Published online Jun 21, 2018. doi: 10.3748/wjg.v24.i23.2501
Peer-review started: February 7, 2018
First decision: February 24, 2018
Revised: March 9, 2018
Accepted: March 25, 2018
Article in press: March 25, 2018
Published online: June 21, 2018
Processing time: 128 Days and 1.3 Hours
To determine the efficacy and safety of transarterial embolization and low-dose continuous hepatic arterial infusion chemotherapy with oxaliplatin and raltitrexed in hepatocellular carcinoma (HCC) with major portal vein tumor thrombus (MPVTT).
Eighty-six patients with MPVTT accepted routine embolization. The catheter was kept in the hepatic artery and oxaliplatin (50 mg in 250 mL of glucose) was infused by pump for 4 h, followed by raltitrexed (2 mg in 100 mL of 0.9% saline) infusion by pump for the next 1 h. The efficacy and safety were evaluated after the transarterial chemoembolization (TACE).
Full or partial embolization was achieved in 86 cases, where all the cases received low dose continuous hepatic arterial infusion chemotherapy. Complete responses (CRs), partial responses (PRs), stable disease (SD), and disease progression (PD) for intrahepatic disease were observed in 0, 45, 20, and 21 patients, respectively. The 1-, 2-and 3-year overall survival rates of the 86 patients were 40.7%, 22.1%, and 8.1% respectively, and the median survival time was 8.7 mo. Complication was limited.
TACE with low dose continuous hepatic arterial infusion of oxaliplatin and raltitrexed could be an option in MPVTT patient; it was shown to be effective in patients with advanced HCC with MPVTT with less toxicity.
Core tip: We analyzed the pharmacokinetic and pharmacodynamic characteristics of continuous hepatic arterial infusion of oxaliplatin and raltitrexed to aid interventional radiologist in determining a more accurate infusion protocol. The clinical protocol based on the pharmacokinetics study in a swine model and the pharmacodynamics study in tumor cell lines was shown to be effective and safe in hepatocellular carcinoma with main portal vein tumor thrombus.
- Citation: Zhu LZ, Xu S, Qian HL. Transarterial embolization and low-dose continuous hepatic arterial infusion chemotherapy with oxaliplatin and raltitrexed for hepatocellular carcinoma with major portal vein tumor thrombus. World J Gastroenterol 2018; 24(23): 2501-2507
- URL: https://www.wjgnet.com/1007-9327/full/v24/i23/2501.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i23.2501
Hepatocellular carcinoma (HCC) is the sixth most prevalent cancer and the third most common cause of cancer-related death worldwide[1,2], particularly in Asian countries[3]. Recent studies have indicated that the overall clinical survival of HCC remains extremely poor, especially among patients with invasion of the portal vein branches[4]. Portal vein tumor thrombosis (PVTT) is a strong predictor of mortality in patients with HCC[5] and is usually associated with an unsatisfactory prognosis[6]. The median survival time is only approximately 2-4 mo compared with 10-24 mo among those without PVTT[7]. When a thrombus involves the main portal vein, the prognosis is much worse than in cases in which a thrombus involves a branch of the portal vein[8].
Treatment of advanced HCC with major portal vein tumor thrombosis (MPVTT) remains a challenge. MPVTT can increase the risk of wide tumor transmission and also increase the pressure of the portal vein (especially when accompanied with arterial portal vein shunt), which results in acute variceal hemorrhaging, intractable ascites, hepatic encephalopathy, and failure. All of these conditions limit treatment choices.
It is well known that transarterial chemotherapy can achieve high local concentrations. Many patients who cannot endure systemic chemotherapy can benefit from transarterial chemoembolization (TACE). We have adopted continuous hepatic arterial infusion chemotherapy technique in our center for the treatment of colon cancer liver metastasis and have demonstrated its safety and advantage[9]. However, PVTT patients cannot endure a regular dose of chemotherapy due to its high risk and high complexity, and we need a more effective and safe protocol that uses the smallest dose possible to provide a sufficient effect while limiting toxicity.
When we sought to create a protocol for transarterial infusion chemotherapy (TAI), we needed to determine a total dose and infusion time, but we first needed to solve following problems: (1) How does a certain dose and infusion time influence the concentration in local tissue? (i.e., pharmacokinetics)? (2) How do high-concentration drugs interact with the tumor (i.e., pharmacodynamics)? And (3) What are the optimum time and concentration for the application of a certain chemotherapy drug, and how can these parameters be realized in the clinic?
We firstly performed a pharmacokinetics study in a swine model and pharmacodynamics studies in different tumor cell lines to learn the real-time drug concentration in local tissue and how tumor cells respond under such an environment during continuous hepatic arterial infusion chemotherapy. Based on our findings, we determined the suitable concentration and infusion time for a certain agent, designed a protocol based on the above mentioned studies, and observed its efficacy and safety for patients with MPVTT.
This was a retrospective study. All patients were admitted to our hospital between May 2010 and March 2017. A total of 86 consecutive patients with unresectable advanced HCC and major PVTT received TACE. The diagnoses of HCC were established using pathology in 64 patients, and the diagnoses were established based on two types of typical HCC imaging, i.e., computed tomography (CT) and magnetic resonance imaging (MRI), plus a serum alpha-fetoprotein (AFP) level of more than 400 ng/mL in 30 patients.
All patients were categorized as American Joint Committee on Cancer stage IIIa (6th edition)[10]. Portal vein thrombosis was evaluated according to criteria of the Liver Cancer Group of Japan[11].
The baseline data are presented in Table 1. Most of the patients were positive for hepatitis B virus (HBV) infection. Seventy-eight patients were male, and 16 were female. Forty-four (46.8%) patients had a tumor larger than 10 cm. Etiologies of HBV and HCV infection were identified in 72 and four cases, respectively. The age range was 31-84 years (mean: 53.7 years). The pre-treatment evaluations consisted of a complete history and physical examination, blood cell count, liver and renal panels, AFP value assessment, and a tri-phase CT scan or dynamic MRI of the liver. All patients had an ECOG performance status ≤ 2, a serum total bilirubin ≤ 3.0 mg/L, a serum aminotransferase ≤ 100 IU/L, a white blood cell count ≥ 3000/μL, a hemoglobin level ≥ 8 g/dL, and a platelet level ≥ 7.5 × 104/μL or corrected to this level via embolization of the spleen. Sixteen patients had gastrointestinal bleeding, and 46 patients had ascites upon admission.
| MPVTT characteristics | Portal trunk + left branch | Portal trunk + right branch | Portal trunk + SMV or SV | Portal trunk + IVC |
| n | 27 | 36 | 12 | 11 |
| Gender | ||||
| Male | 27 | 30 | 8 | 8 |
| Female | 0 | 6 | 4 | 3 |
| Age | 35-84 | 33-72 | 42-70 | 37-74 |
| Child-Pugh grade | ||||
| Child A | 6 | 6 | 3 | 3 |
| Child B | 17 | 22 | 5 | 6 |
| Child C | 4 | 8 | 4 | 2 |
| Tumor Size | ||||
| 5-10 cm | 15 | 16 | 8 | 7 |
| > 10 cm | 12 | 20 | 4 | 4 |
| Gastric esophageal varices | 11 | 30 | 7 | 7 |
| Splenomegaly | 15 | 32 | 7 | 6 |
| Hypersplenism | 12 | 26 | 5 | 3 |
| Ascites | 10 | 22 | 7 | 4 |
| Hepatic artery-portal vein shunt | 10 | 15 | 3 | 7 |
After routine embolization, we kept the catheter in the segmental artery and confirmed its proper position via fluorescence imaging. When the patient was returned to the ward, 50 mg oxaliplatin in 250 mL of glucose was infused with a pump over 4 h. Then, 2 mg raltitrexed in 100 mL of 0.9% normal saline was infused with a pump over the next 1 h. Ondansetron was used to prevent vomiting.
The treatment protocol began with 1-11 sessions of TACE (average interval: 3.2 mo). The patients were followed up every 1-3 mo to check whether new tumor lesions had developed in the liver. When lesions were detected, TACE was performed again to control the intrahepatic lesions.
The acute and late toxicities from the treatments were graded according to the NCI-CTCAE version 4.0[12] Monthly evaluations of the responses to TACE were recommended.
The responses were defined using the mRECIST criteria[13] based on an enhanced CT and MRI of the liver.
SPSS version 19.0 (SPSS Inc., Chicago, IL, United States) for Windows was used for the data analysis. Overall survival (OS) was calculated from the date of HCC diagnosis until death or until the date of the last follow-up visit for all patients who were still alive.
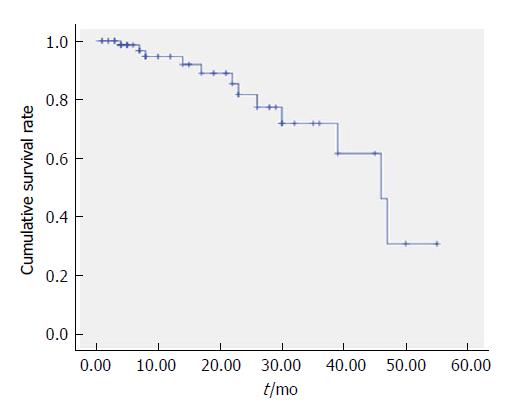
Full embolization was achieved in 68 cases, and partial embolization was achieved in 18 cases. For 86 patients who received embolization, the catheter was kept in the hepatic artery and them low-dose continuous hepatic arterial infusion chemotherapy was administered. Radiologically complete responses (CRs), partial responses (PRs), stable disease (SD), and disease progression (PD) of the intrahepatic disease were observed in 0, 45, 20, and 21 patients, respectively. The following time was 1-55 mo, the 1-, 2- and 3-year overall survival rates of the 86 patients were 40.7%, 22.1% and 8.1%, respectively, and the median survival time was 8.7 mo. Moreover, in 35 patients, we observed the uptake of lipiodol in the MPVTT after TACE, and with the passage of time, collateral circulation was gradually established, and the incidences of bleeding and ascites decreased. These changes may have contributed to the OS benefit (Table 1).
Thirty-five cases died within the first 6 mo, and these deaths were mainly due to bleeding and acute liver failure. Subsequently, the rate of death slowed (Figure 1).
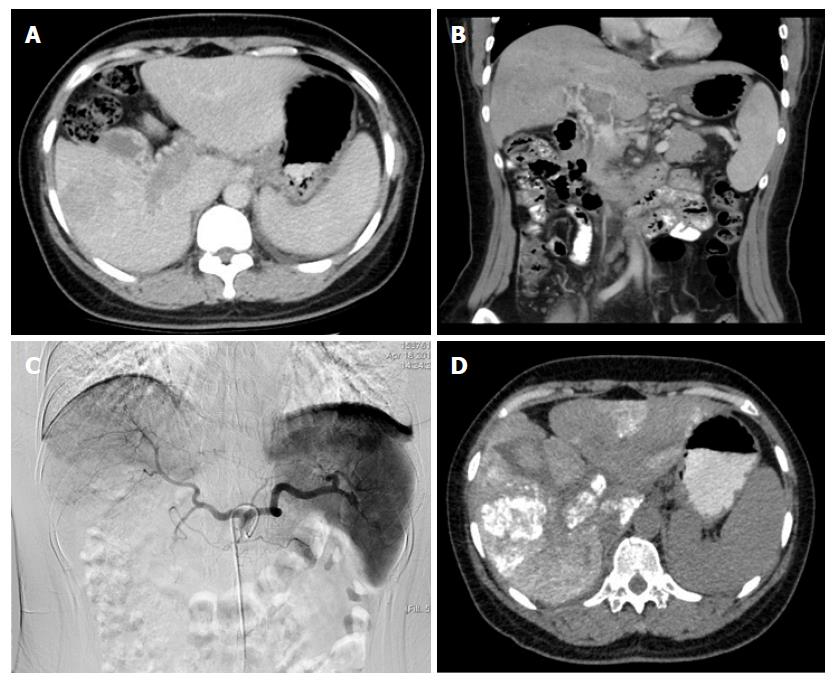
In a 47-year-old man with a HCC and MPVTT, an angiography via the proper hepatic artery revealed a tumor stain in the liver (Figure 2A), a stain in the right branch, and a portal trunk tumor thrombus (Figure 2B). We performed super-selective catheterization and administered embolization and chemotherapy infusion (Figure 2C). A post-operative CT scan revealed scattered deposition of lipiodol inside the MPVTT as well as intra-hepatic lesions (Figure 2D).
In a 51-year-old woman with a HCC and MPVTT, a pre-operative CT scan revealed a tumor thrombus invading into the right branch and portal trunk (Figure 3A and B). Angiography via the celiac hepatic artery revealed a tumor stain in the liver and right branch and a portal trunk tumor thrombus (Figure 3C). A post-operative CT scan revealed the deposition of lipiodol inside the MPVTT (Figure 3D).
The main complications included acute variceal hemorrhaging, intractable ascites, and hepatic encephalopathy and failure. Twenty-eight cases exhibited variceal hemorrhaging after TACE; 19 of these complications occurred within 3 mo, and 14 of these patients died. Fourteen cases had intractable ascites after TACE, and, among the 15 cases with ascites before TACE, the ascites decreased or disappeared after the TACE.
Although a majority of the cases had background cirrhosis, acute liver failure was rare and was observed in six cases. Twenty-eight cases had moderately increased ALT and AST levels, but the T-Bil levels were slightly increased. Eight of these patients exhibited transient abnormal renal function, but function returned to normal within 1 wk in all cases. This phenomenon might have been due to the massive necrosis of the tumor cells.
Postembolization syndrome, which included pain and fever, occurred frequently and was successfully treated with conservative treatment. Twenty-five cases experienced nausea and loss of appetite. However, no cases had severe (grades 3 or 4) adverse effects, and symptoms were controllable with medical treatment. Myelosuppression was very rare; only eight cases exhibited slight reductions of the WBC and PLT count and recovered within 1 week without medical treatment.
The western guidelines of the Barcelona Clinic Liver Cancer (BCLC) staging system are frequently applied for decisions regarding initial treatments[14] for PVTT, but the management options for HCC with PVTT are more limited. To date, radiation and systematic chemotherapy or sorafenib targeted therapy[15] have been used for PVTT. However, the median survival time with sorafenib is only 6.5 mo in Asia[16]. In China, economic conditions restrict the application of sorafenib for most patients. Therefore, consecutive TACE is still used to treat selected patients with PVTT.
TACE has traditionally been considered to be contraindicated in cases of PVTT due to its high embolic effect and the potential for inducing hepatic necrosis and worsening liver dysfunction[6,16]. However, with progress of the technique, the effects of TACE have been confirmed in some randomized controlled trials (RCTs)[17]. A meta-analysis of prospective randomized trials demonstrated that survival is improved and good liver function is preserved after TACE for unresectable HCC[15] TACE can be safely performed in patients with HCCs that invade the main portal vein and may improve overall survival[18]. A meta-analysis of prospective randomized trials[19] demonstrated that TACE is potentially suitable and safe for patients with advanced HCC with PVTT, including patients with MPV obstructions. For selected patients with MPV obstructions, especially those with established collateral circulation and good liver function preservation, TACE treatment may prolong survival[20]. Although PVTTs are located outside of the liver parenchyma, their feeding artery still arises from the hepatic artery branches. We successfully embolized lesions in the liver and PVTTs via the hepatic artery branches in 86 cases; in the remaining eight patients, we still performed infusion chemotherapy via the hepatic artery despite the presence of shunts because we found stains of intra-hepatic lesions.
Hepatic arterial infusion chemotherapy has been investigated for the treatment of advanced HCC with portal vein tumor thrombosis in Asian countries. In this protocol, chemotherapeutic agent is infused into the hepatic artery via an implanted catheter, which reduces systemic side effects by first pass effects and maximizes drug delivery to the tumor. However, for HCC with MPVTT, patients always exhibit accompanying hypertension and hypersplenism. Thus, regular-dose chemotherapy may elicit more serious systemic toxicity, and patients may be more prone to have serious myelosuppression and gastrointestinal reaction and bleeding. Additionally, the nutrition conditions of MPTVV patients are generally poor, which makes a loss of appetite even more dangerous. Therefore, we need a more feasible protocol to ensure effectiveness while limiting the toxicity of chemotherapy.
In interventional therapy, the most feasible methods for reducing systemic toxicity are to reduce the dose or prolong the infusion time. However, doctors may worry that low doses could reduce the effect and wonder how a suitable dose and infusion time can be selected to ensure the effect. Where is the balance point? To find the answer, we must first understand the local plasma concentrations during the infusion process under different doses and whether these concentrations could meet our needs; subsequently, we can make the right decisions.
We performed a pharmacokinetics study with 1 mg/4 mg raltitrexed and 35 mg/150 mg oxaliplatin in a swine model. During infusion, both oxaliplatin and raltitrexed maintained high concentrations in the lobar hepatic artery during the whole infusion process. The highest concentrations of 35 mg and 150 mg of oxaliplatin at 4 h infusion time were 4259 μg/L and 14287 μg/L, respectively. The highest concentrations of 1 mg raltitrexed at 30 min, 60 min, and 120 min infusion times were 405, 212, and 128 μg/L, respectively. The corresponding highest concentrations in the 4-mg raltitrexed group were 1601, 902, and 587 μg/L. These results indicated that, even with the lowest dose and the longest infusion time, relatively high concentrations could be maintained.
However, we still needed to demonstrate how such high concentrations would act on tumor cells. We used the concentrations obtained from the pharmacokinetics study to perform an in vitro pharmacodynamics study in multiple tumor cell models. A MTT assay was used to measure the effects of oxaliplatin and raltitrexed on cell viability using different combinations of concentrations and times. We found that oxaliplatin and raltitrexed increased cell death in a concentration and time dependent manner. For oxaliplatin, a concentration of 5000-10000 μg/L and an infusion time of 4 h was a suitable combination. For raltitrexed, a suitable time was 60-120 min, and a suitable concentration was 250-500 μg/L.
By using super-selective or super super-selective techniques, we advanced a catheter into the segmental branch in most cases, and the blood flow in the target artery ranged from 1-2 mL/s. From this information, we could calculate the dose needed during infusion. For example, if we wanted to achieve a final 5000 μg/L concentration in the target artery, the required oxaliplatin could be calculated as 5000 (μg/L) × 14400 s × 1 (mL/s)/106 = 72 mg; if we wanted to achieve a final concentration of 500 μg/L in the target artery, the required raltitrexed could be calculated as 500 (μg/L) × 3600 s × 1 (mL/s)/106 = 1.8 mg.
Based on the results from these studies, 50 mg oxaliplatin was infused for 4 h, and 2 mg raltitrexed was infused for the next 1 h. This protocol produced good results and fewer complications. The 1-, 2-, and 3-year overall survival rates were 40.7%, 22.1%, and 8.1%, respectively. The complications were acceptable. Myelosuppression was observed in only eight patients. Twenty-eight patients exhibited bleeding, but 19 of these complications occurred within 3 mo after the TACE and may have been due to the establishment of collateral circulation and a decrease of portal vein pressure. Obviously, low-dose continuous hepatic arterial infusion chemotherapy can ensure a sufficient local concentration and an increase in cell death in tumor tissue, and longer infusion times mean that the tumor tissue can take up more of the agent. Additionally, the human body has more time to eliminate the agent, which dramatically reduces the toxicity.
The limitations of this study were the small number of patients and the retrospective design. Multivariate analysis may not precisely identify the factors associated with OS and PFS in such a small study.
In conclusion, this pharmacokinetic and pharmacodynamic study provided important information regarding the characteristics of drugs during continuous hepatic arterial infusion. TACE with low dose oxaliplatin and raltitrexed was effective and less toxic in patients with advanced HCC with MPVTT, making it a viable option for such patients.
We had generated a pharmacokinetics and pharmacodynamics model for transarterial infusion. Here, we aimed to translate these findings into a clinical protocol for hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT).
Although transarterial chemoembolization (TACE) is widely used in the treatment of HCC with PVTT, the incidence of complication is high and overall survival is short, thereby limiting its use. More safe and effective protocols need to be developed to improve therapeutic effects.
We want to provide a simple and effect way to determine a clinical protocol based on pharmacokinetic and pharmacodynamic studies.
After embolization, we kept the catheter in the feeding artery of the tumor and infused chemotherapy: oxaliplatin 50 mg in 250 mL of glucose was infused by pump for 4 h and raltitrexed 2 mg in 100 mL of 0.9% saline for the next 1 h after.
All cases received low dose continuous hepatic arterial infusion chemotherapy without major complications. Complete responses, partial responses, stable disease, and disease progression for intrahepatic disease were observed in 0, 45, 20, and 21 patient, respectively. The 1-, 2-, and 3-year overall survival rates of the 86 patients were 40.7%, 22.1%, and 8.1% respectively, and the median survival time was 8.7 mo.
TACE with low dose continuous hepatic arterial infusion of oxaliplatin and raltitrexed could be safely used in major portal vein tumor thrombosis (MPVTT) patients. It is effective in patients with advanced HCC with MPVTT and is less toxic.
Continuous hepatic arterial infusion chemotherapy was shown to be effective with limited complications. Based on results from pharmacokinetic and pharmacodynamics studies; we were able to choose agents and adjust the protocol with high efficiency.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dulciene DM, Teresa VM, Yuan YF S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 2. | Quirk M, Kim YH, Saab S, Lee EW. Management of hepatocellular carcinoma with portal vein thrombosis. World J Gastroenterol. 2015;21:3462-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Zhu P, Liao Y, Fan J, Li X, Su L, Li J, Yuan S, Yu J, Liao W. Preoperative prediction of hepatocellular carcinoma with portal vein tumor thrombus based on conventional data. Oncotarget. 2017;8:104227-104237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4519] [Article Influence: 347.6] [Reference Citation Analysis (2)] |
| 5. | Suzuki Y, Takeda M. Keratins in the developing olfactory epithelia. Brain Res Dev Brain Res. 1991;59:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 209] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 6. | Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surg Today. 2014;44:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Lin DX, Zhang QY, Li X, Ye QW, Lin F, Li LL. An aggressive approach leads to improved survival in hepatocellular carcinoma patients with portal vein tumor thrombus. J Cancer Res Clin Oncol. 2011;137:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, Brancati FL, Clark JM. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 625] [Article Influence: 52.1] [Reference Citation Analysis (1)] |
| 9. | Guo JH, Zhang HY, Gao S, Zhang PJ, Li XT, Chen H, Wang XD, Zhu X. Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis. World J Gastroenterol. 2017;23:1406-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Henderson JM, Sherman M, Tavill A, Abecassis M, Chejfec G, Gramlich T. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford). 2003;5:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PubMed] |
| 12. | NIH Publication 09-5410: Common Terminology Criteria for Adverse Events (CTCAE) v4. 03: June 14, 2010. United States Department of Health and Human Services, National Institutes of Health, National Cancer Institute. . |
| 13. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3302] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 14. | Lin M, Pellerin O, Bhagat N, Rao PP, Loffroy R, Ardon R, Mory B, Reyes DK, Geschwind JF. Quantitative and volumetric European Association for the Study of the Liver and Response Evaluation Criteria in Solid Tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2012;23:1629-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10265] [Article Influence: 603.8] [Reference Citation Analysis (2)] |
| 16. | Hsu CH, Yang TS, Hsu C, Toh HC, Epstein RJ, Hsiao LT, Chen PJ, Lin ZZ, Chao TY, Cheng AL. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, Li ZK, Qi F, Zhao C. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012;29:2992-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, Tumino E, Ginanni B, Federici G, Cioni R. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |