Published online Jun 7, 2018. doi: 10.3748/wjg.v24.i21.2269
Peer-review started: February 12, 2018
First decision: February 23, 2018
Revised: February 27, 2018
Accepted: March 18, 2018
Article in press: March 18, 2018
Published online: June 7, 2018
Processing time: 111 Days and 23.4 Hours
To investigate the value of multiparameter joint analysis in the early diagnosis of gastric cancer (GC) in clinical practice.
Concentrations of CEA, CA724 and three kinds of cytokines (TNF-α, IL-6 and IL-8) in 176 GC patients, 117 atypical hyperplasia patients, and 204 healthy control individuals were used for building the diagnostic model, then 58 GC patients, 41 atypical hyperplasia patients, and 66 healthy control individuals were enrolled independently. The joints of the indicators were analyzed by binary logistic regression analysis method.
For discriminating the healthy control group and the GC group, IL-6 had the best diagnostic value, and the area under curve (AUC) of joint analysis was 0.95 (0.93-0.97). For the early stage and advanced stage GC, the AUC were 0.95 (0.92-0.98) and 0.95 (0.92-0.97). For discriminating the atypical hyperplasia group and GC group, CA724 had the best diagnostic value, and the AUC of joint analysis was 0.97 (0.95-0.99). For the early stage and advanced stage GC groups, the AUC were 0.98 (0.96-0.99) and 0.96 (0.94-0.98). After evaluation, for discriminating the GC, early stage GC and advanced cancer group from the healthy control group, the diagnostic sensitivity was 89.66%, 84.21% and 92.31%, respectively, and the specificity was 92.42%, 90.91% and 90.91%. For discriminating the GC, early stage GC and advanced cancer groups from the atypical hyperplasia group, the diagnostic sensitivity was 87.93%, 78.95% and 92.31%, respectively, and the specificity was 87.80%, 85.37% and 90.24%.
We have built a diagnostic model including CEA, CA724, IL-6, IL-8, and TNF-α. It may provide potential assistance as a screening method for the early detection of GC.
Core tip: We aimed to use multiparameter joint analysis for improving sensitivity and specificity for detection of gastric cancer. By combining CEA, CA724, IL-6, IL-8 and TNF-α, we built a diagnostic model, which may provide potential assistance as a screening method for the early detection of gastric cancer.
- Citation: Li J, Xu L, Run ZC, Feng W, Liu W, Zhang PJ, Li Z. Multiple cytokine profiling in serum for early detection of gastric cancer. World J Gastroenterol 2018; 24(21): 2269-2278
- URL: https://www.wjgnet.com/1007-9327/full/v24/i21/2269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i21.2269
Gastric cancer is a kind of malignant tumor derived from gastric mucosal epithelial cells[1-3]. It is the fourth most common malignancy worldwide, and it ranks second in terms of the number of deaths[4]. In China, gastric cancer is one of the most malignant tumors, with high morbidity and mortality[5]. Deaths from gastric cancer account for approximately 25% to 30% of the deaths from all cancer types[6]. Its pathogenesis involves aging of the body, eating habits and psychological factors[7-9]. In recent years, more stress, poor diet and overwork have been shown to have greater influence on the incidence of gastric cancer. The occurrence and development of gastric cancer is a multistep process[10]. In current clinical practice, the main treatment for gastric cancer is surgery. The 5-year survival rate is very low[11]; however, if the gastric cancer is detected at an early stage, the 5-year survival rate can be as high as 90%[12]. Early diagnosis and treatment of gastric cancer is extremely important for gastric cancer patients.
At present, many methods of diagnosing gastric cancer are used in scientific research and clinical practice[13]. Serologic biomarkers are important detection methods. In early gastric cancer, the tumor markers [such as carcinoembryonic antigen (CEA) and cancer antigen (CA)724] are increased to some extent in the blood. The levels of these markers have been used as important indicators in gastric cancer screening, early diagnosis and prognosis evaluation[14]. However, no specific tumor marker has been found at present. Diagnosis based on a single tumor marker has some limitations[15]. The detection rate of gastric cancer is still very low.
Cytokines are small molecules secreted by cells in response to various stimuli, and they are involved in biological processes, through their binding to specific receptors on target cells[16]. Many studies have demonstrated that cytokine production and cellular immune function are important regulatory factors in the development of tumors[17-19]. As multifunctional molecules, these inflammatory factors not only directly damage tumor cells but also act as important mediators in the killing of tumor cells by mononuclear cells. The relationship of cytokines and gastric cancer provides a new direction for exploring the pathological mechanism of gastric cancer and may also provide a potential means of diagnosing and treating gastric cancer in the clinical setting.
Studies have confirmed that patients with cancer usually have defects in their immune function, especially having cellular immune dysfunction. TNF-α, IL-6 and IL-8 are important mediators of the inflammatory response and a series of other pathophysiological processes in vivo[20-22]. Their value in the diagnosis of gastric cancer has been evaluated, although their diagnostic value in combination with conventional biomarkers, such as CEA and CA724, has not been studied.
In this study, we first evaluated the diagnostic value of CEA, CA724 and three cytokines (TNF-α, IL-6 and IL-8) for gastric cancer. Then, we analyzed the combinations of the conventional biomarkers with the cytokines by using binary logistic regression. Our aim was to use the multiparameter joint analysis to improve diagnostic sensitivity and specificity and to provide a novel potential method for the early diagnosis of gastric cancer in clinical practice.
Written consent was obtained. The study was reviewed and approved by the Institutional Review Board of the Affiliated Tumor Hospital of Zhengzhou University. This study was conducted from January 2015 to December 2016. There were 176 gastric cancer patients enrolled in our study (63 early-stage and 113 advanced-stage patients). The stages were confirmed by pathological examination. All the gastric cancer patients were enrolled before surgery, chemotherapy, radiotherapy and immunotherapy. In addition, 117 atypical hyperplasia patients were enrolled. The examination results were confirmed by gastroscopy and pathological examination.
Finally, 204 healthy control individuals were also enrolled. The healthy controls were without obvious disease, and the results of the basic tests were checked by B-mode ultrasound and CT examination, including of the heart, brain, kidney and other important organs. After building the diagnostic model, 58 gastric cancer patients (19 early-stage and 39 advanced-stage patients), 41 atypical hyperplasia patients, and 66 healthy control individuals were independently enrolled.
After the collection of whole blood samples, the tubes were centrifuged for 7 min at 3500 r/min and immediately stored at -80 °C. The CEA and CA724 levels were detected by the Roche Modular E170 automatic electrochemiluminescence immunoassay analyzer. The reagents, standards and controls were purchased from Roche. The serum levels of IL-6, IL-8 and TNF-α were detected by Luminex 200, and the detection kits were purchased from Millipore.
Serum samples from the cancer group and the control group were stored in a freezer at -80 °C. Before performing the experiment, the serum samples were thawed, and 100 μL of serum was transferred from each sample to centrifuge tubes. The reagents were allowed to equilibrate to room temperature at 25 °C, and wash buffer was diluted 10 times with deionized water.
The serum concentrations of IL-6, IL-8 and TNF-α were determined by the following protocol. First, 200 μL of assay buffer was added to each reaction well in a 96-well plate. After sealing, the solution was mixed thoroughly on a horizontal shaker, and the assay buffer was vacuumed and then blotted on the bottom of the plate. Second, 25 μL of each standard or control was added to the appropriate wells, and 25 μL of assay buffer was also added to each well, followed by the addition of 25 μL of serum matrix diluent to the standard and control wells. Third, after mixing the microspheres well, 25 μL of hybrid microspheres were added to each well, and the plate was covered with sealing film and foil, before incubation overnight at 4 °C on a horizontal shaker. Fourth, after washing, 25 μL of the detection antibody was added to each well and incubated for 1 h at room temperature. Then, 25 μL of streptavidin-PE was added to each well and incubated for 30 min at room temperature. Fifth, after washing, the 96-well plate was placed in the Luminex reading instrument, and the levels were calculated according to the standard curve.
SPSS 21.0 statistical software was used to analyze the data. The serum levels of CEA, CA724, IL-6, IL-8 and TNF-α in the different groups were compared by one-way ANOVA. The diagnostic value was evaluated by the area under the curve (AUC) of the receiver operator characteristic (ROC) curve, and the cutoff value was determined by the Youden index. The combinations of the indicators were analyzed by the binary logistic regression analysis method[23]. P < 0.05 indicated statistical significance.
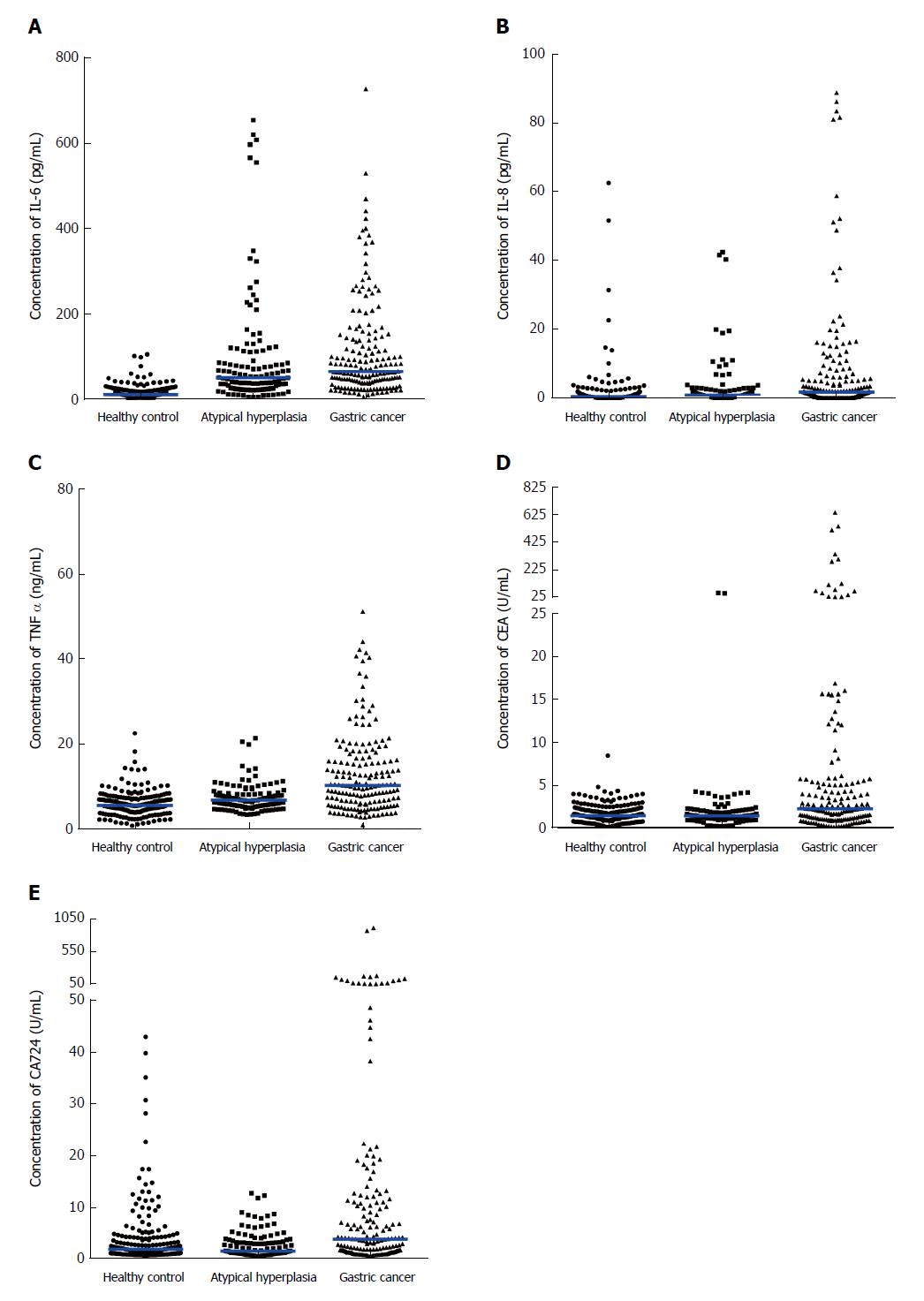
As shown in Figure 1, the concentrations of CEA, CA724, IL-6, IL-8 and TNF-α in the healthy control group, the atypical hyperplasia group and the gastric cancer group were compared. As shown in Figure 1A, the concentrations of IL-6 in the healthy control, atypical hyperplasia and gastric cancer groups were 10.05 (6.47, 18.26), 50.17 (23.93, 110.40) and 63.96 (38.93, 139.10), respectively. The concentrations of IL-8 were 0.48 (0.07, 1.17), 0.85 (0.33, 2.44) and 1.80 (0.11, 6.28), respectively (Figure 1B). The concentrations of TNF-α were 5.49 (4.16, 7.21), 6.73 (5.31, 8.27) and 10.20 (5.88, 16.41), respectively (Figure 1C). The concentrations of CEA were 1.53 (0.91, 2.26), 1.51 (1.15, 2.05) and 2.35 (1.12, 5.22), respectively (Figure 1D). The concentrations of CA724 were 2.02 (1.15, 4.30), 2.21 (1.02, 3.41) and 4.03 (1.52, 11.62), respectively (Figure 1E). The concentrations of IL-6, IL-8, TNF-α, CEA and CA724 in the atypical hyperplasia group and gastric cancer group were significantly different from those in the healthy control group. The concentrations of IL-6, IL-8, TNF-α and CA724 in the gastric cancer group were significantly different compared to those of the atypical hyperplasia group.
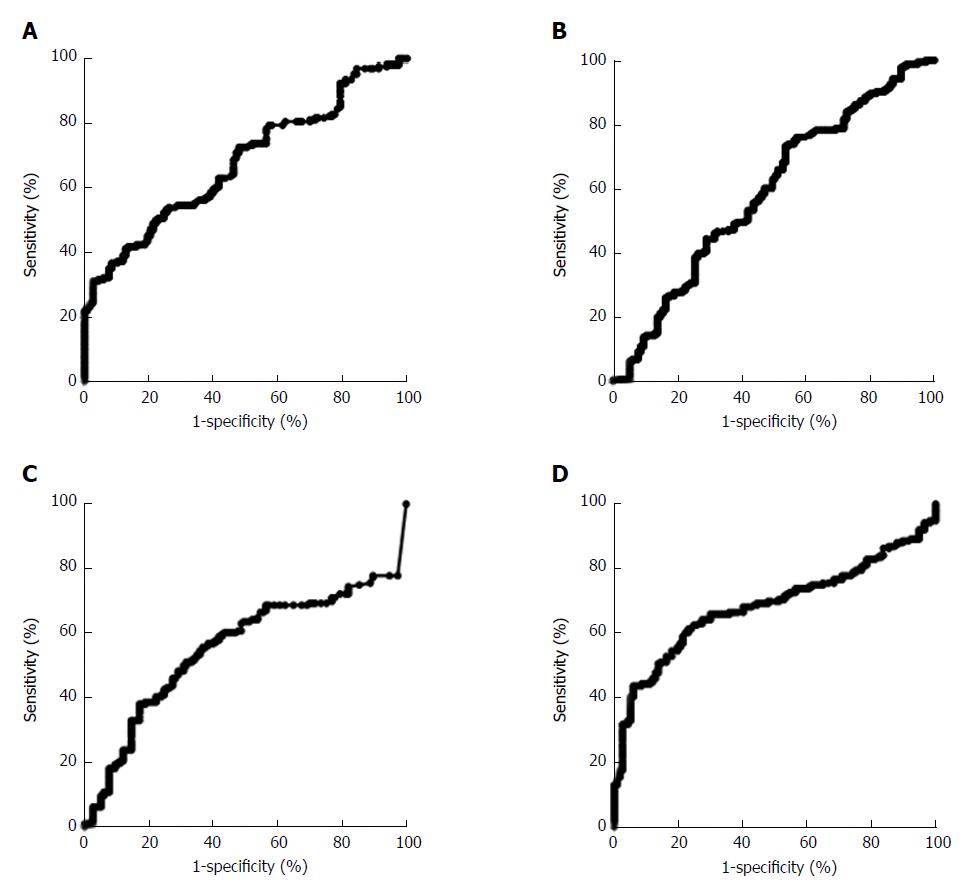
As shown in Table 1, when the concentrations of CEA, CA724, IL-6, IL-8 and TNF-α were used alone to discriminate between the healthy control group and the gastric cancer group, the AUCs of the five indicators ranged from 0.64 to 0.93. The concentration of IL-6 had the best diagnostic value for discriminating between the healthy control group and the gastric cancer group. When the cutoff value was 20.31 pg/mL, the sensitivity and specificity were 92.05% and 78.92%, respectively. For the two conventional biomarkers, CEA and CA724, the AUCs were 0.65 (0.60-0.71) and 0.64 (0.58-0.70), respectively. To discriminate between the atypical hyperplasia group and the gastric cancer group, as shown in Figure 2A, the conventional biomarker CA724 had the best diagnostic value, with an AUC of 0.68 (0.62-0.74). When the cutoff value was 9.13 U/mL, the sensitivity and specificity were 31.25% and 97.44%, respectively. The three cytokines, IL-6, IL-8 and TNF-α, showed poorer diagnostic values, and their AUCs were 0.59 (0.52-0.66), 0.55 (0.49-0.63) and 0.68 (0.62-0.74), respectively (Figure 2B-D).
| Indicator | AUC | 95%CI of AUC | Cutoff value | Sensitivity, % | Specificity, % |
| IL-6 | 0.92 | 0.91-0.94 | 20.31 | 92.05 | 78.92 |
| IL-8 | 0.65 | 0.60-0.71 | 1.45 | 55.68 | 79.41 |
| TNF-α | 0.76 | 0.71-0.81 | 7.82 | 65.91 | 82.84 |
| CEA | 0.65 | 0.60-0.71 | 3.45 | 36.36 | 92.65 |
| CA724 | 0.64 | 0.58-0.70 | 5.80 | 40.91 | 84.34 |
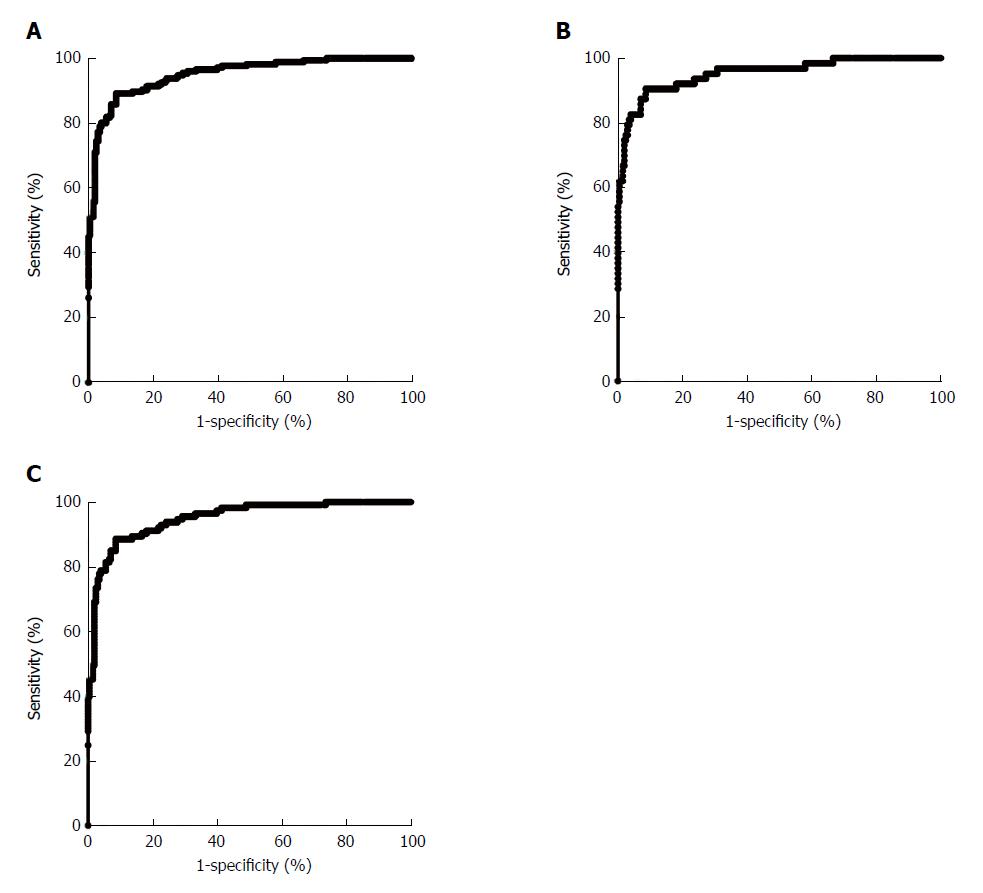
After evaluating the diagnostic value of the concentrations of CEA, CA724, IL-6, IL-8 and TNF-α separately, binary logistic regression was used to analyze the indicators jointly. As shown in Figure 3A, for discriminating between the healthy control group and the gastric cancer group, the AUC was 0.95 (0.93- 0.97). For early-stage gastric cancer, the AUC was 0.95 (0.92- 0.98), and for advanced-stage gastric cancer, it was 0.95 (0.92- 0.97), as shown in Figure 3B and 3C. For discriminating between the healthy control group and the gastric cancer group, our joint analysis method showed similar diagnostic values for early-stage and advanced-stage gastric cancer.
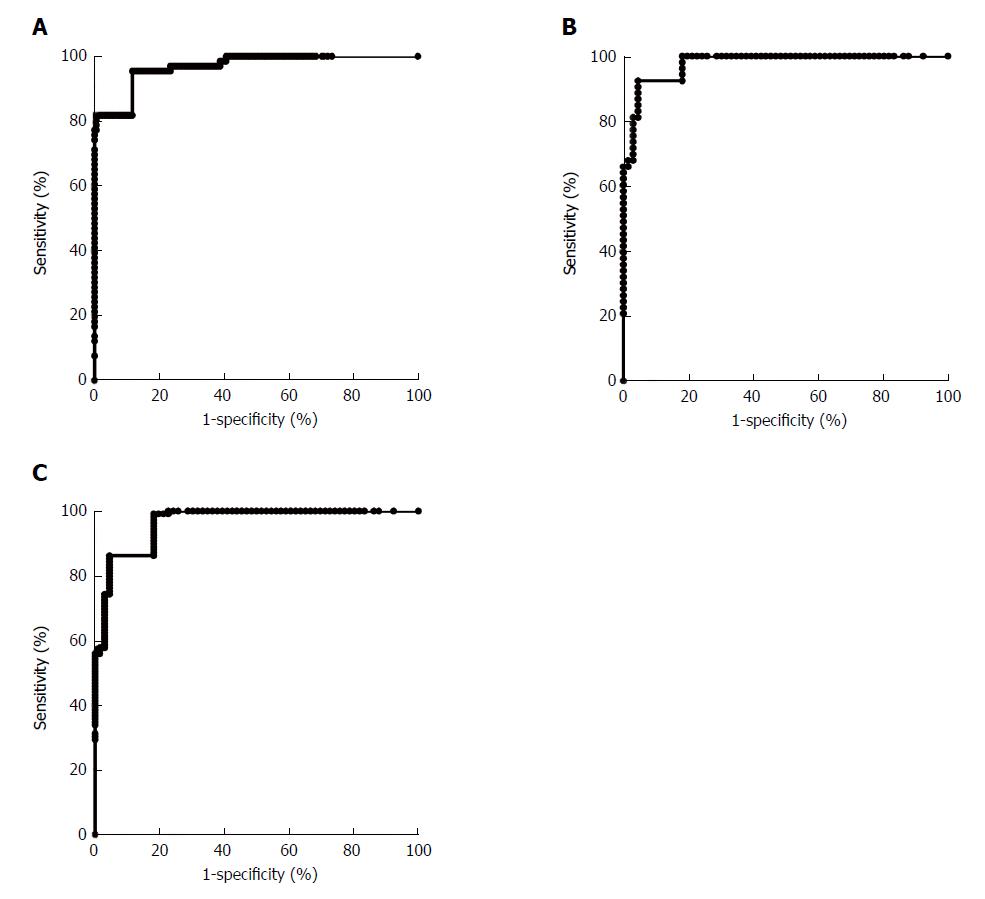
For discriminating between the atypical hyperplasia group and the gastric cancer group, four indicators, namely CA724, IL-6, IL-8 and TNF-α, were used in the joint analysis. As shown in Figure 4A, for discriminating between the atypical hyperplasia group and the gastric cancer group, the AUC was 0.97 (0.95-0.99). For early-stage gastric cancer, the AUC was 0.98 (0.96-0.99), and for advanced-stage gastric cancer, it was 0.96 (0.94-0.98), as shown in Figure 4B and 4C. For discriminating between the atypical hyperplasia group and the gastric cancer group, our joint analysis method also showed similar diagnostic values for early-stage and advanced-stage gastric cancer.
After building the diagnostic model, 58 gastric cancer patients (19 early-stage and 39 advanced-stage patients), 41 atypical hyperplasia patients, and 66 healthy control individuals were independently enrolled. Then, the diagnostic model including CEA, CA724, IL-6, IL-8 and TNF-α for discriminating between the healthy control group and the gastric cancer group and the diagnostic model including CA724, IL-6, IL-8 and TNF-α for discriminating between the atypical hyperplasia group and the gastric cancer group were evaluated. After evaluation, for discriminating between the healthy control group and the gastric cancer group, the early-stage gastric cancer group and the advanced-stage gastric cancer group, the diagnostic sensitivities were 89.66%, 84.21% and 92.31%, respectively. In addition, the specificities were 92.42%, 90.91% and 90.91%, respectively. For discriminating between the atypical hyperplasia group and the gastric cancer group, the early-stage gastric cancer group and the advanced-stage gastric cancer group, the diagnostic sensitivities were 87.93%, 78.95% and 92.31%, respectively. In addition, the specificities were 87.80%, 85.37% and 90.24%, respectively.
According to the estimates of the World Health Organization, nearly 7 million people die from tumors each year worldwide, and that number is increasing annually. Gastric cancer is one of the common malignant tumors that endanger human health. It causes the second highest number of cancer-related deaths. The occurrence and development of gastric cancer is a multistage process, involving multiple gene and molecular level changes. In the pregastric cancer stage there are precancerous lesions, most of which remain unchanged and a small part of which develop into cancer.
The Correa cascade is the most commonly recognized pattern of gastric carcinogenesis[24]. Because most gastrointestinal cancer has no obvious symptoms in the early stage, it cannot be detected in a timely manner; however, when clinical symptoms develop, it is often too late to effectively treat the cancer, resulting in low postoperative survival rates of patients with malignant tumors. Early detection is the key to improving the survival rate of patients and the cure rate[12]. Therefore, early detection of gastric cancer is crucial to the improvement of the treatment of gastric cancer.
CEA is a cell surface antigen. It is a tumor-associated antigen extracted from embryonic tissue and can be detected in a variety of body fluids. As one of the most common tumor markers, it is widely used as a diagnostic and monitoring index for various gastrointestinal tumors, especially gastric adenocarcinoma[25]. CA724 is a high molecular weight glycoprotein, and it is one of the best tumor markers for the diagnosis of gastric cancer. It has high specificity for gastric cancer and has good applicability in digestive system malignant tumors[26]. The results of our study showed that the serum levels of CEA and CA724 in the gastric cancer group were significantly higher than those in the atypical hyperplasia and healthy control groups. The results were consistent with those of previous studies[27,28] and indicated that these markers have certain diagnostic value for gastric cancer.
The inflammation in cancer is a multifactorial process. Phagocytes are effector cells that initiate inflammation. They can use a variety of surface receptors to identify invading foreign microorganisms that they finally kill. In this process, activated phagocytes secrete a large number of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α. The expression levels of these pro-inflammatory cytokines are significantly increased in inflammatory diseases.
As a very important immunosuppressive regulator, IL-8 is a cytokine secreted by fibroblasts, epithelial cells and mononuclear macrophages, and it plays an important role in the growth, differentiation or gene expression of many kinds of cells[29]. In gastric cancer patients, the expression levels of IL-8 are higher in the tumor tissue, serum and malignant effusion of the thoracic and abdominal cavity but lower in normal tissues and serum. In addition, IL-8 also plays an important role in the angiogenesis of gastric tumors. It can act on vascular endothelial cells, inducing large-scale proliferation of endothelial cells to promote angiogenesis[30]. In our experiment, the levels of IL-8 in patients with gastric diseases (gastric cancer group and atypical hyperplasia group) were significantly higher than that in the healthy control group. The results showed that IL-8 was highly expressed in patients with gastric cancer and gastric inflammatory diseases, which was consistent with the results of previous studies.
IL-6 has been demonstrated to play a role in tumor metastasis and tumor angiogenesis[31]. The IL-6 gene is active in many tumor tissues and peripheral blood vessels, and the secretion of various cytokines is increased. Numerous studies have demonstrated that it not only directly stimulates monocyte-derived macrophages and fibroblasts to secrete IL-6 but also that cancer cells can secrete a large amount of IL-1α to promote the proliferation of malignant cells in their own growth process[32]. The imbalance of IL-6 and its receptor affects the stability of the whole environment and leads to disordered immune function, which may induce tumors[33]. In our study, the level of IL-6 was significantly higher in gastric cancer patients than in atypical hyperplasia patients. Previous studies also found that tumors were associated with abnormal expression of IL-6.
TNF-α is a multifunctional cytokine produced by macrophages and activated T cells. It is involved in inducing an acute albumin reaction, activating neutrophils and lymphocytes, regulating the metabolic activity of tissues and promoting the release of other cytokines[11]. Studies have shown that TNF-α can kill a variety of tumor cells and enhance the body’s anti-tumor action, but it can also promote the growth and metastasis of some tumors. It can cause tumor tissue hypoxia and vascular damage around the tumor, promoting the cytotoxic effect of natural killer cells and macrophages and enhancing the body’s immunity, thereby inhibiting tumor growth[34]. When the level of TNF-α is abnormal, the patient’s immune system is disordered, which triggers systemic cytotoxicity, resulting in escape of the tumor cells from host immune surveillance and allowing them to continue to grow[35]. In our study, the levels of TNF-α in the gastric cancer and atypical hyperplasia groups were significantly higher than in the healthy control group, suggesting that TNF-α may be closely related to the occurrence and development of gastric cancer. As an important regulator of inflammation, TNF-α may play a role in tumor-associated inflammatory processes, increasing the risk of inflammation-induced tumors. Our results were consistent with those of previous studies.
Although we have built a potential diagnostic model for the early detection of gastric cancer, there were still some limitations to our study. First, there were only three investigated in our study, and many other kinds of cytokines were excluded. Second, the Luminex 200 detection system may be too sensitive, resulting in a high degree of variance, which may have affected the results of our study. Third, the sample size of our study was relatively small, and the diagnostic model validation was only performed in a small cohort.
In conclusion, we have built a diagnostic model including the levels of CEA, CA724, IL-6, IL-8 and TNF-α. It may provide a potential screening method for the early detection of gastric cancer.
Early diagnosis and treatment of gastric cancer (GC) is extremely important for GC; however, there is still no effective detection method for the early detection of GC.
Many studies have demonstrated that the joint analysis of a panel of indicators may improve the diagnostic value for kinds of cancers. Cytokines have also been demonstrated to play important roles in the development of cancer.
Concentrations of carcinoembryonic antigen (CEA), cancer antigen (CA)724, TNF-α, IL-6 and IL-8 in 176 GC patients, 117 atypical hyperplasia patients and 204 healthy controls were used for building the model; then, 58 GC patients, 41 atypical hyperplasia patients and 66 healthy controls were used for validation. The joints of the indicators were analyzed by binary logistic regression analysis method.
For discriminating the GC, early-stage GC and advanced cancer patients from the healthy control group, the diagnostic sensitivity was 89.66%, 84.21% and 92.31%, respectively. The specificity was 92.42%, 90.91% and 90.91%, respectively. For discriminating the GC, early stage GC and advanced cancer patients from the atypical hyperplasia group, the diagnostic sensitivity was 87.93%, 78.95% and 92.31%, respectively. The specificity was 87.80%, 85.37% and 90.24%, respectively.
We have built a diagnostic model including CEA, CA724, IL-6, IL-8 and TNF-α, and it may represent a potential assistant screening method for the early detection of GC.
Our study provides a simple, effective and noninvasive detection method for the assistant detection of GC. In the future study, multicenter and larger sample size design should be included to validate the diagnostic value.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Goldaracena N, Snowdon VK, Takamatsu S S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 2. | Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20:13842-13862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 247] [Cited by in RCA: 303] [Article Influence: 27.5] [Reference Citation Analysis (2)] |
| 3. | Spence AD, Cardwell CR, McMenamin ÚC, Hicks BM, Johnston BT, Murray LJ, Coleman HG. Adenocarcinoma risk in gastric atrophy and intestinal metaplasia: a systematic review. BMC Gastroenterol. 2017;17:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9:5-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 185] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 5. | Ning FL, Zhang CD, Wang P, Shao S, Dai DQ. Endoscopic resection versus radical gastrectomy for early gastric cancer in Asia: A meta-analysis. Int J Surg. 2017;48:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Zheng Q, Chen C, Guan H, Kang W, Yu C. Prognostic role of microRNAs in human gastrointestinal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:46611-46623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Maleki SS, Röcken C. Chromosomal Instability in Gastric Cancer Biology. Neoplasia. 2017;19:412-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Kalisperati P, Spanou E, Pateras IS, Korkolopoulou P, Varvarigou A, Karavokyros I, Gorgoulis VG, Vlachoyiannopoulos PG, Sougioultzis S. Inflammation, DNA Damage, Helicobacter pylori and Gastric Tumorigenesis. Front Genet. 2017;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Sunakawa Y, Lenz HJ. Molecular classification of gastric adenocarcinoma: translating new insights from the cancer genome atlas research network. Curr Treat Options Oncol. 2015;16:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Akhavan-Niaki H, Samadani AA. Molecular insight in gastric cancer induction: an overview of cancer stemness genes. Cell Biochem Biophys. 2014;68:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Ahn S, Park DY. Practical Points in Gastric Pathology. Arch Pathol Lab Med. 2016;140:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Beeharry MK, Liu WT, Yan M, Zhu ZG. New blood markers detection technology: A leap in the diagnosis of gastric cancer. World J Gastroenterol. 2016;22:1202-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Uedo N, Yao K. Endoluminal Diagnosis of Early Gastric Cancer and Its Precursors: Bridging the Gap Between Endoscopy and Pathology. Adv Exp Med Biol. 2016;908:293-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 267] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, Qu H, Sun G, Li Z, Ma S, Shi Z, Zhao E, Zhang H, He Q. Early postoperative tumor marker responses provide a robust prognostic indicator for N3 stage gastric cancer. Medicine (Baltimore). 2017;96:e7560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Bagheri V, Memar B, Momtazi AA, Sahebkar A, Gholamin M, Abbaszadegan MR. Cytokine networks and their association with Helicobacter pylori infection in gastric carcinoma. J Cell Physiol. 2018;233:2791-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Bockerstett KA, DiPaolo RJ. Regulation of Gastric Carcinogenesis by Inflammatory Cytokines. Cell Mol Gastroenterol Hepatol. 2017;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Jäkel CE, Vogt A, Gonzalez-Carmona MA, Schmidt-Wolf IG. Clinical studies applying cytokine-induced killer cells for the treatment of gastrointestinal tumors. J Immunol Res. 2014;2014:897214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Tye H, Jenkins BJ. Tying the knot between cytokine and toll-like receptor signaling in gastrointestinal tract cancers. Cancer Sci. 2013;104:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Zhang JZ, Liu CM, Peng HP, Zhang Y. Association of genetic variations in IL-6/IL-6R pathway genes with gastric cancer risk in a Chinese population. Gene. 2017;623:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Zhou F, Cheng L, Qiu LX, Wang MY, Li J, Sun MH, Yang YJ, Wang JC, Jin L, Wang YN. Associations of potentially functional variants in IL-6, JAKs and STAT3 with gastric cancer risk in an eastern Chinese population. Oncotarget. 2016;7:28112-28123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Shi J, Li YJ, Yan B, Wei PK. Interleukin-8: A potent promoter of human lymphatic endothelial cell growth in gastric cancer. Oncol Rep. 2015;33:2703-2710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer. 2014;134:2646-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Futawatari N, Fukuyama T, Yamamura R, Shida A, Takahashi Y, Nishi Y, Ichiki Y, Kobayashi N, Yamazaki H, Watanabe M. Early gastric cancer frequently has high expression of KK-LC-1, a cancer-testis antigen. World J Gastroenterol. 2017;23:8200-8206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Shafaghi A, Mansour- Ghanaei F, Joukar F, Nabavi F, Mansour- Ghanaei A, Esrafilian Soltani A. Stage Association of Preoperative Serum Carcinoembryonic Antigen with Gastric Adenocarcinoma in Iranian Patients. Asian Pac J Cancer Prev. 2017;18:2669-2672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Zou L, Qian J. Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma. Chin J Cancer Res. 2014;26:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 27. | Chen XZ, Zhang WK, Yang K, Wang LL, Liu J, Wang L, Hu JK, Zhang B, Chen ZX, Chen JP. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep. 2012;39:9031-9039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Chen XZ, Zhang WH, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Quantitative comparisons of summary receiver operating characteristics (sROC) curves among conventional serological tumor biomarkers for predicting gastric cancer in Chinese population. Tumour Biol. 2014;35:9015-9022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | van Harten-Gerritsen AS, Balvers MG, Witkamp RF, Kampman E, van Duijnhoven FJ. Vitamin D, Inflammation, and Colorectal Cancer Progression: A Review of Mechanistic Studies and Future Directions for Epidemiological Studies. Cancer Epidemiol Biomarkers Prev. 2015;24:1820-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Lee KE, Khoi PN, Xia Y, Park JS, Joo YE, Kim KK, Choi SY, Jung YD. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol. 2013;19:8192-8202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 31. | Wu CW, Wang SR, Chao MF, Wu TC, Lui WY, P’eng FK, Chi CW. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996;91:1417-1422. [PubMed] |
| 32. | Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, Moverley J, Grail D, Jenkins BJ, Ernst M, Giraud AS. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology. 2004;126:196-207. [PubMed] |
| 33. | Lippitz BE, Harris RA. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology. 2016;5:e1093722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 34. | Buell JF, Reed E, Lee KB, Parker RJ, Venzon DJ, Amikura K, Arnold S, Fraker DL, Alexander HR. Synergistic effect and possible mechanisms of tumor necrosis factor and cisplatin cytotoxicity under moderate hyperthermia against gastric cancer cells. Ann Surg Oncol. 1997;4:141-148. [PubMed] |
| 35. | Jiménez FP, Estévez MP. [Role of cytokines in chronic gastritis by Helicobacter pylori]. Acta Gastroenterol Latinoam. 2001;31:137-141. [PubMed] |