Published online Jan 14, 2018. doi: 10.3748/wjg.v24.i2.170
Peer-review started: October 10, 2017
First decision: October 30, 2017
Revised: November 15, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: January 14, 2018
Processing time: 96 Days and 21.9 Hours
To study the molecular effects of three different D-vitamins, vitamin D2, vitamin D3 and calcipotriol, in pancreatic stellate cells (PSCs).
Quiescent PSCs were isolated from mouse pancreas and activated in vitro by seeding on plastic surfaces. The cells were exposed to D-vitamins as primary cultures (early-activated PSCs) and upon re-culturing (fully-activated cells). Exhibition of vitamin A-containing lipid droplets was visualized by oil-red staining. Expression of α-smooth muscle actin (α-SMA), a marker of PSC activation, was monitored by immunofluorescence and immunoblot analysis. The rate of DNA synthesis was quantified by 5-bromo-2’-deoxyuridine (BrdU) incorporation assays. Real-time PCR was employed to monitor gene expression, and protein levels of interleukin-6 (IL-6) were measured by ELISA. Uptake of proline was determined using 18F-proline.
Sustained culture of originally quiescent PSCs induced cell proliferation, loss of lipid droplets and exhibition of stress fibers, indicating cell activation. When added to PSCs in primary culture, all three D-vitamins diminished expression of α-SMA (to 32%-39% of the level of control cells; P < 0.05) and increased the storage of lipids (scores from 1.97-2.15 on a scale from 0-3; controls: 1.49; P < 0.05). No such effects were observed when Dvitamins were added to fully-activated cells, while incorporation of BrdU remained unaffected under both experimental conditions. Treatment of re-cultured PSCs with Dvitamins was associated with lower expression of IL-6 (-42% to -49%; P < 0.05; also confirmed at the protein level) and increased expression of the vitamin D receptor gene (209%-321% vs controls; P < 0.05). There was no effect of Dvitamins on the expression of transforming growth factor-β1 and collagen type 1 (chain α1). The lowest uptake of proline, a main component of collagen, was observed in calcipotriol-treated PSCs.
The three D-vitamins inhibit, with similar efficiencies, activation of PSCs in vitro, but cannot reverse the phenotype once the cells are fully activated.
Core tip: Modulation of the stroma response by vitamin D has been suggested as a concept to treat chronic pancreatitis and pancreatic cancer. Here we show that three derivatives, vitamin D2, vitamin D3 and calcipotriol, with similar efficiencies prevented pancreatic stellate cell (PSC) activation in vitro. Once the cells were fully activated, vitamin D failed to induce a reversal of the myofibroblastic phenotype, but still exerted antifibrotic effects by diminishing the uptake of proline and secretion of interleukin-6, an autocrine mediator of PSC activation. Our findings encourage further studies on the potential of vitamin D derivatives as antifibrotic drugs.
- Citation: Wallbaum P, Rohde S, Ehlers L, Lange F, Hohn A, Bergner C, Schwarzenböck SM, Krause BJ, Jaster R. Antifibrogenic effects of vitamin D derivatives on mouse pancreatic stellate cells. World J Gastroenterol 2018; 24(2): 170-178
- URL: https://www.wjgnet.com/1007-9327/full/v24/i2/170.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i2.170
Pancreatic stellate cells (PSCs) were identified as the main source of extracellular matrix (ECM) proteins in the diseased pancreas, specifically in the context of cancer and chronic pancreatitis (CP), two decades ago. Since then, cellular interactions of PSCs and extracellular as well as intracellular regulators of PSC function have been studied in great detail (reported by Erkan et al[1]). In the healthy pancreas, PSCs exist in a quiescent state and are phenotypically characterized by the presence of abundant vitamin A-containing lipid droplets in their cytoplasm[2,3]. In response to mitogens (such as platelet-derived growth factor) and profibrogenic mediators (e.g., transforming growth factor-β1), PSCs undergo an activation process that involves cell proliferation and an enhanced synthesis of ECM proteins[4]. The cells also lose their vitamin A storages and exhibit stress fibers containing αsmooth muscle actin (α-SMA) protein. In vitro, contact of PSCs with plastic surfaces induces phenotypic changes that mimic the activation process under in vivo conditions[2,3].
In the past years, it has become clear that PSCs play an active and complex role in the progression of pancreatic cancer (PC), a stroma-rich tumor with the worst prognosis of all common human malignancies[5-8]. Most recent publications in the field have shifted the main focus of attention from a stroma deletion, which even displayed deleterious effects in experimental models of PC[9,10], towards a modulation of the stroma response, with the aim to interrupt tumor-promoting interactions between PSCs and PC cells on one hand while preserving the barrier function of the stroma on the other hand[11]. In this regard, vitamin D and its receptor VDR have gained significant interest as master regulators of PSC function and mediators of a transcriptional reprogramming of the tumor stroma that enable an enhanced chemotherapeutic response[12]. Recently, suppression of cell proliferation and inhibition of ECM synthesis have been proposed as important mechanisms of vitamin D action in PSCs[13]. Nevertheless, to this end the precise molecular actions of vitamin D in stellate cells are not fully understood. Furthermore, relative efficiencies of different vitamin D derivatives have not been studied yet.
Here, we have compared the effects of vitamin D2, vitamin D3 and the synthetic vitamin D3 derivative calcipotriol on primary cultures of originally quiescent PSC as well as on fully-activated re-cultured cells. All D-vitamins inhibited PSC activation, but did not reverse the phenotype once the cells were fully activated. Interleukin-6 (IL-6) was identified as an important target of vitamin D action. Although the three D-vitamins displayed slightly different molecular effects in our studies, their general efficiency was found to be similar.
Quiescent PSCs were isolated from the pancreas of healthy C57BL/6 mice (approximately 3 months old) by collagenase digestion followed by Nycodenz® (Nycomed, Oslo, Norway) density gradient centrifugation[14]. Afterwards, they were resuspended in cryopreservation medium [fetal calf serum (FCS) supplemented with 10% dimethyl sulfoxide) and stored at -150 °C until required as previously described[15]. After thawing, the cells were cultured in Iscove’s modified Dulbecco’s medium supplemented with 17% FCS, 1% non-essential amino acids (dilution of a 100 x stock solution), 105 U/L penicillin and 100 mg/L streptomycin (all reagents from Merck Millipore, Darmstadt, Germany). Approximately on day 3 of primary culture, PSCs started to proliferate and reached subconfluency around day 8. Afterwards, the cells were harvested by trypsinization and re-cultured according to the experimental requirements.
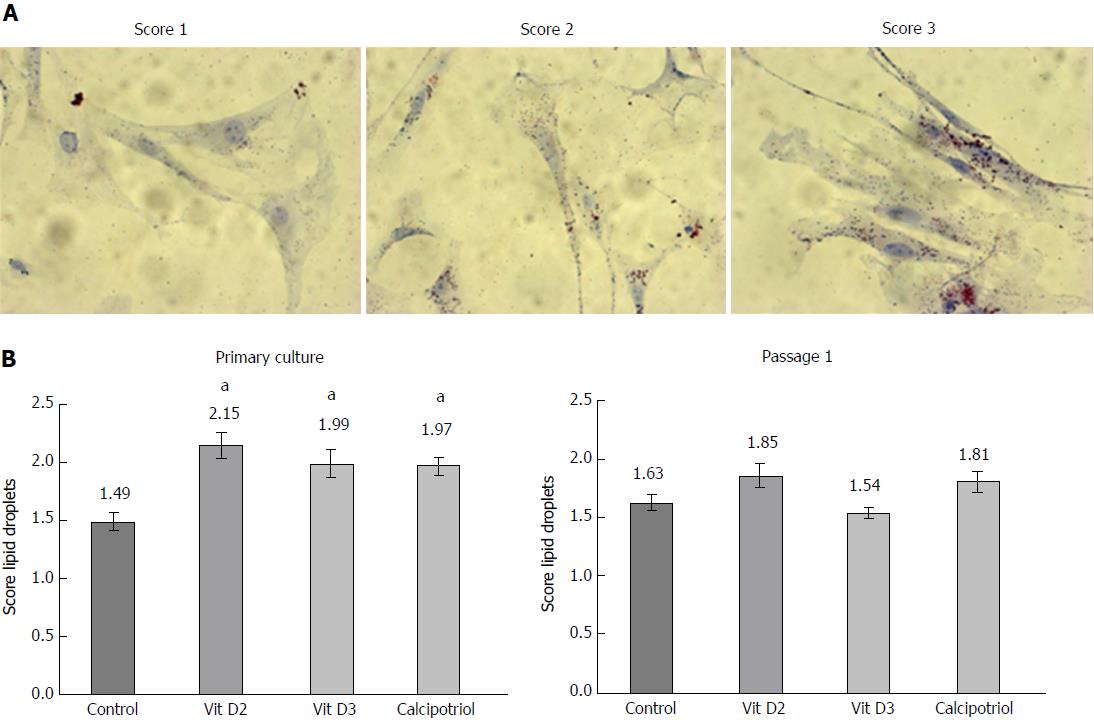
PSCs were grown on glass coverslips as primary cultures or cells of the first passage for the indicated periods of time. Subsequently, they were fixed for 30 min in 2.5% paraformaldehyde, and intracellular fat droplets were visualized by oil red O staining as described before[16]. Briefly, the coverslips were incubated with dye solution [three parts of an oil red O stock solution (1% wt/vol dissolved in isopropanol, mixed with two parts of distilled water)] for 15 min followed by counterstaining with Mayer’s hemalum solution (Merck Millipore). The samples were evaluated by light microscopy and assessed, in a blinded manner, by two independent investigators on a semiquantitative scale from 0 (absence of lipid droplets) to 3 (large and numerous lipid droplets).
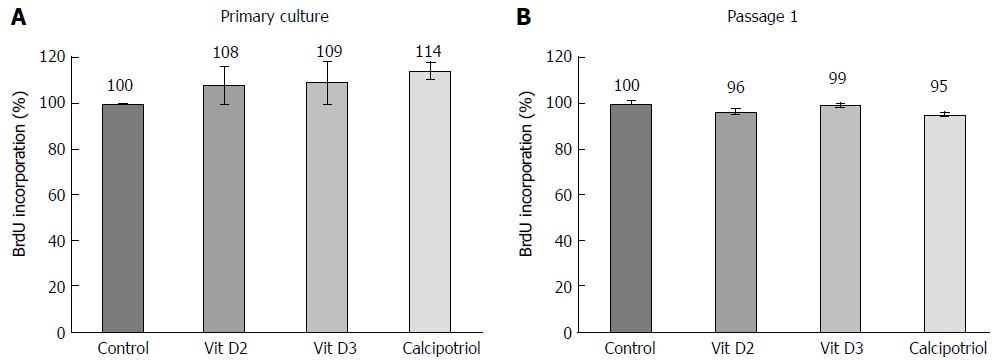
DNA synthesis was quantified employing the 5-bromo-2’-deoxyuridine (BrdU) labelling and detection enzyme-linked immunosorbent assay kit (Roche Diagnostics, Mannheim, Germany). Therefore, quiescent or activated (proliferating) PSCs were plated in 96-well plates at equal seeding densities and allowed to adhere overnight. Afterwards, the cells were exposed to vitamin D2, vitamin D3 (both from Santa Cruz Biotechnologies, Heidelberg, Germany) or calcipotriol (Sigma-Aldrich, Deisenhofen, Germany) for the indicated periods of time. Twenty-four hours prior to cell harvesting, BrdU labelling was initiated by adding labelling solution at a final concentration of 10 μmol/L. Afterwards, labelling was stopped, and BrdU uptake was measured according to the manufacturer’s instructions.
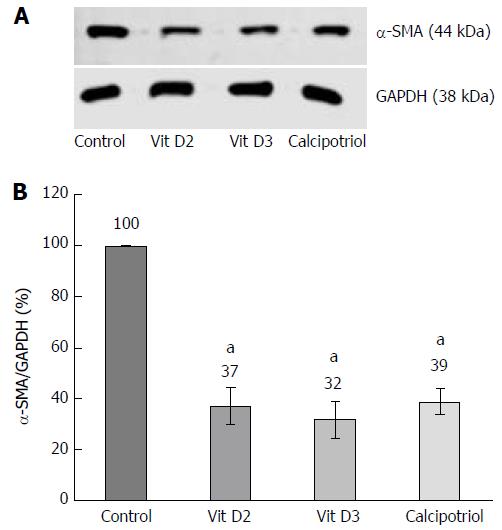
PSCs in primary culture were pretreated as indicated, and protein extracts were prepared and subjected to immunoblot analysis as described before[14], using polyvinylidene fluoride membrane (Merck Millipore) for protein transfer. Primary antibodies were obtained from the following sources: anti-α-SMA; Sigma-Aldrich (#A2547), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH); New England Biolabs, Frankfurt am Main, Germany (#2118). To develop the blots, LI-COR reagents for an Odyssey® Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, United States) were used as described before[17]. Signal intensities were quantified by means of the Image Studio Lite Version 5.2, and normalized to GAPDH by calculating the ratio αSMA/GAPDH.
Immunofluorescence detection of α-SMA
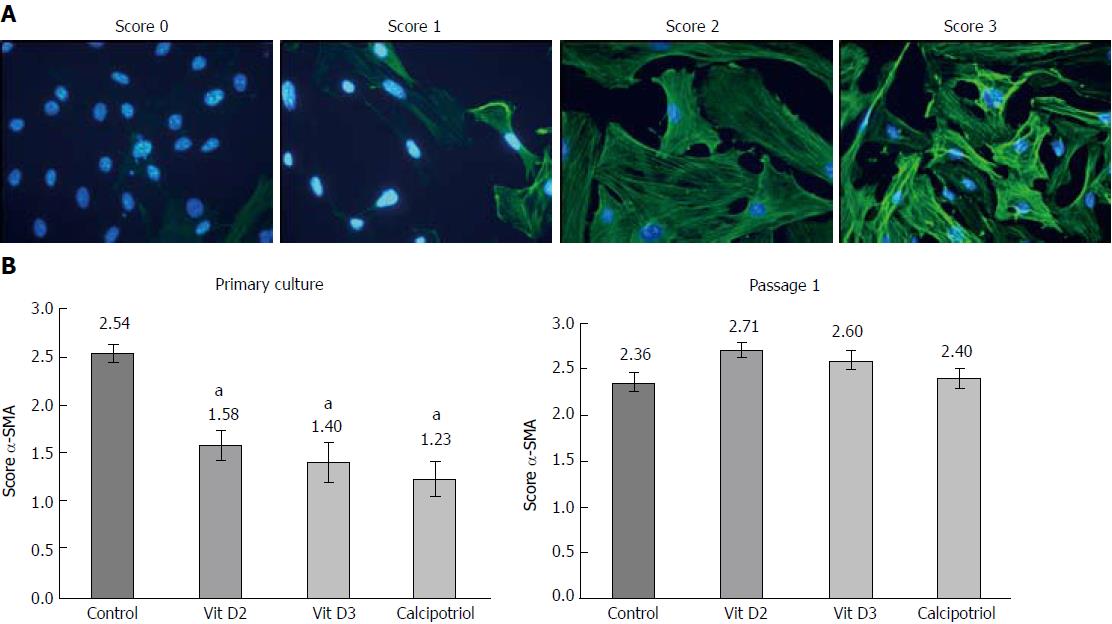
Quiescent PSCs or cells of the first passage were seeded onto glass coverslips and allowed to adhere overnight before they were exposed to D-vitamins at 100 nmol/L. After the indicated periods of time, the cells were fixed with ice-cold methanol, followed by staining of the DNA with 4’,6-diamidino-2-phenylindole. Next, the cells were incubated with a mouse monoclonal antibody to α-SMA (#A2547; Sigma-Aldrich). Antibody binding was determined by a fluorescein-labelled goat anti-mouse IgG (MoBiTec, Göttingen, Germany) and visualized employing a fluorescence microscope (Leica DFC320, Leica Microsystems, Wetzlar, Germany). For further evaluation, αSMA expression in PSCs and organization of the protein in stress fibers were assessed in a semiquantitative manner, using a scoring system as previously established[18]. Therefore, blinded samples were scored by two independent investigators on a scale from 0 (low or undetectable α-SMA expression; absence of stress fibers) to 3 (high expression levels; extensive stress fiber bundles).
PSCs of passage 1 were grown in 12-well plates and treated as indicated, before total RNA was isolated with TriFast reagent (PEQLAB Biotechnologie, Erlangen, Germany). Unless indicated otherwise, reagents from Thermo Fisher Scientific (Karlsruhe, Germany) were used for all subsequent procedures. Traces of genomic DNA were removed employing the DNA-free kit, and 250 ng of RNA per sample was reverse transcribed into cDNA by means of TaqManTM Reverse Transcription Reagents and random priming. Target cDNA levels were quantified by real-time PCR, using a ViiA 7 sequence detection system (Thermo Fisher Scientific). Therefore, qPCR MasterMix (Eurogentec, Seraing, Liège, Belgium) and the following mouse-specific TaqManTM gene expression assays with fluorescently labelled MGB probes were employed: Mm00446190_m1 (Il6), Mm01178820_m1 (Tgfb1), Mm00801666_m1 (α1 type I collagen, Col1a1), Mm00437297_m1 (vitamin D receptor, Vdr) and Mm01545399_m1 (hypoxanthine guanine phosphoribosyl transferase; Hprt; house-keeping gene control). PCR conditions were: 95 °C for 10 min, followed by 40 cycles of 15 s at 95 °C/1 min at 60 °C. The relative amount of target mRNA in untreated PSCs (control) and all other samples was expressed as 2-(∆∆Ct), where ∆∆Ctsample = ∆Ctsample - ∆Ctcontrol.
IL-6 protein levels were determined using a mouse IL-6-specific ELISA (Thermo Fisher Scientific). Therefore, activated PSCs (passage 1) were grown in 24-well plates and treated with D-vitamins as indicated. Cell culture supernatants were collected and stored at 80 °C until assayed. The measurements were performed according to the manufacturer’s instructions.
For this investigation, PSCs were seeded at a low density and grown for a prolonged period of 12 d in primary culture. At this time, the cells, like re-cultured PSCs, displayed a fully activated, myofibroblastic phenotype. Experiments with cells of passage 1 yielded unsatisfactory results due to inconsistent growth rates and low proline uptake (data not shown). 2-[18F] proline (18F-proline) was synthesized according to literature[19] with a modification as described in[20]. After pretreatment with Dvitamins for 48 h, 18F-proline (1.0 GBq/L culture medium) was added to each culture well. Two hours later, incubation was terminated by aspirating the medium and rinsing the cell layer two times with ice-cold PBS. PSCs were solubilized with 100 mmol/L NaOH, and incorporated 18F activity was determined using an automated counter (Wizard 2470, Automatic Gamma Counter, PerkinElmer, Groningen, the Netherlands). The raw data were normalized to the protein content of the sample, which was determined using a commercial Bradford assay (Bio-Rad Laboratories, Munich, Germany).
All data were stored and analyzed using the IBM SPSS Statistics 22.0. Values were expressed as mean ± SE for the indicated number of samples (n) per experimental protocol. Mean group differences were checked using analysis of variance. If data did not meet the assumptions for ANOVA, nonparametric analysis of variance was performed employing the Kruskal-Wallis test or, in case of dependency of samples, the Friedman test, before subgroups were tested pairwise using the Mann-Whitney U test and the Wilcoxon rank sum test, respectively. Here, Bonferroni-adjusted P-values, otherwise P < 0.05 were considered to be statistically significant.
In a first set of experiments, we analyzed the effects of D-vitamins on the activation status of cultured PSCs in vitro. Therefore, PSCs in early primary culture and cells of the first passage were incubated with vitamin D2, vitamin D3 or calcipotriol as indicated, and cytoplasmic lipid droplets (Figure 1) as well as stress fiber bundles of α-SMA (Figure 2) were used as surrogate markers of the cellular phenotype. At the time of analysis, originally quiescent PSCs had undergone 4 d of primary culture on plastic and were considered as a model of early activated stellate cells. Accordingly, re-cultured PSCs corresponded to mature activated cells.
As shown in Figure 1, primary cultures of PSCs contained more lipid droplets after incubation with any of the three vitamin D derivatives than without this treatment. In passaged cells, no such effect was observed. In primary culture as well as upon re-culturing, untreated PSCs expressed high levels of α-SMA protein that was organized in stress fibers (Figure 2). All three D-vitamins significantly diminished the formation of stress fiber bundles in primary cultures, but not in re-cultured cells. The effects of Dvitamins on cells in primary culture were also confirmed by Western blot analysis of α-SMA expression (Figure 3).
High levels of lipid droplets and absence of stress fibers are characteristics of quiescent PSCs[2,3]. Therefore, our data suggest that D-vitamins support the maintenance of a quiescent state when added early in the course of primary culture, but cannot reverse the myofibroblastic phenotype (with high levels of α-SMA and a decline of lipid droplets) once the cells are fully activated.
At concentrations of 100 nmol/L (Figure 4) and below, D-vitamins had no effect on the rate of DNA synthesis, independent of whether primary cultures or passaged cells were exposed to the drugs.
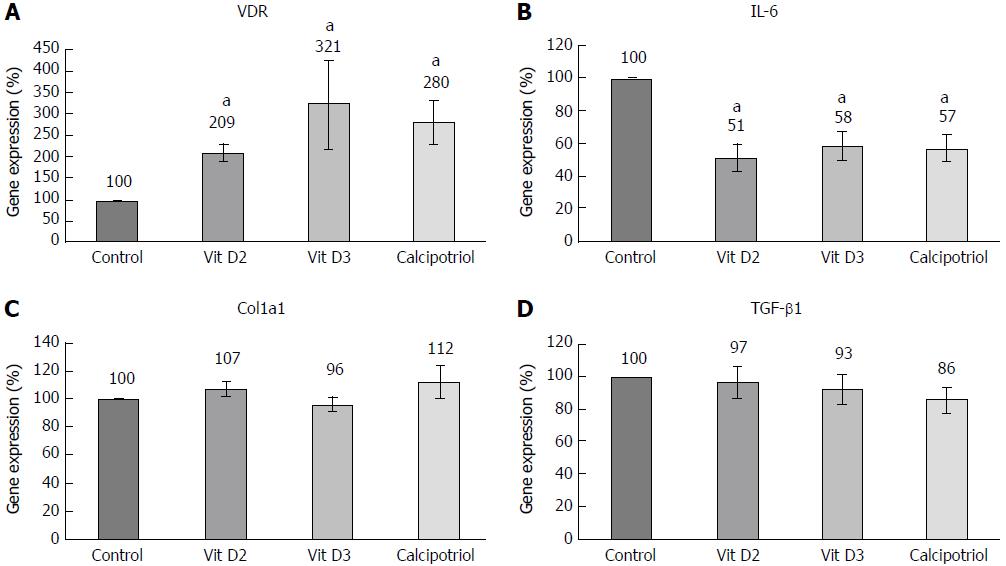
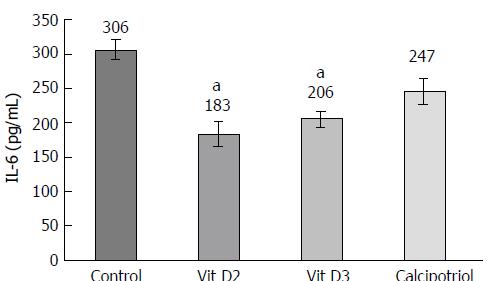
Effects of D-vitamins at the level of gene expression were studied in passaged PSCs only, since the mRNA yield from the small number of cells in primary culture proved too low for reproducible results. As expected, all D-vitamins enhanced the expression of VDR as a known target gene[21] (Figure 5A). Interestingly, vitamins D2 and D3 significantly diminished the mRNA and protein levels of IL-6, an autocrine mediator of PSC activation and major proinflammatory cytokine[22,23] (Figures 5B and 6). Calcipotriol showed similar effects, but only the inhibition of mRNA expression was statistically significant. None of the investigated derivatives inhibited the expression of collagen type 1 (α1chain) and Tgfb1 as the main profibrogenic cytokine[4,24] substantially (Figure 5C and D).
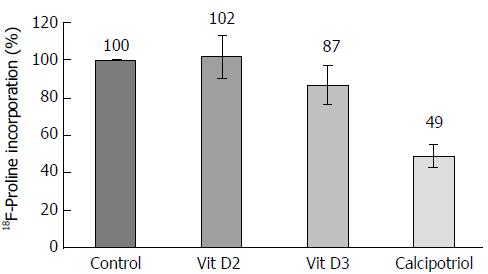
In subsequent experiments, uptake of proline, a main component of collagen, by PSCs was measured. The lowest rate of proline incorporation was observed for calcipotriol-treated cells (Figure 7). Although a Friedman test yielded a P value of 0.007, the differences between treated cells and controls did not reach statistical significance.
To this end, antifibrotic drugs that effectively inhibit the stroma response in the context of CP and PC are largely missing. Undoubtedly, their availability would also facilitate experimental studies on the role of fibrosis in the progression of both diseases, which remains unknown (CP) or controversial (PC). Currently, drugs that block pro-tumorigenic functions of the stroma but maintain, or even enhance, its anti-tumorigenic properties are considered most interesting with respect to their clinical potential. Recently, the vitamin D derivative calcipotriol has been suggested to exert such effects by inducing a transcriptional re-programming of the tumor stroma in PC, and PSCs have been suggested as important targets of vitamin D action[12,13].
The results of this study provide for the first time insights into the relative efficiencies of different vitamin D derivatives in the context of pancreatic fibrogenesis. Using primary cultures of quiescent murine PSCs, we found that vitamin D2, vitamin D3 and calcipotriol all significantly reduced the expression of α-SMA and prevented the loss of vitamin A-containing fat droplets. Once the cells were fully activated (upon re-culture), no such effects were detected anymore. Together, these data suggest that all three vitamin D derivatives inhibit activation of PSCs in vitro, but cannot reverse the myofibroblastic phenotype of fully activated PSCs. Nevertheless, the latter cells remained vitamin D-responsive. Specifically, all D-vitamins reduced the mRNA levels of IL-6. Moreover, vitamins D2 and D3 also significantly diminished IL-6 protein secretion. Here, the effect of calcipotriol was not significant, although this might be due to the small sample size. Furthermore, uptake of proline was affected by vitamin D-treatment. Somewhat unexpectedly, we did not observe significant effects of any vitamin D derivative on DNA synthesis, a finding that is in contrast to a previous report of antiproliferative effects of vitamin D3 on murine PSCs[13]. The possibility that methodological differences account for this discrepancy cannot be excluded at this stage. In the previous study, longer periods of treatment with vitamin D3 and a different assay to monitor cell growth were employed[13]. Interestingly, D-vitamins neither inhibited the expression of collagen type 1 (α1-chain) nor of Tgfb1. These findings suggest that both genes are no direct targets of vitamin D action in PSCs. Since D-vitamins interfered with the exhibition of a myofibroblastic PSC phenotype, we nevertheless suggest an antifibrotic net effect of the three derivatives.
In conclusion, despite small differences, the biological efficiencies of the three D-vitamins were similar. The inability of D-vitamins to reverse the activated PSC phenotype does not necessarily limit their potential as antifibrotic drugs, since (1) they may prevent the further recruitment of still quiescent PSCs; and (2) diminished secretion of the autocrine PSC activator IL-6[20,21]. The reduced uptake of proline, a main compound of collagen, is compatible with a preserved antifibrotic action of D-vitamins even upon completion of PSC activation. We therefore suggest follow-up studies in animal models not only of PC but also of CP to further evaluate the antifibrotic effects of vitamin D under in vivo conditions.
Chronic pancreatitis and pancreatic cancer are accompanied by an extended fibrosis that plays an active role in disease progression. Pancreatic stellate cells (PSCs) are the main source of extracellular matrix proteins in the diseased organ. To this end, there is a lack of specific antifibrotic agents for preclinical evaluation and potential clinical applications.
Vitamin D has recently been suggested to modulate the pancreatic stroma in a way that pancreatitis is suppressed and pancreatic cancer therapy is enhanced. PSCs were identified as a target of vitamin D action. The molecular mechanisms of vitamin D action in PSCs are only partially understood, and the relative efficiencies of different vitamin D derivatives have not been elucidated yet.
The objective of this study was to analyze and to compare the biological and molecular effects of three different D-vitamins, vitamin D2, vitamin D3 and calcipotriol, in PSCs.
Murine PSCs were exposed to D-vitamins as primary cultures (early activated PSCs) and upon re-culturing (fully-activated cells). Exhibition of vitamin A containing lipid droplets and expression of α-smooth muscle actin were used as surrogate markers of PSC activation. Therefore, oil red staining, immunofluorescence studies and immunoblot analyses were performed. Gene expression was monitored by real-time PCR, and interleukin-6 (IL-6) protein levels were quantified by ELISA. Furthermore, 18Fproline was employed to measure the cellular uptake of proline.
The results of this study show for the first time that vitamin D exerts distinct effects on quiescent and activated PSCs in vitro. In quiescent PSCs, vitamin D prevented the exhibition of a myofibroblastic phenotype. Once the cells were fully activated, vitamin D failed to induce a complete reversal of the myofibroblastic phenotype, but still exerted antifibrotic effects on PSCs by inhibiting uptake of proline and expression of IL-6. Three vitamin D derivatives, vitamin D2, vitamin D3 and calcipotriol, displayed very similar biological effects.
D-vitamins are efficient inhibitors of PSC activation in vitro, but cannot reverse the phenotype once the cells are fully activated. In line with other publications in the field, our findings encourage a further evaluation of vitamin D effects in pancreatic cancer and chronic pancreatitis. A modulation of the stroma response by vitamin D might hold potential as part of a multimodal concept for the treatment of both diseases.
These investigations have shown that vitamin D2, vitamin D3 and calcipotriol are similarly effective with respect to the inhibition of PSC activation in vitro. Directions of future research should include both mechanistic studies on the molecular basis of vitamin D action in PSCs, and experimental studies on vitamin D efficiency in the context pancreatic cancer and chronic pancreatitis. Therefore, advanced cell culture models such as human stellate cells and animal models of pancreatic fibrosis need to be employed.
We thank Mrs. Katja Bergmann for expert technical assistance and Dr. Änne Glass for help with the statistical evaluation of the data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chowdhury P, Ker CG S- Editor: Ma YJ L- Editor: A E- Editor: Li D
| 1. | Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 2. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 797] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 3. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 715] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 4. | Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grünert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085-2093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 380] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB, Logsdon CD. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 934] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 7. | Müerköster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Klöppel G, Kalthoff H, Fölsch UR. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 503] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 9. | Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell. 2015;28:831-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1635] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 11. | Wang Z, Li J, Chen X, Duan W, Ma Q, Li X. Disrupting the balance between tumor epithelia and stroma is a possible therapeutic approach for pancreatic cancer. Med Sci Monit. 2014;20:2002-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 888] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 13. | Bläuer M, Sand J, Laukkarinen J. Physiological and clinically attainable concentrations of 1,25-dihydroxyvitamin D3 suppress proliferation and extracellular matrix protein expression in mouse pancreatic stellate cells. Pancreatology. 2015;15:366-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Witteck L, Jaster R. Trametinib and dactolisib but not regorafenib exert antiproliferative effects on rat pancreatic stellate cells. Hepatobiliary Pancreat Dis Int. 2015;14:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Sparmann G, Kruse ML, Hofmeister-Mielke N, Koczan D, Jaster R, Liebe S, Wolff D, Emmrich J. Bone marrow-derived pancreatic stellate cells in rats. Cell Res. 2010;20:288-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Rateitschak K, Karger A, Fitzner B, Lange F, Wolkenhauer O, Jaster R. Mathematical modelling of interferon-gamma signalling in pancreatic stellate cells reflects and predicts the dynamics of STAT1 pathway activity. Cell Signal. 2010;22:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Bülow R, Fitzner B, Sparmann G, Emmrich J, Liebe S, Jaster R. Antifibrogenic effects of histone deacetylase inhibitors on pancreatic stellate cells. Biochem Pharmacol. 2007;74:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Hamacher K. Synthesis of n.c.a. cis- and trans-4-[18F]Fluoro-L-proline, radiotracers for PET-investigation of disordered matrix protein synthesis. J Label Compd Radiopharm. 1999;42:1135-1144. [DOI] [Full Text] |
| 20. | Lavalaye J, Grutters JC, van de Garde EM, van Buul MM, van den Bosch JM, Windhorst AD, Verzijlbergen FJ. Imaging of fibrogenesis in patients with idiopathic pulmonary fibrosis with cis-4-[(18)F]-Fluoro-L: -proline PET. Mol Imaging Biol. 2009;11:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located in the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2007;103:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Sugano K. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem. 2006;99:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Schneider E, Schmid-Kotsas A, Zhao J, Weidenbach H, Schmid RM, Menke A, Adler G, Waltenberger J, Grünert A, Bachem MG. Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2001;281:C532-C543. [PubMed] |