Published online Jun 28, 2018. doi: 10.3748/wjg.v24.i24.2628
Peer-review started: March 13, 2018
First decision: March 30, 2018
Revised: April 5, 2018
Accepted: June 2, 2018
Article in press: June 2, 2018
Published online: June 28, 2018
Processing time: 104 Days and 16.2 Hours
To compare uncut Roux-en-Y (U-RY) gastrojejunostomy with Roux-en-Y (RY) gastrojejunostomy after distal gastrectomy (DG) for gastric cancer.
A literature search was conducted in Pubmed, Embase, Web of Science, Cochrane Library, Science Direct, Chinese National Knowledge Infrastructure, Wanfang, and China Science and Technology Journal Database to identify studies comparing U-RY with RY after DG for gastric cancer until the end of December 2017. Pooled odds ratio or weighted mean difference with 95% confidence interval was calculated using either fixed- or random-effects models. Perioperative outcomes such as operative time, intraoperative blood loss, and hospital stay; postoperative complications such as anastomotic bleeding, stricture and ulcer, reflux gastritis/esophagitis, delayed gastric emptying, and Roux stasis syndrome; and postoperative nutritional status (serum hemoglobin, total protein, and albumin levels) were the main outcomes assessed. Meta-analyses were performed using RevMan 5.3 software.
Two randomized controlled trials and four nonrandomized observational clinical studies involving 403 and 488 patients, respectively, were included. The results of the meta-analysis showed that operative time [weighted mean difference (WMD): -12.95; 95%CI: -22.29 to -3.61; P = 0.007] and incidence of reflux gastritis/esophagitis (OR: 0.40; 95%CI: 0.20-0.80; P = 0.009), delayed gastric emptying (OR: 0.29; 95%CI: 0.14-0.61; P = 0.001), and Roux stasis syndrome (OR: 0.14; 95%CI: 0.04-0.50; P = 0.002) were reduced; and the level of serum albumin (WMD: 0.71; 95%CI: 0.24-1.19; P = 0.003) was increased in patients undergoing U-RY reconstruction compared with those undergoing RY reconstruction. No differences were found with respect to intraoperative blood loss, hospital stay, anastomotic bleeding, anastomotic stricture, anastomotic ulcer, the levels of serum hemoglobin, and serum total protein.
U-RY reconstruction has some clinical advantages over RY reconstruction after DG.
Core tip: No consensus was available in the literature about gastrointestinal reconstruction after distal gastrectomy (DG) for distal gastric cancer. The present study was a novel systematic review and meta-analysis comparing uncut Roux-en-Y (U-RY) and Roux-en-Y (RY) reconstruction after DG for gastric cancer. This study investigated the relationship between the two in terms of perioperative outcomes, postoperative complications, and postoperative nutritional status. U-RY reconstruction was found to have some advantages, such as less operative time, less postoperative complications, and better postoperative nutritional status, over the conventional RY.
- Citation: Sun MM, Fan YY, Dang SC. Comparison between uncut Roux-en-Y and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: A meta-analysis. World J Gastroenterol 2018; 24(24): 2628-2639
- URL: https://www.wjgnet.com/1007-9327/full/v24/i24/2628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i24.2628
Gastric cancer is one of the most common malignant tumors of the gastrointestinal tract, and it poses a serious threat to people’s survival[1]. Epidemiological data show that the number of worldwide new cases of gastric cancer annually has increased to about 951600, ranking fourth among all malignant tumors. Each year, about 723100 patients die of gastric cancer, ranking third in cancer mortality[2].
The most effective method for treating gastric cancer is still radical surgical resection, although alternative methods include adjuvant chemotherapy, radiotherapy, and molecular targeted therapy[3]. At present, the choice of gastrointestinal reconstruction after distal gastrectomy (DG) for distal gastric cancer remains controversial. Reducing the incidence of postoperative complications and improving the quality of life are ideal ways of digestive tract reconstruction[4]. Three widely used reconstruction methods after DG are Billroth I, Billroth II, and Roux-en-Y (RY) anastomosis[5].
Billroth I reconstruction is widely used because of its physiological advantages, but it cannot be applied to all patients due to greater anastomotic tension and greater risk of anastomotic fistula[6]. Billroth II reconstruction solves the problem of anastomotic tension, but it may increase the incidence of postoperative alkaline reflux gastritis, esophagitis, and anastomotic ulcers because of the changes in normal physiological pathways[7,8]. RY reconstruction not only addresses the problem of alkaline bile reflux but also solves the problem of anastomosis; however, it leaves the patient prone to Roux stasis syndrome[9,10]. A new method of digestive tract reconstruction called the “uncut Roux-en-Y (U-RY) anastomosis “was proposed in 1988, and it is an improvement of RY anastomosis[11]. According to the reports of most surgeons, the technique of U-RY reconstruction was summarized in this study. After distal gastrectomy (DG), an end-to-side gastrojejunostomy was established between the remnant stomach and the jejunum, about 25-35 cm distal to the ligament of Treitz. Then, a side-to-side jejunojejunostomy was established between the afferent and efferent jejunal limbs, approximately 10-20 cm distal to the ligament of Treitz and 20-30 cm distal to the gastrojejunostomy site. Finally, the jejunal lumen was occluded using different methods at a site 5 cm proximal to the gastrojejunostomy. A subsequent study[12] showed that the U-RY anastomosis was effective in preventing Roux stasis syndrome, reflux gastritis, and reflux esophagitis. Therefore, U-RY reconstruction is a promising method that may replace the previous type of anastomosis. This meta-analysis was performed to compare the U-RY with RY reconstruction after DG for gastric cancer in terms of perioperative outcomes, postoperative complications, and postoperative nutritional status.
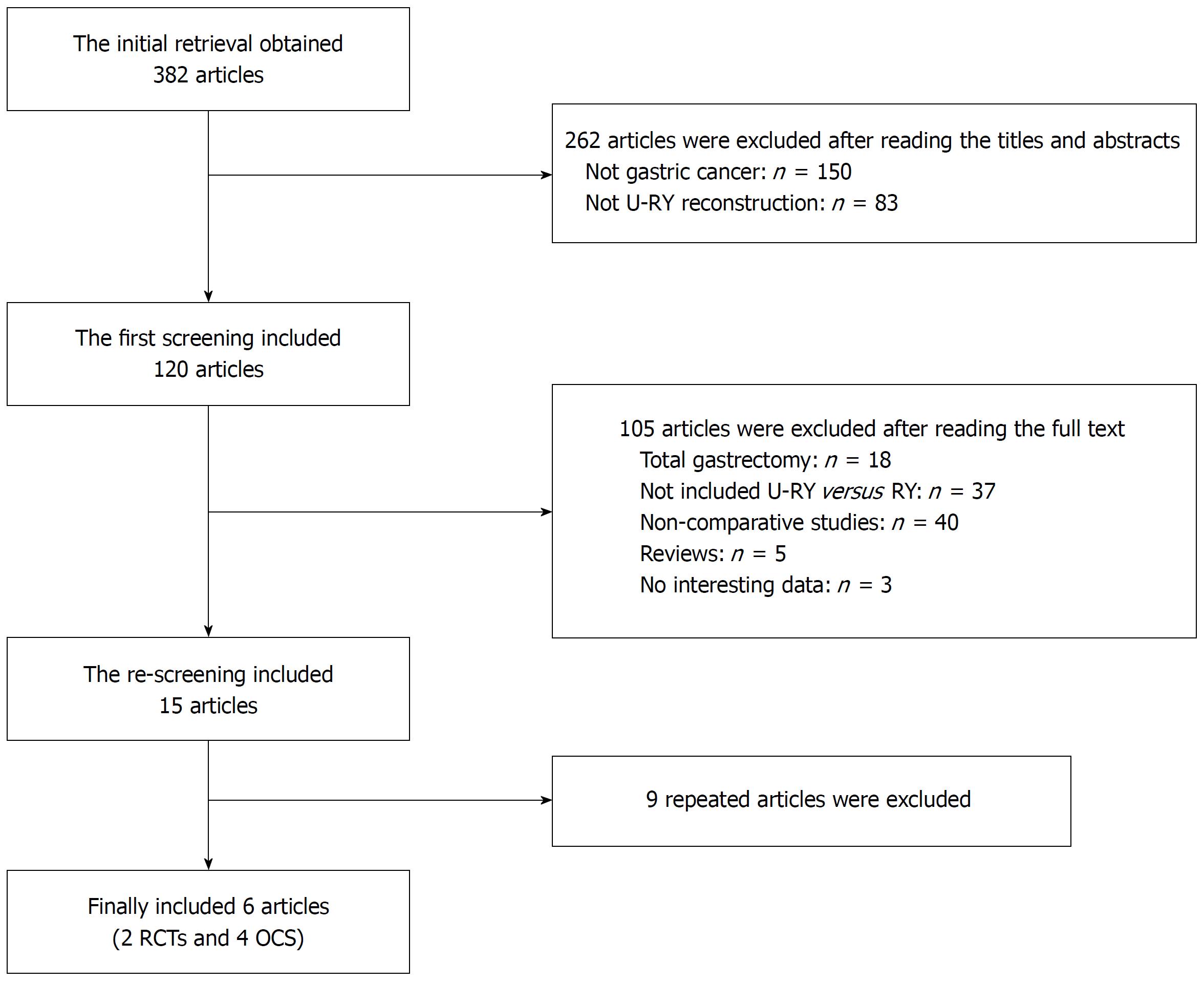
Clinical studies that compared U-RY and RY reconstruction after DG for gastric cancer were collected from PubMed, Embase, Web of Science, Cochrane Library, Science Direct, Chinese National Knowledge Infrastructure, Wanfang, and VIP databases until the end of December 2017. “Uncut Roux-en-Y” was used as the medical subject heading and the key word. A manual screening of the reference lists of all included studies was also performed for extra potentially eligible studies. Randomized controlled trials (RCTs) and nonrandomized observational clinical studies (OCS) with complete full text were included. Two independent reviewers studied the full text of the eligible studies and extracted the research data. In the case of any disagreement, the consensus was reached in consultation with a third researcher. The results of the search strategy are shown in Figure 1.
The inclusion criteria were as follows: (1) Patients with gastric cancer undergoing DG; (2) studies comparing U-RY and RY reconstruction; (3) studies reporting at least one of the outcomes mentioned; (4) original reports with ≥ 10 patients; and (5) studies published in English or Chinese.
The exclusion criteria were as follows: (1) Abstract, case reports, literature review, expert opinions, basic researches, and animal experiments; (2) studies without available data or full text; (3) studies involving patients with gastric cancer undergoing total gastrectomy; and (4) studies including patients with benign disease.
Perioperative outcomes, postoperative complications, and nutritional status were evaluated. Operative time, digestive tract reconstruction time, intraoperative blood loss, and hospital stay were the main perioperative outcomes to be assessed. Postoperative complications included anastomotic bleeding, stricture and ulcer, reflux gastritis/esophagitis, dumping symptoms, delayed gastric emptying, and Roux stasis syndrome. Postoperative nutritional status included serum hemoglobin, total protein, albumin levels, cholesterol, triglyceride, and body weight.
The data were extracted independently by two authors. The RCTs were assessed using the Jadad scoring system[13]. The Newcastle-Ottawa Quality Assessment Scale[14] was used to evaluate the nonrandomized OCS. The quality of the studies was assessed by two reviewers independently and is displayed in Tables 1 and 2.
The data extracted from the studies included population characteristics (study year, country, design, gender, and mean age) and outcome indexes (perioperative outcomes, postoperative complications, and postoperative nutritional status).
Meta-analyses were performed using Review Manager Version 5.3 software. Dichotomous variables were analyzed by estimating the risk ratio with 95% confidence interval (CI), and continuous variables were analyzed using the weighted mean difference (WMD) with 95%CI. A P-value < 0.05 was considered a statistically significant difference.
A chi-square test was used to assess the homogeneity of effect sizes to decide the I² value before meta-analysis. A random-effects model was used when significant heterogeneity existed (I² > 50%). If the heterogeneity was not significant (I² < 50%), a fixed-effects statistical model was used.
Moreover, if the heterogeneity was high, subgroup and sensitivity analyses were performed to find the source of the heterogeneity. The funnel plot was constructed to detect potential publication bias.
A total of 382 studies relevant to the search terms were included. Six studies met the inclusion criteria. Finally, two RCTs[15,16] and four OCS[17-20] with 403 and 488 patients, respectively, were included. The characteristics of studies included in the meta-analysis are shown in Tables 3 and 4. The definition of postoperative complications in the included studies is shown in Table 5.
| Ref. | Country | Design | Group | Patients | Male/female | Mean age (yr) |
| Noh[15] 2000 | South Korea | RCT | U-RY RY | 54 51 | 38/16 31/20 | 59 53 |
| Xu et al[16] 2010 | China | RCT | U-RY RY | 193 105 | 102/91 73/32 | 61.5 ± 7.5 63.2 ± 6.3 |
| He et al[17] 2017 | China | Retro | U-RY RY | 80 51 | 50/30 24/27 | 58.9 ± 5.4 58.7 ± 6.3 |
| Li et al[18] 2011 | China | Retro | U-RY RY | 127 46 | 70/57 31/15 | 58.1 ± 9.1 58.8 ± 11.3 |
| Park et al[19] 2014 | South Korea | Retro | U-RY RY | 41 55 | N/A N/A | N/A N/A |
| Huang et al[20] 2017 | China | Retro | U-RY RY | 34 54 | 28/6 38/16 | 58.7 ± 15.0 59.8 ± 10.3 |
| Ref. | Country | Design | Group | Operation type | Pathology stage | ||
| I | II | III | |||||
| Noh[15] 2000 | South Korea | RCT | U-RY RY | Open | 19 | 22 | 13 |
| 32 | 8 | 11 | |||||
| Xu et al[16] 2010 | China | RCT | U-RY RY | Open | 23 | 63 | 107 |
| 12 | 34 | 59 | |||||
| He et al[17] 2017 | China | Retro | U-RY RY | Open | 10 | 31 | 39 |
| 4 | 21 | 26 | |||||
| Li et al[18] 2011 | China | Retro | U-RY RY | Open | 13 | 37 | 77 |
| 5 | 13 | 28 | |||||
| Park et al[19] 2014 | South Korea | Retro | U-RY RY | Laparoscopic/ Laparoscopy-assisted | N/A | ||
| N/A | |||||||
| Huang et al[20] 2017 | China | Retro | U-RY RY | Laparoscopic/ Open | 22 | 10 | 2 |
| 28 | 21 | 5 | |||||
| Ref. | Anastomotic | Reflux gastritis/esophagitis | Delayed gastric emptying | Roux stasis syndrome |
| bleeding/stricture/ulcer | ||||
| Noh[15] 2000 | -/ND/ND | ND | ND | Definition D |
| Xu et al[16] 2010 | ND/ND/- | ND | - | ND |
| He et al[17] 2017 | -/ND/ND | Definition A | Definition B | Definition D |
| Li et al[18] 2011 | ND/ND/- | Definition A | Definition C | Definition D |
| Park et al[19] 2014 | -/-/- | ND | ND | ND |
| Huang et al[20] 2017 | -/-/- | ND | ND | ND |
According to the Newcastle-Ottawa scoring system, four OCS were found to be of high quality. One RCT was found to be of high quality while another RCT was of low quality according to the Jadad scoring system. All the results are presented in Table 6.
| Outcome of interest | Studies | Patients (n) | OR/WMD | 95%CI | P value |
| Perioperative outcomes | |||||
| Operative time | 4 | 488 | -12.95 | -22.29, -3.61 | 0.007 |
| Intraoperative blood loss | 4 | 488 | -46.38 | -91.93, -0.83 | 0.05 |
| Hospital stay | 4 | 488 | -0.71 | -1.69, 0.27 | 0.16 |
| Postoperative complications | |||||
| Anastomotic bleeding | 2 | 471 | 0.41 | 0.07, 2.50 | 0.33 |
| Anastomotic stricture | 4 | 707 | 0.41 | 0.12, 1.42 | 0.16 |
| Anastomotic ulcer | 2 | 236 | 0.25 | 0.03, 2.49 | 0.24 |
| Reflux gastritis/esophagitis | 6 | 891 | 0.40 | 0.20, 0.80 | 0.009 |
| Delayed gastric emptying | 5 | 593 | 0.29 | 0.14, 0.61 | 0.001 |
| Roux stasis syndrome | 6 | 891 | 0.14 | 0.04, 0.50 | 0.002 |
| Postoperative nutritional status | |||||
| Serum hemoglobin | 2 | 227 | 0.51 | -0.58, 1.60 | 0.36 |
| Serum total protein | 2 | 227 | 0.55 | -1.77, 2.86 | 0.64 |
| Serum albumin | 2 | 227 | 0.71 | 0.24, 1.19 | 0.003 |
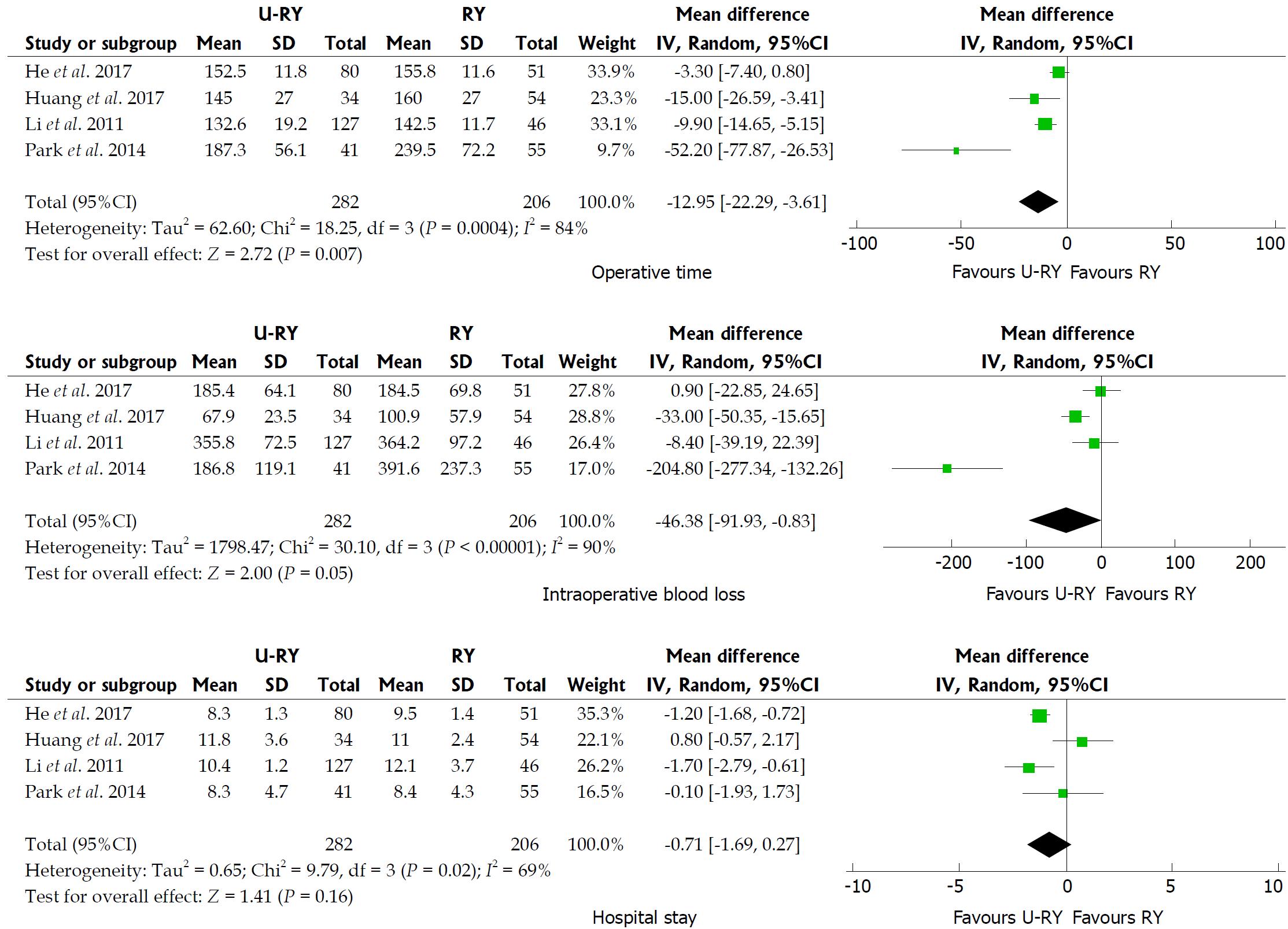
Comparison of perioperative outcomes: The detailed results of meta-analysis are given in Figure 2. The meta-analysis revealed that U-RY reconstruction was associated with a significant shortening in the duration of operative time (WMD: -12.95; 95%CI: -22.29 to -3.61; P = 0.007) compared to RY reconstruction. No significant difference was observed between the groups in terms of intraoperative blood loss (WMD: -46.38; 95%CI: -91.93 to -0.83; P = 0.05) and hospital stay (WMD: -0.71; 95%CI: -1.69 to 0.27; P = 0.16).
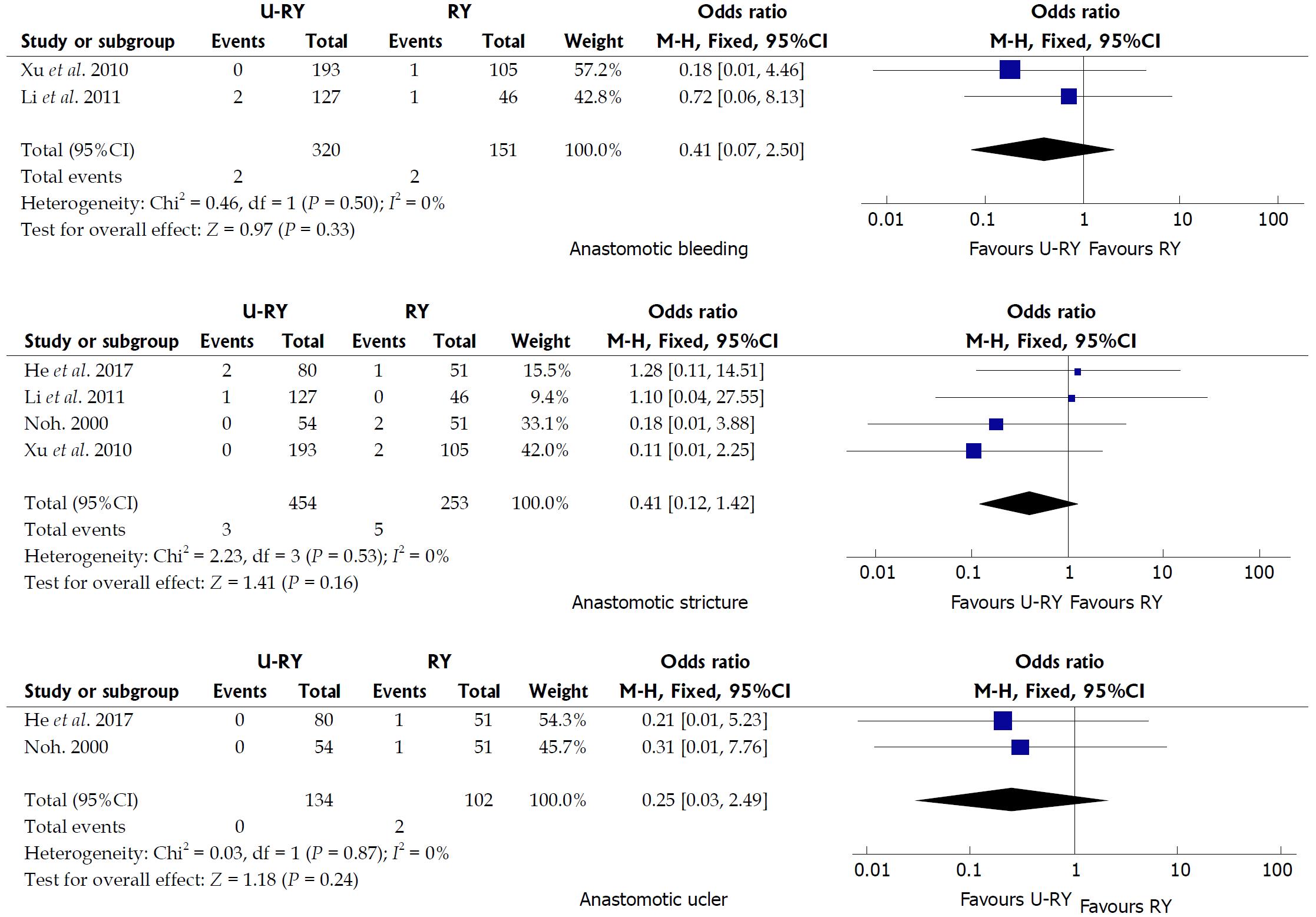
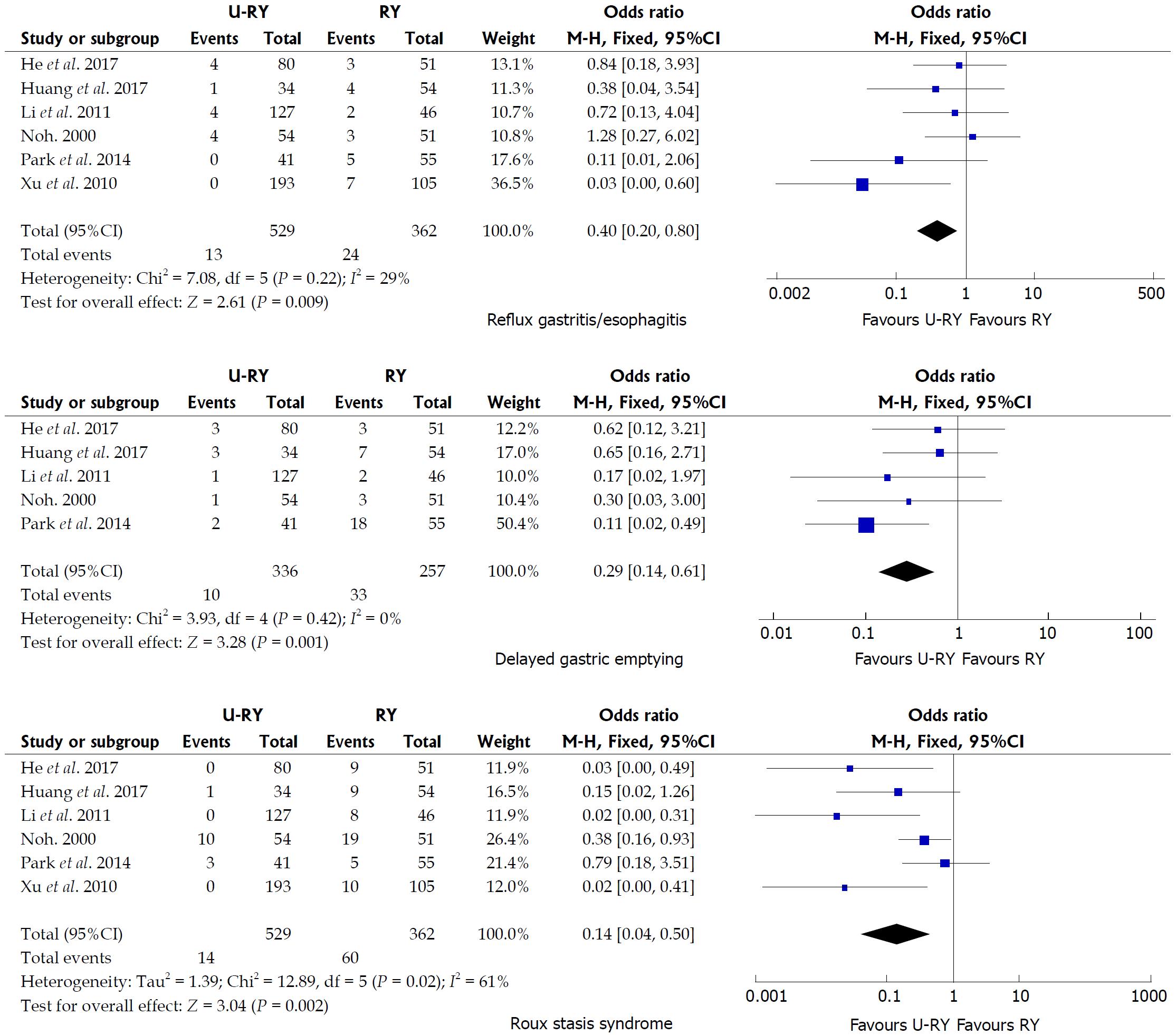
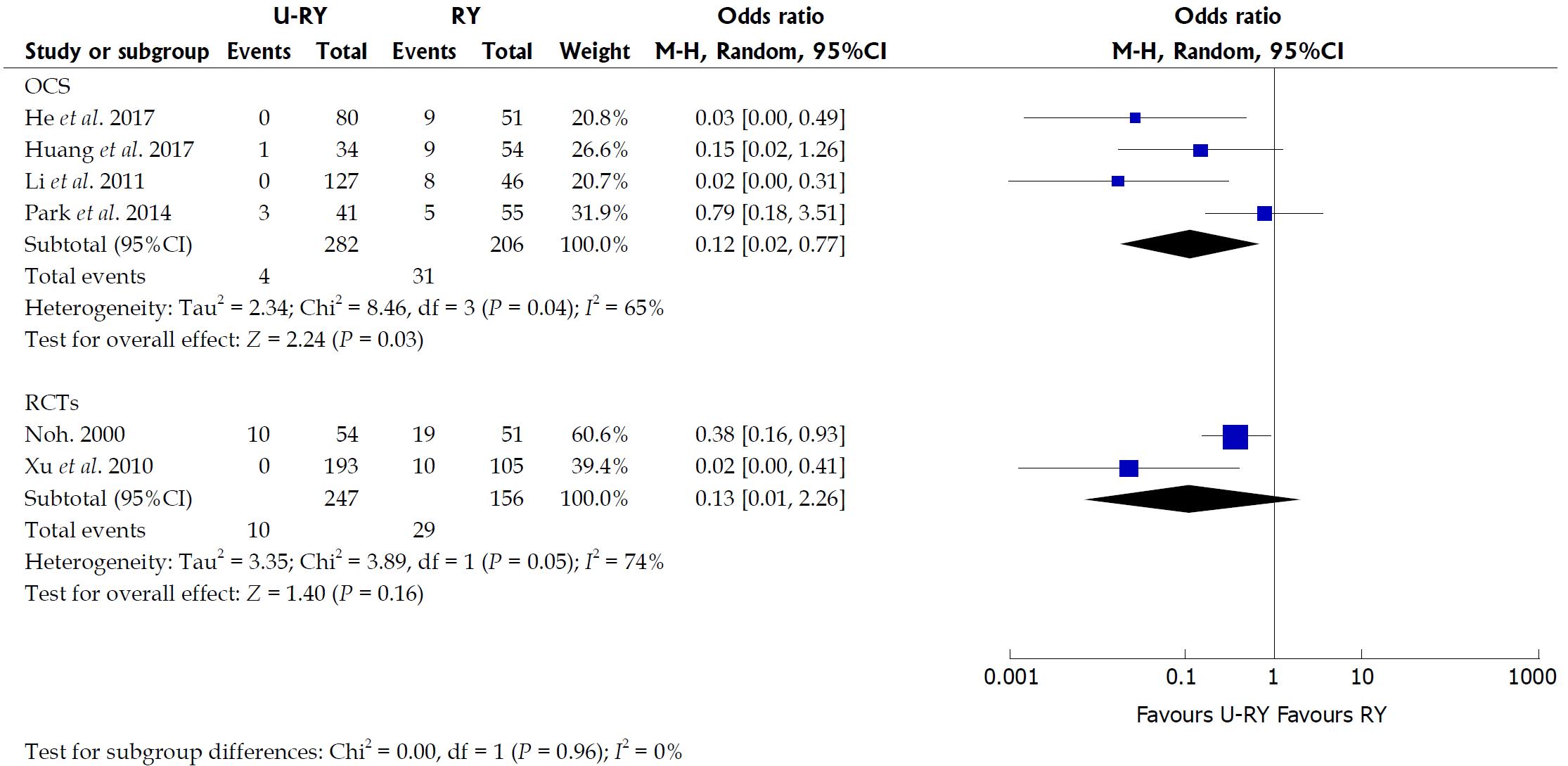
Comparison of postoperative complications: The detailed results of the meta-analysis are given in Figures 3 and 4. The results suggested that U-RY reconstruction had significantly lower incidence of reflux gastritis/esophagitis (OR: 0.40; 95%CI: 0.20-0.80; P = 0.009), delayed gastric emptying (OR: 0.29; 95%CI: 0.14-0.61; P = 0.001), and Roux stasis syndrome (OR: 0.14; 95%CI: 0.04-0.50; P = 0.002) compared to RY reconstruction. No significant differences were found between the two reconstructive methods in terms of anastomotic bleeding (OR: 0.41; 95%CI: 0.07-2.50; P = 0.33), anastomotic stricture (OR: 0.41; 95%CI: 0.12-1.42; P = 0.16), and anastomotic ulcer (OR: 0.25; 95%CI: 0.03-2.49; P = 0.24).
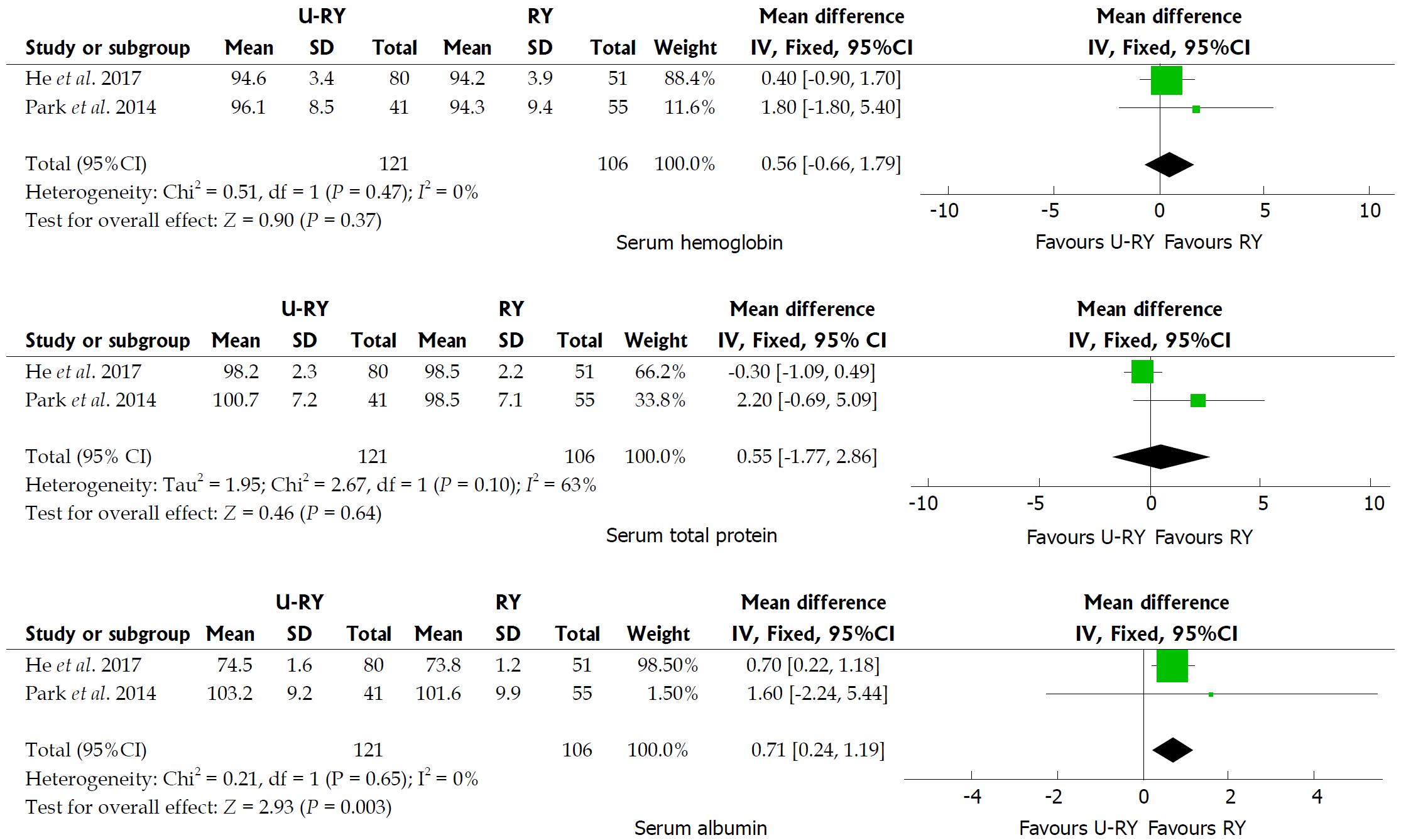
Comparison of postoperative nutritional status: The detailed results of meta-analysis are given in Figure 5. The meta-analysis results suggested that U-RY reconstruction had a significantly higher level of serum albumin (WMD: 0.71; 95%CI: 0.24-1.19; P = 0.003) than RY reconstruction. No significant differences were found between the two reconstructive methods in terms of serum hemoglobin (WMD: 0.56; 95%CI: -0.66 to 1.79; P = 0.37) and serum total protein (WMD: 0.55; 95%CI: -1.77 to 2.86; P = 0.64).
The results of the meta-analysis revealed a significant heterogeneity in some outcomes, such as operative time (I² = 84%; P = 0.0004), intraoperative blood loss (I² = 90%; P < 0.00001), hospital stay (I² = 69%; P = 0.02), and incidence of Roux stasis syndrome (I² = 61%; P = 0.02). Sensitivity analysis of operative time and intraoperative blood loss showed no heterogeneity (Table 7). Sensitivity analysis of hospital stay indicated no heterogeneity after excluding the study by Huang et al[20] (Table 7). However, no reason for excluding the aforementioned study was found after checking it carefully. Considering that significant heterogeneity might be derived from the different types of studies, a subgroup analysis was performed according to the classification of the study types. The subgroup analysis showed that both OCS and RCT groups had significant heterogeneity (Figure 6), suggesting that heterogeneity was not derived from the combination of different study types. Therefore, it was considered that the heterogeneity was caused by differences in operative technique, experience of the surgeons, postoperative management, and so on.
| Sensitivity analysis | Heterogeneity test | Overall effect | ||
| I² (%) | Tau² | OR/WMD and 95%CI | P value | |
| Operative time | ||||
| Include all studies | 84 | 62.60 | -12.95 (-22.29 to -3.61) | 0.007 |
| Exclude "He et al 2017" | 81 | 142.92 | -20.41 (-36.14 to -4.68) | 0.01 |
| Exclude "Huang et al 2017" | 88 | 70.94 | -12.75 (-23.99 to -1.52) | 0.03 |
| Exclude "Park et al 2014" | 69 | 18.50 | -8.03 (-14.13 to -1.94) | 0.01 |
| Exclude "Li et al 2011" | 88 | 235.46 | -19.13 (-38.42 to 0.16) | 0.05 |
| Intraoperative blood loss | ||||
| Include all studies | 90 | 1798.47 | -46.38 (-91.93 to -0.83) | 0.05 |
| Exclude "He et al 2017" | 92 | 3010.33 | -69.86 (-136.73 to -2.99) | 0.04 |
| Exclude "Huang et al 2017" | 93 | 4274.27 | -60.75 (-139.17 to 17.68) | 0.13 |
| Exclude "Park et al 2014" | 65 | 259.64 | -15.15 (-37.95 to 7.64) | 0.19 |
| Exclude "Li et al 2011" | 93 | 2690.80 | -64.63 (-127.90 to -1.37) | 0.05 |
| Hospital stay | ||||
| Include all studies | 69 | 0.65 | -0.71 (-1.69 to 0.27) | 0.16 |
| Exclude "Huang et al 2017" | 9 | 0.03 | -1.22 (-1.71 to -0.73) | < 0.00001 |
We noticed that laparoscopic technique was used in two studies as well as the definitions of complications were different in the included studies. Subgroup analyses were undertaken for relevant outcome measures by including studies with laparoscopic technique and different definitions (Table 8). The results showed that there were no statistically significant differences in outcomes between patients undergoing U-RY reconstruction and those receiving RY reconstruction using laparoscopic surgery. When pooling studies using the definition D, patients undergoing U-RY reconstruction had a lower Roux stasis syndrome rate than those receiving RY reconstruction (OR: 0.08; 95%CI: 0.01 to 0.85; P = 0.04). However, there were no statistically significant differences in any other defined outcomes.
| Outcome of interest | Studies | Patients (n) | OR/WMD | 95%CI | P value |
| Laparoscopic/laparoscopy-assisted | |||||
| Operative time | 2 | 184 | -31.76 | -68.04, 4.51 | 0.09 |
| Intraoperative blood loss | 2 | 184 | -115.14 | -283.34, 53.06 | 0.18 |
| Hospital stay | 2 | 184 | 0.48 | -0.62, 1.57 | 0.39 |
| Reflux gastritis/esophagitis | 2 | 184 | 0.22 | 0.04, 1.23 | 0.08 |
| Delayed gastric emptying | 2 | 184 | 0.27 | 0.04, 1.62 | 0.15 |
| Roux stasis syndrome | 2 | 184 | 0.39 | 0.12, 1.23 | 0.11 |
| Definition A | |||||
| Reflux gastritis/esophagitis | 2 | 304 | 0.79 | 0.25, 2.49 | 0.68 |
| Definition B | |||||
| Delayed gastric emptying | 1 | 131 | 0.62 | 0.12, 3.21 | 0.57 |
| Definition C | |||||
| Delayed gastric emptying | 1 | 173 | 0.17 | 0.02, 1.97 | 0.16 |
| Definition D | |||||
| Roux stasis syndrome | 3 | 409 | 0.08 | 0.01, 0.85 | 0.04 |
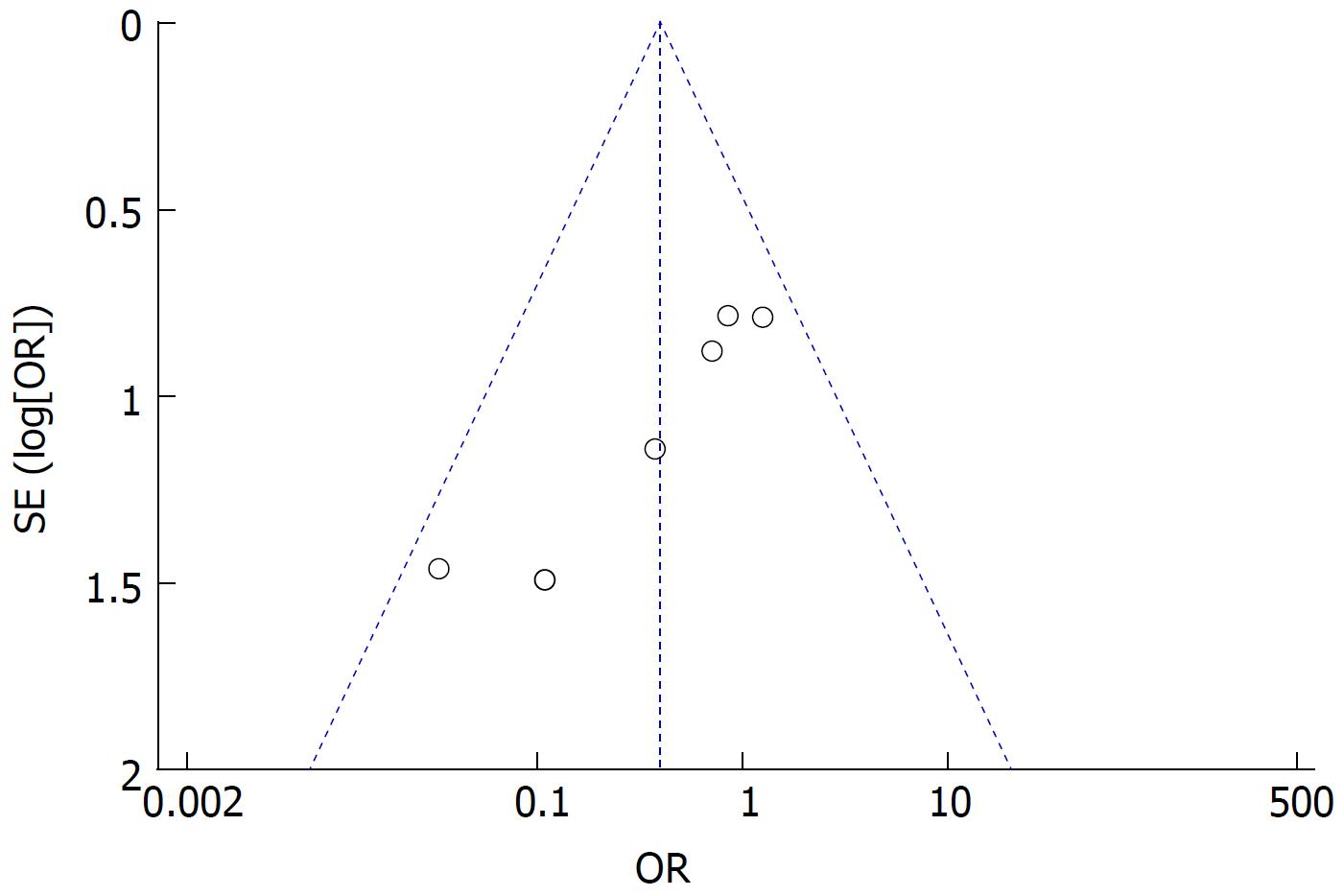
Funnel plot analysis showed no obvious publication bias for the incidence of reflux gastritis/esophagitis (Figure 7).
Digestive tract reconstruction is an important aspect of gastric cancer surgery. However, surgeons have not yet reached a consensus on the choice of reconstruction method after DG. They are seeking the best method of reconstruction that can reduce complications and improve postoperative quality of life[21]. Recent studies revealed that RY reconstruction after DG for gastric cancer was advantageous in terms of postoperative long-term functional outcomes compared with the two Billroth methods[21-23]. Moreover, a series of studies confirmed the feasibility, practicability, and superiority of the U-RY anastomosis after DG in clinical practice[24,25]. However, the choice of U-RY or RY gastrojejunostomy is still controversial. The related outcomes of both methods of reconstruction after DG were analyzed in this study[26-28].
Four studies reported operative time, intraoperative blood loss, and hospital stay. The results of the meta-analysis showed that the operative time was reduced in patients undergoing U-RY reconstruction compared to RY reconstruction. Recent studies[22,24] reported that U-RY significantly reduced operative time and intraoperative blood loss compared with the traditional RY because cutting off the mesentery and closing mesentery holes were not required. These views supported the conclusion of the present study. No significant difference was found in the intraoperative blood loss between the two groups largely due to the smaller sample size of the included studies. The traditional RY anastomosis required cutting off the bowel, even separating off the part of the mesentery and handling the mesenteric vessels, thereby increasing the difficulty in the operation. Thus, U-RY had an advantage in shortening the operative time.
The postoperative complication was an important outcome to assess the safety of the operation type. This study found that U-RY reconstruction had an advantage in reducing the incidence of reflux gastritis/esophagitis, delayed gastric emptying, and Roux stasis syndrome, whereas no significant advantage was found in preventing anastomotic bleeding, stricture, and ulcer.
Noh et al[15] reported that Roux stasis syndrome, alkaline reflux gastritis, and esophagitis were significantly reduced in the U-RY reconstruction compared with the conventional RY reconstruction. In their study, the incidence of Roux stasis syndrome was 18.5% in the uncut RY group (10 out of 51 patients) and 37.3% in the conventional RY group (19 out of 51 patients). A recent clinical study by Park and Kim[19] indicated that, according to postoperative endoscopic findings, U-RY anastomosis had advantages in improving gastritis, duodenal secretion reflux, and gastric residue. Thus, they claimed that U-RY was superior to conventional RY gastrojejunostomy in keeping the function of the gastric remnants. Ma[29] reported that the U-RY anastomosis reduced the occurrences of stasis syndrome because the jejunum was transversed while preserving the advantages of RY surgery. The aforementioned opinions were consistent with the conclusion of the present study.
The present study suggested that the bile and pancreatic juice were forced into the efferent jejunal limb through the Braun anastomosis between the afferent and efferent jejunal limbs because the afferent jejunal limb near the gastrointestinal anastomosis was closed. The smaller bile and pancreatic juice could be refluxed to the residual stomach. Hence, U-RY reconstruction could prevent reflux gastritis and esophagitis. The U-RY reconstruction could effectively prevent delayed gastric emptying and Roux stasis syndrome by preserving jejunum continuity, integrity of myoelectrical conduction, direction of muscle contraction in the gastrointestinal tract, and normal intestinal peristalsis[30,31]. No significant difference was found in anastomosis-related complications between the two groups. It might be due mainly to the use of gastrointestinal stapling devices and the refinement of technique. Theoretically, the U-RY anastomosis maintained mesenteric continuity and ensured good perfusion of the anastomotic site. Therefore, it could reduce the formation of anastomotic stenosis. However, further clinical studies are still needed to verify the aforementioned conclusions.
Only two studies evaluated the changes in the nutritional status at 1 year of follow-up after surgery. The levels of serum hemoglobin, serum total protein, and serum albumin were calculated to assess the nutritional status. The present meta-analysis revealed that two reconstructive procedures showed similar changes in serum hemoglobin and total protein levels. However, the level of serum albumin was higher in the U-RY group than in the RY group. However, several important outcomes, including cholesterol, triglyceride, and body weight, were not reported adequately in the included studies. Therefore, postoperative nutritional status could not be fully assessed due to lack of sufficient data. Consequently, further clinical studies, including various nutritional indicators and long follow-up, to assess the nutritional status after the U-RY procedure are required to validate the findings.
Related studies[25-28] reported the occurrence of recanalization at the site of afferent closure after U-RY reconstruction, which may increase the incidence of alkaline reflux gastritis and esophagitis and decrease quality of life. Yang et al[31] concluded in their study that the incidence of partial recanalization after U-RY reconstruction reached 13.0%. In the included literature, three articles mentioned the problem of recanalization of the obliterated afferent jejunal limb. No recanalization was found in the two studies[18,19] after follow-up for 6 mo and 1 year, respectively. However, the incidence of recanalization at 1 and 2 years after surgery in one study[20] was 2.9% and 5.9%, respectively. Some modified procedures were used that could reinforce the occlusion and reduce the recanalization rate. These include using nonbladed six-row linear staplers to staple[32], using four or five seromuscular stitches annularly around the jejunal wall with tightly tied 3-0 polypropylene[33], and suturing the serosal layers of the upper and lower jejunum at the occlusion site after the ligature of the jejuna[34]. This study recommended improving the jejunal occlusion method to ensure that jejunal occlusion was permanently closed but not transected.
In conclusion, the present meta-analysis indicated that U-RY reconstruction after DG for gastric cancer was secure and feasible. It has several advantages, such as shorter operative time and reduced incidence of reflux gastritis/esophagitis, delayed gastric emptying, and Roux stasis syndrome. However, this study also had several limitations. Only two RCTs were included, and the relatively small sample size likely had a certain effect on the results. Lastly, the studies included were only single-center studies conducted in China and Korea. Therefore, high-quality RCTs in multiple centers are still needed for further confirmation of our findings.
Gastric cancer is the fourth most common cancer worldwide and the third most frequent cause of death from cancer. At present, the choice of gastrointestinal reconstruction after distal gastrectomy (DG) for gastric cancer remains controversial. Uncut Roux-en-Y (U-RY) reconstruction is an improvement of the Roux-en-Y (RY) reconstruction, which is a promising method that may replace the previous type of anastomosis. This systematic review and meta-analysis aimed to compare the clinical efficacy and safety of U-RY vs RY reconstruction after DG for gastric cancer.
A method of digestive tract reconstruction called “U-RY anastomosis” was first proposed in 1988. It has been a research hotspot for years since then. Some surgeons consider U-RY reconstruction superior to RY reconstruction, while others do not. Therefore, its use remains controversial.
This novel meta-analysis compared U-RY and RY reconstruction after DG for gastric cancer. It compared U-RY and RY reconstruction in terms of perioperative outcomes, postoperative complications, and postoperative nutritional status.
A literature search was conducted to identify studies comparing U-RY with RY after DG for gastric cancer. Using either fixed- or random-effects models, pooled odds ratios or weighted mean difference with 95% confidence interval was calculated. Meta-analyses were performed using RevMan 5.3 software.
Some clinical advantages were provided by U-RY reconstruction, such as shorter operative time and lowered incidence of reflux gastritis/esophagitis, delayed gastric emptying, and Roux stasis syndrome.
The present study showed that U-RY reconstruction after DG for gastric cancer was secure and feasible, providing a guideline for clinical practice. However, high-quality RCTs in multiple centers are still needed for further confirmation.
U-RY anastomosis maintained mesenteric continuity and ensured good perfusion of the anastomotic site. It could reduce the formation of anastomotic stenosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen L, Noshiro H, Wang YH S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21370] [Article Influence: 2137.0] [Reference Citation Analysis (3)] |
| 3. | Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A. Treatment of gastric cancer. World J Gastroenterol. 2014;20:1635-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 461] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (5)] |
| 4. | Piessen G, Triboulet JP, Mariette C. Reconstruction after gastrectomy: which technique is best? J Visc Surg. 2010;147:e273-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M, Doki Y; Osaka University Clinical Research Group for Gastroenterological Study. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Hoya Y, Mitsumori N, Yanaga K. The advantages and disadvantages of a Roux-en-Y reconstruction after a distal gastrectomy for gastric cancer. Surg Today. 2009;39:647-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | In Choi C, Baek DH, Lee SH, Hwang SH, Kim DH, Kim KH, Jeon TY, Kim DH. Comparison Between Billroth-II with Braun and Roux-en-Y Reconstruction After Laparoscopic Distal Gastrectomy. J Gastrointest Surg. 2016;20:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Shim JH, Oh SI, Yoo HM, Jeon HM, Park CH, Song KY. Roux-en-Y gastrojejunostomy after totally laparoscopic distal gastrectomy: comparison with Billorth II reconstruction. Surg Laparosc Endosc Percutan Tech. 2014;24:448-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Xiong JJ, Altaf K, Javed MA, Nunes QM, Huang W, Mai G, Tan CL, Mukherjee R, Sutton R, Hu WM. Roux-en-Y versus Billroth I reconstruction after distal gastrectomy for gastric cancer: a meta-analysis. World J Gastroenterol. 2013;19:1124-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Zong L, Chen P. Billroth I vs. Billroth II vs. Roux-en-Y following distal gastrectomy: a meta-analysis based on 15 studies. Hepatogastroenterology. 2011;58:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Van Stiegmann G, Goff JS. An alternative to Roux-en-Y for treatment of bile reflux gastritis. Surg Gynecol Obstet. 1988;166:69-70. [PubMed] |
| 12. | Mon RA, Cullen JJ. Standard Roux-en-Y gastrojejunostomy vs. “uncut” Roux-en-Y gastrojejunostomy: a matched cohort study. J Gastrointest Surg. 2000;4:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12887] [Article Influence: 444.4] [Reference Citation Analysis (1)] |
| 14. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12664] [Article Influence: 844.3] [Reference Citation Analysis (0)] |
| 15. | Noh SM. Improvement of the Roux limb function using a new type of “uncut Roux” limb. Am J Surg. 2000;180:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Xu J, Ye Z Y, Wang Y Y, Shao Q S, Sun Y S, Zhao Z K. Application of uncut Roux-en-Y anastomosis in radical distal gastrectomy for gastric carcinoma. Zhejiang Yixue. 2010;32:1325-1326, 1332. [DOI] [Full Text] |
| 17. | He SQ, Luo YC, Zeng DQ, Li XW. Effect of uncut Roux-en-Y Reconstruction after Distal Gastrectomy for Gastric Cancer. Medical Innovation of China. 2017;14:127-130. |
| 18. | Li FX, Zhang RP, Zhao JZ, Wang XJ, Xue Q, Liang H. [Use of uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Park JY, Kim YJ. Uncut Roux-en-Y Reconstruction after Laparoscopic Distal Gastrectomy Can Be a Favorable Method in Terms of Gastritis, Bile Reflux, and Gastric Residue. J Gastric Cancer. 2014;14:229-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Huang Y. A Comparative Study of Uncut Roux-en-Y and Traditional Roux-en-Y Anastomosis after Distal Gastrectomy for Gastric Cancer. MSc Thesis. 2017; Available from: http://kns.cnki.net/kns/detail/detail.aspx?FileName=1017124843.nh&DbName=CMFDTEMP. |
| 21. | Huang Y, Wang S, Shi Y, Tang D, Wang W, Chong Y, Zhou H, Xiong Q, Wang J, Wang D. Uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Expert Rev Gastroenterol Hepatol. 2016;10:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 22. | Kim CH, Song KY, Park CH, Seo YJ, Park SM, Kim JJ. A comparison of outcomes of three reconstruction methods after laparoscopic distal gastrectomy. J Gastric Cancer. 2015;15:46-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, Yang HK, Kim N, Lee WW. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Yun SC, Choi HJ, Park JY, Kim YJ. Total laparoscopic uncut Roux-en-Y gastrojejunostomy after distal gastrectomy. Am Surg. 2014;80:E51-E53. [PubMed] |
| 25. | Shibata C, Kakyo M, Kinouchi M, Tanaka N, Miura K, Naitoh T, Ogawa H, Yazaki N, Haneda S, Watanabe K. Results of modified uncut Roux-en-Y reconstruction after distal gastrectomy for gastric cancer. Hepatogastroenterology. 2013;60:1797-1799. [PubMed] |
| 26. | Tu BN, Sarr MG, Kelly KA. Early clinical results with the uncut Roux reconstruction after gastrectomy: limitations of the stapling technique. Am J Surg. 1995;170:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Richardson WS, Spivak H, Hudson JE, Budacz MA, Hunter JG. Teflon buttress inhibits recanalization of uncut stapled bowel. J Gastrointest Surg. 2000;4:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Morton JM, Lucktong TA, Trasti S, Farrell TM. Bovine pericardium buttress limits recanalization of the uncut Roux-en-Y in a porcine model. J Gastrointest Surg. 2004;8:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Ma JJ, Zang L, Yang A, Hu WG, Feng B, Dong F, Wang ML, Lu AG, Li JW, Zheng MH. A modified uncut Roux-en-Y anastomosis in totally laparoscopic distal gastrectomy: preliminary results and initial experience. Surg Endosc. 2017;31:4749-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Zhang YM, Liu XL, Xue DB, Wei YW, Yun XG. Myoelectric activity and motility of the Roux limb after cut or uncut Roux-en-Y gastrojejunostomy. World J Gastroenterol. 2006;12:7699-7704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (3)] |
| 31. | Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. 2017;23:6350-6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 32. | Uyama I, Sakurai Y, Komori Y, Nakamura Y, Syoji M, Tonomura S, Yoshida I, Masui T, Inaba K, Ochiai M. Laparoscopy-assisted uncut Roux-en-Y operation after distal gastrectomy for gastric cancer. Gastric Cancer. 2005;8:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 33. | Jangjoo A, Mehrabi Bahar M, Aliakbarian M. Uncut Roux-en-y esophagojejunostomy: A new reconstruction technique after total gastrectomy. Indian J Surg. 2010;72:236-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Sun YS, Ye ZY, Shao QS, Zhang Q, Xu XD, Hu JF, Shi D. [The application of uncut Roux-en-Y esophagojejunostomy with distal jejunal pouch on behalf of the stomach surgery in the digestive tract reconstruction after total gastrectomy]. Zhonghua Wai Ke Za Zhi. 2012;50:699-703. [PubMed] |