Published online Jul 14, 2018. doi: 10.3748/wjg.v24.i26.2844
Peer-review started: March 29, 2018
First decision: May 16, 2018
Revised: May 23, 2018
Accepted: June 16, 2018
Article in press: June 16, 2018
Published online: July 14, 2018
Processing time: 106 Days and 2.1 Hours
A gallbladder polyp is an elevation of the gallbladder mucosa that protrudes into the gallbladder lumen. Gallbladder polyps have an estimated prevalence in adults of between 0.3%-12.3%. However, only 5% of polyps are considered to be “true” gallbladder polyps, meaning that they are malignant or have malignant potential. The main radiological modality used for diagnosing and surveilling gallbladder polyps is transabdominal ultrasonography. However, evidence shows that other modalities such as endoscopic ultrasound may improve diagnostic accuracy. These are discussed in turn during the course of this review. Current guidelines recommend cholecystectomy for gallbladder polyps sized 10 mm and greater, although this threshold is lowered when other risk factors are identified. The evidence behind this practice is relatively low quality. This review identifies current gaps in the available evidence and highlights the necessity for further research to enable better decision making regarding which patients should undergo cholecystectomy, and/or radiological follow-up.
Core tip: Evidence for the optimum management of gallbladder polyps is lacking. The main imaging modality used for diagnosis and follow-up is transabdominal ultrasound, but some studies suggest improved accuracy with endoscopic ultrasound. Other imaging modalities lack evidence. Surgical management involves cholecystectomy and the general consensus is that polyps 10 mm and greater should undergo surgery. However, this is an arbitrary cut-off and high-quality evidence to support this is lacking. Lowering the threshold for cholecystectomy when patients have additional risk factors for gallbladder malignancy may improve the cancer detection rate in polyps smaller than 10 mm, but again, the evidence behind this is lacking.
- Citation: McCain RS, Diamond A, Jones C, Coleman HG. Current practices and future prospects for the management of gallbladder polyps: A topical review. World J Gastroenterol 2018; 24(26): 2844-2852
- URL: https://www.wjgnet.com/1007-9327/full/v24/i26/2844.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i26.2844
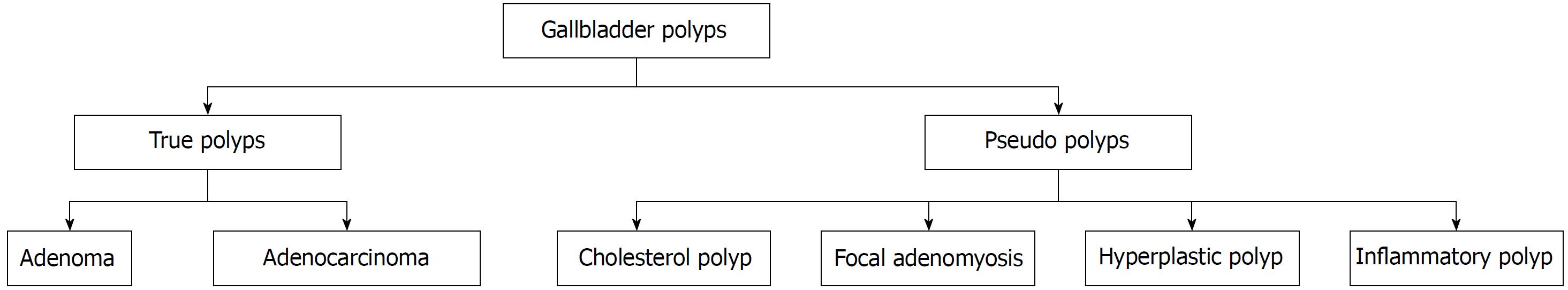
A gallbladder polyp is an elevation of the gallbladder mucosa that protrudes into the gallbladder lumen[1,2]. Gallbladder polyps have an estimated prevalence of approximately 5% in the global population, but only 5% of these are considered to be “true” gallbladder polyps[3,4]. The majority of gallbladder polyps are detected incidentally on radiological imaging or histological examination after cholecystectomy. However, a small number of patients with gallbladder polyps may be symptomatic and present with acute cholecystitis due to the polyp obstructing the cystic duct, or cholangitis due to fragments of the polyp breaking off and travelling down into in the bile duct[2,5]. The majority of gallbladder polyps are classified as “pseudo”-polyps, as displayed in Figure 1. “Pseudo”-polyps have no malignant potential and do not require any follow-up or intervention, whereas “true” gallbladder polyps, which include adenocarcinomas or adenomas require surgical removal[2]. Although adenomas are benign, they have malignant potential and there is some evidence to suggest they may follow the adenoma-carcinoma sequence as seen in colorectal cancer[6,7].
Gallbladder cancer is the 20th most common cancer in the world and there are an estimated 178100 new cases diagnosed each year[8]. The highest incidences of gallbladder cancer are seen in South America and Asia, whilst lower incidences are seen in developed regions such as North America and the United Kingdom[9]. For example, the incidence of gallbladder cancer in Chile and Bolivia is 12.8 and 10.9 per 100000 population respectively, whereas in the United Kingdom and North America the incidence is 1.6 and 1.5 per 100000 people[9,10]. The staging of gallbladder cancer as per the American Joint Committee on Cancer 8th edition, ranges from stage 0 to stage 4b. Stage 0 describes carcinoma in-situ when the cancer involves the mucosa only as seen in early polyp cancers, while stage 4b indicates lymph node involvement of 4 or more lymph nodes (N2 disease) or the presence of metastatic disease[11]. Survival in gallbladder cancer patients varies significantly from an 80% 5-year survival in those with in-situ disease, declining to only 8% when lymph nodes are involved, and 2% for patients with stage 4b disease[11]. These figures demonstrate the importance of identifying malignant and pre-malignant polyps to enable early treatment to prevent cancer spread or development of malignancy.
It should be noted that once detected, surgical removal of all gallbladder polyps is not appropriate, given that the majority of polyps are “pseudo”-polyps with no malignant potential and there is a significant risk associated with surgery. In patients with “true” gallbladder polyps, laparoscopic cholecystectomy is the surgical option preferred, although in patients with larger polyps, open cholecystectomy is recommended[12,13]. The risks associated with surgery include damage to intra-abdominal structures during port insertion, bile duct injury (between 0.3% and 1%) and bile leak[14,15]. Furthermore, surgical intervention to repair a bile duct injury and endoscopic retrograde cholangio-pancreatography (ERCP) to manage a bile leak are associated with significant mortality, cholangitis, biliary cirrhosis, pancreatitis, perforation and haemorrhage[16,17].
This review discusses the current evidence that exists regarding the management of gallbladder polyps. Given the low incidence of true polyps within all gallbladder polyps identified, coupled with the high mortality associated with gallbladder cancer and the risk of complications associated with cholecystectomy, it is essential to differentiate between “pseudo”- polyps and true polyps to enable appropriate management. The use of imaging modalities assists with the decision-making process and this review discusses the benefits and shortcomings of the imaging modalities used for identifying and following up gallbladder polyps.
Radiological imaging plays the main role in the diagnosis and decision making for the management of gallbladder polyps. The ideal imaging modalities should have three key features. Firstly, they should be able to accurately diagnose polyps and differentiate them from gallstones, sludge, or folds of the gallbladder mucosa. Secondly, “true” polyps need to be differentiated from “pseudo”- polyps, as the latter are benign with no malignant potential and therefore do not require any intervention or follow-up. Thirdly, the size of polyps need to be measured accurately as this is currently the most important factor which determines if patients should undergo cholecystectomy, radiological follow up or cease to be followed up. Given that some patients with gallbladder polyps will require follow-up for many years, it is also important that the imaging modality is acceptable to patients and incurs minimal radiation exposure.
Accurate imaging will prevent unnecessary surgery and ensure true polyps which do not fall into the size criteria for surgical removal category are identified during follow-up. The benefits and shortcomings of different imaging modalities are discussed below. The main modalities discussed include ultrasonography, computed tomography and magnetic resonance imaging.
Trans abdominal ultrasound (TAUS), encompasses conventional ultrasound (CUS), high-resolution ultrasound (HRUS), three-dimensional ultrasound and contrast enhanced ultrasound (CEUS). CUS and HRUS are easily accessible, cheap, non-invasive tests[18] and are the most widely used modalities for diagnosing and following up gallbladder polyps. However, other studies have been performed to assess the effectiveness of the other forms of ultrasonography mentioned above[18,19].
Ultrasonography is operator dependent and results can be limited by increased body mass index, in particular truncal obesity[20]. Polyp echogenicity is examined to distinguish between “true” polyps and “pseudo”- polyps and the presence of a fixed lesion helps to distinguish between polyps and gallstones. However, in some cases gallstones may be impacted in the gallbladder wall and be incorrectly labelled as a polyp[2]. Features that suggest the presence of a “pseudo”- polyp include a “comet tail” which arises posterior to the lesion but this is not identifiable in all “pseudo”- polyps[21].
CUS uses a low-frequency transducer between 2 and 5MHz but despite this has demonstrated good specificity (71%-98%) and sensitivity (50%-90%) for diagnosing all types of gallbladder polyps[22]. In the same systematic review, CUS had a sensitivity of 47%-67% and specificity of 36-100% for diagnosing malignancy[22] and in polyps 10mm or greater in size, the sensitivity and specificity for identifying malignancy was 78%-100% and 52%-87%, respectively[22].
However, shortcomings in CUS have been reported, for example in a single study by French et al[23] which compared histopathology reports from cholecystectomy specimens with findings from the CUS report found that imaging only identified 50% of polyps. This group concluded that CUS should not be used for following up gallbladder polyps[23] .
HRUS operates at a higher frequency than CUS (5-7 MHz) but a lower frequency than endoscopic ultrasound (EUS) (5-12 MHz) and therefore theoretically has a better diagnostic accuracy than CUS but is less accurate than EUS[24]. It does however have the benefit over EUS, in that it is a non-invasive procedure. Kim et al[24] demonstrated that HRUS is more accurate than CUS at staging the T-stage of gallbladder cancer and was more accurate for identifying hypoechoic foci in neoplastic polyps which has previously been shown to be a strong predictive factor for neoplastic gallbladder polyps[24,25]. More studies however are required which compare the sensitivity and specificity of CUS and HRUS.
One study has compared HRUS, endoscopic ultrasound (EUS), and computed tomography (CT) in diagnosing and staging gallbladder polyps in 144 patients who all had a polyp greater than 10 mm in size[26]. Diagnostic sensitivities for malignancy were highest in HRUS, compared to the other two modalities and specificity was the same when using EUS and HRUS[26]. The drawback from this study however is that the applicability of this technique to smaller gallbladder polyps remains unknown and polyps of less than 10 mm are diagnostically most difficult group to assess. Furthermore, HRUS was not compared to CUS, which is currently the most commonly used imaging modality.
3D-US is an emerging modality which eliminates the operator dependency seen in 2-dimensional CUS. Research for this imaging modality is minimal but a study of 80 patients with gallbladder polyps found that there was agreement in the diagnosis in 89% of cases when both techniques were applied[27]. This study however found that 3D-US did have difficulty detecting polyps less than 4mm, but it is predicted that as technology continues to evolve this issue will decline in future[27]. Current research therefore does not support the routine use of 3D-US for evaluating gallbladder polyps.
Several small studies have looked at the use of contrast media to improve the diagnostic accuracy of CUS. Contrast aids radiologists to differentiate normal from abnormal conditions. Numata et al[28] used galactose palmitic acid contrast injection to assess 35 polyps which were larger than 10 mm in size. Using the criteria of tumour enhancement and tortuous type tumour vessels, this technique had 91% accuracy at identifying malignancy. The downside to this study however, is that it did not compare contrast-enhanced ultrasonography with CUS[28]. Zheng et al[29] did compare the two modalities in a study of 116 patients with gallbladder polyps, and found that CEUS was useful for improving diagnostic accuracy in polyps greater than 10 mm, but not less than 10 mm in size.
EUS works at a higher frequency as described above and enables the transducer to be in closer proximity to the target tissue therefore, hypothetically improving diagnostic accuracy[24]. It is however, an invasive examination associated with a small risk of bleeding and upper gastrointestinal perforation and presents a higher risk of complications than all forms of TAUS[30].
A systematic review has found EUS to have a greater sensitivity (67%-86%) and specificity (84%-91%) for diagnosing malignancy in polyps than CUS[22]. A single study by Sugiyama et al[31] compared EUS and CUS in 58 patients who had undergone cholecystectomy. All polyps were 20 mm or less in size, and EUS was more accurate at differentiating between true and “pseudo”- polyps than CUS (97% vs 76%). Cheon et al[32] however, found that although EUS was more successful at identifying true polyps in those with diameters of 11 mm and greater (83% vs 64%), there was not the same success in polyps of diameter 10 mm and less (80% vs 72%). Therefore, this imaging technique may play a role in decreasing the number of unnecessary cholecystectomies in larger gallbladder polyps, but more research needs to be done investigating its role in smaller polyps, for which the management is most controversial.
Two studies have been performed looking at the role of contrast- enhanced EUS (CE-EUS) in diagnosing gallbladder polyps. Park studied 34 patients who had a cholecystectomy for gallbladder polyps and found that CE-EUS when attempting to distinguish adenomatous polyps from cholesterol polyps had a sensitivity of 75% and specificity of 66.6%. Unfortunately, in this study CE-EUS was not compared to any other imaging modality. Choi et al[33] however compared EUS with CE-EUS and found that diagnostic accuracy was slightly improved with the latter.
Other methods including the use of real time colour Doppler flow EUS has been used to try and improve the diagnostic accuracy of EUS. Kim et al[34] found that the presence of a strong colour Doppler flow in a study 115 patients who underwent cholecystectomy for gallbladder polyps may help predict the presence of neoplastic polyps and therefore further research is warranted.
CT imaging is widely used in the staging of gallbladder adenocarcinoma[2]. However, some research has been performed to assess if it may also play a role in differentiating between true and “pseudo”- polyps and for long-term surveillance[35]. The accuracy of CT imaging was assessed in 31 patients with polypoid lesions of the gallbladder of 3cm or less. The CT diagnosis was accurate in 87% of cases however, only 5 polyps were less than 11 mm and therefore this study provides us with limited evidence regarding the role of CT in this group of patients[35]. Lou et al[36] assessed the accuracy of CT biliary cystoscopy in 32 patients and found that CUS accurately detected polyps in 96.9% of cases compared to 93.8% for CT.
This evidence would suggest that CT imaging is best used in staging larger, suspicious malignant polyps, rather than for diagnostic purposes and follow-up, due to lack of superiority to CUS demonstrated in studies to date.
Minimal research has been performed looking at the role of MRI in differentiating between benign and malignant gallbladder polyps. In a small study, Irie at al[37] demonstrated in 10 benign polyps and 13 malignant polyps that the ADC values of the malignant lesions were significantly lower than that seen in the benign lesions. They concluded that diffusion-weighted MR imaging may play a role in diagnosing benign and malignant polyps[37]. However, further research is warranted to establish if MRI can improve the accuracy of diagnosing gallbladder polyps.
Other imaging modalities have been considered in small single studies. One study has shown that positive emission tomography can differentiate between benign and malignant disease but more research is needed[2]. Results from a study examining the role of percutaneous transhepatic cholecystoscopy were promising but this is an invasive procedure with significant risk and is difficult for patients to tolerate[2]. Finally, intravenous cholecystography has shown to be of no benefit to date, compared with current imaging modalities[18].
After studying the evidence, TAUS and in particular CUS and HRUS would appear to be the most appropriate imaging modality for detecting gallbladder polyps. Although some studies looking at the role of other forms of ultrasonography in managing gallbladder polyps appear promising, there is still not enough evidence to introduce these modalities into routine practice for the management of gallbladder polyps. Evidence for smaller gallbladder polyps is of particularly low quality. In cases of clear uncertainty however, additional imaging modalities may be deployed to help the clinician in their decision-making process. The role of CT is evident in staging gallbladder cancer but due to a lack of high-quality studies examining a role in gallbladder polyps and the high radiation exposure associated with this imaging, it is not appropriate for either the diagnosis or follow-up of gallbladder polyps.
Studies have shown that malignant polyps in general tend to be larger than benign polyps[5,22]. Kwon et al[5] reported in their study of 291 patients that malignant polyps had a mean size of 27.97+/-2.46 mm compared to 8.56+/-0.36 mm in the benign group. Currently, the polyp size on radiological imaging is the biggest contributing factor to the management plan for gallbladder polyps. Multiple retrospective studies have found the risk of malignancy rises sharply from 10 mm and upwards, and the general consensus is that patients with polyps of 10 mm or greater should be treated with cholecystectomy[19,22,38]. Although this is the accepted practice, evidence for this recommendation lacks quality. The most up-to-date guidelines published by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) support this approach but two recent systematic reviews demonstrate that although the majority of malignant polyps are over 10 mm in diameter, there are a significant number of both malignant polyps or polyps with malignant potential under this sizing threshold[19,22,38].
Babu et al[22] performed a systematic review which included 43 studies, of which 20 provided information on the size and histology of 2347 polyps. Of these, 356 were classified as true polyps, of which 228 were malignant - and 29 of these were between 5-10 mm but none below the 5 mm size. Bhatt et al[38] in their systematic review also demonstrated that there were a significant number of malignant polyps under 10 mm in size but the probability of malignancy when a polyp was 4.15 mm or smaller was approximately zero. These two large studies demonstrate that although the majority of true polyps are over 10 mm there are a significant number of true polyps under this cut off which will be missed if cholecystectomy is only performed for polyps greater than 10 mm.
Several authors have suggested a change in this cut off with some suggesting polyps of 6 mm and larger should undergo cholecystectomy whilst others have felt that the cut off should be increased to 12mm[39,40]. The argument for lowering the threshold carries more weight, as demonstrated by the findings in the systematic reviews discussed above. The counter-argument of lowering the threshold is that by offering cholecystectomy to those patients with polyps below 10 mm, a greater number of patients may be put through an unnecessary operation associated with significant risk of complications. It has therefore been proposed that polyps under 10 mm should undergo surveillance, based on their size unless significant risk factors are present in which case cholecystectomy should be offered[19].
Polyp surveillance aims to provide a safety net for those patients with true polyps that cannot be differentiated from “pseudo”- polyps on radiological investigations and are under 10 mm in diameter. It is hypothesised that “true” polyps will undergo faster growth, and by careful follow-up these can be identified early and removed[22]. Guidelines state that polyps which reach 10 mm in size or increase in size by 2 mm at follow up transabdominal ultrasonography are recommended to be removed surgically[19]. However, evidence to support this practice is lacking.
There is no consensus on the size of polyps that require follow up, or the frequency or duration of follow up. The most recent set of guidelines published by ESGAR states that patients with polyps of 6-9 mm should be followed up more extensively than patients with polyps of less than 6 mm[19]. Several studies support 6 mm as a lower limit cut-off for less extensive follow up, but go a step further by suggesting the cessation of follow up in polyps less than 6 mm[41,42]. However, this has been contradicted by multiple studies which have found true polyps to be less than 6 mm in size and a single case report that has shown that a 5 mm polyp transformed into a 20 mm carcinoma over a period of two years[22,38,43]. The evidence would suggest that all polyps between 4-10 mm should be followed up equally as although the risk reduces with size, there is still a significant number of true polyps between 4 mm and 6mm. Although no malignant polyps have been shown to be below 4 mm there is still a risk of adenomas and these polyps therefore would still require follow up but on a less frequent basis[22].
The recommended follow up for patients with gallbladder polyps depends on the size of the polyps and the presence of risk factors for malignancy, but opinions differ and the evidence base informing these guidelines is relatively limited. For example, Babu et al[22] recommend that the follow up of polyps 5-10 mm should be two scans at six month intervals and following this the surveillance plan should be tailored for individual patients. The ESGAR group recommend that in polyps of 6-9 mm, after two initial six monthly scans there should be yearly scans up to 5 years. However, in polyps under 6 mm there should be imaging at 1, 3 and 5 years but if he patient has risk factors for malignancy there should be more extensive follow-up as those seen for polyps of 6-9 mm with no risk factors[22].
Follow up imaging may have a limited benefit as only a small number of polyps actually change in size during follow up. Babu et al[22] identified 10 studies which looked at the follow up of gallbladder polyps between six months and seven years. They found that only 7.6% of polyps increased in size and Bhatt et al[38] also found that that 93% of polyps did not change in size during follow up. Neither study stated if growth was more likely to be seen in in pseudo or true polyps and this was supported in a third systematic review[44]. Although there is a lack of evidence comparing growth patterns between pseudo-polyps and true polyps, small individual studies have shown that both can undergo sudden growth[2].
As discussed above the main determining factor for gallbladder malignancy is the presence of a polyp greater than 10 mm in size. However, not all polyps under 10 mm are benign and therefore it is important to identify risk factors to enable the clinician to have a higher suspicion for malignancy and therefore perform cholecystectomy below the 10 mm threshold. These potential risk factors are discussed below and summarised in Table 1.
| Risk factor | Direction of association | Strength of association | Related notable findings | Key references |
| Age | Positive | Probability of malignancy was 20.7% in those patients older than 50 | This systematic review studied polyps less than 10 mm only | [38] |
| Sessile morphology | Positive | Probability of malignancy was 13.9% in sessile compared to pedunculated polyps | This systematic review studied polyps less than 10 mm only | [38] |
| Presence of gallstones | Inconclusive | Aldouri et al[47] found increased risk of malignancy with gallstones (HR = 3.2, 95%CI: 1.42-7.22) but Park et al[39] found no difference (P = 0.27) | There is no strong evidence to suggest there is a definite association | [39,47] |
| Indian Ethnicity | Positive | HR = 12.92 (95%CI: 3.77-44.29) This shows a significant HR but the width of the CI’s are noted. | This is the only study to compare risk between Indian ethnicity and Caucasian race | [47] |
| Primary sclerosing cholangitis | Positive | 40%-60% of polyps in patients with PSC were malignant | 33% of those with benign polyps had associated dysplasia | [56] |
Evidence is mixed on whether solitary polyps are more likely to be malignant compared to the presence of multiple polyps. In a systematic review by Bhatt et al[38], the probability of malignancy in a polyp under 10 mm if it was solitary was 4.3% higher compared to when multiple polyps were present. The authors did not deem this to incur a high enough risk to suggest cholecystectomy in all patients with a solitary polyp under 10mm. Perhaps this is the most useful study as the authors look at the risk exclusively in the 5-9 mm group and it is this cohort in which the evidence is weakest[38]. A study by Kwon et al[5] also found that malignant polyps were more likely to be solitary (P = 0.02), but this study only patients who had gallbladder polyps greater than 10 mm. Several other studies however have demonstrated no association between a solitary polyp and malignancy. For example, Park et al[39] in a study of 689 patients found that 60% of benign polyps were solitary and 76% of malignant polyps were benign and this was not significantly different (P = 0.11).
Although the probability of malignancy is not high enough to recommend cholecystectomy in all solitary polyps, the presence of a solitary polyp should be considered in combination with other risk factors for malignancy as discussed below.
Single studies such as that performed by Kwon et al[5] have demonstrated that patients with gallbladder polyps of sessile morphology have a higher risk of malignancy compared to those with pedunculated polyps (OR: 7.70; 95%CI: 2.48-23.95). In the systematic review by Bhatt et al[38], malignant polyps under 10 mm were also more likely to be sessile in nature and the probability of malignancy was 13.9% in these patients but cholecystectomy was not recommended. However, if there was a solitary sessile polyp, the probability of malignancy was 24.8% and cholecystectomy was recommended[38]. Although Bhatt et al[38] do not recommend cholecystectomy based on sessile morphology alone, the most recent guidelines by the ESGAR group use the strength of this evidence to recommend cholecystectomy for all sessile polyps under between 6 mm and 9 mm.
The risk of most cancers increases with age and a similar pattern is seen for gallbladder cancer. Multiple case series support this but the cut off for an increased risk of malignancy varies significantly between 50 and 65 years old[13,38,39,45]. For example, Park et al[39] identified age 57 years and older as a risk factor for malignancy, but in this study one patient who was only 37 years old had a malignant polyp of 10 mm and the one patient who had a malignant polyp under 10 mm in size was only 50 years old. Furthermore, Sarkut et al[46] found that there was an increased likelihood of malignancy in patients aged 50 and over, but again this was not exclusive as one patient under 50 had a malignant polyp. The only study to date that looks at the contribution of age to risk of malignancy in polyps solely under 10 mm was performed by Bhatt et al[38]. They found that when the polyp was less than 10 mm and the patient was over 50 that the probability of malignancy was 20.7%, and therefore cholecystectomy was recommended[38]. The ESGE group used this evidence to conclude that if patients are aged 50 and have polyps of 6-9 mm they should undergo cholecystectomy[19].
The evidence considering the impact of concurrent gallstones and the risk of malignancy in gallbladder polyps varies significantly and is of relatively low quality. Aldouri et al[47] found that if gallstones were present there was an increased risk of malignancy (HR: 3.2; 95%CI: 1.42-7.22) but Park et al[39] found that there was no association between the presence of gallstones and malignancy (P = 0.27). In those patients with symptoms due to gallstones, cholecystectomy is already recommended and therefore the decision-making process is simple. However, the evidence is not strong enough to suggest cholecystectomy should be performed in all cases with dual pathology.
As discussed earlier, gallbladder cancer incidence varies significantly between countries. A study by Aldouri et al[47] carried out in the United Kingdom demonstrated that in 5391 patients who underwent cholecystectomy, the risk of malignancy was almost 13 times higher in the Indian population compared to the Caucasian population (HR: 12.92; 95%CI: 3.77-44.29). This is the only study to date which compares risk between different ethnic groups, however the ESGAR felt the evidence was so compelling that their guidelines state that in patients of Indian ethnicity and a polyp between 6-9 mm they should undergo cholecystectomy[19]. Further research needs to be performed comparing other ethnic groups to determine if there should be a lower threshold for cholecystectomy in different ethnicities.
Primary sclerosing cholangitis (PSC) is a recognised risk factor for a gallbladder polyp malignancy, and cholecystectomy is currently recommended in these patients who have a gallbladder polyp irrespective of the polyp size[48]. The largest study to date including 286 PSC patients, found that in 18 patients with a gallbladder polyp, 10 had a malignancy in polyps as small as 5 mm whilst in 9 patients who had no mass lesion they still had dysplasia of the gallbladder[49]. Furthermore, in a case series of 4 patients with PSC and gallbladder polyps, all were shown to have malignant disease including in two polyps under 10 mm in size[50]. Other evidence is less compelling, including a study by Eaton et al[51] who found that in 14 patients with PSC and polyps only two were malignant. This group concluded that polyps under 8 mm were less likely to be malignant and in this group and follow up should be applied. Given the presence of research such as this further research would be justified. The difficulty will be recruiting enough patients with both pathologies.
Limited research has been performed to assess if there is a role for tumour markers in the pre-operative evaluation of gallbladder polyps. The two markers focused on to date has been CEA and CA19-9 but no correlation between malignancy and elevated markers has been found. In a case series of 291 patients, Kwon et al[5] found no difference in pre-operative CEA or CA19-9 levels in the benign or malignant groups. Indeed, the CEA level was elevated in more benign cases (5.7%) than malignant cases (2.9%). When comparing the CA19-9 levels, there were 4.9% of benign group who had a raised level and 8.6% of malignant group had a raised level[5]. There is no sufficient evidence to show that tumour markers will assist in the decision-making process for gallbladder polyps.
To our knowledge, no research has studied genetic risk factors for gallbladder polyps, despite multiple studies having investigated genetic contributions to gallbladder cancer. For example, studies from Shanghai and Sweden have noted significantly increased risks of gallbladder cancer in patients with a family history of gallbladder cancer[52,53]. It has also been shown in a recent review that approximately one quarter of cases diagnosed in a Utah cohort study were familial[54]. However, the difficulty with evaluating family history as a proxy for genetic factors is that it may also reflect exposure to similar environmental exposures. A recent review has highlighted the paucity of research on specific genetic polymorphisms with respect to gallbladder cancer risk, and extrapolated some biologically plausible hypotheses from gallstone aetiology[54-56]. Overall, there is only low quality evidence for genetic predisposition to gallbladder cancer, and no studies have been conducted for gallbladder polyps. Robust, genome-wide association studies are required to confirm or deny any potential associations.
The gaps in the available evidence to support the current guidelines on the management of gallbladder polyps are outlined above. TAUS is the current mainstay for radiological investigation of gallbladder polyps. EUS and HRUS have shown some promise as an adjunct to TAUS but more work is required to assess the exact role and the category of polyps that they may provide diagnostic accuracy. Although polyps of 10 mm and greater are more likely to be true polyps, this cut-off will miss a significant number of true polyps below this threshold and cholecystectomy will also be performed unnecessarily for pseudopolyps when they are greater than 10 mm. The factoring in of the risk factors discussed above to lower the threshold for cholecystectomy will no doubt decrease the number of missed true polyps in the under 10 mm category but cholecystectomy will also be performed when it is not required. No research has been performed to assess the impact of following these guidelines and therefore larger retrospective and prospective case series need to be performed to assess the success of managing gallbladder polyps as per the current guidelines.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Barreto SG, Cho JH, Lee KG, Sharma KL, Wang J S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Chattopadhyay D, Lochan R, Balupuri S, Gopinath BR, Wynne KS. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: a nine year experience. World J Gastroenterol. 2005;11:2171-2173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 2. | Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N Am J Med Sci. 2012;4:203-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Okamoto M, Okamoto H, Kitahara F, Kobayashi K, Karikome K, Miura K, Matsumoto Y, Fujino MA. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am J Gastroenterol. 1999;94:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Lin WR, Lin DY, Tai DI, Hsieh SY, Lin CY, Sheen IS, Chiu CT. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: analysis of 34 669 cases. J Gastroenterol Hepatol. 2008;23:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24:481-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 6. | Aldridge MC, Bismuth H. Gallbladder cancer: the polyp-cancer sequence. Br J Surg. 1990;77:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Kozuka S, Tsubone N, Yasui A, Hachisuka K. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50:2226-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | IARC. Globocan 2012;. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. |
| 9. | World Cancer Research Fund International. Gallbladder cancer statistics. Available from: https://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/gallbladder-cancer-statistics. |
| 10. | Cancer Research UK. Cancer Research UK Cancer incidence statistics. 2014; Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/leukaemia-cll/incidence. |
| 11. | Amin MB, Greene FL, Edge SB. AJCC Cancer Staging Manual. Sprin Inter Publ. 2017;. [DOI] [Full Text] |
| 12. | Keus F, de Jong JA, Gooszen HG, van Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database Syst Rev. 2006;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Lee KF, Wong J, Li JC, Lai PB. Polypoid lesions of the gallbladder. Am J Surg. 2004;188:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Gurusamy KS, Samraj K. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Cochrane Database of Systematic Reviews. Vol 67. Chichester, UK: John Wiley & Sons, Ltd; 2006; 381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Krähenbühl L, Sclabas G, Wente MN, Schäfer M, Schlumpf R, Büchler MW. Incidence, risk factors, and prevention of biliary tract injuries during laparoscopic cholecystectomy in Switzerland. World J Surg. 2001;25:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Gurusamy KS, Abu-Amara M, Farouk M, Davidson BR. Cholecystectomy for gallbladder polyp. Cochrane Database Syst Rev. 2009;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004;60:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Matos AS, Baptista HN, Pinheiro C, Martinho F. [Gallbladder polyps: how should they be treated and when?]. Rev Assoc Med Bras (1992). 2010;56:318-321. [PubMed] |
| 19. | Wiles R, Thoeni RF, Barbu ST, Vashist YK, Rafaelsen SR, Dewhurst C, Arvanitakis M, Lahaye M, Soltes M, Perinel J. Management and follow-up of gallbladder polyps: Joint guidelines between the European Society of Gastrointestinal and Abdominal Radiology (ESGAR), European Association for Endoscopic Surgery and other Interventional Techniques (EAES), International Society of Digestive Surgery - European Federation (EFISDS) and European Society of Gastrointestinal Endoscopy (ESGE). Eur Radiol. 2017;27:3856-3866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 20. | Gallahan WC, Conway JD. Diagnosis and management of gallbladder polyps. Gastroenterol Clin North Am. 2010;39:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 21. | Shapiro RS, Winsberg F. Comet-tail artifact from cholesterol crystals: observations in the postlithotripsy gallbladder and an in vitro model. Radiology. 1990;177:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Babu BI, Dennison AR, Garcea G. Management and diagnosis of gallbladder polyps: a systematic review. Langenbecks Arch Surg. 2015;400:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | French DG, Allen PD, Ellsmere JC. The diagnostic accuracy of transabdominal ultrasonography needs to be considered when managing gallbladder polyps. Surg Endosc. 2013;27:4021-4025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kim JH, Lee JY, Baek JH, Eun HW, Kim YJ, Han JK, Choi BI. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am J Roentgenol. 2015;204:W150-W159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Cho JH, Park JY, Kim YJ, Kim HM, Kim HJ, Hong SP, Park SW, Chung JB, Song SY, Bang S. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest Endosc. 2009;69:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Jang JY, Kim SW, Lee SE, Hwang DW, Kim EJ, Lee JY, Kim SJ, Ryu JK, Kim YT. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009;250:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Stenberg B, Elliott S. Diagnosis of gallbladder problems using three-dimensional ultrasound. Eur Radiol. 2010;20:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Numata K, Oka H, Morimoto M, Sugimori K, Kunisaki R, Nihonmatsu H, Matsuo K, Nagano Y, Nozawa A, Tanaka K. Differential diagnosis of gallbladder diseases with contrast-enhanced harmonic gray scale ultrasonography. J Ultrasound Med. 2007;26:763-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Zheng SG, Xu HX, Liu LN, Lu MD, Xie XY, Wang WP, Hu B, Yan K, Ding H, Tang SS. Contrast-enhanced ultrasound versus conventional ultrasound in the diagnosis of polypoid lesion of gallbladder: a multi-center study of dynamic microvascularization. Clin Hemorheol Microcirc. 2013;55:359-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Jenssen C, Alvarez-Sánchez MV, Napoléon B, Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659-4676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Sugiyama M, Xie XY, Atomi Y, Saito M. Differential diagnosis of small polypoid lesions of the gallbladder: the value of endoscopic ultrasonography. Ann Surg. 1999;229:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Cheon YK, Cho WY, Lee TH, Cho YD, Moon JH, Lee JS, Shim CS. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol. 2009;15:2361-2366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Choi JH, Seo DW, Choi JH, Park DH, Lee SS, Lee SK, Kim MH. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest Endosc. 2013;78:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Kim SY, Cho JH, Kim EJ, Chung DH, Kim KK, Park YH, Kim YS. The efficacy of real-time colour Doppler flow imaging on endoscopic ultrasonography for differential diagnosis between neoplastic and non-neoplastic gallbladder polyps. Eur Radiol. 2018;28:1994-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Furukawa H, Kosuge T, Shimada K, Yamamoto J, Kanai Y, Mukai K, Iwata R, Ushio K. Small polypoid lesions of the gallbladder: differential diagnosis and surgical indications by helical computed tomography. Arch Surg. 1998;133:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Lou MW, Hu WD, Fan Y, Chen JH, E ZS, Yang GF. CT biliary cystoscopy of gallbladder polyps. World J Gastroenterol. 2004;10:1204-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Irie H, Kamochi N, Nojiri J, Egashira Y, Sasaguri K, Kudo S. High b-value diffusion-weighted MRI in differentiation between benign and malignant polypoid gallbladder lesions. Acta Radiol. 2011;52:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Bhatt NR, Gillis A, Smoothey CO, Awan FN, Ridgway PF. Evidence based management of polyps of the gall bladder: A systematic review of the risk factors of malignancy. Surgeon. 2016;14:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH, Yu SJ, Kang HY, Lee JY, Park MJ. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008;2:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Zielinski MD, Atwell TD, Davis PW, Kendrick ML, Que FG. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg. 2009;13:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Pedersen MR, Dam C, Rafaelsen SR. Ultrasound follow-up for gallbladder polyps less than 6 mm may not be necessary. Dan Med J. 2012;59:A4503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Corwin MT, Siewert B, Sheiman RG, Kane RA. Incidentally detected gallbladder polyps: is follow-up necessary?--Long-term clinical and US analysis of 346 patients. Radiology. 2011;258:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 43. | Lu D, Radin R, Yung E, Tchelepi H. Malignant transformation of a 5-mm gallbladder polyp over 2 years: a case report and review of current literature. Ultrasound Q. 2015;31:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Wiles R, Varadpande M, Muly S, Webb J. Growth rate and malignant potential of small gallbladder polyps--systematic review of evidence. Surgeon. 2014;12:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Cha BH, Hwang JH, Lee SH, Kim JE, Cho JY, Kim H, Kim SY. Pre-operative factors that can predict neoplastic polypoid lesions of the gallbladder. World J Gastroenterol. 2011;17:2216-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Sarkut P, Kilicturgay S, Ozer A, Ozturk E, Yilmazlar T. Gallbladder polyps: factors affecting surgical decision. World J Gastroenterol. 2013;19:4526-4530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 47. | Aldouri AQ, Malik HZ, Waytt J, Khan S, Ranganathan K, Kummaraganti S, Hamilton W, Dexter S, Menon K, Lodge JP. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol. 2009;35:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1203] [Article Influence: 75.2] [Reference Citation Analysis (1)] |
| 49. | Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Leung UC, Wong PY, Roberts RH, Koea JB. Gall bladder polyps in sclerosing cholangitis: does the 1-cm rule apply? ANZ J Surg. 2007;77:355-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol. 2012;107:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Hsing AW, Bai Y, Andreotti G, Rashid A, Deng J, Chen J, Goldstein AM, Han TQ, Shen MC, Fraumeni JF Jr, Gao YT. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2007;121:832-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Hemminki K, Li X. Familial liver and gall bladder cancer: a nationwide epidemiological study from Sweden. Gut. 2003;52:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Liebe R, Milkiewicz P, Krawczyk M, Bonfrate L, Portincasa P, Krawczyk M. Modifiable Factors and Genetic Predisposition Associated with Gallbladder Cancer. A Concise Review. J Gastrointestin Liver Dis. 2015;24:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Lee SE, Jang JY, Lee YJ, Choi DW, Lee WJ, Cho BH, Kim SW; Korean Pancreas Surgery Club. Choledochal cyst and associated malignant tumors in adults: a multicenter survey in South Korea. Arch Surg. 2011;146:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Buckles DC, Lindor KD, Larusso NF, Petrovic LM, Gores GJ. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol. 2002;97:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |