Published online Jun 14, 2018. doi: 10.3748/wjg.v24.i22.2392
Peer-review started: February 27, 2018
First decision: March 9, 2018
Revised: March 29, 2018
Accepted: May 5, 2018
Article in press: May 5, 2018
Published online: June 14, 2018
Processing time: 103 Days and 23.1 Hours
To investigate the location to which a pancreatic stent should be inserted to prevent post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP).
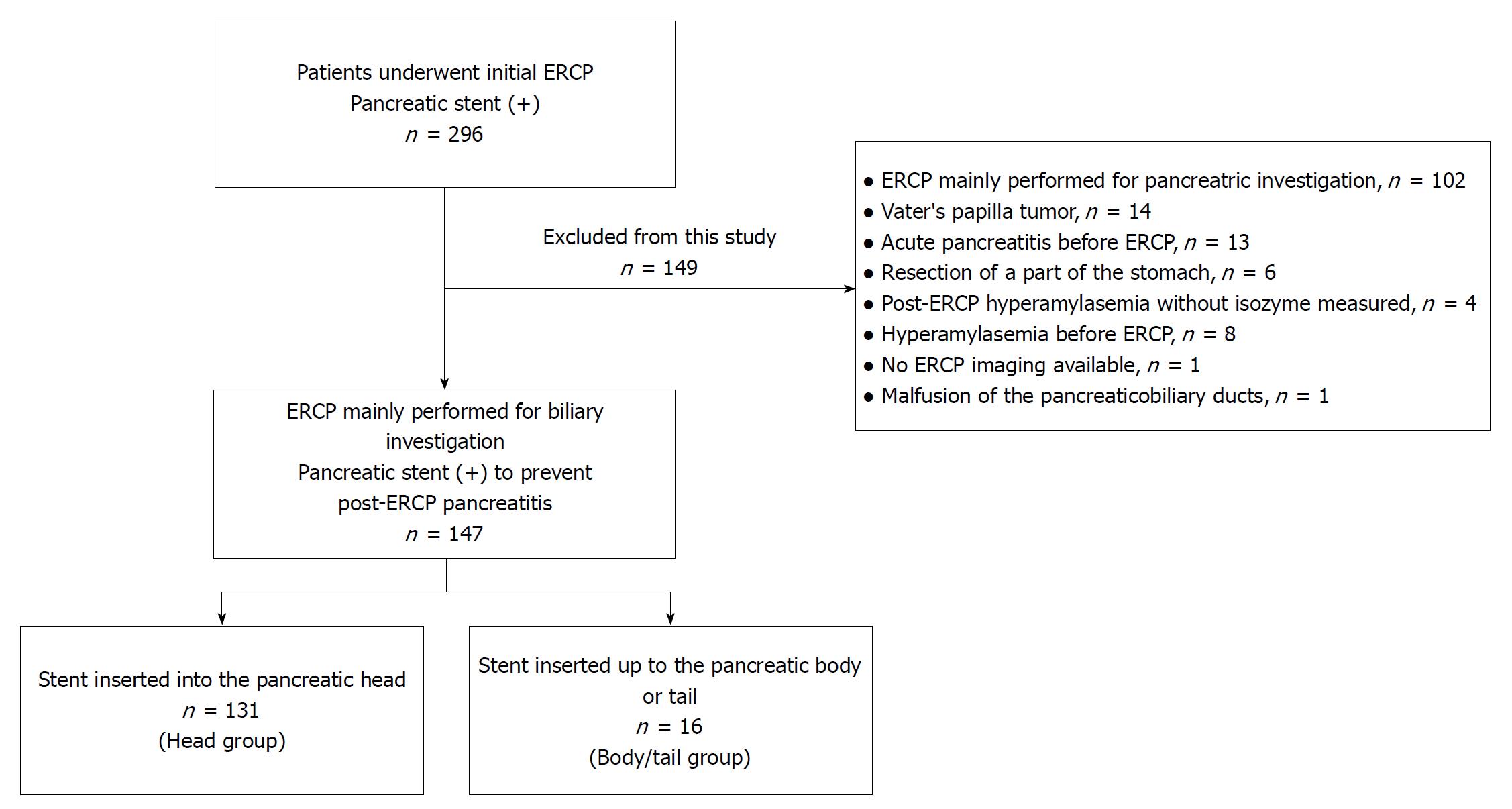
Over a ten-year period at our hospital, 296 patients underwent their first ERCP procedure and had a pancreatic stent inserted; this study included 147 patients who had ERCP performed primarily for biliary investigation and had a pancreatic stent inserted to prevent PEP. We divided these patients into two groups: 131 patients with a stent inserted into the pancreatic head (head group) and 16 patients with a stent inserted up to the pancreatic body or tail (body/tail group). Patient characteristics and ERCP factors were compared between the groups.
Pancreatic amylase isoenzyme (p-AMY) levels in the head group were significantly higher than those in the body/tail group [138.5 (7.0-2086) vs 78.5 (5.0-1266.5), P = 0.03] [median (range)]. No cases of PEP were detected in the body/tail group [head group, 12 (9.2%)]. Of the risk factors for post-ERCP hyperamylasemia (≥ p-AMY median, 131 IU/L), procedure time ≥ 60 min [odds ratio (OR) 2.65, 95%CI: 1.17-6.02, P = 0.02) and stent insertion into the pancreatic head (OR 3.80, 95%CI: 1.12-12.9, P = 0.03) were identified as independent risk factors by multivariate analysis.
Stent insertion up to the pancreatic body or tail reduces the risk of post-ERCP hyperamylasemia and may reduce the risk of PEP.
Core tip: We investigated whether the location of the inserted pancreatic stent rather than pancreatic stent length influenced the frequency of post-endoscopic retrograde cholangiopancreatography (ERCP) hyperamylasemia and post-ERCP pancreatitis (PEP). Pancreatic amylase isoenzyme levels after ERCP were significantly higher in the head group than in the body/tail group. PEP did not occur in the body/tail group. Stent insertion into the pancreatic head was an independent risk factor for hyperamylasemia after ERCP, while stent insertion up to the pancreatic body or tail reduced the risk of post-ERCP hyperamylasemia and may reduce the risk of PEP.
- Citation: Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Sato Y, Irie H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Hikichi T, Ohira H. Pancreatic stents for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis should be inserted up to the pancreatic body or tail. World J Gastroenterol 2018; 24(22): 2392-2399
- URL: https://www.wjgnet.com/1007-9327/full/v24/i22/2392.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i22.2392
Endoscopic retrograde cholangiopancreatography (ERCP) plays a significant role in endoscopic investigation and treatment for cholangiopancreatic diseases. However, a serious adverse effect of ERCP is pancreatitis. Previous studies have found that post-ERCP pancreatitis (PEP) occurs in 0.4%-5.6% of cases[1-8], and that mortality from PEP occurs in 0-0.1% of cases[4,6-8]. Pancreatic stents have been shown to be useful for preventing PEP[9-22]. The adaptation of pancreatic stent insertion to prevent PEP has been reported in the contexts of sphincter of Oddi dysfunction, a history of pancreatitis or PEP, difficulty with biliary duct cannulation, pancreatic duct cannulation, and others[23]. Despite pancreatic ductal stent insertion, PEP was reported to occur in 1.7%-20% of these cases[12-18]. The pancreatic stent that should be used is unclear (size, with flap or without flap, insertion location, etc.). One study found that a 5-Fr pancreatic stent was easier to insert than a 3-Fr pancreatic stent[24], and another reported that a 5-Fr pancreatic stent was similarly effective as a 4-Fr pancreatic stent in preventing PEP[25]. An additional report stated that insertion of a pancreatic stent with a diameter > 5 Fr was effective in preventing PEP[22]. The incidence of PEP, however, was not reduced in most reports. Meanwhile, two reports have discussed the length of the pancreatic stents. Fujisawa et al[26] compared pancreatic stent lengths (unflapped straight stent, 5 Fr at 3 cm vs 5 Fr at 5 cm) and reported that the PEP rate and the median time until stent migration were lower in the 3-cm group than in the 5-cm group. However, pancreatic sizes varied, and 3-cm or 5-cm pancreatic stents were primarily inserted at the pancreatic head. Olsson et al[22] reported that a pancreatic stent with a length > 5 cm and a diameter > 5 Fr is most effective in preventing PEP. The independent efficacy of a longer pancreatic stent to prevent PEP remains unknown.
Therefore, we investigated whether the location of an inserted pancreatic stent rather than the pancreatic stent length influenced the frequency of post-ERCP hyperamylasemia and PEP.
In this retrospective study, we compared the efficacy of pancreatic ductal stent insertion between patients with a stent inserted into the pancreatic head and patients with a stent inserted up to the pancreatic body or tail. This study was approved by the Institutional Review Board of Fukushima Medical University.
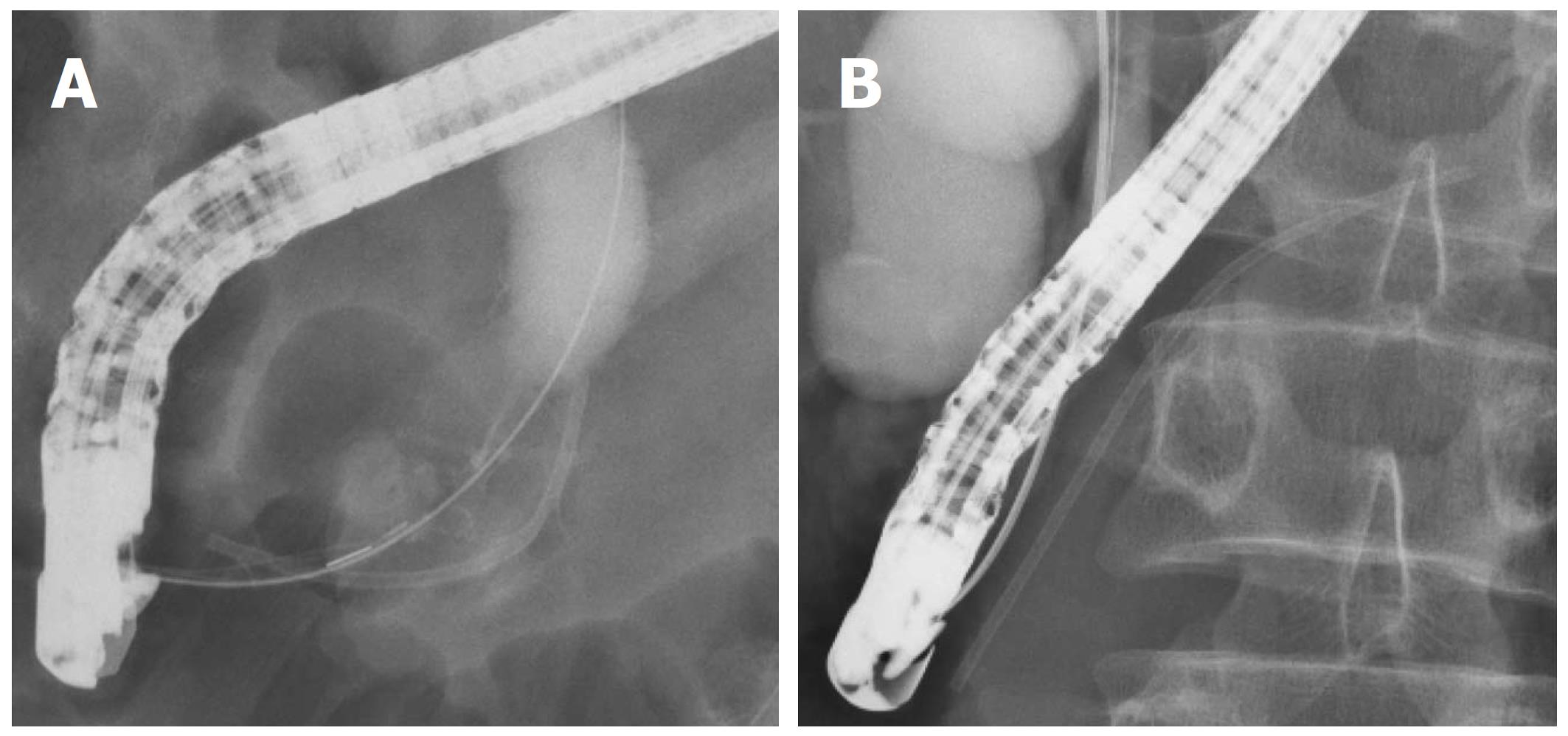
In all, 296 patients underwent their first ERCP procedure and had a pancreatic stent inserted at our hospital between January 2007 and November 2017 (Figure 1). Among these patients, 147 underwent ERCP primarily for biliary investigation and had a stent inserted to prevent PEP and were included in this study. We divided these patients into two groups: 131 patients with a stent inserted into the pancreatic head (head group) and 16 patients with a stent inserted up to the pancreatic body or tail (body/tail group). The location of the inserted pancreatic stent was determined by X-ray during ERCP (Figure 2).
In total, 149 patients were excluded from this study because 102 patients underwent ERCP primarily to investigate the pancreatic duct, 14 patients had a Vater’s papilla tumor or pancreatic cancer invasion to Vater’s papilla, 13 patients contracted acute pancreatitis before ERCP, eight patients had hyperamylasemia before ERCP, six patients had part of the stomach resected, four patients did not have amylase isozyme measurements after ERCP hyperamylasemia, one patient did not have images from the ERCP, and one patient had malfusion of the pancreaticobiliary ducts.
All the patients had an endoscope inserted after they were sufficiently sedated with midazolam. After the endoscope reached the descending part of the duodenum, biliary cannulation was initiated. When a guidewire was passed into the pancreatic duct or contrast media was injected, the guidewire was placed in the main pancreatic duct (MPD) as deep as possible by pancreatography. We injected contrast media as sparingly as possible to confirm MPD placement. After biliary cannulation was achieved, a pancreatic stent was inserted. If biliary cannulation was difficult, we inserted a pancreatic stent and performed a precut of Vater’s papilla. The lengths of the pancreatic stents were determined randomly by the endoscopists. If the patient was diagnosed with PEP or elevated serum pancreatic amylase isoenzyme (p-AMY) (≥ 500 IU/L) with abdominal pain, then the pancreatic stent was removed 1 d after ERCP. In other cases, pancreatic stents with a flap were removed 2-3 d after ERCP, but pancreatic stents without a flap were not removed. JF260V, JF240, and TJF240 ERCP endoscopes (Olympus, Tokyo, Japan) were used. A Tandem XL (Boston Scientific Japan, Tokyo, Japan), MTW ERCP catheter taper (MTW Endoskopie, Wesel, Germany), or PR-233Q (Olympus) was used as the ERCP catheter. A CleverCut 3V (Boston Scientific Japan, Tokyo, Japan) was used for endoscopic sphincterotomy (EST). An RX Needle Knife (Boston Scientific Japan, Tokyo, Japan) was used to precut Vater’s papilla. A Zimmon 5-Fr, 2-cm single pigtail stent without an inner flap (Cook Japan, Tokyo, Japan), a Zimmon 5-Fr, 4-cm single pigtail stent with an inner flap (Cook Japan, Tokyo, Japan), a Geenen 5-Fr, 3-cm stent with outer flaps and without an inner flap (Cook Japan, Tokyo, Japan), or a Geenen 5-Fr, 5-, 7-, or 9-cm stent with inner flaps and outer flaps was used as the pancreatic stent.
Patient characteristics (age, gender, pancreatic calcification, parapapillary diverticulum, and diameter of the MPD) and ERCP factors (EST, endoscopic papillary balloon dilation (EPBD), precut of Vater’s papilla, procedure time, serum levels of p-AMY, PEP, and pancreatic stent migration within 1 wk) were compared between the head group and the body/tail group. The p-AMY value peaked within 1 wk of ERCP. The level of p-AMY was measured 3 h and 24 h after ERCP. If the level of serum amylase was more than three times the normal level 24 h after ERCP, with p-AMY accounting for the majority of the change in serum amylase, we measured p-AMY every day until the serum amylase level decreased to approximately two times the normal level or to a normal level. PEP was diagnosed by hyperamylasemia more than three times the normal level at more than 24 h after ERCP and abdominal pain[27]. In addition, we confirmed peripancreatic inflammation by contrast computed tomography (CT) imaging in all PEP patients. The severity of PEP was determined as proposed by Cotton et al[27] (mild: hospitalization prolonged for 2-3 d; moderate: 4-10 d; and severe: more than 10 d, or pseudocyst requiring intervention (percutaneous drainage or surgery), or hemorrhagic pancreatitis). Pancreatic stent migration was confirmed randomly by X-ray or CT.
Kolmogorov-Smirnov tests and Shapiro-Wilk normality tests were used to test normality. Mann-Whitney U tests were used to compare continuous variables. Fisher’s exact tests were used to compare nominal variables. Logistic regression was used to investigate post-ERCP hyperamylasemia factors. A P value < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using the EZR platform (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R commander that was designed to perform functions frequently used in biostatistics[28].
Patient characteristics were not significantly different between the two groups (Table 1). Regarding aspects of the ERCP procedures, the p-AMY levels after ERCP in the head group were significantly higher than those in the body/tail group [138.5 (7.0-2086) IU/L vs 78.5 (5.0-1266.5), P = 0.03] [median (range)] (Table 2). Other factors were not different between the two groups, and PEP did not occur in the body/tail group. However, PEP occurred in 12 patients in the head group (mild 2; moderate 8; and severe 2).
| Head group (n = 131) | Body/tail group (n = 16) | P value | |
| Age, median (range), yr | 71.0 (29-97) | 66.5 (26-81) | 0.052 |
| Male/female | 69/62 | 11/5 | 0.29 |
| Pancreatic calcification | 4 (3.1)1 | 1 (6.3) | 0.45 |
| Parapapillary diverticulum | 38 (29.5)2 | 5 (31.2) | 1.0 |
| Diameter of the MPD, median (range), mm | 3.1 (0.7-22.0) | 3.7 (1.5-13.8) | 0.15 |
| Diagnosis | |||
| Left hepatic duct extension | 1 | ||
| Biliary stricture | |||
| Benign | 5 | ||
| Malignant | |||
| Pancreatic cancer | 24 | 7 | |
| Biliary tract cancer | 33 | 3 | |
| Hepatocellular carcinoma | 3 | ||
| Obstructive jaundice by metastatic cancer | |||
| Barrett’s esophageal cancer | 1 | ||
| Colon cancer | 4 | ||
| Gastric cancer | 2 | ||
| Uterine cancer | 1 | ||
| Bladder cancer | 1 | ||
| Ovarian cancer | 1 | ||
| Suppression of lymph node swelling | 1 | ||
| Central bile duct stone | 47 | 4 | |
| Primary sclerosing cholangitis | 1 | 1 | |
| Gallbladder adenomyosis | 3 | ||
| IPNB | 1 | ||
| Sphincter of Oddi dysfunction | 1 | ||
| Biliary cysts | 1 | ||
| Lemmel syndrome | 1 |
| Head group (n = 131) | Body/tail group (n = 16) | P value | |
| EST | 98 (75.4) | 15 (93.8) | 0.12 |
| Precut of Vater’s papilla | 43 (33.1) | 5 (31.3) | 1 |
| EPBD | 8 (6.2) | 0 (0) | 0.6 |
| Pancreatic stent migration within 1 wk | 26 (19.8) | 0 (0) | 0.076 |
| Procedure time, median (range), min | 60 (20-150)1 | 60 (40-120)2 | 0.31 |
| Type of stent | |||
| Zimmon 5 Fr, 2 cm | 3 | ||
| Zimmon 5 Fr, 4 cm | 1 | ||
| Geenen 5 Fr, 3 cm | 59 | ||
| Geenen 5 Fr, 5 cm | 58 | ||
| Geenen 5 Fr, 7 cm | 11 | 12 | |
| Geenen 5 Fr, 9 cm | 4 | ||
| p-AMY after ERCP, median (range), IU/L | 138.5 (7.0-2086)3 | 78.5 (5.0-1266.5) | 0.03 |
| PEP | 12 (9.2) | 0 (0) | 0.363 |
| Mild | 2 | ||
| Moderate | 8 | ||
| Severe | 2 |
Regarding the risk factors of post-ERCP hyperamylasemia (≥ p-AMY median, 131 IU/L), age ≥ 71 years, parapapillary diverticulum, stent insertion into the pancreatic head, and procedure time ≥ 60 min were identified as significant factors in the univariate analysis (factors with P < 0.15 were selected for multivariate analysis) (Table 3). Logistic regression with backward stepwise selection was performed using these four items, and the independent risk factors included procedure time ≥ 60 min [odds ratio (OR) 2.65, 95%CI: 1.17-6.02, P = 0.02] and stent insertion into the pancreatic head (OR 3.80, 95%CI: 1.12-12.9, P = 0.032) (Table 4).
| p-AMY < 131 IU/L1 (n = 70) | p-AMY ≥ 131 IU/L1 (n = 70) | P value | |
| Age (≥ 71 yr) | 29 | 41 | 0.063 |
| Gender (male/female) | 40/30 | 35/35 | 0.5 |
| Pancreatic calcification | 3 | 2 | 1 |
| Parapapillary diverticulum | 27 | 16 | 0.043 |
| MPD ≥ 3.2 mm | 36 | 36 | 1.0 |
| EST | 52 | 55 | 0.69 |
| EPBD | 3 | 3 | 1.0 |
| Precut of Vater’s papilla | 18 | 26 | 0.20 |
| Stent inserted pancreatic head | 58 | 66 | 0.06 |
| Pancreatic stent migration within a week | 11 | 12 | 1.0 |
| Procedure time ≥ 60 min | 45 | 57 | 0.048 |
| OR | 95%CI | P value | |
| Procedure time ≥ 60 min | 2.65 | 1.17-6.02 | 0.020 |
| Stent insertion into the pancreatic head | 3.80 | 1.12-12.9 | 0.032 |
In this study, we investigated how far a pancreatic stent should be inserted. We compared several factors between the head group and the body/tail group. P-AMY levels after ERCP were significantly higher in the head group than in the body/tail group. PEP did not occur in the body/tail group. Stent insertion into the pancreatic head was an independent risk factor for hyperamylasemia after ERCP.
Several reports are available regarding the diameter of pancreatic stents. Lawrence et al[29] reported that 3-Fr pancreatic stents passed spontaneously and did not induce changes in the pancreatic duct. Zolotarevsky et al[24] reported that placement of a 5-Fr pancreatic stent was easier, faster, and required fewer wires compared to a 3-Fr pancreatic stent. Pahk et al[25] reported that 4-Fr pancreatic stents migrated more frequently than 5-Fr pancreatic stents; therefore, the need for additional endoscopy to retrieve the pancreatic stent was reduced by using a 4-Fr pancreatic stent. However, PEP was no less common with the slimmer stents in these reports.
This report showed that pancreatic stent placement in the pancreatic body or tail could reduce the rates of post-ERCP hyperamylasemia and PEP. Difficulty with pancreatic duct drainage leads to proteinase activation, which exacerbates pancreatitis[30]. Pancreatic stent placement up to the pancreatic body or tail could allow greater pancreatic duct drainage than stent placement in the pancreatic head. Therefore, stent placement up to the pancreatic body or tail may reduce the risks of PEP and post-ERCP hyperamylasemia. In addition, no pancreatic stent migration was observed in the body/tail group. Therefore, the risk of pancreatic stent obstruction by contact with the duodenal wall was considered to be low.
This study has some limitations. First, this was a retrospective study with a small number of patients at a single institution. In addition, we generally insert stents into the pancreatic head, as described in past reports; we only recently began inserting pancreatic stents up to the body or tail of the pancreas. Therefore, the number of patients in the body/tail group was small. We hope to conduct larger prospective studies to verify the findings of this study. We performed a multivariate analysis, including post-ERCP serum p-AMY levels, and were able to identify the risk factors for post-ERCP hyperamylasemia. Second, many patients were excluded from this study. However, the amount of contrast media used in 102 patients to investigate the pancreatic duct by pancreatography was much higher than the target amount of this study. The risk of PEP in the 102 excluded patients was very different from that in the general population, as many of these excluded patients had chronic pancreatitis. In addition, many patients with pancreatic stricture underwent pancreatic stenting as treatment rather than for the prevention of PEP. Therefore, the patients included in the present study and the 102 patients excluded from the study could not be compared. Third, due to the retrospective nature of this study, ERCP procedures were not performed by specific endoscopists. In this study, ERCP was performed by specialists in pancreaticobiliary endoscopy who had experience in performing at least 2000 ERCP procedures or by trainees under the guidance of these specialists. Therefore, the quality of the ERCP procedure was considered to be consistent. Fourth, stents without an inner flap (Geenen 5 Fr, 3 cm and Zimmon 5 Fr, 2 cm) were used in only the head group. Consequently, the stent migration rate was higher in the head group than in the body/tail group. In past reports, however, incidence of spontaneous pancreatic stent migration did not subsequently result in increased incidence of PEP[25,26,31]. Therefore, using a stent without an inner flap was not considered disadvantageous in the head group.
In conclusion, stent insertion up to the pancreatic body or tail reduced the incidence of post-ERCP hyperamylasemia and may reduce the incidence of PEP.
Pancreatic stents are reported to be useful for preventing pancreatitis (PEP).
We wanted to determine the appropriate length of a pancreatic stent for preventing PEP.
To investigate whether a stent should be inserted into the pancreatic head, body, or tail to prevent PEP.
Patient characteristics and endoscopic retrograde cholangiopancreatography (ERCP) factors were compared between 131 patients with a stent inserted into the pancreatic head (head group) and 16 patients with a stent inserted up to the pancreatic body or tail (body/tail group).
Pancreatic amylase isoenzyme (p-AMY) levels after ERCP were significantly higher in the head group than in the body/tail group. PEP did not occur in the body/tail group. Stent insertion into the pancreatic head was an independent risk factor for hyperamylasemia after ERCP.
Stent insertion up to the pancreatic body or tail reduced the incidence of post-ERCP hyperamylasemia and may reduce the incidence of PEP.
Prophylactic stent insertion up to the pancreatic body or tail may be the main method for preventing PEP. We hope that a future prospective, multicenter study will confirm the conclusion of this study.
I am grateful to the staff at the Department of Gastroenterology of Fukushima Medical University School of Medicine, the medical staff in the Department of Endoscopy at Fukushima Medical University Hospital, and the medical staff of the Gastroenterology Ward at Fukushima Medical University Hospital.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Gonzalez-Ojeda AG, Gupta R, Han JH, Isik ARDA, Luglio G, Lorenzo-Zuniga V, Rabago LR, Tallon-Aguilar L S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Reiertsen O, Skjøtø J, Jacobsen CD, Rosseland AR. Complications of fiberoptic gastrointestinal endoscopy--five years’ experience in a central hospital. Endoscopy. 1987;19:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Sherman S, Hawes RH, Rathgaber SW, Uzer MF, Smith MT, Khusro QE, Silverman WB, Earle DT, Lehman GA. Post-ERCP pancreatitis: randomized, prospective study comparing a low- and high-osmolality contrast agent. Gastrointest Endosc. 1994;40:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Johnson GK, Geenen JE, Bedford RA, Johanson J, Cass O, Sherman S, Hogan WJ, Ryan M, Silverman W, Edmundowicz S. A comparison of nonionic versus ionic contrast media: results of a prospective, multicenter study. Midwest Pancreaticobiliary Study Group. Gastrointest Endosc. 1995;42:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1689] [Article Influence: 58.2] [Reference Citation Analysis (2)] |
| 5. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 779] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 6. | Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, Pilotto A, Forlano R. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 772] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 7. | Glomsaker T, Hoff G, Kvaløy JT, Søreide K, Aabakken L, Søreide JA; Norwegian Gastronet ERCP Group. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br J Surg. 2013;100:373-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Katsinelos P, Lazaraki G, Chatzimavroudis G, Gkagkalis S, Vasiliadis I, Papaeuthimiou A, Terzoudis S, Pilpilidis I, Zavos C, Kountouras J. Risk factors for therapeutic ERCP-related complications: an analysis of 2,715 cases performed by a single endoscopist. Ann Gastroenterol. 2014;27:65-72. [PubMed] |
| 9. | Shi QQ, Ning XY, Zhan LL, Tang GD, Lv XP. Placement of prophylactic pancreatic stents to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a meta-analysis. World J Gastroenterol. 2014;20:7040-7048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Takenaka M, Fujita T, Sugiyama D, Masuda A, Shiomi H, Sugimoto M, Sanuki T, Hayakumo T, Azuma T, Kutsumi H. What is the most adapted indication of prophylactic pancreatic duct stent within the high-risk group of post-endoscopic retrograde cholangiopancreatography pancreatitis? Using the propensity score analysis. J Hepatobiliary Pancreat Sci. 2014;21:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Nakahara K, Okuse C, Suetani K, Michikawa Y, Kobayashi S, Otsubo T, Itoh F. Need for pancreatic stenting after sphincterotomy in patients with difficult cannulation. World J Gastroenterol. 2014;20:8617-8623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Kawaguchi Y, Ogawa M, Omata F, Ito H, Shimosegawa T, Mine T. Randomized controlled trial of pancreatic stenting to prevent pancreatitis after endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2012;18:1635-1641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Sofuni A, Maguchi H, Itoi T, Katanuma A, Hisai H, Niido T, Toyota M, Fujii T, Harada Y, Takada T. Prophylaxis of post-endoscopic retrograde cholangiopancreatography pancreatitis by an endoscopic pancreatic spontaneous dislodgement stent. Clin Gastroenterol Hepatol. 2007;5:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Tsuchiya T, Itoi T, Sofuni A, Itokawa F, Kurihara T, Ishii K, Tsuji S, Kawai T, Moriyasu F. Temporary pancreatic stent to prevent post endoscopic retrograde cholangiopancreatography pancreatitis: a preliminary, single-center, randomized controlled trial. J Hepatobiliary Pancreat Surg. 2007;14:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Ito K, Fujita N, Noda Y, Kobayashi G, Obana T, Horaguchi J, Takasawa O, Koshita S, Kanno Y, Ogawa T. Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J Gastroenterol. 2010;45:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Pan XP, Dang T, Meng XM, Xue KC, Chang ZH, Zhang YP. Clinical study on the prevention of post-ERCP pancreatitis by pancreatic duct stenting. Cell Biochem Biophys. 2011;61:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Sofuni A, Maguchi H, Mukai T, Kawakami H, Irisawa A, Kubota K, Okaniwa S, Kikuyama M, Kutsumi H, Hanada K. Endoscopic pancreatic duct stents reduce the incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients. Clin Gastroenterol Hepatol. 2011;9:851-8; quiz e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Cha SW, Leung WD, Lehman GA, Watkins JL, McHenry L, Fogel EL, Sherman S. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc. 2013;77:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Elmunzer BJ, Serrano J, Chak A, Edmundowicz SA, Papachristou GI, Scheiman JM, Singh VK, Varadurajulu S, Vargo JJ, Willingham FF, Baron TH, Coté GA, Romagnuolo J, Wood-Williams A, Depue EK, Spitzer RL, Spino C, Foster LD, Durkalski V; SVI study group and the United States Cooperative for Outcomes Research in Endoscopy (USCORE). Rectal indomethacin alone versus indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials. 2016;17:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Li GD, Jia XY, Dong HY, Pang QP, Zhai HL, Zhang XJ, Guo R, Dong YC, Qin CY. Pancreatic Stent or Rectal Indomethacin-Which Better Prevents Post-ERCP Pancreatitis: A Propensity Score Matching Analysis. Medicine (Baltimore). 2016;95:e2994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Yin HK, Wu HE, Li QX, Wang W, Ou WL, Xia HH. Pancreatic Stenting Reduces Post-ERCP Pancreatitis and Biliary Sepsis in High-Risk Patients: A Randomized, Controlled Study. Gastroenterol Res Pract. 2016;2016:9687052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Olsson G, Lübbe J, Arnelo U, Jonas E, Törnqvist B, Lundell L, Enochsson L. The impact of prophylactic pancreatic stenting on post-ERCP pancreatitis: A nationwide, register-based study. United European Gastroenterol J. 2017;5:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Zolotarevsky E, Fehmi SM, Anderson MA, Schoenfeld PS, Elmunzer BJ, Kwon RS, Piraka CR, Wamsteker EJ, Scheiman JM, Korsnes SJ. Prophylactic 5-Fr pancreatic duct stents are superior to 3-Fr stents: a randomized controlled trial. Endoscopy. 2011;43:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Pahk A, Rigaux J, Poreddy V, Smith J, Al-Kawas F. Prophylactic pancreatic stents: does size matter? A comparison of 4-Fr and 5-Fr stents in reference to post-ERCP pancreatitis and migration rate. Dig Dis Sci. 2011;56:3058-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Fujisawa T, Kagawa K, Ochiai K, Hisatomi K, Kubota K, Sato H, Nakajima A, Matsuhashi N. Prophylactic Efficacy of 3- or 5-cm Pancreatic Stents for Preventing Post-ERCP Pancreatitis: A Prospective, Randomized Trial. J Clin Gastroenterol. 2016;50:e30-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 2036] [Article Influence: 59.9] [Reference Citation Analysis (1)] |
| 28. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13300] [Article Influence: 1108.3] [Reference Citation Analysis (0)] |
| 29. | Lawrence C, Cotton PB, Romagnuolo J, Payne KM, Rawls E, Hawes RH. Small prophylactic pancreatic duct stents: an assessment of spontaneous passage and stent-induced ductal abnormalities. Endoscopy. 2007;39:1082-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Kingsnorth A. Role of cytokines and their inhibitors in acute pancreatitis. Gut. 1997;40:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Chahal P, Tarnasky PR, Petersen BT, Topazian MD, Levy MJ, Gostout CJ, Baron TH. Short 5Fr vs long 3Fr pancreatic stents in patients at risk for post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2009;7:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |