrospective, case-control studies have confirmed this hypothesis, showing a number of changes in the site of anastomosis as high as 25% and a reduction in the leakage rate from 18% to 3% and from 15% to 6% in the series reported by Karampinis and Kitagawa, respectively[14,15]. In a consecutive series of 40 patients undergoing the Ivor-Lewis procedure, Koyanagi et al[16] compared the blood flow using ICG in the gastric conduit and in the greater curve; by employing ROC analysis, they identified a speed of 1.76 cm/s as the cut-off value predicting leakage. However, even in esophageal surgery, the lack of prospective randomized trials hampers the possibility of reaching a definitive conclusion supporting evaluation of the perfusion of the gastric conduit using ICG-based fluorescence.
The same principles for checking the perfusion of the upper extremity of the stomach transection line apply to sleeve gastrectomy in bariatric surgery. Identifying the less vascularized proximal area would allow a targeted resection and reduce the leakage rate along the suture line[17].
In many other clinical settings, the estimation of visceral perfusion may turn out to be a useful and relatively inexpensive intraoperative tool, for example, to assess the small bowel trophism during urgent interventions for intestinal ischemia, after mistaken mesocolon interruption, and/or small bowel mesenteric division[18].
Identification of the major vascular structures in the early phase of dissection from the surrounding fat might be an additional and potentially very useful development from a theoretical point of view. Small intravenous boluses of ICG can facilitate navigation of the vascular structure. However, the penetration depth of the fluorescence is limited to 5-6 mm; furthermore, after the injection, the tissues surrounding both the large arteries and veins become fluorescent, causing a background fluorescence field that reduces visualization. Finally, from then on, the bowel exhibits background staining, which could reduce the clarity of stump perfusion as evaluated at the time of anastomosis. For these reasons, repeated intraoperative angiographies for visualizing the main vascular structure are not possible with ICG.
BILIARY ANATOMY
ICG-based fluorescence imaging to visualize and study biliary anatomy represents one of the most established applications of this technique in abdominal surgery. The reason is simple: the exclusively hepatic metabolism of ICG, which results in biliary excretion starting approximately 30 min after i.v. injection, enables clear visualization of the biliary anatomy, which is very useful during difficult cholecystectomies[19] and from a theoretical point of view during the resection of centrally located liver tumors and hilar (Klatskin’s) tumors[20].
There is more than one comparative study that, although always retrospective, enables us to conclude that ICG-based fluorescence imaging is an extremely useful tool when applied to visualize and study the biliary anatomy[21]. In a systematic review published in 2017, 19 studies were analyzed. There was moderate-quality evidence that ICG-assisted visualization of the extrahepatic bile duct is superior to intraoperative cholangiogram (RR = 1.16; 95%CI: 1.00-1.35)[22]. While the difference was not significant in that study, it was significant in some other single-center series[23]. Considering the relative ease and speed of the procedure, in the future, ICG-based visualization of the biliary anatomy might replace intraoperative cholangiography, which requires a preliminary dissection of the confluence between the cystic and bile ducts and cannulation of the structures.
Recently, in an experimental study with a limited number of cases (7 pigs), ICG injection directly into the gallbladder enabled superior biliary anatomy visualization compared to i.v. injection[24]. The same study group translated their analysis to patients in a subsequent series of 46 cases[25].
With the aim of overcoming the limits of retrospective data, a prospective randomized controlled trial (FALCON) was set up in 2016 to compare near-infrared fluorescence cholangiography-assisted laparoscopic cholecystectomy versus conventional laparoscopic cholecystectomy. The results are expected in 2 years[26].
The main point of debate remains the ideal injection time. Although fluorescence of the bile duct can be detected shortly after i.v. injection, the high fluorescence of the liver parenchyma makes it less evident in contrast. In our view, the ideal timing for this injection is 3 to 5 h before surgery. However, a recent series demonstrated that an injection 24 h before surgery could still be possible[27].
LIVER SURGERY
In liver surgery, the state of the art still considers ICG mainly as a simple reagent for the preoperative evaluation of hepatic function. The comparatively limited use of ICG-based fluorescence imaging in liver surgery with respect to other surgical specialties is slightly paradoxical, considering the pathophysiological assumption that ICG exhibits only hepatic metabolism and only bile excretion. In addition, many centers devoted to liver surgery use the ICG clearance rate as a reliable liver function indicator[28,29].
During the development of fluorescence cholangiography, it was discovered that ICG accumulates in the cancerous tissues of hepatocellular carcinoma (HCC) and in the noncancerous hepatic parenchyma around adenocarcinoma foci. The first application of this imaging technique was reported in 2009 by Ishizawa et al[30] and Gotoh et al[31].
From a theoretical point of view, three remarks should be taken into consideration. First, in HCC (which obviously contains hepatocytes), ICG can be captured and retained by malignant cells; moreover, the secretion of ICG may be altered due to architectural disorders reducing the possibility of excretion[32]. Second, if the tumor is formed by non-hepatocellular cells, as in the case of metastases, ICG could be retained by a group of hepatocytes surrounding the nodule and compressed by the nodule itself[33]. Third, if the nodule contains predominantly epithelial cells that are normally part of the biliary ducts (cholangiocarcinoma), they should not absorb the dye and should behave in a manner similar to metastases but with greater alterations in biliary delivery processes[34].
In clinical practice, however, these theoretical insights may not be taken into consideration because there is currently no clearly defined timing for i.v. injection. Indeed, especially at an early stage, increased vascularization of the neoplasm could result in greater exposure to the dye, regardless of the type of cells contained therein. The nodule behavior in the portal and tardive phases (with more or less washout) is also relevant in determining the amount of retained ICG. These factors translate into the absence of widely accepted guidelines for the intraoperative use of ICG-based fluorescence to differentiate benign from malignant nodules and among the different types of malignant tumors.
The key objective of ongoing studies (some of which are being performed in our medical institutions) is to enable differential diagnosis. ICG-based fluorescence may then be extremely helpful, as follows: (1) In the detection of small superficial malignant nodules not detected in the preoperative study (especially for managing metastases)[35]; and (2) in excluding the malignant nature of small superficial nodules of uncertain contrast medium behavior (especially useful for HCC in cirrhotic liver tissue)[36].
To date, however, some consolidated uses in liver surgery exist. First, it is common practice that metastases in healthy livers are well visualized by the presence of an ICG capture ring due to the compression of the surrounding cells. This can be an advantage during laparoscopic surgery, where tactile sensation is not available as a guide for resection, and continuous monitoring with intraoperative ultrasound is both difficult and time consuming[37]. The presence of the nontumor tissue ring can also be a useful indicator of the extent of resection required to obtain an adequate free margin[38].
Second, there is an increasing need to treat patients with liver metastases after they have undergone multiple chemotherapy cycles; in these cases, nontumor parenchyma is hardened and metastases are largely necrotic, and, hence, softer. For this reason, the normal, easy tactile perception of metastasis is more difficult, and finding a fluorescent area can be of great help.
The third application pertains to some primary liver neoplasms without a capsule, for which it is difficult to assess the optimum extent of the resection. In these cases, a good rule should be to check the absence of complete residual fluorescence at the end of the surgical transection.
Fourth, there are neoplasms with peripheral biliary infiltration and peribiliary spreading, which are not clearly recognizable by preoperative study, except for data indicating a dilatation of the upstream biliary structures[39]. In these cases, ICG retention affects the whole area that drains the Glisson segment and not just the nodule, suggesting the need for a wider resection.
Moreover, some authors have assessed the use of ICG for detecting biliary leaks in the hepatic transection surface after liver resection[40]. Finally, experimental studies involving intraportal and intrahepatic artery ICG injection (by celiac trunk catheterization in experimental models and by femoral puncture in hybrid operating rooms) have been published with the aim of achieving precise, real-time definitions of liver segmentation[41-43].
The lesion depth represents the major limitation in applying ICG-based fluorescence imaging to liver surgery. Further technological improvements are needed to deepen the fluorescence penetration more than the current 5-6 millimeters[44,45]. In addition, few data exist regarding extrahepatic HCC metastases, but this may represent a promising clinical application for FGS[46].
ONCOLOGICAL SURGERY OF THE UPPER AND LOWER GI TRACTS
The simplest use of ICG to surgically treat cancers affecting the digestive tract consists of endoscopically marking tumors to see the tumor site, which is necessary for small and nonserous infiltrating cancers. In this context, ICG is similar to other dyes used to date (China, indigo carmine)[47,48].
However, the most intriguing and potentially powerful application of FGS is currently one involving nodal navigation in esophageal, gastric, and colorectal cancers[49]. Injection of the dye into peritumoral tissues, ideally performed endoscopically in the submucosa, should allow for the intraoperative evaluation of lymphatic diffusion in real time. Multicentric prospective series are already available on the use of ICG to identify the sentinel lymph node in gastric cancer. These studies demonstrate that ICG is superior to both radioactive tracers and dyestuffs used to date[50,51]. In a recent multicentric Japanese series of 44 EGCs, the identification of sentinel node basins and the accuracy of metastatic node identification were both 100%[52].
The same strategy has been used for assessing colorectal tumors, investigating both the sentinel node basin and the enlarged mesocolic excision area draining the tumor site[53,54]. This latter principle is indeed the opposite of the sentinel node strategy: the goal would be to remove all lymph nodes that have received ICG as possible metastatic sites originating from the specific tumor site. The method is primarily useful for anatomical studies. Indeed, the ideal injection timing for the visualization of lymph nodes is not yet clear. Likely, after many hours, the dye may pass local lymph node drainage stations and move up to the central lymphatic pathways. The ideal study should therefore include (1) a preoperative tumor injection at different times before surgery up to an intraoperative injection; (2) the subsequent removal of individual lymph nodes; (3) a fluorescence evaluation for each node; and (4) a comparison of the final histological data.
In clinical practice, the problems are mainly related to the feasibility of the injection. For cancer of the esophagus, stomach, and rectum, the injection can be endoscopic and intraoperative (despite causing an increase in time and costs), while in the proximal colon, it is easier to proceed with a serous injection during the initial stages of the intervention. Clinical experience shows that the injection should be limited to a few cc, as staining rapidly spreads in lymphatic and adipose tissues. If, during the injection, a few drops of dye come out of the serosa on the peritoneum, they stain the surrounding structures and the surgical instruments and cannot be washed away.
Despite the above limitations, the possible applications of this method for navigating lymph nodes appear to be of great interest and have very important clinical consequences. It is clearly emerging that the ideal lymphadenectomy needs to be individualized based on preoperative staging. However, in many cases, imaging is not able to discern with high probability, for example, between T1 and T2. On the one hand, limiting lymphadenectomy to stations of real potential interest could allow considerable time savings and reduce most of the major postoperative complications, often related to vascular lesions secondary to an excessive nodal dissection. On the other hand, for example, in rectal surgery, extending lymphadenectomy to stations, such as obturatory and inguinal nodes, that are not usually removed could reduce the rate of lymph node recurrence[55].
The same applies to periaortic stations in cardia and proximal stomach cancer, to the right colic artery basin in cecum and ascending colon cancer, and to flexural cancers. A small series of 10 hepatic and 10 splenic flexure colon cancers reported a 25% rate of changes to the mesocolon resection line due to ICG lymphatic flow visualization[56]. A subsequent series of 31 patients with splenic flexure cancer from the same study group confirmed that all metastatic nodes were included in the area determined by ICG lymphatic flow visualization and that interesting insights into the lymphatic spread of those tumors can be derived[57].
In the near future, it is important to search for a factor predicting the positivity of perigastric and extraperigastric nodes based on their ICG load. To date, a relationship between ICG quantities in dissected nodes and the likelihood of the nodes bearing metastases has not been demonstrated. Studies focusing on the sentinel node strategy have shown that the radioisotope count was a significant predictive factor for metastasis[58]. In contrast, some very limited series recently described patients with unexpected nodal metastases from colorectal cancer discovered by fluorescence visualization after i.v. ICG injection while searching for peritoneal carcinomatosis[59]; this report suggests that a particular trophism of intravenously injected ICG for cancer cells may be discovered.
Few studies have taken into account the same type of lymph node navigation in pancreatic cancers, which have extremely rich and complex lymph node spread patterns. A recent series of 10 patients undergoing laparoscopic pancreaticoduodenectomy for periampullary cancers with i.v. ICG injection during retroportal fat tissue dissection revealed a possible advantage in obtaining an R0 margin at this level[60]. However, at this stage, it is not possible to hypothesize and assess the clinical usefulness of this strategy in pancreatic surgery[61]. Interestingly, a small series of pancreatic neuroendocrine tumors (NETs) has demonstrated a clear affinity of ICG for NET cells, which enables the removal of some residual tissue at the end of the first resection in laparoscopic distal pancreatectomy[62].
PERITONEAL CARCINOMATOSIS
This is an area where few studies have been conducted[63]. Peritoneal carcinomatosis remains a mode of metastatic diffusion that is largely devoid of real therapeutic strategies. Thus, finding it at an early stage while it is too small to be visible to the human eye, could represent a major clinical breakthrough. Some current studies are trying to link ICG to epithelial cell markers that should not be present on the peritoneum, which contains only mesothelial cells. No clinical outcomes of these studies are yet available, but some experimental settings have been developed in animal models. For instance, Cheng and colleagues have shown that a surgical navigation system combining optical molecular targets with an ICG-linked molecular probe is able to identify a peritoneal carcinomatosis site as small as 1.8 mm[64]. On the other hand, reports of some very limited series have shown that after i.v. injection, ICG is retained by cancerous cells in the peritoneum[65,66]. There is no clear explanation for these clinical observations, which, if confirmed in larger studies, may have very high clinical value as a comprehensive modality for staging visceral cancers.
CONCLUSION AND PERSPECTIVE
The key take-home messages from this overview are as follows. (1) The most well-established use of fluorescence imaging is for checking anastomotic stump perfusion in visceral surgery (Figure 1); Some prospective uncontrolled series and retrospective controlled studies, mainly dealing with colorectal and esophageal surgery, have confirmed the usefulness of this technique; (2) The second field of application for which there is a unanimous consensus in the literature pertains to visualization of the biliary anatomy during cholecystectomy (Figure 2). A comparison with intraoperative cholangiography demonstrated the superiority of fluorescence in terms of simplicity, timing, and efficacy; (3) In liver surgery, multiple possible uses of fluorescence have been described (tumor visualization, especially in laparoscopic surgery, vascular segmentation, biliary leak identification) (Figure 3). However, no comparative studies are available; (4) With regard to lymph node navigation in gastrointestinal tumors, most studies in the literature have focused on the sentinel node technique, whereas the use of fluorescence for complete lymphadenectomy has been described only by a few very experienced centers (Figure 4); and (5) Finally, there are some interesting preliminary findings regarding the use of fluorescence for the early detection of peritoneal carcinomatosis.
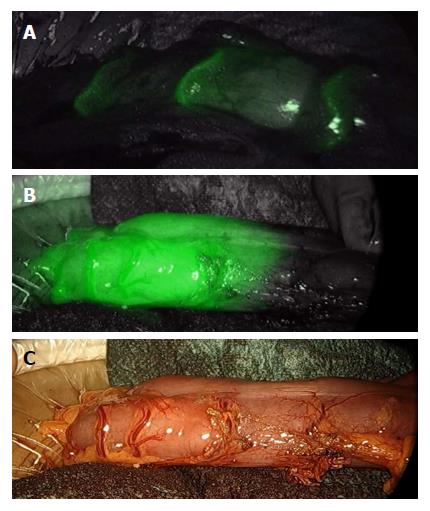
Figure 1 Colon perfusion before anastomosis during left colectomy.
A few seconds after the i.v. injection of 0.3 mg/kg indocyanine green, bowel arteries clearly appear (A); thereafter, the bowel perfusion cut-off area becomes evident (B and C).
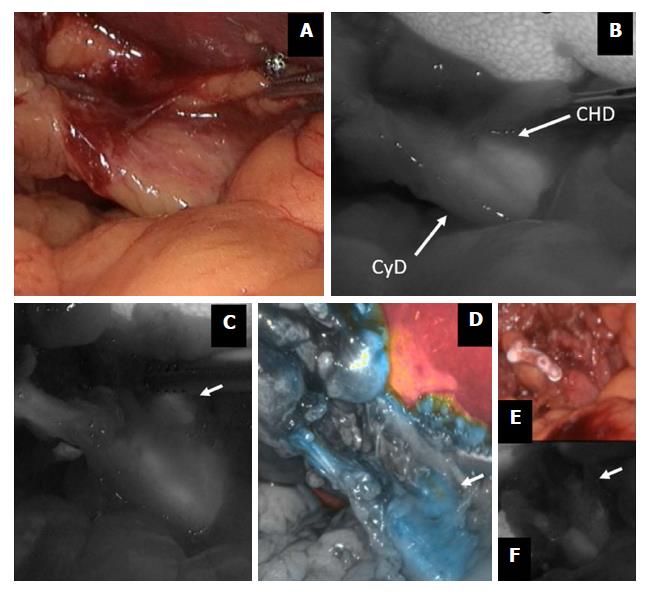
Figure 2 Indocyanine green-enhanced biliary anatomy.
During a difficult cholecystectomy for acute cholecystitis (A), the confluence between the cystic duct (CyD) and the common hepatic duct (CHD) is shown by fluorescence imaging (B); common hepatic duct (arrow) is further visualized before (C and D) and after (E and F) cystic duct division. ICG: Indocyanine green.
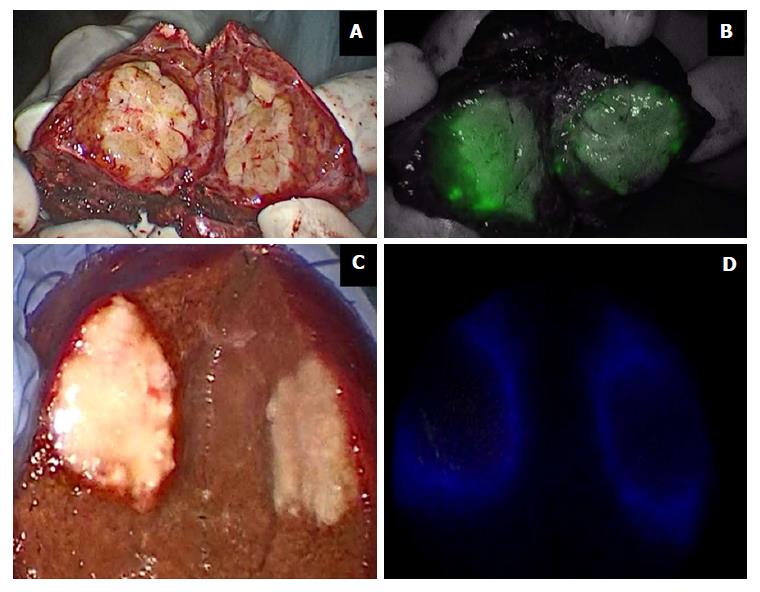
Figure 3 Indocyanine green in liver surgery.
Primary liver tumors show intense and complete staining because their hepatocytes take up ICG but do not secrete it (A and B); liver metastases show a ring appearance because their cells do not take up ICG but hepatocytes surrounding the nodule are compressed (C and D). ICG: Indocyanine green.
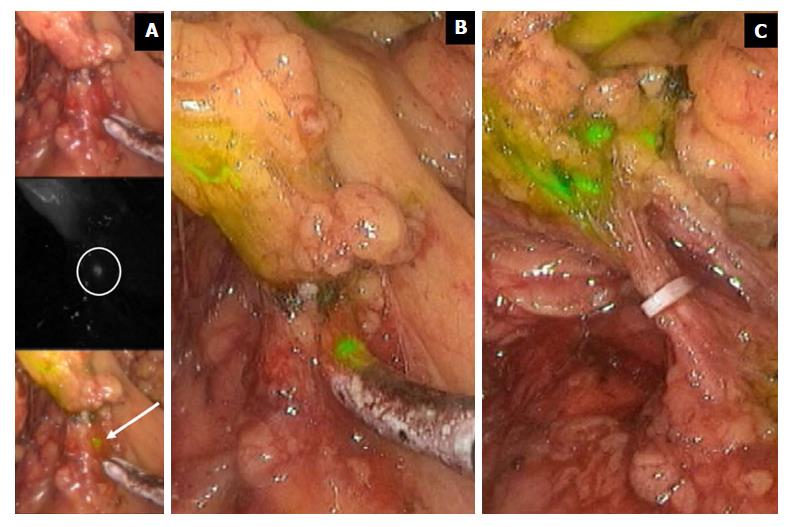
Figure 4 Indocyanine green fluorescence imaging in extended right hemicolectomy.
The figure displays the right branches of middle colic vessel division during extended right hemicolectomy for transverse colon cancer. ICG injected in the tumor site spreads in nodes at the very proximal root of the artery. ICG fluorescence imaging allows a radical lymphadenectomy, including very small nodes (A and B). Only when all the stained nodes are removed may the nodal dissection be considered radical (C). ICG: Indocyanine green.
An important issue pertains to the complexity and costs of this technology. The major positive feature is that the required training is minimal and the learning curve is extremely rapid, because the visualization is activated by either clicking a button on the camera or hitting a pedal. When considering the costs of ICG-based fluorescence imaging, the relevant distinction is between the fixed costs of the equipment versus the variable costs of the injections. The highest costs lie in obtaining a NIR fluorescence camera system with dedicated optical devices, cameras, and light cables. Depending on the system chosen (goggles, handheld, or stand alone), the cost of the system varies from several thousands to hundreds of thousands of USD (and similarly in other currencies). Nowadays, many major companies sell these tools; therefore, at the fast pace at which cost-saving technological change and updating are occurring, it is very likely that in the next 5 to 10 years most operating theaters will be equipped with the technology required to display the ICG-generated fluorescence. Once the NIR fluorescence camera system is available in a hospital, the additional cost of each surgical procedure utilizing ICG-based fluorescence imaging is relatively limited: as a matter of example, in Italy, Japan, and the United States, a 25 mg vial of ICG costs 80 euros, 588 yen, and 65 USD, respectively[41].
The imaging of fluorescence emitted by ICG is a simple, fast, and relatively inexpensive tool without side effects that has numerous different applications in surgery not only for treating cancers affecting the visceral and hepatobiliary systems but also for visualizing the biliary tree during difficult cholecystectomies. The fast pace at which technological development is occurring in this area and the ever-increasing availability of more powerful visual systems that can utilize this tool will transform some of these applications into the standard of care in the near future.
ACKNOWLEDGMENTS
The authors are grateful to RicerChiAmo onlus (wwww.ricerchiamobrescia.it) for supporting the international workshop “Intraoperative ICG Fluorescence Imaging in Hepatobiliary and Visceral Surgery: State of the Art and New Frontiers” held in Brescia (Italy) on October 21, 2017, which was attended by all three authors and turned out to be the driver of the conceptual development of this review.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aktekin A, Aosasa S, Hussain A, Mastoraki A S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y