Published online May 14, 2018. doi: 10.3748/wjg.v24.i18.1942
Peer-review started: March 20, 2018
First decision: April 10, 2018
Revised: April 12, 2018
Accepted: April 23, 2018
Article in press: April 23, 2018
Published online: May 14, 2018
Processing time: 53 Days and 1.6 Hours
Gastric cancer (GC) is one of the most lethal and aggressive cancers, being the third cause of cancer related death worldwide. Even with radical gastrectomy and the latest generation of molecular chemotherapeutics, the numbers of recurrence and mortality remains high. This is due to its biological heterogeneity based on the interaction between multiple factors, from genomic to environmental factors, diet or infections with various pathogens. Therefore, understanding the molecular characteristics at a genomic level is critical to develop new treatment strategies. Recent advances in GC molecular classification provide the unique opportunity to improve GC therapy by exploiting the biomarkers and developing novel targeted therapy specific to each subtype. This article highlights the molecular characteristics of each subtype of gastric cancer that could be considered in shaping a therapeutic decision, and also presents the completed and ongoing clinical trials addressed to those targets. The implementation of the novel molecular classification system will allow a preliminary patient selection for clinical trials, a mandatory issue if it is desired to test the efficacy of a certain inhibitor to the given target. This will represent a substantial advance as well as a powerful tool for targeted therapy. Nevertheless, translating the scientific results into new personalized treatment opportunities is needed in order to improve clinical care, the survival and quality of life of patients with GC.
Core tip: A new molecular classification of gastric cancer (GC) became available after publication of three landmark studies: The Cancer Genome Atlas, “Singapore-Duke” study, and Asian Cancer Research Group, allowing patient selection for specific targeted therapy. The Epstein-Barr virus positive, microsatellite stable TP53-active or microsatellite-unstable tumors subtypes presents tumour infiltrating patterns with overexpression of PD-1, PD-L1, PDL-2. Preliminary data show high response rate to immunotherapy and open new avenues to gene therapy. Currently effective therapies for diffuse GC are lacking. Mutations in e-cadherin and Ras homolog family member A genes, or Claudin-18-ARHGAP6/26 fusions may be exploited as therapeutic targets. The only targeted therapies approved so far for chromosomal instability and microsatellite stable TP53-inactive subtypes of GC are trastuzumab and ramucirumab (HER2 and VEGFR2 inhibitors).
- Citation: Chivu-Economescu M, Matei L, Necula LG, Dragu DL, Bleotu C, Diaconu CC. New therapeutic options opened by the molecular classification of gastric cancer. World J Gastroenterol 2018; 24(18): 1942-1961
- URL: https://www.wjgnet.com/1007-9327/full/v24/i18/1942.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i18.1942
Gastric cancer (GC) is a common and deadly cancer worldwide, with over 1000000 patients being diagnosed and over 723000 dying each year[1]. Five-year survival for advanced or metastatic GC is 5%-20%, and median overall survival less than 1 year[2]. As in all cancer subtypes, angiogenesis, fibrosis and inflammation are critical processes in local progression and metastasis. These factors do so in part by creating a tumour microenvironment that is characterized by hypoxia and immunosuppression, which thwarts immune system’s ability to fight the cancer. As a result, no single chemotherapy or molecularly targeted drug, or even combination regimen, consistently leads to objective and durable tumour shrinkage in GC.
Surgical resection still represents the only potentially curative treatment in gastric cancer patients. However, in most cases, patients are diagnosed with advanced disease and therapeutic approach, include, beside surgery, adjuvant or neoadjuvant chemotherapy and radiotherapy. Molecular targeted therapy in advanced gastric cancer it is currently limited to trastuzumab, as first-line therapy in patients with HER-2 positive tumours and ramucirumab, alone or in combination with chemotherapy, as second-line therapy[3].
In the last years, several studies have attempted, on the basis of microarray and meta-analyses, to highlight a genetic signature for gastric cancer linked to either tumour stage[4,5] or prognosis[6,7]. Furthermore, those genetic signatures were the basis for further studies of targeting and inhibition by means of RNA interference technology of overexpressed genes in order to validate them as therapeutic targets in gastric cancer[8-10].
An important step in obtaining more effective therapeutic responses is identifying subsets of patients that are suitable candidates to benefit from specific therapeutic agents, knowing that differences in gene expression profile are correlated with different treatment response[11,12]. This has become possible in gastric cancer due to results from three landmark studies that provided new genetic and epigenetic molecular classifications of GC: the Cancer Genome Atlas (TCGA), “Singapore-Duke” study, and Asian Cancer Research Group (ACRG)[1,13,14]. These findings are offering an unprecedented opportunity to make substantial progress in GC therapy. Moreover, based on the promising results obtained in other solid tumors[15], new treatment strategies, like immunotherapy, are emerging in the field of gastric cancer therapeutics. Preliminary results showed that targeting the immune checkpoint pathways may represent a promising strategy that can lead to better outcomes in gastric cancer patients.
Traditionally, gastric cancers were histologically divided into main types: intestinal and diffuse, according to Lauren classification[16]. In 2010, World Health Organization (WHO), distribute gastric carcinomas in four groups of tumors: papillary, tubular, mucinous and poorly cohesive. Beside adenocarcinoma, the WHO classification also included less frequent histologic variants[7].
Latest advances in molecular methods such as next-generation sequencing (NGS), including DNA sequencing, RNA sequencing, whole-exome sequencing, copy number variation analysis, and DNA methylation arrays, have increased our understanding of GC biology, and led to the development of a new comprehensive molecular classification.
One of the first studies aimed to identify molecular gastric cancer subtypes was the study undertaken by Tan et al[17], who analysed patterns of gene expression for 28 gastric cell lines and proposed two major distinct subtypes of GC: intestinal (G-INT) and diffuse (G-DIF) that overlaps with Lauren’s intestinal or diffuse-type. These intrinsic subgroups were validated in 4 independent cohort totalized 521 primary tumors. Moreover, the authors showed that the G-INT cell lines are more responsive to treatment with 5-fluorouracil and oxaliplatin but more resistant to cisplatin than the G-DIF cell lines, and the patients with G-INT cancers have better overall survival in comparison to patients with G-DIF tumors.
In 2013, in a subsequent study Lei et al[18] using consensus hierarchical clustering with selection by repetitive features on 248 gastric tumors identified three subtypes of gastric adenocarcinoma: mesenchymal, proliferative and metabolic. Each subtype display distinct genomic and epigenetic properties and drug sensitivities. The proliferative subtype includes tumors with a high level of genomic instability, DNA hypomethylation and TP53 mutations. For this tumour subgroup, frequent mutations in CCNE1, MYC, ERBB2 and KRAS genes, were also described. The metabolic subtype comprises cancer cells with a gene expression pattern similar to cells from normal mucosa that are more sensitive to 5-fluorouracil treatment than mesenchymal or proliferative tumors subtypes. Tumors in the mesenchymal subtype contain cells with stem cell-like properties, with high activity of the epithelial-mesenchymal transition pathway. The in vitro studies show that the cell lines of this subtype are sensitive to PI3K/Akt/mTOR inhibitors.
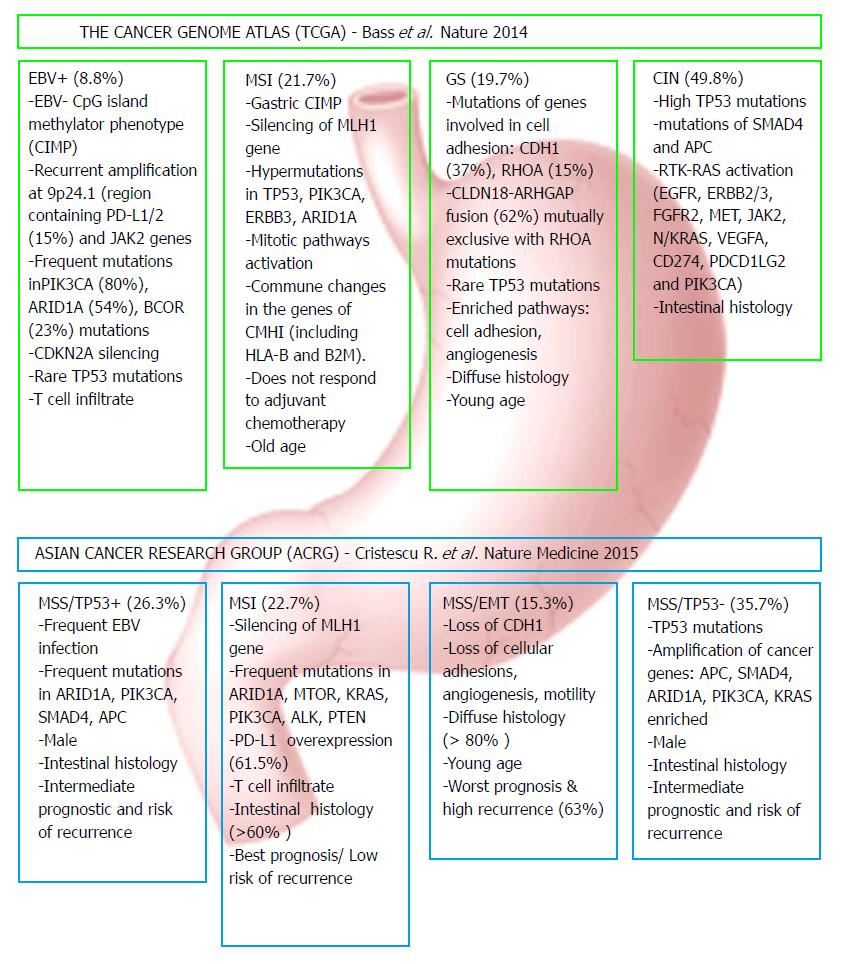
In 2014, as part of The Cancer Genome Atlas (TCGA) project, Adam Bass et al[1] realize a comprehensive molecular characterization of 295 primary gastric adenocarcinomas and proposed a new molecular classification system for gastric cancer which comprises four subtypes: tumors positive for Epstein-Barr virus (EBV), microsatellite unstable tumours (MSI), genomically stable tumours (GS) and tumours with chromosomal instability (CIN).
A similar approach had researchers from Asian Cancer Research Group (ACRG), who analysed gene expression data from 300 primary gastric tumors. Their findings have led to a novel proposal of gastric cancer molecular classification that includes four tumors subtypes: with microsatellite stability (MSS)/epithelial-mesenchymal transition (EMT), microsatellite-unstable tumors (MSI), microsatellite stable TP53-active (MSS/TP53+) and microsatellite stable TP53-inactive (MSS/TP53-)[14].
Both molecular classification systems highlight the main molecular alterations specific to each subtype, together with their frequency which can provide a new orientation in targeted therapy. In addition, the ACRG classification model provides useful information about disease progression and prognosis.
Although there are not equivalent, the subgroups proposed by the two research teams share common features and are partially overlapping. The similarities were observed between MSI subtypes, the MSS/TP53+ and EBV positive subgroups, the MSS/EMT subtype and the GS subgroup, and also in the MSS/TP53- and CIN. Figure 1 presents the major features and genomic alterations associated with each GC subtype according to TCGA and ACRG studies.
The EBV-infected tumours represents around 9% of GC according to TCGA classification and are characterized by high level of DNA hypermethylation, non-silent mutations in phosphatidylinositol 3-kinase PIK3CA (80% of the current subtype cases), AT-rich interactive domain-containing protein 1A (ARID1A) (54%), B-cell lymphoma 6 Corepressor (BCOR) (23%), and recurrent amplification at 9p24.1, a chromosomal region that contains Janus-associated kinase 2 (JAK2) gene and two other genes that encodes for programmed death-ligand 1 and 2 (PD-L1, PDL-2) proteins (15%)[1,19].
The EBV subtype have some overlaps with the MSS/TP53+ subtype. The microsatellite stable TP53 active subtype appears to have a greater prevalence of APC, ARID1A, KRAS, PI3KCA and SMAD4 mutations compared with MSS/TP53- subtype and presents an intermediate rate of relapse and prognosis. All of these genetic alterations may have therapeutic value and must be exploited for the treatment of GC patients.
The MSI subtypes are mainly associated with hypermethylation of the MutL homolog 1 (MHL1) promoter, one of the genes involved in DNA mismatch repair (MMR) system. Due to MMR mechanism deficiency, this GC subtype has the highest rate of mutations compared to the others. Frequent recurrent mutations were observed in PIK3CA, ARIDA1, Erb-B2 receptor tyrosine kinase 3 and 2 (ERBB3, ERBB2), and epidermal growth factor receptor (EGFR) genes[1,20,21]. In the TCGA cohort, this subtype was associated with 23% of tumors and moreover with advanced age, female gender and less advanced tumoral stages. According to ACRG classification, the MSI group (22%) present recurrent mutations in KRAS, ALK, ARID1A, ERBB2, ERBB3 genes as well in genes involved in PI3K/PTEN/mTOR signaling. Usually occurs in the antral region and have the lowest recurrence rate (22%) and the best prognosis from all subgroups.
The GS subtype correspond to MSS/EMT subtype in that early age of appearance, association with diffuse type of GC and displaying low frequency of mutations compared to other gastric cancer subtypes. In the TCGA cohort 21.56% of cases were associated with GS subtype. Mutations in E-cadherin (CDH1) and Ras homolog family member A (RHOA) genes, together with the fusion between Claudin-18 (CLDN18) and Rho GTPase activating protein-6 or 26 (ARHGAP6, ARHGAP26), are the main feature of GS subtype of GC. The same genomic alterations were associated with MSS/EMT subtype in the ACRG classification, which represents 15.33% of cases. It has the highest recurrence rate (63%) and the worst prognosis among the four subtypes.
CDH1 mutations and EMT are representative features of this GC subtype. Both somatic and germline mutations, were identified. Somatic mutation have been detected in approximately 30% of GC and were related to poor prognosis[22]. Germline alterations in CDH1 gene are the main cause of hereditary diffuse gastric cancer and occur in about 40% of patients with this pathology[23]. E-cadherin, which is encoded by the CDH1 gene, is an adhesion molecule widely involved in carcinogenesis. E-cadherin deficiency has been linked to early tumor initiation in a large proportion of diffuse GC like signet ring adenocarcinoma, which is very resisting to all therapies, and hereditary diffuse GC, both with very poor survival[24,25].
Another hallmark of GS subtype are mutations in RHOA gene. RHOA is known to modulate Rho signalling downstream effectors and its mutation can prevent programmed cell death[26]. In activated form, RHOA controls actin-myosin-dependent cell contractility and motility, and its mutations promote a diffuse growth pattern.
The third characteristic feature of this subtype is the fusion between CLDN18-ARHGAP6/ARHGAP26, a GTPase-activating protein that facilitates conversion of RHO GTPases to GDP. A recent study reported that in gastric epithelial cells, CLDN18-ARHGAP26 fusion might be involved in the epithelial-mesenchymal transition and cancer progression[27].
In genomically stable tumours, RHOA mutations and CLDN18-ARHGAP6 or ARHGAP26 fusions are mutually exclusive. Thereby, RHOA gene mutations and the gene fusions specific for GS subtype could become therapeutic targets in this group of gastric adenocarcinoma.
CIN tumors represent 45% of TCGA cases and are frequently located at the gastroesophageal junction/cardia region. Are commonly related to intestinal type of gastric cancer and are associated with MSS/TP53- subtype from ACRG classification. The CIN tumors display genomic amplifications of receptor tyrosine kinases (RTKs) from ERBB, vascular endothelial growth factor (VEGF), phosphatidylinositol-3-kinase (PI3K)/Akt, mammalian target of rapamycin (mTOR) and Met/hepatocyte growth factor (HGF) signaling pathways. Recurrent amplifications of cell cycle mediators: Cyclins E1, D1 (CCNE1, CCND1) and cell division protein kinase 6 (CDK6), VEGFA amplification, frequent TP53 mutation and high levels of phosphorylated epithelial growth factor receptor (EGFR) were also described for CIN tumors[1]. In the ACRG classification, the correspondent microsatellite stable TP53-inactive (MSS/TP53-) subtype - include tumours with high-grade aneuploidy, with frequent focal amplifications in Mouse double minute 2 homolog (MDM2), MYC, ERBB2, EGFR, CCNE1 and CCND1 genes and the highest TP53 mutations frequency[28]. Most of these aberrant expressed proteins involved in signaling pathways are investigated as possible therapeutic targets for GC anti-tumour therapy and are currently being tested in clinical trials[21,29].
The novel classification system, based on the molecular characteristics, have allowed the identification of pathways that contribute to carcinogenesis and underline several driver genes relevant for each GC subtype, that can be used as potential therapeutic targets. In Table 1, the most advanced clinical trials of novel therapeutic strategies are presented, divided for each GC subgroup and molecular target[30-69].
| GC subtype | Molecular target | Therapeutic agents | Clinical trial name (ID) | Phase | Patients | Aditional treatments | ESCD | Clinical efficiency | Condition | Study (citation) | ||
| ORR | mOS | mPFS | ||||||||||
| EBV | PD-1 | Pembrolizumab | KEYNOTE-059 (NCT02335411) | II | 316 | Cis+5-FU | May-19 | 11.60% | 5.6 | 2 | Rec/Met GC | [37] |
| KEYNOTE-061 (NCT02370498) | III | 592 | Paclitaxel | Jul-19 | Failed | Adv GC | [38] | |||||
| KEYNOTE-062 (NCT02494583) | III | 764 | Cis+5-FU(X) | Jun-20 | Adv GC | |||||||
| KEYNOTE-585 (NCT03221426) | III | 860 | Cis+X(5-FU) or FLOT | Jul-23 | GC | |||||||
| Nivolumab +/-Ipilimumab (CTLA4 inhibitor) | ONO-4538-38 (NCT03006705) | III | 700 | S-1 or XOX | Jun-10 | Stage III GC | ||||||
| CheckMate649 (NCT02872116) | III | 1266 | XOX or FOLFOX | Oct-21 | Adv/Met GC | |||||||
| ONO-4538-37 (NCT02746796) | II/III | 680 | SOX or XOX | Aug-20 | Adv/Rec GC | |||||||
| ONO-4538-12 (NCT02267343) | III | 480 | Nivolumab vs Placebo | Aug-17 | 11.20% | 5.32/4.14 P < 0.0001 | 1.61/1.45 | Adv/Rec GC | [40] | |||
| PD-L1 | Avelumab | JAVELIN Gastric 300 (NCT02625623) | III | 371 | Avelumab + BSC vs Irinotecan + Paclitaxel | Sep-20 | Rec/Met GC | |||||
| JAVELIN Gastric 100 (NCT02625610) | III | 466 | vs OX + 5-FU(X)(LV) | Mar 202 4 | Adv/Met GC | |||||||
| Durvalumab | NCT02572687 | I | 114 | Ramucirumab | Sep-18 | 36% | 2.6 | Adv/Met GC | [43,45] | |||
| NCT02340975 | Ib/II | 135 | +/- Tremelimumab (CTLA4 inhibitor) | Aug-19 | Rec/Met GC | [46] | ||||||
| NCT02678182 | II | 770 | Cis, X | Aug-10 | Adv/Met HER2 neg. GC | |||||||
| Atezolizumab | DANTE (NCT03421288) | II | 295 | FLOT | Feb-25 | Adv GC | ||||||
| ICONIC (NCT03399071) | II | 40 | FLOT-A | Aug-25 | T1-T3 GC | |||||||
| PIK3CA | BYL719 | NCT01613950 | I | 18 | AUY922 | Mar-14 | NA | Adv/Met GC | ||||
| AZD5363 | NCT02451956 | II | 25 | Paclitaxel | Dec-18 | Adv GC with PIK3CA mutation | ||||||
| ARID1A | PLX2853 | NCT03297424 | II | 166 | May-21 | ARID1A mutations | ||||||
| AZD2281 (Olaparib) | NCT02576444 | II | 64 | Sep-18 | PIK3CA, AKT, or ARID1A mutations | |||||||
| MSI | PD-1 | Pembrolizumab | KEYNOTE-016 (NCT01876511) | II | 171 | Jun-21 | 40% | Not reached | 5.4 | MSI | [47] | |
| GS | CDH1 | NA | - | Prophylactic gastrectomy | ||||||||
| RHOA | NA | NA | ||||||||||
| CLDN18-ARHGAP fusion | NA | NA | ||||||||||
| CIN | EGFR | Cetuximab | NCT00183898 | II | 75 | XOX | Jun-18 | Adv GC | ||||
| Panitumumab | NEOPECX (NCT01234324) | II | 171 | ECX, placebo | Aug-17 | Adv GC incl. GEJ | ||||||
| MEGA (NCT01443065) | II | 162 | FOLFOX/FOLFOX + panitumumab/FOLFOX + AMG102 | Jan-19 | 13.1/8.3/11.5 | 5.8/5.2/7.6 | Adv GC | [48] | ||||
| Nimotuzumab | NCT01813253 | III | 400 | Irinotecan, placebo | Jan-18 | EGFR overexpr. Adv GC or GEJ | ||||||
| NIEGA (NCT03400592) | II | 55 | Irinotecan | Jun-18 | Rec/Met GC with overexpr. EGFR | |||||||
| HER2 | Trastuzumab | NCT01260194 | IV | 4 | Jan-15 | GC | ||||||
| HELOISE (NCT01450696) | IIIb | 248 | Cis + X + Herceptin (6 mg/kg or 10 mg/kg) | Aug-15 | 12.5/10.6 P = 0.2401 | GC | [49] | |||||
| PETRARCA/FLOT6) (NCT02581462) | II/III | 404 | FLOT or FLOT + Herceptin/Pertuzumab | Mar-21 | HER2+ GC or GEJ | |||||||
| NCT02954536 | II | 37 | Pembrolizumab or Pembrolizumab + X/Cis | Nov-19 | HER2+ GC | |||||||
| Her + XELOX (NCT01396707) | II | 55 | Herceptin + XELOX | Mar-18 | 21 | 9.8 | Met/Rec HER2 + GC | [50] | ||||
| EVIDENCE (NCT01839500) | 95 | Feb-18 | 30 | 9.5 | GC | [51] | ||||||
| Trastuzumab emtansine | GATSBY (NCT01641939) | II/III | 415 | or Standard Taxane Therapy | Apr-16 | 7.9/8.6 P = 0.8589 | 2.66/2.89 | Prev. treated for HER2+ Adv GC | [52] | |||
| Pertuzumab | JACOB trial (NCT01774786) | III | 780 | Trastuzumab + 5-FU/X/Cis, placebo | Dec-21 | 17.5/14.2 P = 0.0565 | 8.5/7.0 | HER2+ Met GC and GEJ | [53] | |||
| NCT01461057 | II | 30 | X + Cis + Trastuzumab | Sep-17 | Met HER2 + GC or GEJ | [54] | ||||||
| INNOVATION (NCT02205047) | II | 220 | Cis/X or Cis/5-FU +/- trastuzumab, placebo | Sep-24 | GC, EGFR overexpress. | [55] | ||||||
| Margetuximab | NCT02689284 | Ib/II | 72 | Pembrolizumab | Mar-20 | Adv/Met HER2 + GC or GEJ | [56] | |||||
| NCT01148849 | I | 67 | Dec-17 | HER2+ GC | [57] | |||||||
| EGFR/ HER2 | Lapatinib | LOGiC/TRIO-013 (NCT00680901) | III | 545 | XOX, Placebo | Dec-19 | 11.9/10.4; P = 0.3244 | 6.0/5.4; P = 0.0381 | GC | [58,59] | ||
| NCT02015169 | II | 32 | XELOX | Nov-17 | HER2 + GC with liver metastasis | |||||||
| VEGF | Bevacizumab (Avastin) | NCT01471470 | II | 31 | Docetaxel + X + Cis | Dec-19 | 38.6 | 13.1 | Adv GC | [60] | ||
| AGMT_GASTRIC-3 (NCT00952003) | II | 40 | 1 OX + Irinotecan + Avastin; 2 Docetaxel + Avastin; 3 Avastin | Apr-18 | 11 | GC | [61] | |||||
| NCT00911820 | II | 88 | Cis+Irinotecan or Docetaxel + Cis + Irinotecan | Jun-18 | 0.57/0.51; P = 0.605 | 11.7/13.4; P = 0.714 | 7.9/8.4; P = 0.721 | Met GC | ||||
| NCT01191697 | II | 35 | XOX + trastuzumab | Apr-17 | 26.92 | 13.93 | HER2 + Met GC | [62] | ||||
| VEGFR2 | Ramucirumab (IMC-1211B) | Rainbow trial (NCT01170663) | III | 665 | Paclitaxel Placebo | Feb-17 | 9.6/7.4; P = 0.0169 | 4.4/2.9; P < 0.0001 | GC | [63,64] | ||
| ARMANI (NCT02934464) | III | 280 | Ramucirumab + Paclitaxel or FOLFOX4/mFOLFOX6/XELOX | Oct-19 | Adv/ Met HER2- GC or GEJ | |||||||
| RAINFALL (NCT02314117) | III | 128 | X + Cis, Placebo | May-18 | Met GC | [65] | ||||||
| NCT02898077 | III | 450 | Paclitaxel, Placebo | Mar-21 | GC | |||||||
| RAMSES/FLOT7 (NCT02661971) | II/III | 908 | FLOT, Placebo | Oct-19 | GC or GEJ | |||||||
| VEGFR2/ TIE2 | Regorafenib | INTEGRATE II (NCT02773524) | III | 350 | Placebo | Apr-19 | GC | [66] | ||||
| NCT01913639 | II | 36 | FOLFOX, Placebo | Jul-18 | GC | [67] | ||||||
| mTOR | Everolimus (RAD001) | AIO-STO-0111 (NCT01248403) | III | 300 | Paclitaxel, Placebo | Jul-17 | 8.0%/7.3%; P = 0.4 | 6.1/5.0; P = 0.54 | 2.2/2.07; P = 0.3 | GC | [68] | |
| AZD2014 | NCT03082833 | II | 25 | Feb-19 | TSC1/2 mut. or null GC | |||||||
| MET | Onartuzumab | NCT01662869 | III | 562 | mFOLFOX6, Placebo | Dec-15 | 11.0/11.3 | 6.7/6.8 | Met HER2-/+ GC or GEJ | [69,70] | ||
The EBV and MSS/TP53+ subtypes present similar features such as EBV infection, PD-L1/PD-L2, JAK2 overexpression, non-silent mutations in PIK3CA, ARID1A, BCOR and high prevalence of cyclin-dependent kinase inhibitor 2A (CDKN2A) promoter methylation[1,19]. These data suggest that EBV-positive tumors could be targeted with PI3-kinase, JAK2 inhibitors or even better with a combination of those two types of inhibitors which proved strong synergistic effect in other systems[30]. Using checkpoint inhibitors is another therapeutic strategy that is currently evaluated in a series of clinical trials in patients with gastric cancer.
This GC subtype is particularly associated with an enhanced survival due to a strong inflammatory response induced by a major CD8+ cytotoxic T-cell infiltrate as a result of EBV infection. Several studies have shown that EBV can directly increase PD-L1 promoter activity as a result of EBV latent membrane protein 1 (LMP1) binding to JAK3, via STAT signaling and AP-1 activation[31,32]. The immune checkpoint ligands PD-L1/PD-L2 overexpression can be a valuable way to block the PD-1 pathway, attenuating the immune response and therefore helping the EBV+ GC to evade immune attack. Additional, IFN-γ released by tumour infiltrating T cells as a result of EBV infection, can directly induce PD-L1 expression in tumour cells[33,34]. Together, these data suggest that EBV+ GC may benefit from anti-PD-1 directed therapy.
Currently there are several PD-1/PD-L1 inhibitors approved by FDA for cancers like non-small-cell lung carcinoma (NSCLC) and melanoma, which are tested for efficacy in ongoing clinical trials on GC. The most known PD-1 inhibitors are: Pembrolizumab (Keytruda®) and Nivolumab (Opdivo®). Additional, PD-L1 approved inhibitors are: Avelumab (Bavencio®), Durvalumab (Imfinzi®) and Atezolizumab (Tecentriq®).
Pembrolizumab is a humanized monoclonal IgG4 antibody directed against human cell surface receptor PD-1 (programmed death-1 or programmed cell death-1) with potential immune checkpoint inhibitory and antineoplastic activities. At this time there are 29 ongoing studies involving pembrolizumab treatment in GC: 13 in phase I, 12 in phase II and 4 in phase III. Based on previous encouraging results obtained by two phase Ib clinical trials: KEYNOTE-012 (NCT01848834) and KEYNOTE-028 (NCT02054806)[35] that evaluated the efficacy of pembrolizumab as single-agent in patients with solid cancers, with a partial response rate of 22%-30%, ongoing efforts are testing pembrolizumab combined with cytotoxic chemotherapy in an attempt to improve the response rate. The phase II KEYNOTE-059 study (NCT02335411) is testing the efficacy of pembrolizumab associated with cisplatin and 5-fluorouracil. Full data from the KEYNOTE-059 trial were presented at the 2017 ASCO Annual Meeting[36]. The general overall response rate (ORR) with pembrolizumab was 11.6%, and higher in patients who specifically received 2 prior lines of therapy (16.4%). The median PFS was 2.0 mo and the median overall survival (OS) was 5.6 mo, with a 12 mo OS rate of 23.4%. Based on these results, the FDA accelerated the approval of pembrolizumab in September 2017 for the treatment of patients with PD-L1-positive recurrent or advanced GC who has received 2 or more lines of chemotherapy. The accelerated approval of pembrolizumab for this indication was contingent on the results of a confirmatory trial. The ongoing phase III KEYNOTE-062 (NCT02494583) trial is evaluating pembrolizumab alone and in combination with Cis and Capecitabine (5-FU) as the first line therapy for PD-L1-positive GC. KEYNOTE-585 (NCT03221426), another phase III trial, is studying the combination of pembrolizumab and chemotherapy (Cis + Capecitabine (5-FU) or FLOT (docetaxel+oxaliplatin+5FU+leucovorin) as neoadjuvant and adjuvant therapy. Recently, results from a different phase III trial KEYNOTE-061 (NCT02370498), testing the association of pembrolizumab and paclitaxel, were announced. On Dec 2017, Merck Company, reveal that pembrolizumab (Keytruda®) did not improve survival as a second-line treatment for PD-L1-positive patients with advanced GC[37].
Nivolumab (ONO-4538/BMS-936558) is another human monoclonal IgG4 antibody which blocks the PD-1 receptor. Currently, there are 17 clinical trials on GC involving nivolumab: 8 in phase I, 6 in phase II and 3 in phase III. The activity and safety of nivolumab was first tested in a phase I/II study CheckMate-032 (NCT01928394), as standalone agent or in combination with ipilimumab, an inhibitor for CTLA4, a protein receptor expressed on T-cells that function as an immune checkpoint. This study reported that ORR was 14% in patients treated with nivolumab alone N3 (nivolumab 3 mg/kg corp), 26% in N1+I3 (nivolumab 1mg/kg corp plus ipilimumab 3 mg/kg corp) cohort, and 10% in N3+I1 (nivolumab 3 mg/kg corp plus ipilimumab 1 mg/kg corp) group. The mOS were 6.9 mo in N1+I3 group, followed by 5.0 mo in N3, and 4.8 mo in N3+I1 groups. However the most active regimenN1+I3 had the highest toxicity (84%), compared with N3 (70%) and N3+I1 (75%). Another important outcome of the study was the evaluation of the response rate for nivolumab alone or in combination with ipilimumab depending on the PD-L1 expression. As a result, the response rate for PD-L1 positive tumors was 27% for N3 alone and 44% for N1+I3. For patients with PD-L1 negative tumors the response rate was 12% for N3 and 21% for N1+I3 regimen[38].
More recently, results from ONO-4538-12 (NCT02267343) study were made available. This was a phase III clinical trial aiming to evaluate the efficacy and safety of nivolumab as rescue treatment after failure of the standard chemotherapy for GC. The results showed that nivolumab was effective with significantly improved OS, PFS and ORR compared to placebo. Median OS was 5.32 mo with nivolumab vs 4.14 mo with placebo. The ORR was 11.2% with nivolumab vs 0 with placebo (P < 0.0001). Median progression-free survival (mPFS) was 1.61 mo with nivolumab vs 1.45 mo with placebo[39].
Avelumab is a human monoclonal IgG1 antibody directed against the human PD-L1 protein, with potential immune checkpoint inhibitory and antineoplastic activities. Avelumab was approved by FDA as Bavencio® for metastatic Merkel cell carcinoma (MCC) based on multi-center clinical trial (JAVELIN Merkel 200 trial, NCT02155647) that reported a ORR of 29.5% and a mPFS of 2.6 mo, shoing that avelumab has a manageable safety profile with durable responses[40]. Five clinical trials are currently testing avelumab in GC: 1 in phase I, 2 in phase II, and 2 in phase III. In the phase III clinical trials, the avelumab efficacy as single agent is being compared with different chemotherapeutic regimens: Irinotecan + paclitaxel in the JAVELIN Gastric 300 (NCT02625623), and OX + 5-FU(X) (LV) in JAVELIN Gastric 100 (NCT02625610).
Durvalumab (MEDI4736) is an Fc optimized monoclonal antibody directed against PD-L1. It was accepted by FDA and European Medicines Agency (EMA) as Imfinzi® for the treatment of patients with metastatic urothelial carcinoma and locally-advanced (stage III), unresectable non-small cell lung cancer whose disease has not progressed following platinum-based chemoradiation therapy in a phase III PACIFIC trial[41]. Durvalumab is not currently approved for the treatment of patients with gastric or GEJ adenocarcinoma. There are 4 phase Ib/II studies that are currently enrolling patients for treatment with durvalumab as single agent, or in combination with tremelimumab - a CTLA4 inhibitor (NCT02340975) or ramucirumab - a VEGFR-2 inhibitor (NCT02572687). Preliminary results from the last study, presented at the 2018 Gastrointestinal Cancers Symposium, suggests that blocking VEGFR-2 and the PD-1/PD-L1 pathway induces synergic antitumor effects[42].
Atezolizumab is a monoclonal antibody designed to bind with PD-L1. It was approved by FDA and EMA under the trade name Tecentriq® for the treatment of patients with advanced or metastatic non-small cell lung cancer or metastatic urothelial carcinoma after they have been previously treated with chemotherapy. In the field of gastric cancer, there are three studies (1 in phase I and 2 in phase II) that are currently enrolling patients to evaluate the safety and efficacy of atezolizumab in combination with FLOT chemotherapy vs FLOT alone, for operable adenocarcinoma of the stomach, the ICONIC (NCT03399071) and DANTE (NCT03421288) studies.
As an important alternative strategy addressing T cell receptors/Ligands interactions is knocking-out the gene for PD-1 receptor by CRISPR-Cas9 DNA-editing technology that might be more effective than using inhibitors or antibodies against it or against the ligands. It is postulated that editing PD-1 gene will maintain T cell activity in the presence of the checkpoint ligands, PD-L1 and PD-L2, however ethical aspects of the in-human use of the technology are still debated[43].
PIK3CA inhibitors: A number of PI3K inhibitors are under clinical investigation by pharmaceutical companies and academic institutions, including the pan-PI3K inhibitor buparlisib (BKM120) and the PI3Kα-selective inhibitor alpelisib (BYL719). Buparlisib and alpelisib are currently in Phase III and Phase II clinical trials, respectively for HER2-negative breast cancer (NCT00863655, NCT01610284), HER2-positive breast cancer (NCT01007942), head and neck squamous cell carcinoma (NCT01737450), non-small cell lung carcinoma (NCT01297491), lymphoma (NCT01719250), glioblastoma multiforme (NCT01934361, NCT01349660), preclinical results and initial clinical findings sowing great promise[70].
In GC filed, clinical trials are currently underway, often with PI3K inhibitors in combination with other drugs. The association between BYL719 and HSP90 inhibitor AUY 922, is tested specific in gastric cancer in NCT01613950 trail, and BKM120 with hedgehog pathway inhibitor LDE 225 in advanced solid tumors through NCT01576666 study. The AKT inhibitor AZD5363 is also being tested in the second line in combination with paclitaxel, in gastric cancer patients with and without PIK3CA mutations or amplifications (NCT02451956, NCT02449655)[71].
ARID1A inhibitors: ARID1A protein is a subunit of the SWI/SNF (BAF) chromatin remodelling complex that regulates gene expression by controlling gene accessibility. ARID1A shows one of the highest mutation rates across different human cancer types[13,72-76].
Currently there are no clinical trials in gastric cancer patients involving ARID1A inhibitors. There are however two clinical trials involving patients with solid tumors harbouring ARID1A mutation. First one, NCT03297424 a phase II trial, is testing the efficacy of PLX2853, an inhibitor of the bromodomain-containing protein 4 (BRD4), in subjects with advanced malignancies and ARID1A mutations. The second one NCT02576444, also a phase II trial, is testing patients with solid tumors harbouring PIK3CA, AKT, or ARID1A mutations for response at Olaparib (AZD2281) a poly ADP ribose polymerase (PARP) inhibitor. Both studies are ongoing.
Due to MMR mechanism deficiency, the MSI subtype has the highest rate of mutations compared to the other groups. A study published by An et al[77] demonstrated that patients with an increased number of mutations in MMR genes do not respond to 5-FU chemotherapy. Consequently, chemotherapy may be beneficial only for MSI patients with low mutational burden. This situation is similar to that of MSI colon cancer[78]. Based on the positive results recorded in the treatment of patients with PD-1-positive MSI colon cancer[79], a similar therapy with pembrolizumab was attempted in patients with GC MSI high. Recent results of a Phase II clinical trial KEYNOTE-016 (NCT01876511) showed that this treatment was beneficial[46]. The results can be explained by the findings of a recent study by Cho et al[80], which reported an overexpression of PD-L1 in 61.5% of MSI-GC samples that is associated with long-term survival of the patients. Based on this finding, we can conclude that MSI+ status is associated with good prognosis and may be a candidate for immune checkpoint inhibitors therapy due to the sustained response. These results, together with other outcomes from four clinical trials KEYNOTE-164 (NCT02460198), KEYNOTE-012 (NCT01848834), KEYNOTE-028 (NCT02054806), and KEYNOTE-158 (NCT02628067), determined in May 2017 the FDA to grant accelerated approval to pembrolizumab (Keytruda) for patients with solid tumors that have the microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR)[81].
This group is best described as diffuse type of GC, with a low number of mutations that are developed of early age. This form of cancer includes early-onset gastric carcinoma which due its association with some early triggers that impair genome stability, could be considered the best model of gastric cancerogenesis and the best model for testing new treatment options, in the pressing need for new therapeutic for diffuse GC[82]. Another One key finding was that the MSS/EMT subtype showed a higher recurrence rate with peritoneal seeding, and very poor survival compared to other subtypes. EMT is an important mechanism in tissue fibrosis that is characterized by the loss of cell-cell adhesion. Various studies have indicated transforming growth factor (TGF)-b1, secreted by gastric cancer cells and cancer-associated fibroblasts (CAFs), as common initiator of EMT[83,84]. Saito et al[85] successfully used tranilast, an inhibitor of TGF-b/Smad pathway to inhibit interactions between cancer cells and stroma, preventing fibrous tumor establishment represented by peritoneal dissemination.
Currently effective targeted therapies for diffuse GC are lacking. A significant key in our understanding of diffuse GC was the discovery of hereditary form carried out by missense mutation in CDH1 gene, which encodes E-cadherin. Additional, novel recurrent mutations of RHOA and CLDN18-ARHGAP6 fusion were identified and linked with diffuse GC pathogenesis.
CDH1: Currently there are only three clinical trials regarding CDH1 in gastric cancer: Two of them are observational, aiming to determine the incidence of CDH1 germline mutations among individuals with early onset or familial gastric cancer and their relatives (NCT00582257, NCT03030404), and third one is prospective, aiming to investigate the association between the level of CDH1 methylation (among other genes) and metastasis/recurrence of gastric carcinomas (NCT02159339). At the moment, the primary clinical approach is curative prophylactic gastrectomy at early age, since the lifetime risk of developing GC for CDH1 mutation carriers with familial history of hereditary diffuse gastric cancer is 60%-80%, with a mean age of onset of 37 years[86].
RHOA: RHOA mutations were found in 14%-25% of diffuse GC patients[87,88]. RHOA, as a member of the Ras GTPase superfamily, is a critical transducer of extracellular signals, which leads to organization of actin cytoskeleton, motility, adhesion and gene regulation. Overexpression of RHOA or constitutive activation was observed in various cancers, and was associated with implicated in tumorigenesis and tumor cell invasion[89]. Also, several mutations were found in diffuse GC with tumor promoting activity, as demonstrated by small interfering RNA (siRNA)-mediated silencing studies[90]. There are no currently available clinical trials or directed treatment for RHOA mutations. A study performed by Kang et al[91]. aimed to explore whether RHOA influences the susceptibility of gastric cancer cells to chemotherapeutic drugs founded that RHOA significantly enhanced the resistance to lovastatin, 5-FU, taxol and vincristine, but did not affect the sensitivity to cisplatin or etoposide in SNU638 human carcinoma cell line.
CLDN18-ARHGAP6 or 26 fusions are another genomic alterations associated with GS type of GC. It is mutually exclusive with RHOA and CDH1 mutations among GS tumors[92]. ARHGAP 26 gene is encoding a GTP-ase protein that modulates RHOA activation. Moreover, this genomic fusion may affect CLDN18 function, a protein involved in cell adhesion.
Currently, there are no targeted therapies for the gene mutations and alterations involved in this GC subtype. However, these known mutations may facilitate the development of novel therapies.
Characterization of GC at the molecular level allowed also the identification of the intracellular pathways that might contribute to carcinogenesis. Studies to date point out that several signaling cascades are implicated in gastric carcinogenesis including: ERBB, VEGF, PI3K/AKT/mTOR, and HGF/MET signaling pathways. There are currently numerous studies using inhibitors of ERBB, MET, PI3K, AKT or mTOR signaling molecules, but often attempting to block one these paths leads to activation of other cascade due to compensatory signaling[93]. However, Trastuzumab and Ramucirumab (targeting HER2 and VEGFR2 respectively) are the only targeted therapies approved so far, and most Phase III clinical trials evaluating molecular drugs targeting signaling molecules in gastric cancer have failed[94].
ERBB signaling pathways: ERBB receptors (1-4) are members of the RTK superfamily, localized to the cell surface, with high-affinity for many growth factors, cytokines, and hormones. These molecules have a predominantly regulatory role in nearly every aspect of cell biology and also a critical role in the development and progression of many types of cancer, including gastric cancer. Activation of ERBB1 (EGFR) and ERBB2 (HER2) due to amplification or mutations has been reported in almost 30% of gastric cancer patients and overexpression of these receptors has been associated with advanced stages of gastric cancer, more aggressive disease and a poor prognosis[95-97].
Several strategies for the targeting of EGFR signaling using small molecules tyrosine kinase inhibitors and monoclonal antibodies have been developed and used in preclinical and early phase clinical studies, like Cetuximab, Panitumumab, Trastuzumab, Pertuzumab, and also EGFR tyrosine kinase inhibitors such as Lapatinib[98].
Cetuximab is a chimeric human-murine IgG1 monoclonal antibody which binds to the extracellular domain of EGFR leading to the internalization of antibody-receptor complex and downregulation of EGFR expression. Cetuximab-EGFR complex formation inhibits proliferation and enhances apoptosis, as well as reduces angiogenesis, invasiveness and metastasis in tumours[99]. Also, cetuximab induces antibody-dependent cellular cytotoxicity (ADCC)[100] through NK and CD8+ T cells[101]. Cetuximab is approved by FDA[102] and EMA[103] as Erbitux® for the treatment of K-ras wild-type, EGFR-expressing metastatic colorectal cancer and for squamous cell carcinoma of the head and neck (SCCHN). Regarding GC, cetuximab is reported, alone or in combination with other drugs, in 20 clinical trials on clinicaltrials.gov: 1 in phase III, 14 in phase II, 2 in phase I/II, and three in phase I. In the phase II EXTRA trial (NCT00477711), 47 patients were enrolled with unresectable or metastatic GC from China. After administration of cisplatin, capecitabine and cetuximab, the ORR was 53.2%, mPFS 5.2 mo, and OS 10.8 mo. In the case of patients with EGFR strong expression longer PFS (7.1 mo) and OS (16.6 mo) were registered, as well as tumour reduction. Moreover, patients with high levels of transforming growth factor-alpha (TGF-α) presented better response and longer PFS (6.0 mo vs 2.7 mo, P = 0.001) and OS (12.9 mo vs 7.0 mo, P = 0.001) compared to patients with lower levels[104]. Still, comparing the administration of cetuximab with capecitabine and cisplatin and of capecitabine and cisplatin alone in EXPAND trial (NCT00678535), the addition of cetuximab to first-line chemotherapy provided no additional benefit in the treatment of GC. EXPAND is a phase III trial financed by Merck KGaA, in which were enrolled 904 patients with histologically confirmed locally advanced unresectable or metastatic GC and tested regimens were capecitabine and cisplatin with or without cetuximab. The progression-free survival (PFS) of patients receiving cetuximab comparing with those receiving only first-line chemotherapy was 4.4 mo vs 5.6 mo (P = 0.32), while overall survival (OS) was 9.4 mo vs 10.7 mo (P = 0.9547). Moreover, 54% of patients in the cetuximab group had any grade of serious adverse event vs 44% in the control group[105]. At present, cetuximab is implicated in several ongoing trials on GC involving NK cells (NCT03319459) or combinations with other monoclonal antibodies (NCT02318901).
Panitumumab is a human IgG2 monoclonal antibody that binds to EGFR and, similar to cetuximab, induce its internalization leading to increased apoptosis, reduced proliferation and reduced angiogenesis and metastasis[106,107]. Panitumumab is approved by FDA[108] and EMA[109] as Vectibix™ for treatment of colorectal cancer. In GC, panitumumab was tested in several clinical trials. In the open-label phase III REAL trial (NCT00824785) were enrolled 553 patients with untreated, metastatic, or locally advanced GC and a combination of epirubicin, oxaliplatin, and capecitabine (EOC) with/without panitumumab was tested. OS in patients that received EOC plus panitumumab was 8.8 mo vs 11.3 mo in patients that were allocated only EOC (P = 0.013). Moreover, EOC plus panitumumab induced more severe adverse effect. The study was interrupted in 2011 by independent data monitoring committee due to lack of efficacy, the addition of panitumumab to EOC chemotherapy being not recommended for use in an unselected population[110].
In phase II study (VEGA trial) were included 65 patients with metastatic GC. After allocation of oxaliplatin, leucovorin, and 5-fluorouracil (FOLFOX) plus panitumumab, registered ORR was 42%, PFS-5.6 mo, and mOS-11 mo[111].
The phase II study, reported by Tomasello et al[112], explored panitumumab in combination with docetaxel, cisplatin, folinic acid and 5-fluorouracil (DCF) administrated to 52 patients with HER2 negative locally advanced or metastatic GC. The registered ORR was 64% and mOS was 10 mo, still, the toxicity profile of this regimen limits its further development[113]. At present, on clinicaltrials.gov, panitumumab is used in 4 clinical trials on GC: 1 in phase III, 2 in phase II, and 1 in phase I/II from which one is ongoing.
Nimotuzumab (h-R3) is a humanized IgG1 monoclonal antibody[114] that binds to the extracellular domain of EGFR and inhibits EGFR-dependent transformation of cells proving also anti-proliferative, pro-apoptotic, and antiangiogenic activity[63]. Although nimotuzumab is less toxic than other anti-EGFR antibodies[64], it is still not approved by FDA or EMA. At present, in GC, nimotuzumab is reported in four clinical trials on clinicaltrials.gov: 1 in phase III and 3e in phase II. In a phase II randomized trial (NCT02370849) were enrolled 62 patients with untreated unresectable or metastatic GC which received S-1 and cisplatin with or without nimotuzumab. The ORR was 54.8% for patients receiving S-1, cisplatin, and nimotuzumab compared with 58.1% for those receiving S-1 and cisplatin alone (P = 0.798), mPFS were 4.8 mo vs 7.2 mo (P = 0.011), while OS were 10.2 mo vs 14.3 mo (P = 0.062). The addition of nimotuzumab to S-1 and cisplatin regimen offered no additional benefit in the first-line treatment of GC[115]. Also, nimotuzumab proved no advantage in combination with irinotecan (a phase II study on 82 irinotecan-naive patients with Adv GC), with registered PFS of 73.0 d vs 85.0 d (P = 0.5668), and mOS of 250.5 d vs 232.0 d (P = 0.9778) for the patients receiving irinotecan with nimotuzumab vs those receiving irinotecan alone. Still, this combination presented a potential improvement in the EGFR 2+/3+ group (PFS: 118.5 d vs 59.0 d and OS: 358.5 d vs 229.5 d)[116].
Trastuzumab is a humanised IgG1 monoclonal antibody that targets the extracellular domain of HER2 and prevents HER2 shedding, inhibits MAPK and PI3K/Akt pathways, and induces ADCC by activating NK cells[117,118]. Trastuzumab is approved by FDA[119] and by EMA[120] for treatment of breast cancer and of metastatic GC. At present, on clinicaltrials.gov, there are 67 studies on GC including trastuzumab: 2 in phase IV, 5 in phase III, 2 in phase II/III, 29 in phase II, 8 in phase I/II (including 2 ongoing studies that imply NK cells) and 10 studies in phase I. In ToGA study (phase III, NCT01041404), trastuzumab administrated with fluoropyrimidine/cisplatin improved PFS (6.7 vs 5.5, P = 0.0002) and mOS (13.8 vs 11.1, P = 0.0046) in patients with HER2+ advanced GC[121]. In a phase II study including patients with HER2+ advanced GC (NCT01228045), the use of trastuzumab with S-1 and cisplatin induced mOS of 14.6 mo and mPFS of 7.4 mo, at 6 mo 62.6% of patients being free from disease progression, this combination proving good activity and being well tolerated[122]. The combination of trastuzumab with oxaliplatin/capecitabine in patients with HER2+ advanced GC was evaluated in other phase II clinical trial (CGOG1001, NCT01364493). The registered PFS and mOS were 9.2 and 19.5 mo, respectively. Patients with a greater HER2/CEP17 ratio proved a better OS (20.9 mo vs 19.5 mo, P = 0.001)[123].
In a retrospective study reported by Palle et al[124] were evaluated 104 patients with HER2 + GC who received after first-line chemotherapy (fluoropyrimidine and cisplatin or oxaliplatin, plus trastuzumab), one of the following second line chemotherapy regimens: FOLFIRI, taxane (paclitaxel or docetaxel) or platinum-based chemotherapy (different from that used in first-line), with or without trastuzumab. The continuation of trastuzumab was associated with an increased PFS (4.4 mo vs 2.3 mo, P = 0.002) and OS (12.6 mo vs 6.1 mo, P = 0.001). Therefore the administration of trastuzumab in combination with second line chemotherapy proves clinical benefit in patients with HER2+ advanced GC[124].
Pertuzumab is a humanized monoclonal antibody that inhibits HER2 by preventing its dimerization and acts at different epitope than trastuzumab[125]. Pertuzumab is approved by FDA[126] and by EMA[127] for treatment of HER2-positive breast cancer. Regarding gastric cancer pertuzumab is implicated in 5 clinical trials (4 ongoing) reported on clinicaltrials.gov: one in phase III, one in phase II/III and 3 in phase II, but still proved no benefit, alone or in combination with other drugs.
Lapatinib is a tyrosine kinase inhibitor that inhibits both HER2 and EGFR[128]. It is approved by FDA as Tykerb®[129] and by EMA as Tyverb[130] for breast cancer treatment. In gastric cancer, lapatinib is included in 10 clinical trials on clinicaltrials.gov: 2 in phase III, 6 in phase II and 2 in phase I. In the phase III TyTAN trial (NCT00486954), including Asian patients with Her2+ GC, mOS/PFS were 11.0/5.4 mo in patients receiving lapatinib plus paclitaxel vs 8.9/4.4 mo in patients receiving paclitaxel alone (P = 0.1044), the combination of lapatinib and paclitaxel proving activity in the second-line treatment of patients with HER2+ advanced GC[131].
VEGF signaling pathway: VEGF signaling represents an essential factor for tumour angiogenesis and lymphangiogenesis being involved in growth, invasion, and metastatic spread of solid neoplasms. VEGF family consists of 7 members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF (placental growth factor), which act through specific tyrosine kinase receptors (VEGF VEGFR1, VEGFR2, and VEGFR3). VEGF expression and serum levels are correlated with advanced stage disease and poor outcome in gastric cancer patients. Preclinical studies showed that EGFR2 inhibition decreased tumor growth and angiogenesis[132,133]. The most important approaches for targeting VEGF signaling use the monoclonal antibodies Bevacizumab (anti-VEGF-A mAb), and Ramucirumab (anti-VEGFR-2 mAb) and also VEGFR-targeted tyrosine kinase inhibitors: Sunitinib, Sorafenib, Cediranib, and Apatinib[134].
Bevacizumab (Avastin) is a humanized monoclonal antibody that targets VEGF proving an anti-angiogenic effect[135]. It is approved by FDA[136] and EMA[137] for treatment of metastatic cancer of the colon (large intestine) or rectum, metastatic breast cancer, advanced non-small cell lung cancer, advanced or metastatic kidney cancer, epithelial cancer of the ovary, cancer of the fallopian tube or the peritoneum) and persistent, recurrent or metastatic cervix cancer. Regarding GC, bevacizumab is implicated in 23 clinical trials reported on clinicaltrials.gov: 1 in phase IV (status unknown), 2 in phase III, 16 in phase II, 2 in phase I/II and 2 in phase I. In the phase III AVAGAST trial (NCT00548548) the addition of bevacizumab to capecitabine, 5-fluorouracil and cisplatin regiment improved PFS (6.7 mo vs 5.3 mo) and mOS (12.1 mo vs 10.1 mo) of patients with locally advanced or metastatic GC. The obtained results highlighted the influence of regional healthcare environment on overall survival differences observed in this study[138]. Based on these conclusions, in the phase III AVATAR study (NCT00887822), bevacizumab was tested in combination with capecitabine and cisplatin in Chinese patients with advanced gastric cancer. PFS and OS were similar in both tested arms (with/without bevacizumab): 6.3 vs 6.1 (P = 0.3685) and 10.5 vs 11.4 (P = 0.8636) respectively, and no new safety signals were reported comparing with AVAGAST study[139]. In a phase II study reported by Kim et al[60], bevazicumab was tested in combination with docetaxel, capecitabine, and cisplatin on patients with unresectable locally advanced or metastatic GC. The registered mPFS and OS were 13.1 mo and 38.6 mo, respectively[60]. In GASTRIC-3 trial, the combination of bevacizumab with oxaliplatin and irinotecan followed by docetaxel, followed by bevacizumab maintenance, was tested on patients with advanced GC. PFS was 7.0 mo and mOS was 11 mo. Notable, two patients continued on bevacizumab maintenance for more than 5 years showing long-term tumour remission. As such, the regimen formed of oxaliplatin/irinotecan and docetaxel plus bevacizumab followed by bevacizumab maintenance is feasible for metastatic GC treatment[61].
Ramucirumab is a humanized monoclonal antibody that targets selectively VEGFR2 and inhibits angiogenesis[140]. It is approved by FDA[141] and EMA[142] for advanced GC, metastatic colorectal cancer, and non-small cell lung cancer treatment. The clinical phase III studies REGARD (NCT00917384) (best-supportive care with/without ramucirumab) and RAINBOW (NCT01170663) (paclitaxel with/without ramucirumab) demonstrated significant survival benefits and manageable toxicity of ramucirumab administration in patients with advanced GC[63,64,114,143,144]. Based on the results obtained in RAINBOW trial, Ramucirumab has been approved by FDA as a single agent or in association with paclitaxel for the treatment of the patients with advanced or metastatic gastric and gastroesophageal junction[95]. At present, on clinicaltrials.gov ramucirumab is implicated in 19 studies: 5 in phase III, one in phase II/III, 6 in phase II, 3 in phase I/II, and 4 in phase I.
Apatinib (YN968D1) is a selective inhibitor for VEGFR2, also slightly inhibiting c-Kit and c-Src tyrosine kinases[145]. Currently it is involved in 39 clinical trials on GC according to clinicaltrials.gov: 3 in phase IV, 4 in phase III, 5 in phase II/III, 17 in phase II, 1 in phase I/II and 3 in phase I. In a phase III clinical trial (NCT01512745) involving patients with advanced GC, apatinib showed an improved PFS (2.6 mo vs 1.8 mo, P = 0.0156) and mOS (6.5 mo vs 4.7 mo, P = 0.0149) comparing with placebo[146,147]. Administration of apatinib induces progression-free survival rather than post-progression survival[148]. In an observational study, apatinib seems to be more effective in patients previously treated with antiangiogenic therapy[149].
In conclusion, considering all the studies presented, it can be ascertain that drugs directed against VEGFR-2 are more effective than those targeting VEGF-A
PI3K/AKT/mTOR signaling pathway: This signaling cascade is frequently perturbed in human cancers being associated with therapy resistance. Mutations and/or amplifications of PIK3CA gene or loss of function of tumour suppressor protein PTEN were identified in GC, especially in EBV and the MSI subtypes[1,150]. However, phase III and II studies evaluating inhibitors of mTOR (Everolimus) and AKT (MK-2206) respectively, reported negative results. It should be noted that in these studies the patients were not selected based on PI3K signaling pathway activation[151,152]. The AKT inhibitor AZD5363 effects are being evaluated in combination with paclitaxel in patients with advanced gastric adenocarcinoma harboring PIK3CA mutation or amplification as a second line chemotherapy (NCT02451956). As well, AZD2014, an mTOR inhibitor that targets both mTORC1 and mTORC2 complexes[153], is implicated in phase II clinical studies as second-line chemotherapy.
Everolimus (RAD001) is a rapamycin analog that targets mTOR[154]. It is approved by FDA[155] and by EMA[156] as Afinitor® for breast cancer, pancreatic neuroendocrine tumours, neuroendocrine tumors originating in the lungs or gut, and for advanced renal cell carcinoma treatment. In GC everolimus is tested in 14 clinical trials on clinicaltrials.gov: 2 in phase III, 5 in phase II, 3 in phase I/II and 4 in phase I. Everolimus was evaluated in combination with paclitaxel in a phase III trial (NCT01248403) in patients with advanced GC pretreated with fluoropyrimidines-containing regimen, the obtained PFS and mOS in patients that received paclitaxel with everolimus compared to those that received only paclitaxel being 2.2 mo vs 2.07 mo (P = 0.3) and 6.1 vs 5.1 mo (P = 0.48), respectively[68]. As a result, the addition of everolimus to paclitaxel does not improve the outcomes in pretreated metastatic GC. In other phase III study (GRANiTE-1, NCT00879333), everolimus efficacy and safety was compared with that of best supportive care (BSC) in patients with previously treated advanced GC. PFS were 1.7 mo vs 1.4 mo for patients that received everolimus vs placebo group, while mOS were 5.4 mo vs 4.3 mo (P = 0.124), respectively[151].
HGF/HGFR signaling: Hepatocyte growth factor (HGF) and its receptor encoded by the MET gene, play key roles in tumour growth through activated signaling pathways in GC cells. Genomic amplification of MET was identified in 4% of patients with gastric cancer and is related to survival. Moreover, preclinical studies suggest that inhibition of MET expression using antibodies or small-molecule inhibitors suppress tumor-cell proliferation and tumor progression in MET-amplified GC cells. Several molecules targeting the MET/HGF signaling have been developed, including monoclonal antibodies [against either HGF (Rilotumumab), or MET (Onartuzumab)] and MET small kinase inhibitors (Foretinib). These inhibitors were evaluated in clinical trials as target therapies for metastatic or unresectable gastric cancer[157]. Unfortunately, negative results were obtained in several phases II and III clinical trials due to a higher rate of serious toxicities or an increase in the number of deaths[158]. The failure of these trials could be explained by an inappropriate selection of the patients that could benefit from the therapy, emphasizing the need to identify new inhibitors for this molecule.
Onartuzumab is an E. coli-derived humanized monoclonal antibody that targets MET by binding to the extracellular domain of the receptor and inhibits HGF binding[159]. At the moment it is involved in two clinical trials on GC on clinicaltrials.gov: one in phase III and one in phase II. In the phase II study (NCT01590719) onartuzumab was investigated in combination with mFOLFOX6 in patients with metastatic EGFR2-negative GC. In mFOLFOX6 plus onartuzumab group, the PFS and mOS were 6.77 and 10.61 mo, respectively, while in the mFOLFOX6 plus placebo group, the PFS and mOS were 6.97 (P = 0.71) and 11.27 mo (P = 0.83), respectively. In the MET+ population, PFS were 5.95 mo vs 6.80 mo (P = 0.45) and mOS were 8.51 mo vs 8.48 mo (P = 0.80) in the onartuzumab vs placebo group. Therefore, the addition of onartuzumab to mFOLFOX6 regiment did not improve the outcome of GC patients, including MET+ ones[160].
Rilotumumab (AMG102) is a human IgG2 monoclonal antibody that targets HGF and inhibits HGF/MET signaling[161]. Althought at present on clinicaltrials.gov are mentioned 5 trials on GC that use rilotumumab, only one of them is ongoing - a phase II study that evaluate the combination of FOLFOX with AMG 102 or Panitumumab. Two studies (phase III), RILOMET 1 (NCT01697072) and RILOMET 2 (NCT02137343) were stopped early by Amgen (rilotumumab developer) due to higher number of deaths in the rilotumumab group than in the placebo group[161,162].
Even if there are a lot of clinical trials testing a wide range of inhibitory molecules in GC patients, Trastuzumab and Ramucirumab (targeting HER2 and VEGFR2 respectively) are the only targeted therapies approved so far.
Given the large number of clinical trials currently taking place, one may ask why only two compounds have been validated for GC therapy. How can be explain these many failures? First of all, these drawbacks may be due to the high degree of intra-tumour heterogeneity frequently displayed by GC and to the fact that most often clinical trials did not performed a preliminary patient selection for the molecular alteration of the target, a mandatory issue if it is desired to test the efficacy of certain inhibitor to the given target. From this point of view, the recent in-depth molecular studies that allowed stratification of patients in GC subtypes based on genomic alteration, represent a substantial advance as well as a powerful tool for targeting therapy. The implementation of the novel system in which GC patients can be classified in molecular subtypes will bring a real progress in clinical trials, allowing the evaluation of the efficiency of each compound in specific molecular subtypes. Moreover, the new perspective opened by the successful use of immune checkpoints inhibitors in PD-1 and PD-L1/2 positive GC like EBV positive, MSS/TP53+ or MSI subtypes, can open the way for combination of emerging immunotherapies with molecularly targeted drugs, and these findings could have important translational relevance in the field of GC therapy. Finally, we can conclude that in the attempt to improve the quality of medical care, we have to increase the speed in GC research fields and to develop new therapeutic approaches with high clinical benefits and minimum adverse effects.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Skierucha M, Sitarz R S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4800] [Article Influence: 436.4] [Reference Citation Analysis (2)] |
| 2. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4571] [Article Influence: 240.6] [Reference Citation Analysis (0)] |
| 3. | Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 4. | Cui J, Li F, Wang G, Fang X, Puett JD, Xu Y. Gene-expression signatures can distinguish gastric cancer grades and stages. PLoS One. 2011;6:e17819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Chivu Economescu M, Necula LG, Dragu D, Badea L, Dima SO, Tudor S, Nastase A, Popescu I, Diaconu CC. Identification of potential biomarkers for early and advanced gastric adenocarcinoma detection. Hepatogastroenterology. 2010;57:1453-1464. [PubMed] |
| 6. | Wang Z, Chen G, Wang Q, Lu W, Xu M. Identification and validation of a prognostic 9-genes expression signature for gastric cancer. Oncotarget. 2017;8:73826-73836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 8. | Zhou Z, Zhu JS, Gao CP, Li LP, Zhou C, Wang H, Liu XG. siRNA targeting YAP gene inhibits gastric carcinoma growth and tumor metastasis in SCID mice. Oncol Lett. 2016;11:2806-2814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Gencer S, Cebeci A, Irmak-Yazicioglu MB. Silencing of the MMP-3 gene by siRNA transfection in gastric cancer AGS cells. J Gastrointestin Liver Dis. 2011;20:19-26. [PubMed] |
| 10. | Chivu-Economescu M, Dragu DL, Necula LG, Matei L, Enciu AM, Bleotu C, Diaconu CC. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Das K, Chan XB, Epstein D, Teh BT, Kim KM, Kim ST, Park SH, Kang WK, Rozen S, Lee J. NanoString expression profiling identifies candidate biomarkers of RAD001 response in metastatic gastric cancer. ESMO Open. 2016;1:e000009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Jamieson NB, Maker AV. Gene-expression profiling to predict responsiveness to immunotherapy. Cancer Gene Ther. 2017;24:134-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 510] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 14. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1559] [Article Influence: 155.9] [Reference Citation Analysis (0)] |
| 15. | Ascierto PA, Addeo R, Cartenì G, Daniele B, De Laurentis M, Ianniello GP, Morabito A, Palmieri G, Pepe S, Perrone F. The role of immunotherapy in solid tumors: report from the Campania Society of Oncology Immunotherapy (SCITO) meeting, Naples 2014. J Transl Med. 2014;12:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 17. | Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011;141:476-485, 485.e1-485.11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, Chua C, Feng Z, Guan YK, Ooi CH. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 19. | Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7:32925-32932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 20. | Falchetti M, Saieva C, Lupi R, Masala G, Rizzolo P, Zanna I, Ceccarelli K, Sera F, Mariani-Costantini R, Nesi G. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Riquelme I, Saavedra K, Espinoza JA, Weber H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC, Bizama C. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget. 2015;6:24750-24779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Corso G, Carvalho J, Marrelli D, Vindigni C, Carvalho B, Seruca R, Roviello F, Oliveira C. Somatic mutations and deletions of the E-cadherin gene predict poor survival of patients with gastric cancer. J Clin Oncol. 2013;31:868-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Shepard B, Yoder L, Holmes C. Prophylactic Total Gastrectomy for Hereditary Diffuse Gastric Cancer. ACG Case Rep J. 2016;3:e179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (3)] |
| 25. | Voron T, Messager M, Duhamel A, Lefevre J, Mabrut J-Y, Goere D, Meunier B, Brigand C, Hamy A, Glehen O. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer. 2016;19:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Yao F, Kausalya JP, Sia YY, Teo AS, Lee WH, Ong AG, Zhang Z, Tan JH, Li G, Bertrand D. Recurrent Fusion Genes in Gastric Cancer: CLDN18-ARHGAP26 Induces Loss of Epithelial Integrity. Cell Rep. 2015;12:272-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 28. | Garattini SK, Basile D, Cattaneo M, Fanotto V, Ongaro E, Bonotto M, Negri FV, Berenato R, Ermacora P, Cardellino GG. Molecular classifications of gastric cancers: Novel insights and possible future applications. World J Gastrointest Oncol. 2017;9:194-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Sever R, Brugge JS. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 602] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 30. | Choong ML, Pecquet C, Pendharkar V, Diaconu CC, Yong JW, Tai SJ, Wang SF, Defour JP, Sangthongpitag K, Villeval JL. Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J Cell Mol Med. 2013;17:1397-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 1997;16:6478-6485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, Neuberg D, Shipp MA. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 567] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 33. | Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140-1145. [PubMed] |
| 34. | Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin Cancer Res. 2016;22:2329-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 35. | Doi T, Piha-Paul SA, Jalal SI, Mai-Dang H, Saraf S, Koshiji M, Csiki I, Bennouna J. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol. 2016;34:7-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Fuchs CS, Doi T, Jang RW-J, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol. 2017;35:4003-4003. [RCA] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 37. | Merck. Merck Provides Update on KEYNOTE-061. A Phase 3 Study of KEYTRUDA® (pembrolizumab) in Previously Treated Patients with Gastric or Gastroesophageal Junction Adenocarcinoma. 2017; Available from: http://investors.merck.com/news/press-release-details/2017/Merck-Provides-Update-on-KEYNOTE-061-a-Phase-3-Study-of-KEYTRUDA-pembrolizumab-in-Previously-Treated-Patients-with-Gastric-or-Gastroesophageal-Junction-Adenocarcinoma/default.aspx.. |
| 38. | Janjigian YY, Bendell JC, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Bono P, Jaeger D, Evans TRJ. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). J Clin Oncol. 2016;34:4010-4010. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Kang YK, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yoshikawa T. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol. 2017;35:2-2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Kaufman H, Russell JS, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, Shih KC, Lebbe C, Linette GP, Milella M. Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic Merkel cell carcinoma previously treated with chemotherapy: Results of the phase 2 JAVELIN Merkel 200 trial. J Clin Oncol. 2016;34:9508-9508. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3202] [Article Influence: 400.3] [Reference Citation Analysis (0)] |
| 42. | Bang YJ, Golan T, Lin CC, Kang YK, Wainberg ZA, Wasserstrom H, Jin J, Mi G, McNeely S, Laing N. Interim safety and clinical activity in patients (pts) with locally advanced and unresectable or metastatic gastric or gastroesophageal junction (G/GEJ) adenocarcinoma from a multicohort phase I study of ramucirumab (R) plus durvalumab (D). J Clin Oncol. 2018;36:92-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Baylis F, McLeod M. First-in-human Phase 1 CRISPR Gene Editing Cancer Trials: Are We Ready? Curr Gene Ther. 2017;17:309-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Lin CC, Golan T, Corral J, Moreno V, Chung HC, Wasserstrom H, Yang J, Mi G, Bang YJ. 2OPhase 1 study of ramucirumab (R) plus durvalumab (D) in patients (pts) with locally advanced and unresectable or metastatic gastrointestinal or thoracic malignancies (NCT02572687); Phase 1a results. Ann Surg Oncol. 2016;27:mdw525.501-mdw525.501. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Kelly RJ, Chung K, Gu Y, Steele KE, Rebelatto MC, Robbins PB, Tavakkoli F, Karakunnel JJ, Lai DW, Almhanna K. Phase Ib/II study to evaluate the safety and antitumor activity of durvalumab (MEDI4736) and tremelimumab as monotherapy or in combination, in patients with recurrent or metastatic gastric/gastroesophageal junction adenocarcinoma. J ImmunoTher Cancer. 2015;3:P157-P157. |
| 46. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7191] [Article Influence: 719.1] [Reference Citation Analysis (0)] |
| 47. | Malka D, Castan F, Francois E, Bouche O, Bennouna J, Ghiringhelli F, Fouchardiere CDL, Borg C, Samalin E, Bachet JB. FOLFOX alone or combined to rilotumumab or panitumumab as first-line treatment in patients (pts) with advanced gastroesophageal adenocarcinoma (AGEA): An open-label, randomized phase II trial (PRODIGE 17 ACCORD 20 MEGA). J Clin Oncol. 2015;33:4013-4013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Shah MA, Xu RH, Bang YJ, Hoff PM, Liu T, Herráez-Baranda LA, Xia F, Garg A, Shing M, Tabernero J. HELOISE: Phase IIIb Randomized Multicenter Study Comparing Standard-of-Care and Higher-Dose Trastuzumab Regimens Combined With Chemotherapy as First-Line Therapy in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma. J Clin Oncol. 2017;35:2558-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 49. | Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS, Park SR, Han HS, Chung IJ, Song EK, Lee KH. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer. 2015;51:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 50. | Qin S, Lin S, Xu R, Su W, Tang Y, Wang X, Xu J-m, Zhou W, Cui T, Luo S, Wu X, Ji J. Treatment patterns, effectiveness, and safety of Trastuzumab in Chinese patients with metastatic gastric cancer: Interim analysis of the EVIDENCE registry study. J Clin Oncol. 2017;35:e15595-e15595. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Thuss-Patience PC, Shah MA, Ohtsu A, Van Cutsem E, Ajani JA, Castro H, Mansoor W, Chung HC, Bodoky G, Shitara K. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 52. | Tabernero J, Hoff P, Shen L, Ohtsu A, Shah M, Cheng K, Song C, Wu H, Eng-Wong J, Kang Y. 616OPertuzumab (P)+ trastuzumab (H)+ chemotherapy (CT) for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (mGC/GEJC): Final analysis of a Phase III study (JACOB). Ann Surg Oncol. 2017;28. |
| 53. | Kang YK, Rha SY, Tassone P, Barriuso J, Yu R, Szado T, Garg A, Bang YJ. A phase IIa dose-finding and safety study of first-line pertuzumab in combination with trastuzumab, capecitabine and cisplatin in patients with HER2-positive advanced gastric cancer. Br J Cancer. 2014;111:660-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Wagner AD, Kang Y-K, Dieren Jv, Mauer ME, Grabsch HI, Lia M, Atasoy A, Cho JY, Moehler MH, Roth A, Salto-Tellez M, Schumacher C, Grieken NCTv, Sandick JWv, Lordick F, on behalf of the EORTC GI Tract Cancer Group KCSG, Group tDUGC. EORTC-1203: Integration of trastuzumab (T), with or without pertuzumab (P), into perioperative chemotherapy (CT) of HER-2 positive stomach cancer—INNOVATION trial. J Clin Oncol. 2016;34:TPS4133-TPS4133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Catenacci DVT, Park H, Lockhart AC, Gold PJ, Enzinger PC, Nordstrom JL, Hong S, Hochster HS, Kelly RJ, Uronis HE. Phase 1b/2 study of margetuximab (M) plus pembrolizumab (P) in advanced HER2+ gastroesophageal junction (GEJ) or gastric (G) adenocarcinoma (GEA). J Clin Oncol. 2018;18. |
| 56. | Bang YJ, Giaccone G, Im SA, Oh DY, Bauer TM, Nordstrom JL, Li H, Chichili GR, Moore PA, Hong S. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 57. | Hecht JR, Bang YJ, Qin SK, Chung HC, Xu JM, Park JO, Jeziorski K, Shparyk Y, Hoff PM, Sobrero A. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol. 2016;34:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 58. | Chu MP, Hecht JR, Slamon D, Wainberg ZA, Bang YJ, Hoff PM, Sobrero A, Qin S, Afenjar K, Houe V. Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 2017;3:767-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Kim JH, Park SR, Ryu MH, Ryoo BY, Kim KP, Kim BS, Yoo MW, Yook JH, Kim BS, Kim J. Phase II Study of Induction Chemotherapy with Docetaxel, Capecitabine, and Cisplatin Plus Bevacizumab for Initially Unresectable Gastric Cancer with Invasion of Adjacent Organs or Paraaortic Lymph Node Metastasis. Cancer Res Treat. 2018;50:518-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Woll E, Thaler J, Keil F, Gruenberger B, Hejna M, Eisterer W, Fridrik MA, Ulmer H, Trommet V, Huemer F. Oxaliplatin/Irinotecan/Bevacizumab Followed by Docetaxel/Bevacizumab in Inoperable Locally Advanced or Metastatic Gastric Cancer Patients - AGMT_GASTRIC-3. Anticancer Res. 2017;37:5553-5558. |
| 61. | Enzinger PC, Abrams TA, Chan JA, McCleary NJ, Zheng H, Kwak EL, Yurgelun M, Blaszkowsky LS, Cleary JM, Wolpin BM. Multicenter phase 2: Capecitabine (CAP) + oxaliplatin (OX) + bevacizumab (BEV) + trastuzumab (TRAS) for patients (pts) with metastatic esophagogastric cancer (MEGCA). J Clin Oncol. 2015;33:4038-4038. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 62. | Al-Batran SE, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov ON, Kim TY, Cunningham D. Quality-of-life and performance status results from the phase III RAINBOW study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated gastric or gastroesophageal junction adenocarcinoma. Ann Oncol. 2016;27:673-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Shitara K, Muro K, Shimada Y, Hironaka S, Sugimoto N, Komatsu Y, Nishina T, Yamaguchi K, Segawa Y, Omuro Y. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer. 2016;19:927-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 64. | Fuchs CS, Tabernero J, Al-Batran S-E, Chau I, Ilson DH, Cutsem EV, Shitara K, Ferry D, Emig M, Vanvoorden V. A randomized, double-blind, placebo-controlled phase III study of cisplatin plus a fluoropyrimidine with or without ramucirumab as first-line therapy in patients with metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma (RAINFALL, NCT02314117). J Clin Oncol. 2016;34:TPS178-TPS178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Sjoquist KM, Pavlakis N, Martin AJ, Tsobanis E, Yip S, Bang Y-J, Alcindor T, O’Callaghan CJ, Shitara K, Bekaii-Saab TS. Integrate II: A randomised phase 3 double-blind placebo-controlled study of regorafenib in refractory advanced gastro-oesophageal cancer (AGOC)—An international study organized by the Australasian Gastrointestinal Trials Group (AGITG). J Clin Oncol. 2017;35:TPS4136-TPS4136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Janjigian YY, Ku GY, Chou JF, Capanu M, Siebel M, Chalasani SB, Boyar MS, Goldberg Z, Desai AM, Kelsen DP. Phase II study of FOLFOX plus regorafenib (REGO) in patients with unresectable or metastatic esophagogastric (EG) cancer. J Clin Oncol. 2015;33:4053-4053. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Al-Batran S-E, Riera-Knorrenschild J, Pauligk C, Goetze TO, Hegewisch-Becker S, Seraphin J, Thuss-Patience PC, Kopp H-G, Dechow TN, Vogel A. A randomized, double-blind, multicenter phase III study evaluating paclitaxel with and without RAD001 in patients with gastric cancer who have progressed after therapy with a fluoropyrimidine/platinum-containing regimen (RADPAC). J Clin Oncol. 2017;35:4-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Shah MA, Bang Y-J, Lordick F, Tabernero J, Chen M, Hack SP, Phan S-C, Shames DS, Cunningham D. METGastric: A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC). J Clin Oncol. 2015;33:4012-4012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 69. | Shah MA, Bang YJ, Lordick F, Alsina M, Chen M, Hack SP, Bruey JM, Smith D, McCaffery I, Shames DS. Effect of Fluorouracil, Leucovorin, and Oxaliplatin With or Without Onartuzumab in HER2-Negative, MET-Positive Gastroesophageal Adenocarcinoma: The METGastric Randomized Clinical Trial. JAMA Oncol. 2017;3:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 70. | Massacesi C, Di Tomaso E, Urban P, Germa C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Oncotargets Ther. 2016;9:203-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 71. | Ang YLE, Yong WP, Tan P. Translating gastric cancer genomics into targeted therapies. Crit Rev Oncol Hemat. 2016;100:141-146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Takeda T, Banno K, Okawa R, Yanokura M, Iijima M, Irie-Kunitomi H, Nakamura K, Iida M, Adachi M, Umene K. ARID1A gene mutation in ovarian and endometrial cancers (Review). Oncol Rep. 2016;35:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Wu RC, Wang TL, Shih IeM. The emerging roles of ARID1A in tumor suppression. Cancer Biol Ther. 2014;15:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 74. | Giulino-Roth L, Wang K, MacDonald TY, Mathew S, Tam Y, Cronin MT, Palmer G, Lucena-Silva N, Pedrosa F, Pedrosa M. Targeted genomic sequencing of pediatric Burkitt lymphoma identifies recurrent alterations in antiapoptotic and chromatin-remodeling genes. Blood. 2012;120:5181. |
| 75. | Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat genet. 2012;44:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1146] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 76. | Balbás-Martínez C, Rodríguez-Pinilla M, Casanova A, Domínguez O, Pisano DG, Gómez G, Lloreta J, Lorente JA, Malats N, Real FX. ARID1A Alterations Are Associated with FGFR3-Wild Type, Poor-Prognosis, Urothelial Bladder Tumors. PLoS One. 2013;8:e62483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 77. | An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Gatalica Z, Vranic S, Xiu J, Swensen J, Reddy S. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. FAM CANCER. 2016;15:405-412. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Diaz LA, Marabelle A, Delord J-P, Shapira-Frommer R, Geva R, Peled N, Kim TW, Andre T, Cutsem EV, Guimbaud R. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J Clin Oncol. 2017;35:3071-3071. [RCA] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 80. | Cho J, Lee J, Bang H, Kim ST, Park SH, An JY, Choi MG, Lee JH, Sohn TS, Bae JM. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. 2017;8:13320-13328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 81. | Cavallo J. FDA’s First Site-Agnostic Drug Approval Marks a Paradigm Shift in Regulatory Criteria. The ASCO Post. 2017;. |
| 82. | Skierucha M, Milne AN, Offerhaus GJ, Polkowski WP, Maciejewski R, Sitarz R. Molecular alterations in gastric cancer with special reference to the early-onset subtype. World J Gastroenterol. 2016;22:2460-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011;1:482-497. [PubMed] |
| 84. | Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res. 2000;20:4489-4493. [PubMed] |
| 85. | Saito H, Fushida S, Harada S, Miyashita T, Oyama K, Yamaguchi T, Tsukada T, Kinoshita J, Tajima H, Ninomiya I. Importance of human peritoneal mesothelial cells in the progression, fibrosis, and control of gastric cancer: inhibition of growth and fibrosis by tranilast. Gastric Cancer. 2018;21:55-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Hebbard PC, Macmillan A, Huntsman D, Kaurah P, Carneiro F, Wen X, Kwan A, Boone D, Bursey F, Green J. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol. 2009;16:1890-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 827] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 88. | Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 89. | Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 90. | Zhou J, Hayakawa Y, Wang Timothy C, Bass Adam J. RhoA Mutations Identified in Diffuse Gastric Cancer. Cancer Cell. 2014;26:9-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 91. | Kang WK, Lee I, Park C. Characterization of RhoA-mediated chemoresistance in gastric cancer cells. Cancer Res Treat. 2005;37:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Choi YY, Noh SH, Cheong JH. Molecular Dimensions of Gastric Cancer: Translational and Clinical Perspectives. J Pathol Transl Med. 2016;50:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget. 2012;3:1068-1111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 94. | Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget. 2017;8:57654-57669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 95. | Kim JW, Im SA, Kim M, Cha Y, Lee KH, Keam B, Kim MA, Han SW, Oh DY, Kim TY. The prognostic significance of HER2 positivity for advanced gastric cancer patients undergoing first-line modified FOLFOX-6 regimen. Anticancer Res. 2012;32:1547-1553. [PubMed] |
| 96. | Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 97. | Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M; ACTS-GC Group. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992-6000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 98. | Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 665] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 99. | Harding J, Burtness B. Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today (Barc). 2005;41:107-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39:271-278. [PubMed] |
| 101. | Wang L, Wei Y, Fang W, Lu C, Chen J, Cui G, Diao H. Cetuximab Enhanced the Cytotoxic Activity of Immune Cells during Treatment of Colorectal Cancer. Cell Physiol Biochem. 2017;44:1038-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | FDA Approval for Cetuximab 2012. Updated: 2 Jul 2013. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/fda-cetuximab#Anchor-Hea-5647. |
| 103. | EMA. Erbitux 2009, Updated:19 Jul 2017. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000558/human_med_000769.jsp. |
| 104. | Zhang X, Xu J, Liu H, Yang L, Liang J, Xu N, Bai Y, Wang J, Shen L. Predictive biomarkers for the efficacy of cetuximab combined with cisplatin and capecitabine in advanced gastric or esophagogastric junction adenocarcinoma: a prospective multicenter phase 2 trial. Med Oncol. 2014;31:226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 672] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 106. | Dubois EA, Cohen AF. Panitumumab. Br J Clin Pharmacol. 2009;68:482-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Messersmith WA, Hidalgo M. Panitumumab, a Monoclonal Anti-Epidermal Growth Factor Receptor Antibody in Colorectal Cancer: Another One or the One? Clin Cancer Res. 2007;13:4664-4666. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | FDA Approval for Panitumumab 2006. Updated: 3 Jul 2013. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/fda-panitumumab. |
| 109. | EMA. Vectibix 2009, Updated:27 Feb 2018. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000741/human_med_001128.jspmid=WC0b01ac058001d124. |
| 110. | Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 575] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 111. | Formica V, Casaretti R, Comella G, Carlomagno C, Maiorino L, Greco E, Russo A, Sanna G, Barzelloni Maria L, Massidda B. P-096The panitumumab with FOLFOX4 in metastatic gastric or gastroesophageal junction adenocarcinoma (mGA) - VEGA trial. Efficacy and safety outcomes of a phase II S.I.C.O.G. study. Ann Oncol. 2017;28:mdx261.095-mdx261.095. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 112. | Tomasello G, Valeri N, Ghidini M, Smyth EC, Liguigli W, Toppo L, Mattioli R, Curti A, Hahne JC, Negri FM. First-line dose-dense chemotherapy with docetaxel, cisplatin, folinic acid and 5-fluorouracil (DCF) plus panitumumab in patients with locally advanced or metastatic cancer of the stomach or gastroesophageal junction: final results and biomarker analysis from an Italian oncology group for clinical research (GOIRC) phase II study. Oncotarget. 2017;8:111795-111806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 113. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1752] [Article Influence: 159.3] [Reference Citation Analysis (0)] |
| 114. | Du F, Zheng Z, Shi S, Jiang Z, Qu T, Yuan X, Sun Y, Song Y, Yang L, Zhao J. S-1 and Cisplatin With or Without Nimotuzumab for Patients With Untreated Unresectable or Metastatic Gastric Cancer: A Randomized, Open-Label Phase 2 Trial. Medicine (Baltimore). 2015;94:e958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 115. | Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, Yasui H, Kim TY, Yamaguchi K, Fuse N. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric Cancer. 2015;18:824-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 116. | Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977-984. [RCA] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 117. | Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol. 2012;2:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 385] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 118. | FDA Approval for Trastuzumab 2010. Updated: 3 Jul 2013. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/fda-trastuzumab.. |
| 119. | EMA. Herceptin (trastuzumab) 2009, Updated: 13 Dec 2017. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000278/human_med_000818.jspmid=WC0b01ac058001d124.. |
| 120. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5269] [Article Influence: 351.3] [Reference Citation Analysis (3)] |
| 121. | Chua C, Tan IB, Yamada Y, Rha SY, Yong WP, Ong WS, Tham CK, Ng M, Tai DW, Iwasa S. Phase II study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol. 2015;76:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, Wang J, Xu N, Cheng Y, Bai Y. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 123. | Palle J, Tougeron D, Pozet A, Soularue E, Artru P, Leroy F, Dubreuil O, Sarabi M, Williet N, Manfredi S. Trastuzumab beyond progression in patients with HER2-positive advanced gastric adenocarcinoma: a multicenter AGEO study. Oncotarget. 2017;8:101383-101393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 124. | Harbeck N, Beckmann MW, Rody A, Schneeweiss A, Müller V, Fehm T, Marschner N, Gluz O, Schrader I, Heinrich G. HER2 Dimerization Inhibitor Pertuzumab - Mode of Action and Clinical Data in Breast Cancer. Breast Care (Basel). 2013;8:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 125. | FDA. FDA grants regular approval to pertuzumab for adjuvant treatment of HER2-positive breast cancer 2017, Updated: 21 Dec 2017;. Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm590005.htm. |
| 126. | EMA. Perjeta (pertuzumab) 2013, Updated: 04 Jan 2018. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002547/human_med_001628.jspmid=WC0b01ac058001d124. |
| 127. | Segovia-Mendoza M, González-González ME, Barrera D, Díaz L, García-Becerra R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence. Am J Cancer Res. 2015;5:2531-2561. [PubMed] |
| 128. | FDA Approval for Lapatinib Ditosylate 2010. Updated: 14 Jan 2011. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/fda-lapatinib. |
| 129. | EMA. Tyverb (lapatinib) 2008, Updated:19 Oct 2017. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000795/human_med_001120.jspmid=WC0b01ac058001d124. |
| 130. | Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, Tsuji A, Omuro Y, Li J, Wang JW. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 487] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 131. | Macedo F, Ladeira K, Longatto-Filho A, Martins SF. Gastric Cancer and Angiogenesis: Is VEGF a Useful Biomarker to Assess Progression and Remission? J Gastric Cancer. 2017;17:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 132. | Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, Hicklin DJ, Ellis LM. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133-1140. [PubMed] |
| 133. | Abdel-Rahman O. Targeting vascular endothelial growth factor (VEGF) pathway in gastric cancer: preclinical and clinical aspects. Crit Rev Oncol Hematol. 2015;93:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 134. | Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33:S1-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 135. | FDA Approval for Bevacizumab 2004. Updated: 4 Dec 2014. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/fda-bevacizumab. |
| 136. | Avastin (bevacizumab) 2009. Updated: 15 Dec 2017. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000582/human_med_000663.jspmid=WC0b01ac058001d124. |
| 137. | Sawaki A, Yamada Y, Yamaguchi K, Nishina T, Doi T, Satoh T, Chin K, Boku N, Omuro Y, Komatsu Y. Regional differences in advanced gastric cancer: exploratory analyses of the AVAGAST placebo arm. Gastric Cancer. 2018;21:429-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Shen L, Li J, Xu J, Pan H, Dai G, Qin S, Wang L, Wang J, Yang Z, Shu Y. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. 2015;18:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 139. | Wadhwa R, Taketa T, Sudo K, Blum-Murphy M, Ajani JA. Ramucirumab: a novel antiangiogenic agent. Future Oncol. 2013;9:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 140. | FDA Approval for Ramucirumab 2014. Updated: 30 Apr 2015. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/fda-ramucirumab. |
| 141. | EMA. Cyramza (ramucirumab) 2015, Updated: 29 Jan 2018. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002829/human_med_001825.jspmid=WC0b01ac058001d124. |
| 142. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1566] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 143. | Muro K, Cho JY, Bodoky G, Goswami C, Chao Y, Santos LVd, Shimada Y, Topuzov E, Cutsem EV, Tabernero J, Zalcberg JR, Chau I, Cheng R, Hsu Y, Emig M, Orlando M, Wilke H, Fuchs CS. Efficacy and safety of ramucirumab (RAM) for metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma across age subgroups in two global phase 3 trials. J Clin Oncol. 2017;35:3-3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 144. | Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075-6081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 145. | Qin S. Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2014;32:4003-4003. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 728] [Article Influence: 80.9] [Reference Citation Analysis (1)] |
| 147. | Huang L, Wei Y, Shen S, Shi Q, Bai J, Li J, Qin S, Yu H, Chen F. Therapeutic effect of apatinib on overall survival is mediated by prolonged progression-free survival in advanced gastric cancer patients. Oncotarget. 2017;8:29346-29354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 148. | Zhang Y, Han C, Li J, Zhang L, Wang L, Ye S, Hu Y, Bai L. Efficacy and safety for Apatinib treatment in advanced gastric cancer: a real world study. Scientific Reports. 2017;7:13208. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 149. | Singh SS, Yap WN, Arfuso F, Kar S, Wang C, Cai W, Dharmarajan AM, Sethi G, Kumar AP. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J Gastroenterol. 2015;21:12261-12273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 150. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 151. | Ramanathan RK, McDonough SL, Kennecke HF, Iqbal S, Baranda JC, Seery TE, Lim HJ, Hezel AF, Vaccaro GM, Blanke CD. Phase 2 study of MK-2206, an allosteric inhibitor of AKT, as second-line therapy for advanced gastric and gastroesophageal junction cancer: A SWOG cooperative group trial (S1005). Cancer. 2015;121:2193-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 152. | Guichard SM, Curwen J, Bihani T, D’Cruz CM, Yates JW, Grondine M, Howard Z, Davies BR, Bigley G, Klinowska T. AZD2014, an Inhibitor of mTORC1 and mTORC2, Is Highly Effective in ER+ Breast Cancer When Administered Using Intermittent or Continuous Schedules. Mol Cancer Ther. 2015;14:2508-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 153. | Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368-1372. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 154. | FDA. Everolimus (Afinitor) 2016, Updated:26 Feb 2016 [cited 05 Mar 2018]. Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm488028.htm. |
| 155. | EMA. Afinitor (everolimus) 2010, Updated:13 Sept 2017. [cited 05 Mar 2018]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001038/human_med_000633.jspmid=WC0b01ac058001d124. |
| 156. | Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Clinical significance of MET in gastric cancer. World J Gastrointest Oncol. 2015;7:317-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 157. | Chae YK, Arya A, Chiec L, Shah H, Rosenberg A, Patel S, Raparia K, Choi J, Wainwright DA, Villaflor V. Challenges and future of biomarker tests in the era of precision oncology: Can we rely on immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) to select the optimal patients for matched therapy? Oncotarget. 2017;8:100863-100898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 158. | Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, Huang A, Yang NY, Nishimura M, Greve J. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci USA. 2013;110:E2987-E2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 159. | Shah MA, Cho JY, Tan IB, Tebbutt NC, Yen CJ, Kang A, Shames DS, Bu L, Kang YK. A Randomized Phase II Study of FOLFOX With or Without the MET Inhibitor Onartuzumab in Advanced Adenocarcinoma of the Stomach and Gastroesophageal Junction. Oncologist. 2016;21:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 160. | Zhang Y, Doshi S, Zhu M. Pharmacokinetics and pharmacodynamics of rilotumumab: a decade of experience in preclinical and clinical cancer research. Br J Clin Pharmacol. 2015;80:957-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 161. | Catenacci DVT, Tebbutt NC, Davidenko I, Murad AM, Al-Batran SE, Ilson DH, Tjulandin S, Gotovkin E, Karaszewska B, Bondarenko I. Rilotumumab plus epirubicin, cisplatin, and capecitabine as first-line therapy in advanced MET-positive gastric or gastro-oesophageal junction cancer (RILOMET-1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1467-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 162. | Amgen. Amgen Announces Termination Of All Amgen-Sponsored Clinical Studies Of Rilotumumab In Advanced Gastric Cancer 2014. [cited 07 Mar 2018]. Available from: https://www.prnewswire.com/news-releases/amgen-announces-termination-of-all-amgen-sponsored-clinical-studies-of-rilotumumab-in-advanced-gastric-cancer-300000103.html. |