Published online May 7, 2018. doi: 10.3748/wjg.v24.i17.1825
Peer-review started: April 2, 2018
First decision: April 19, 2018
Revised: April 21, 2018
Accepted: April 23, 2018
Article in press: April 23, 2018
Published online: May 7, 2018
Processing time: 34 Days and 19.3 Hours
Chronic hepatitis B (CHB) remains a challenging global health problem, with nearly one million related deaths per year. Nucleos(t)ide analogue (NA) treatment suppresses viral replication but does not provide complete cure of the hepatitis B virus (HBV) infection. The accepted endpoint for therapy is the loss of hepatitis B surface antigen (HBsAg), but this is hardly ever achieved. Therefore, indefinite treatment is usually required. Many different studies have evaluated NA therapy discontinuation after several years of NA treatment and before HBsAg loss. The results have indicated that the majority of patients can remain off therapy, with some even reaching HBsAg seroconversion. Fortunately, this strategy has proved to be safe, but it is essential to consider the risk of liver damage and other comorbidities and to ensure a close follow-up of the candidates before considering this strategy. Unanswered questions remain, namely in which patients could this strategy be effective and what is the optimal time point at which to perform it. To solve this enigma, we should keep in mind that the outcome will ultimately depend on the equilibrium between HBV and the host’s immune system. Viral parameters that have been described as good predictors of response in HBeAg(+) cases, have proven useless in HBeAg(-) ones. Since antiviral immunity plays an essential role in the control of HBV infection, we sought to review and explain potential immunological biomarkers to predict safe NA discontinuation in both groups.
Core tip: Nucleos(t)ide analogue (NA) treatment efficiently suppress hepatitis B virus replication. However, hepatitis B surface antigen loss, the optimal endpoint of NA therapy, is rarely achieved. Thus, a major unmet need in the management of chronic hepatitis B is the definition of earlier and safe treatment stopping points. There is growing clinical evidence that the majority of patients can benefit from this strategy after long-term NA therapy; yet, no criteria that distinguish which cases can safely stop treatment is established. We review here different biomarkers that could serve as a prognostic tool to safely discontinue therapy, focusing on host antiviral immunity.
- Citation: Moreno-Cubero E, Arco RTSD, Peña-Asensio J, Villalobos ES, Míquel J, Larrubia JR. Is it possible to stop nucleos(t)ide analogue treatment in chronic hepatitis B patients? World J Gastroenterol 2018; 24(17): 1825-1838
- URL: https://www.wjgnet.com/1007-9327/full/v24/i17/1825.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i17.1825
According to recent data from the World Health Organization, about 257 million people suffer from chronic hepatitis B (CHB) worldwide. Hepatitis B virus (HBV) infection remains a major global health concern, as the disease itself and its complications, mainly hepatocellular carcinoma (HCC) and cirrhosis, caused 887000 deaths in 2015 alone. The estimated worldwide incidence of HCC in 2012 was 782000 cases, representing the fifth and the ninth most common cancer in males and females respectively. Moreover, HCC was the second cause of global cancer mortality, as it tends to have very poor prognosis with an overall ratio of mortality to incidence of 0.95[1].
Although the actual HBV vaccine is 95% effective, vaccination coverage is still suboptimal in many highly endemic areas. Besides, most of the current HBV-infected persons were born before the vaccine was widely accessible[2-4]. HBV infection chronification is not fully understood. The HBV genome assembly into a stable mini-chromosome, known as covalently closed circular (ccc)DNA, which can integrate into and persist in the hepatic cell nucleus. In addition, the immune response against HBV is profoundly impaired[5,6]. Both are, in fact, the main reasons why indefinite treatment is usually necessary.
Immune modulators were the first approach to CHB treatment. The first one, interferon (IFN)-α was approved in 1991, being afterwards substituted by its pegylated form (Peg-IFN-α) as the latter provides a safer profile. The principal mechanism of Peg-IFN-α therapy relies on the induction of long-term immune control, which occurs in almost half of the responders and with limited treatment duration. However, it poses significant drawbacks, including an adverse safety profile and a high response variableness, the reasons why a number of patients are ineligible, unsuitable or reluctant to partake in this treatment alternative[7,8].
At present, nucleos(t)ide analogues (NAs) constitute the lynchpin of CHB therapy, as they facilitate achievement of viral suppression in almost all adherent patients, while having an overall favourable safety profile[7-9]. The currently approved NAs for CHB treatment in the United States and Europe include lamivudine (LMV), telbivudine (TBV), adefovir dipivoxil (ADV), tenofovir (disoproxil fumarate, TDF; alafenamide, TAF) and entecavir (ETV). The NA mechanism of action comprises viral polymerase inhibition, which leads to decreased virion assembly and ultimately a hypothetical cccDNA downturn that would only be appreciated after an extended period of treatment[10,11].
Nonetheless, NAs are not able to stop de novo cccDNA synthesis in recently infected hepatocytes; thus, lingering viremia could perpetuate the viral repository. That is the reason why “complete cure” is not a realistic endpoint of NAs to date. “Functional cure”, understood as HBV DNA and hepatitis B surface antigen (HBsAg) seroclearance with or without seroconversion, constitutes a more plausible goal. However, it is achieved only in a small proportion of the treated patients. Lifelong NA therapy is usually necessary, especially in hepatitis B e antigen-negative [HBeAg(-)] cases[7,12,13].
Since indefinite treatment is mandatory, development of viral resistance is a paramount concern, especially with the first- and second-generation oral NAs such as LMV, TBV and ADV. Fortunately, that problem seems to have been overcome by the new agents TDF/TAF and ETV, as they present low resistance rates and high efficacy with a very favourable safety profile[14,15].
Combination therapy has also been proposed as a strategy for HVB eradication, but results are still under evaluation and intense debate. Its rationale comprises attacking the virus in different parts of its life cycle, and follows practical successes observed in other infectious diseases, like hepatitis C virus and human immunodeficiency virus. Potential objectives regarding this approach include viral targeting (viral entry, cccDNA, RNA interference, encapsidation, DNA replication, etc) as well as innate and adaptive immunomodulation (IFN, Toll-like receptor/RIG-1 agonists; and checkpoint inhibitors, T cell modification and vaccination respectively).
Other trials that have evaluated the synergies between innate immunity potentiation and NAs have already shown promising results. A preclinical phase study that combined a woodchuck hepatitis virus DNA vaccine, a programmed cell death protein 1 (PD-1) inhibitor and ETV showed restoration of the cytolytic capacities of HBV-specific T cells and better control of viral replication. Another study that associated a DNA vaccine with any NA revealed no differences in relapse after NA cessation. A third study that blended an HBsAg vaccine with LMV did not find clinical differences[16-19].
If theoretically attractive, current guidelines do not recommend combination therapy for clinical practice[9,20]. The ultimate and thus optimal target of HBV therapy for HBeAg(-) and HBeAg(+) patients comprises viral eradication. Such would involve HBsAg seroconversion or seroclearance and cccDNA elimination from hepatocytes[21]. Unfortunately, it is not a likely outcome, and recommended goals point towards sustained inhibition of replication and maintenance of alanine aminotransferase (ALT) enzyme levels within the normal range[9,20,22]. Achievement of these objectives has been shown to stop the inflammation cascade and fibrosis progression[23,24], with e consequent improvement in life-quality and survival[25]. The weight of the beneficial effects of the latest generation NAs over the risk of HCC are still controversial, as the latter develops even despite therapy[26-28].
It seems, then, reasonable to bear in mind that any strategy involving NA treatment withdrawal must guarantee the patient’s safety and, therefore, the maintenance of the aforementioned objectives.
There is a growing body of evidence that helped to elucidate whether NA therapy cessation is safe and effective. A relevant study by Hadziyannis et al[29] must be pointed out as a point of inflexion regarding the NA cessation approach. It showed a significantly higher HBsAg clearance rate (almost reaching 40%) in the HBeAg(-) CHB patients that stopped after 5 years under ADV therapy, in comparison to that reported for their equals under NA. Since then, a set of investigations have attempted to clarify whether stopping treatment with NA may have an additional benefit in the loss of HBsAg, showing achievement of rates between 20%-24%[30,31].
Some parameters have been pointed out as possible predictors of both sustained viral response and HBsAg loss. Among these, it is worth highlighting the decrease of quantitative (q)HBsAg. Other biomarkers that could permit identification of patients in which NA cessation will be safe will be reviewed broader, later on.
The recent FINITE study[32] was the first randomized controlled trial that compared standard TDF therapy continuation against its interruption in HBeAg(-) patients that had been under treatment for at least 3.5 years. In line with the previous commented work, 13 out of 21 patients in the cessation arm remained off-therapy and 4 of them even achieved HBsAg seroclearance after 3 years of follow-up. No unexpected safety issues were reported. These and the other studies about NA interruption that have been published to date are summarised in Table 1.
| Patients off NA, n | Treatment characteristics | Outcomes | ||||||||||
| Study | Total | HBeAg(+) | HBeAg(-) | Cirrhosis | Age in year | Sex, male | Ethnicity | NA | Treatment duration in mo | Durable virologic response, n | HBsAg loss, n | Deaths, n |
| Fung et al[100] (2004) | 27 | 0 | 27 | 7 | 45 | 40 | Asian | LMV | 24 | 15 | NR | 0 |
| Enomoto et al[101] (2008) | 22 | 0 | 22 | 3 | 49 | 15 | Asian | LMV | NR | 5 | NR | 0 |
| Yeh et al[102] (2009) | 71 | 71 | 0 | 11 | 41 | 55 | Asian | LMV | NR | 52 | 0 | 0 |
| Fung et al[103] (2009) | 22 | 22 | 0 | NR | 28 | 16 | Asian | LMV | 74 | 8 | NR | 0 |
| Wang et al[104] (2010) | 125 | 125 | 0 | 0 | 26/32 | 95 | Asian | LMV | 24-36 | 87 | NR | 0 |
| Kuo et al[105] (2010) | 124 | 124 | 0 | NR | NR | NR | Asian | LMV | 14 | 42 | NR | 1 |
| Cai et al[106] (2010) | 11 | 11 | 0 | NR | 29 | 12 | Asian | TBV | 24 | 4 | NR | 0 |
| Liu et al[40] (2011) | 61 | 0 | 61 | 0 | 32 | 50 | Asian | LMV | 27 | 30 | 8 | 0 |
| Jung et al[107] (2011) | 19 | 10 | 9 | 4 | 37 | 12 | Asian | ADV | 33 | 13 | 0 | 0 |
| Chan et al[30] (2011) | 53 | 0 | 53 | 18 | 56 | 43 | Asian | LMV | 27 | 16 | 9 | NR |
| Chaung et al[108] (2012) | 39 | 39 | 0 | NR | 34 | 24 | Asian | LMV, ADV, ETV | 21 | 4 | 0 | 0 |
| Hadziyannis et al[29] (2012) | 33 | 0 | 33 | 0 | 53 | 38 | Caucasian | ADV | 56 | 18 | 14 | 0 |
| Ha et al[41] (2012) | 145 | 0 | 145 | NR | 33 | 101 | Asian | ADV | 26 | 50 | NR | 0 |
| Song et al[109] (2012) | 48 | 48 | 0 | 0 | 42 | 29 | Asian | ETV, CLE | 26 | 28 | NR | NR |
| He et al[110] (2013) | 66 | 0 | 66 | 0 | 35 | 50 | Asian | LMV, ADV, ETV, TBV | 37 | 47 | 2 | 0 |
| Kim et al[111] (2013) | 45 | 0 | 45 | 9 | 45 | 33 | Asian | LMV, ADV, ETV | 38 | 12 | NR | NR |
| Jeng et al[44] (2013) | 95 | 0 | 95 | 39 | 52 | 83 | Asian | ETV | 24 | 40 | 0 | 0 |
| Kwon et al[112] (2013) | 16 | NR | NR | NR | NR | NR | Asian | LMV | 79 | 12 | 2 | 0 |
| Ridruejo et al[113] (2014) | 35 | 33 | 2 | 0 | NR | NR | Caucasian | ETV | 42 | 26 | 18 | NR |
| Sohn et al[114] (2014) | 95 | 41 | 54 | 44 | 47 | 53 | Asian | LMV, ETV, CLE | 22 | 16 | 0 | 0 |
| Patwardhan et al (2014) | 33 | 0 | 33 | 0 | 42 | 24 | Mixed | LMV, ADV, ETV, TDF | 64 | 12 | 0 | 0 |
| He et al[115] (2014) | 97 | 97 | 0 | NR | 26 | 53 | Asian | LMV, ADV, ETV, TBV | 35 | 89 | 11 | 0 |
| Chen et al[31] (2014) | 188 | 83 | 105 | 12 | 38/49 | 143 | Asian | LAM | 20-22 | 63 | 23 | NR |
| Jiang et al[116] (2015) | 72 | 33 | 39 | 8 | 36 | 53 | Asian | LMV, LMV + ADV, ADV, ETV, TBV | 33 | 25 | NR | 0 |
| Seto et al[117] (2015) | 184 | 0 | 184 | 34 | 54 | 125 | Asian | ETV | 37 | 15 | 0 | 0 |
| Huang et al[118] (2003) | 32 | 0 | 32 | NR | 46 | 29 | Asian | LMV | 9 | 14 | NR | NR |
| Marcellin et al[119] (2004) | 181 | 0 | 181 | 53 | 40 | 156 | Asian | LMV | 12 | 53 | 0 | 0 |
| Lai et al[120] (2006)1 | 325/313 | 0 | 325/313 | 16/31 | 44/44 | 248/236 | Mixed | ETV/ LMV | ≥13 | 124/78 | 1/1 | 22 |
| Marcellin et al[121] (2009)3 | 181/85 | 0 | 181/85 | 40/39 | 156/74 | Asian | LMV | 12 | 52/33 | 0/0 | 12 | |
| Paik et al[122] (2010) | 50 | 0 | 50 | 15 | 39 | 43 | Asian | LMV | 24 | 25 | NR | 0 |
| Liang et al[123] (2011) | 84 | 41 | 43 | 0 | 37 | 56 | Asian | LMV, ADV, ETV or LMV +ADV | 33 | 47 | 5 | NR |
| Jin et al[124] (2012) | 138 | 102 | 36 | 17 | 39 | 82 | Asian | LMV | 35 | 116 | 82 | 0 |
| Berg et al[32] (2017) | 21 | 0 | 21 | 0 | 45 | 33 | Caucasian | TDF | ≥ 48 | 13 | 4 | 0 |
| Van Hees et al[35] (2018) | 62 | 62 | 0 | 11 | 43 | 45 | Caucasian | LMV, TDF, ETV, LMV + ADV | 70 | 32 | 6 | 2 |
| Rivino et al[43] (2018)4 | 21/27 | 0/0 | 21/27 | 0/0 | 43/51 | 14/19 | Caucasian/Asian | TDF, LMV | ≥ 24/≥ 24 | 4/14 | 0/0 | NR |
Some of the current HBV management guidelines[9,22] have begun to consider treatment cessation in other selected populations of patients. The most accepted election criteria include cirrhosis absence, treatment for at least 2 or 3 years, sustained viral suppression and guaranteed patient monitoring (Table 2). Concerns regarding treatment cessation include virological and clinical relapse but also the possibility of dangerous complications, such as hepatic decompensation, liver failure and, ultimately, death. Serious complications are uncommon, and some meta-analyses have shown a decompensation rate of less than 1% in patients that presented baseline cirrhosis[33,34]. Therapy reestablishment proved to be effective in most cases, but also cases of death after liver failure have been reported.
| Society | HBeAg(+) | HBeAg(-) | Cirrhosis |
| EASL (2017)[9] | HBsAg clearance (safest) HBeAg seroconversion and HBV DNA undetectability with 6-12 mo of ensuing consolidation therapy | HBsAg clearance Selected patients with ≥ 3 yr virological suppression if guaranteed close postNA monitoring for at least 1 yr | Not recommended |
| AASLD (2016)[20] | HBsAg clearance HBeAg seroconversion with at least 12 mo of persistently normal ALT levels and undetectable serum HBV DNA levels (close monitoring for at least 1 yr) | HBsAg clearance | Not recommended |
| APASL (2016)[22] | HBeAg seroconversion with undetectable HBV DNA and persistently normal ALT levels with 1-3 yr of consolidation therapy | HBsAg clearance with antiHBs seroconversion HBsAg loss with at least 12 mo of consolidation period After treatment for at least 2 yr with undetectable HBV DNA documented on 3 separate occasions, 6 mo apart | Could be considered in compensated cirrhosis with careful monitoring |
A recent study provided alert to the risk of relapse and potentially fatal effects among Caucasian cirrhotic patients with HBeAg(+) HBV virus infection[35]. Two patients died of liver-related events: one after decompensation and sepsis, and the other one after developing a multicentric HCC 10 years after the NA treatment cessation. Nevertheless, both of those patients had presented with advanced fibrosis and cirrhosis, respectively, at the time of therapy discontinuation. Furthermore, although a few studies have claimed benefits of long-term treatment regarding HCC incidence, as previously stated, it is not doubtlessly prevented by NA therapy[26-28,36,37].
Hence, considering that the treatment withdrawal could lead to severe flares and even death in a few cases it should be avoided in patients with advanced fibrosis or cirrhosis, and a close follow-up must always be guaranteed for the rest of the cases[38]. However, severe complications are rare, and research must continue to address the optimal NA cessation point. The identification of reliable factors capable of predicting clinical, virological and biochemical relapse, or the maintenance of the viral response, would be of vital importance for clinical practice.
Once the safety of NA treatment cessation has been addressed, and keeping in mind that severe complications are rare, the vast benefit may be considered. Notwithstanding that NA treatment has an overall positive safety profile in the general population, some issues arise.
Lifelong NA treatment is an unaffordable burden for healthcare systems. That is why a significant advantage of its cessation would be cost reduction[22,34]. However, increase in the incidence of some chronic conditions, such as metabolic syndrome, diabetes mellitus and renal failure, may limit NA applicability in the future. Furthermore, we are not aware of potential concerns of NA therapy in elder individuals and research must address this subject. There are some other potential concerns about long-term NA therapy[39]. The most common side effects involve nephrological and bone toxicity, which are associated with TDF, perhaps the most widely used drug. However, the new tenofovir formulation TAF seems to have a better safety profile regarding these points. Other side effects appear to be related to mitochondrion impairment derived from human DNA polymerase function alteration and resulting in bone, renal and neurologic toxicity.
NAs have a relatively benign safety profile for pregnancy, with telbivudine and tenofovir being the most favourable ones, rated B category by the Federal Drug Administration. On the other hand, ETV has shown deleterious effects for the embryo, being rated C category. Nevertheless, there is a lack of information about foetal safety in humans, so NA treatment during pregnancy should only be considered if benefits overwhelm risks.
Despite all the above-mentioned issues, NA counts on its excellent safety profile for almost the totality of the patients eligible for this therapy. To sum up, life-long therapy is usually necessary for the majority of CHB patients because the functional cure is rarely achieved. Therefore, identification of biomarkers to safely stop treatment remains an unmet need in the management of the disease.
Taking into account that CHB outcome relies on equilibrium between the virus and the host, in the next paragraphs it will be explained how different virological and immunological parameters could be considered or not as predictors to safely discontinue NA treatment.
Sex: Female sex was identified as an independent predictor for sustained virological response after NA discontinuation in HBeAg(-) patients[31].
Age: Older age has been correlated to higher relapse rates[31,40,41], possibly reflecting the enhanced immune response in younger individuals.
NA treatment duration: A more extended therapy time would mean more time for an exhausted immune system to recover its response efficacy. Logistic regression has revealed that sustained virologic remission is more likely in HBeAg(-) patients after long periods of treatment, at least over 2 years. Nevertheless, this parameter has not proven to be useful in HBeAg(+) CHB[33].
ALT: The predictive role of ALT is controverted. Although it was classically accepted that ALT flares were associated with the virologic response after NA treatment cessation[42], lower ALT baseline levels have been correlated with higher rates of HBsAg loss[31]. Also, it has recently been demonstrated that patients who do not flare upon treatment withdrawal are those who remain off-therapy[43]. Given the observed disparities, more research is needed to elucidate the role of ALT as a biomarker.
DNA: Lower baseline HBV DNA titres were reported as associated independently with lower relapse rates[44], whereas elevated HBV DNA titres and its persistence after NA interruption also seem to be useful for relapse prediction[45].
Serum qHBsAg: Decrease in serum qHBsAg has been correlated to HBsAg clearance and has been spotlighted as a possible predictor of sustained response and flares after NA withdrawal[46-48]. The interest in qHBsAg has been limited, however, due to the low level required for consideration of NA cessation (100-700 IU/mL)[31,49-51], and which is rarely achieved. Taking into account that these qHBsAg levels are not adequately good predictors to safely discontinue NA therapy, because they would only represent a small portion of cases, more research has been performed to improve the prognostic accuracy.
Noncytopathic viruses, such as HBV, have developed evolutionary mechanisms to remain hidden from the immune system, which is an advantage for their persistence. HBV virus is not highly infectious but produces long-lasting disease that allows it to spread the infection over time. The host/HBV relationship is a dynamic process in which the virus tries to decrease its visibility, whereas the host attempts to prevent and eradicate infection with minimal collateral damage to itself[52].
Several viral markers have been proposed as potential biomarkers for a safe NA discontinuation, and they are discussed below.
Serum HBV RNA reflects the transcriptional activity of liver cccDNA, and its decline seems to be a good predictor of HBeAg seroconversion[53]. Nevertheless, it is commonly undetectable in HBeAg(-) cases[54], making it useless as a biomarker for stoppage of NA treatment in this increasing population. Moreover, improvement of the HBV RNA assay to make it more sensitive and reproducible, as well as studies in bigger cohorts, are essential before considering it as a potential biomarker for monitoring safe discontinuation of NA therapy in HBeAg(+) patients.
Hepatitis B core (HBc) is an inner nucleocapsid surrounding the viral DNA and is the target of specific T cell response against the virus. AntiHBc is the first antibody to appear after HBV exposure and it represents a classical serological marker for HBV infection[55]. The role of antiHBc as a predictor of NA discontinuation, however, has not been fully examined, but it was recently reported that baseline antiHBc level is a strong predictor for HBeAg seroconversion during PEG-IFN-α or NA therapy[56]. Moreover, there was a trend for an inverse association between antiHBc and clinical relapse after long-term ETV treatment cessation in an Asiatic CHB cohort[57]. AntiHBc, as a predictor, needs to be further assessed and validated in non-Asiatic cohorts, to verify if it could be useful.
HBV core-related antigen (HBcrAg) includes HBcAg, HBeAg and a pre-core protein (p22cr), and its quantification closely correlates with intrahepatic cccDNA level[58,59]. In HBeAg(+) CHB patients, the dynamics of HBcrAg accurately predict spontaneous HBeAg seroconversion[60] and the combination of HBsAg together with HBcrAg quantification help to predict safe discontinuation after NA treatment cessation[61]. However, most of this research has been performed in Japan with first-generation NAs, so further validation with the currently available NAs and different areas of study is lacking.
In summary, some virological markers could be useful predictors of response in HBeAg(+) patients, but improvement of the assays together with further cohort validation is still needed for HBeAg(-) cases. The other side of the balance is the host’s immune defence against the virus, presented in the next section.
To achieve control of the HBV infection, a functional adaptive immune response, in particular the cellular immune response, is essential; meanwhile, whether and how HBV triggers the components of the innate immune system remain controversial topics. Even though the humoral response is an effective line of defence against reinfection, in the setting of CHB, the virus persists despite high levels of HBV-specific antibodies[62] due to antigen overload, and only hepatitis B surface antibody is associated with disease resolution.
Primed HBV-specific CD4 T cells are crucial to allow the adequate activation of HBV-specific CD8 T cells by secretion of proinflammatory cytokines, including IFN-α[63]. Afterwards, HBV-specific CD8 T cells play a major role in the resolution of spontaneous infection because they can specifically recognise the infected hepatocytes. Moreover, they can clear the virus by inducing apoptosis of the infected cell as well as by proinflammatory cytokine production to eliminate the virus without causing cell death[64].
CD4- and CD8-specific HBV responses are vigorous, polyclonal and multispecific in acute-resolving cases, whereas are profoundly impaired in chronically infected patients[6,65-68]. During CHB, HBV-specific T cell responses gradually lose their functionality and are finally deleted[69] due to the high and persistent antigen exposure, in order to avoid host-induced tissue damage, in a process called T cell exhaustion. T cell exhaustion is characterised by high and sustained expression of several negative pathways (i.e., PD-1, immunoregulatory cytokines and so on)[70-75].
The role of HBV-specific CD4 T cell features as a predictor for NA cessation has not been intensely studied. It could be explained mainly by two reasons. First, the frequency of these cells in the chronic setting of the disease is very low[76]. Second, due to the nature of CD4 responses, in vitro stimulation assays are difficult because these cells are only successfully stimulated by professional antigen-presenting cells. Even though a robust HBV-specific CD4 T cell response is observed in acute resolving cases, and they are essential to support HBV-specific CD8 T cells, the difficulty of assessment makes them less useful than HBV-specific CD8 T cells or other surrogates when trying to find an easy and reproducible immunological marker to stop NA therapy safely.
CHB is one of the best models to study CD8 T cell exhaustion. In the different stages of the natural history of HBV infection, there are different virus-host interactions, reflected by different immune features of HBV-specific CD8 T cells. Bearing in mind that several studies have shown that after long-term NA treatment interruption the majority of patients remain with a viral response after long follow-up[29,32], we could infer that the host’s immunity is controlling HBV replication.
After a long-term NA treatment cessation, HBV-specific CD8 T cells could be given a second chance to fight the virus. If these cells have been restored by the reduced viremia that had been induced by the antiviral therapy at that point, these cases would be able to control the infection in a similar way to chronic infection cases. Therefore, patients with viral control are likely to have a good immune response against the virus, whereas cases with virologic rebound may have a dysfunctional response.
Thus, changes in HBV-specific CD8 T cell phenotype may predict acquisition of antiviral control before HBsAg loss. Taking into account the vital role of HBV-specific CD8 T cells during the natural history of the disease, and its in-depth characterisation achieved over the last two decades, it is presumable that those different features according to viral control could give hints to answer one of the most critical questions regarding CHB management: What kind of patients could benefit from NA therapy interruption?
Boni et al[77] have extensively studied several immune subsets in different groups of chronically infected patients, including those under NA therapy. In the LMV treated patients, they found an initial improvement of HBV-specific T cell effector capacities against different HBV epitopes (HBcAg, HBeAg) after DNA fall[77], followed by a decline at 6 mo after the treatment has been stopped; this biphasic behaviour is irrespective of clinical outcome[78]. It appears that the first-generation NAs lack the potency needed for HBV-specific T cell restoration.
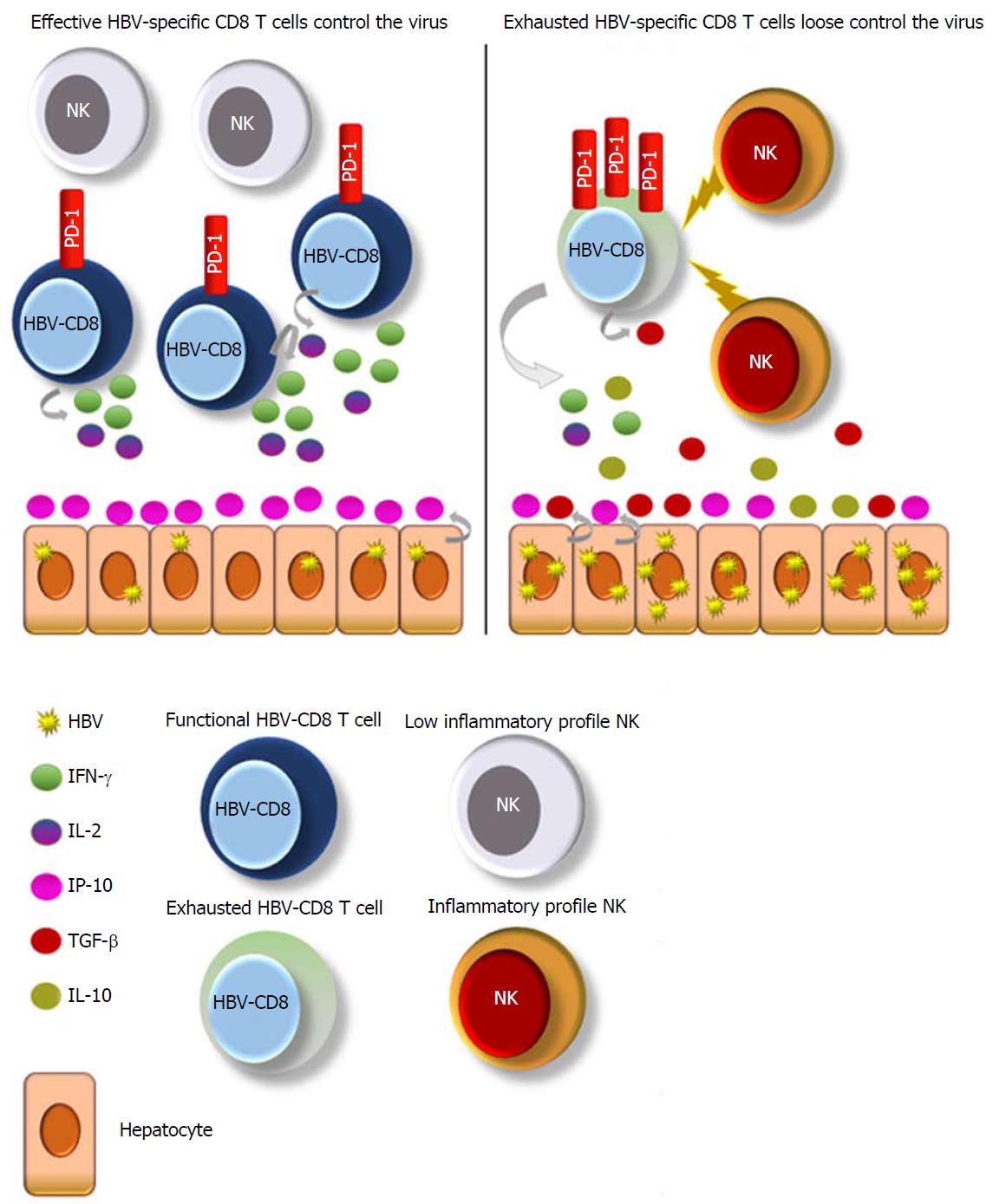
Succeeding experiments in larger cohorts under the first- and second-generation NA therapies demonstrated that HBV-specific CD8 T cell effector abilities were similar between patients after several years of antiviral treatment and acute resolving cases featured by a PD-1+ phenotype[79] (Figure 1). PD-1 up-regulation arises on HBV-specific T cells following acute and chronic infection. In the setting of acute infection, PD-1 up-regulation is transient, returning to low levels after viral clearance. However, in chronic infection, PD-1 up-regulation is sustained, and the blockade of PD-1/PD-L1 interaction has shown promising results in restoring virus-specific T cell functionality[80-83]. Therefore, a PD-1+ phenotype could mean both activation before clearance or exhaustion after persistent and high antigenemia.
The most recent work studying HBV-specific T cell response as a biomarker for HBV therapy discontinuation demonstrated that the patients who did not relapse to NA stoppage featured, during NA treatment, an increased frequency of functional PD-1+ HBV-specific T cells directed against nucleocapsid and polymerase HBV proteins[43] (Figure 1). The PD-1+ expression on functional HBV-specific T cells may reflect an activated, nonexhausted phenotype. Along these lines, patients with functional HBV-specific CD8 T cells, positive for PD-1, may no longer need NA treatment and should be considered for treatment cessation.
However, the current method is complicated to move from bench to bedside because it involves the study of rare populations by multicolour flow cytometry. Hence, the development of an assay to directly quantify PD-1+ HBV-specific CD8 T cells would be of great interest. Even though the final effectors to clear HBV are the HBV-specific CD8 T cells, it is essential to consider the interplay between them and other components of the immune system to fully understand immunity against HBV and their potential as surrogate biomarkers.
The natural enrichment of natural killer (NK) lymphocytes in the human liver underscores their potential importance in the control of hepatotropic viruses, such as HBV[84]. During CHB, NK cells express an inhibitory phenotype with altered functionality[85,86] and have predilection for apoptosis of HBV-specific T cells, resulting in HBV-specific T cell deletion after death ligand-death receptor interaction[87]. Boni et al[88] showed a low inflammatory profile of NKs after successful NA therapy, similar to healthy controls. In line with the previously commented work, this lower inflammatory status of NKs correlated with a better HBV-specific T cell response[88] (Figure 1). Moreover, a partial restoration of blood NK cells was shown following long-term ETV, in terms of antiviral cytokine production compared to naïve CHB[89].
So, why should study of NKs - instead of HBV-specific CD8 T cells - be useful? The study of NK cell inflammation does not involve multimers nor intracellular cytokine staining, as used to assess HBV-specific CD8 T cell responses, resulting in more easily reproducible experiments. A low inflammatory profile of NK cells can be evaluated by surface staining and may reflect an HBV-specific T cell restoration and subsequent control without the need of therapy. Studies in bigger cohorts after stoppage of NA treatment are needed to address if successful NA discontinuation correlates with a lower inflammation phenotype of NK cells.
The third signal of T cell activation requires an adequate cytokine profile, and long-term NA therapy has been shown to modulate it. Successful viral repression leads to antiviral response stimulation by promoting proinflammatory cytokines such as IFN-γ[90,91] and IL-2[92,93], as well as by decreasing regulatory effectors such as IL-10[91,94] and TGF-β[95]. At least theoretically, the measurement of these cytokines together with HBV-specific T cells or NK cells could also give us clues to establish a good cessation point for therapy (Figure 1).
Not only are the phenotype and functionality of the different immune subsets important components of an adequate milieu during CHB but also the trafficking of HBV-specific T cells to the infected liver. The migration of lymphocytes to the liver is a complicated process involving adhesion, rolling, triggering and transendothelial migration. Chemokines and their receptors play an essential role in this multistep pathway[96,97].
After the analysis of several plasma chemokines, the one that appears to be a promising surrogate of HBsAg loss under NA therapy is CXCL10 (IP-10)[47]. IP-10 is a small protein, secreted by hepatocytes in response to viruses and the subsequent recruitment of proinflammatory CD4 and CD8 T cells to the infected liver[97] (Figure 1). It was previously reported that baseline serum IP-10 levels were higher in patients with HBsAg loss during NA therapy[98] and, in line with those findings, another work examined the serum IP-10 kinetics during ETV therapy. Interestingly, they found that IP-10 levels started to significantly increase after the 3rd year of treatment with ETV[99], which is in line with the timing observed to be necessary to achieve a sustained virological response in the different stopping-treatment studies[29,32].
It is likely that after a prolonged and effective viral replication suppression under NA treatment, the migration process to the liver is restored and HBV-specific T cells are functional and able to clear the remaining infected hepatocytes, thus reflecting the HBsAg decline.
The study of different immune features against HBV, especially HBV-specific CD8 T cells, is a promising strategy to characterise which patients could benefit from NA treatment cessation. Surveying HBV-specific CD8 T cells is complex, as it involves rare population assays. However, different, easier to perform surrogates of this response have been explored recently, providing a more suitable application for clinical use. NA withdrawal is still an active and attractive research field. Nevertheless, even if a considerable number of studies have tried to address this point, their methods have shown marked heterogenicity. Furthermore, although results of some randomised controlled trials are becoming available, more high-quality clinical evidence is needed. It is possible that in the future, therapies able to completely clear cccDNA will be accessible. In the meantime, advantages in the management of CHB may be achieved by using this strategy. Fortunately, the field of immunology shows how basic science can improve the health of our patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sirin G, Zhong JH S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Petruzziello A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma. Open Virol J. 2018;12:26-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW; Centers for Disease Control and Prevention (CDC). Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20. [PubMed] |
| 3. | Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE. Surveillance of Vaccination Coverage among Adult Populations - United States, 2015. MMWR Surveill Summ. 2017;66:1-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 4. | Cohen C, Holmberg SD, McMahon BJ, Block JM, Brosgart CL, Gish RG, London WT, Block TM. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Allweiss L, Dandri M. The Role of cccDNA in HBV Maintenance. Viruses. 2017;9:pii: E156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 734] [Article Influence: 40.8] [Reference Citation Analysis (1)] |
| 7. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2169] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 8. | Papatheodoridis GV, Manolakopoulos S, Dusheiko G, Archimandritis AJ. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect Dis. 2008;8:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3775] [Article Influence: 471.9] [Reference Citation Analysis (1)] |
| 10. | Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593-1608.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 539] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 11. | Gish R, Jia JD, Locarnini S, Zoulim F. Selection of chronic hepatitis B therapy with high barrier to resistance. Lancet Infect Dis. 2012;12:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Papatheodoridis GV. Why do I treat HBeAg-negative chronic hepatitis B patients with nucleos(t)ide analogues? Liver Int. 2013;33 Suppl 1:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Viganò M, Mangia G, Lampertico P. HBeAg-negative chronic hepatitis B: why do I treat my patients with nucleos(t)ide analogues? Liver Int. 2014;34 Suppl 1:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, Aguilar Schall R, Flaherty JF, Martins EB, Charuworn P. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 15. | Lam YF, Seto WK, Wong D, Cheung KS, Fung J, Mak LY, Yuen J, Chong CK, Lai CL, Yuen MF. Seven-Year Treatment Outcome of Entecavir in a Real-World Cohort: Effects on Clinical Parameters, HBsAg and HBcrAg Levels. Clin Transl Gastroenterol. 2017;8:e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Emery JS, Feld JJ. Treatment of hepatitis B virus with combination therapy now and in the future. Best Pract Res Clin Gastroenterol. 2017;31:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Liu J, Zhang E, Ma Z, Wu W, Kosinska A, Zhang X, Möller I, Seiz P, Glebe D, Wang B. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10:e1003856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 18. | Fontaine H, Kahi S, Chazallon C, Bourgine M, Varaut A, Buffet C, Godon O, Meritet JF, Saïdi Y, Michel ML. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial--ANRS HB02 VAC-ADN. Gut. 2015;64:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Vandepapelière P, Lau GK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, Merican MI, Win KM, Trepo C, Cooksley G. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine. 2007;25:8585-8597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 20. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1581] [Article Influence: 175.7] [Reference Citation Analysis (2)] |
| 21. | Papatheodoridis GV, Hadziyannis SJ. Review article: current management of chronic hepatitis B. Aliment Pharmacol Ther. 2004;19:25-37. [PubMed] |
| 22. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1950] [Article Influence: 216.7] [Reference Citation Analysis (0)] |
| 23. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 463] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 24. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1364] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 25. | Lampertico P, Invernizzi F, Viganò M, Loglio A, Mangia G, Facchetti F, Primignani M, Jovani M, Iavarone M, Fraquelli M. The long-term benefits of nucleos(t)ide analogs in compensated HBV cirrhotic patients with no or small esophageal varices: A 12-year prospective cohort study. J Hepatol. 2015;63:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Papatheodoridis GV, Dalekos GN, Yurdaydin C, Buti M, Goulis J, Arends P, Sypsa V, Manolakopoulos S, Mangia G, Gatselis N. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J Hepatol. 2015;62:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol. 2016;22:319-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 407] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 29. | Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629-636.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 30. | Chan HL, Wong GL, Chim AM, Chan HY, Chu SH, Wong VW. Prediction of off-treatment response to lamivudine by serum hepatitis B surface antigen quantification in hepatitis B e antigen-negative patients. Antivir Ther. 2011;16:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 32. | Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, Eisenbach C, Welzel TM, Zachoval R, Felten G. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 33. | Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, Petersen J. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology. 2016;63:1481-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 34. | Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2015;42:243-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 35. | Van Hees S, Bourgeois S, Van Vlierberghe H, Sersté T, Francque S, Michielsen P, Sprengers D, Reynaert H, Henrion J, Negrin Dastis S. Stopping nucleos(t)ide analogue treatment in Caucasian hepatitis B patients after HBeAg seroconversion is associated with high relapse rates and fatal outcomes. Aliment Pharmacol Ther. 2018;47:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 36. | Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, van Boemmel F, Calleja JL, Sypsa V, Goulis J, Manolakopoulos S. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017;66:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 37. | Wei L, Kao JH. Benefits of long-term therapy with nucleos(t)ide analogues in treatment-naïve patients with chronic hepatitis B. Curr Med Res Opin. 2017;33:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Lim SG, Wai CT, Rajnakova A, Kajiji T, Guan R. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. Gut. 2002;51:597-599. [PubMed] |
| 39. | Fung J, Seto WK, Lai CL, Yuen MF. Extrahepatic effects of nucleoside and nucleotide analogues in chronic hepatitis B treatment. J Gastroenterol Hepatol. 2014;29:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Liu F, Wang L, Li XY, Liu YD, Wang JB, Zhang ZH, Wang YZ. Poor durability of lamivudine effectiveness despite stringent cessation criteria: a prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients. J Gastroenterol Hepatol. 2011;26:456-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Ha M, Zhang G, Diao S, Lin M, Sun L, She H, Kuan C, Shen L, Huang C, Shen W. A prospective clinical study in hepatitis B e antigen-negative chronic hepatitis B patients with stringent cessation criteria for adefovir. Arch Virol. 2012;157:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Nagaoka S, Abiru S, Komori A, Sasaki R, Bekki S, Hashimoto S, Saeki A, Yamasaki K, Migita K, Nakamura M. Hepatic flares promote rapid decline of serum hepatitis B surface antigen (HBsAg) in patients with HBsAg seroclearance: A long-term follow-up study. Hepatol Res. 2016;46:E89-E99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, Becht E, Hansi NK, Foster GR, Su TH. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 44. | Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, Liaw YF. Off-therapy durability of response to entecavir therapy in hepatitis B e antigen-negative chronic hepatitis B patients. Hepatology. 2013;58:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 45. | Cao J, Chi H, Yu T, Li Z, Hansen BE, Zhang X, Zhong C, Sun J, Hou J, Janssen HLA. Off-Treatment Hepatitis B Virus (HBV) DNA Levels and the Prediction of Relapse After Discontinuation of Nucleos(t)ide Analogue Therapy in Patients With Chronic Hepatitis B: A Prospective Stop Study. J Infect Dis. 2017;215:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Qiu YW, Huang LH, Yang WL, Wang Z, Zhang B, Li YG, Su TT, Zhou HY, Xu W, Wang XD. Hepatitis B surface antigen quantification at hepatitis B e antigen seroconversion predicts virological relapse after the cessation of entecavir treatment in hepatitis B e antigen-positive patients. Int J Infect Dis. 2016;43:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Höner Zu Siederdissen C, Rinker F, Maasoumy B, Wiegand SB, Filmann N, Falk CS, Deterding K, Port K, Mix C, Manns MP. Viral and Host Responses After Stopping Long-term Nucleos(t)ide Analogue Therapy in HBeAg-Negative Chronic Hepatitis B. J Infect Dis. 2016;214:1492-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Yao CC, Lee CM, Hung CH, Wang JH, Hu TH, Lu SN, Changchien CS, Hsu MC, Chen CH. Combining age and HBsAg level predicts post-treatment durability of nucleos(t)ide analogue-induced HBeAg seroconversion. J Gastroenterol Hepatol. 2015;30:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Wang CC, Tseng KC, Hsieh TY, Tseng TC, Lin HH, Kao JH. Assessing the Durability of Entecavir-Treated Hepatitis B Using Quantitative HBsAg. Am J Gastroenterol. 2016;111:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Hara T, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir therapy results in falls in serum hepatitis B surface antigen levels and seroclearance in nucleos(t)ide-naïve chronic hepatitis B patients. J Viral Hepat. 2014;21:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Clearance of hepatitis B surface antigen during long-term nucleot(s)ide analog treatment in chronic hepatitis B: results from a nine-year longitudinal study. J Gastroenterol. 2013;48:930-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Nowak MA, Bangham CR. Population dynamics of immune responses to persistent viruses. Science. 1996;272:74-79. [PubMed] |
| 53. | van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, Edelmann A. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 54. | Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, Zhang R, Chen R, Li T, Zhang T. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 342] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 55. | Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 980] [Article Influence: 61.3] [Reference Citation Analysis (1)] |
| 56. | Fan R, Sun J, Yuan Q, Xie Q, Bai X, Ning Q, Cheng J, Yu Y, Niu J, Shi G. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues. Gut. 2016;65:313-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 57. | Tseng CH, Hsu YC, Chang CY, Tseng TC, Wu MS, Lin JT, Kao JH. Quantification of serum hepatitis B core antibody to predict off-entecavir relapse in patients with chronic hepatitis B. J Formos Med Assoc. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Kimura T, Rokuhara A, Sakamoto Y, Yagi S, Tanaka E, Kiyosawa K, Maki N. Sensitive enzyme immunoassay for hepatitis B virus core-related antigens and their correlation to virus load. J Clin Microbiol. 2002;40:439-445. [PubMed] |
| 59. | Lin CL, Kao JH. New perspectives of biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2016;22:423-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Song G, Yang R, Rao H, Feng B, Ma H, Jin Q, Wei L. Serum HBV core-related antigen is a good predictor for spontaneous HBeAg seroconversion in chronic hepatitis B patients. J Med Virol. 2017;89:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 61. | Tanaka E, Matsumoto A. Guidelines for avoiding risks resulting from discontinuation of nucleoside/nucleotide analogs in patients with chronic hepatitis B. Hepatol Res. 2014;44:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1202] [Cited by in RCA: 1212] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 63. | Larrubia JR, Benito-Martínez S, Miquel-Plaza J, Sanz-de-Villalobos E, González-Mateos F, Parra T. Cytokines - their pathogenic and therapeutic role in chronic viral hepatitis. Rev Esp Enferm Dig. 2009;101:343-351. [PubMed] |
| 64. | Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol. 2014;20:3418-3430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 65. | Maini MK, Reignat S, Boni C, Ogg GS, King AS, Malacarne F, Webster GJ, Bertoletti A. T cell receptor usage of virus-specific CD8 cells and recognition of viral mutations during acute and persistent hepatitis B virus infection. Eur J Immunol. 2000;30:3067-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195:1089-1101. [PubMed] |
| 67. | Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386-1396. [PubMed] |
| 68. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. [PubMed] |
| 69. | Lopes AR, Kellam P, Das A, Dunn C, Kwan A, Turner J, Peppa D, Gilson RJ, Gehring A, Bertoletti A. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J Clin Invest. 2008;118:1835-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205-2213. [PubMed] |
| 71. | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 281] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 72. | Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, Wächtler M, Spannagl M, Denk G, Ulsenheimer A. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One. 2014;9:e105703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol. 2014;61:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 74. | Isogawa M, Chung J, Murata Y, Kakimi K, Chisari FV. CD40 activation rescues antiviral CD8+ T cells from PD-1-mediated exhaustion. PLoS Pathog. 2013;9:e1003490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 75. | Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 76. | Park JJ, Wong DK, Wahed AS, Lee WM, Feld JJ, Terrault N, Khalili M, Sterling RK, Kowdley KV, Bzowej N. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology. 2016;150:684-695.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 77. | Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 369] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 78. | Boni C, Penna A, Bertoletti A, Lamonaca V, Rapti I, Missale G, Pilli M, Urbani S, Cavalli A, Cerioni S. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J Hepatol. 2003;39:595-605. [PubMed] |
| 79. | Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963-73.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 299] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 80. | Moreno-Cubero E, Subirá D, Sanz-de-Villalobos E, Parra-Cid T, Madejón A, Miquel J, Olveira A, González-Praetorius A, García-Samaniego J, Larrubia JR. According to Hepatitis C Virus (HCV) Infection Stage, Interleukin-7 Plus 4-1BB Triggering Alone or Combined with PD-1 Blockade Increases TRAF1low HCV-Specific CD8+ Cell Reactivity. J Virol. 2018;92:pii: e01443-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 82. | Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 83. | Fisicaro P, Valdatta C, Massari M, Loggi E, Biasini E, Sacchelli L, Cavallo MC, Silini EM, Andreone P, Missale G. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138:682-693, 693.e1-693.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 84. | Maini MK, Peppa D. NK cells: a double-edged sword in chronic hepatitis B virus infection. Front Immunol. 2013;4:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 85. | Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, Emerson SS, Shuhart MC, Gretch DR. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 86. | Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K, Markova A, Bremer B, Schlaphoff V, Cornberg M. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis. 2014;209:1362-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 87. | Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A, Micco L, Nebbia G, Singh HD, Adams DH. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210:99-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 277] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 88. | Boni C, Lampertico P, Talamona L, Giuberti T, Invernizzi F, Barili V, Fisicaro P, Rossi M, Cavallo MC, Vecchi A. Natural killer cell phenotype modulation and natural killer/T-cell interplay in nucleos(t)ide analogue-treated hepatitis e antigen-negative patients with chronic hepatitis B. Hepatology. 2015;62:1697-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 90. | Meng N, Gao X, Yan W, Wang M, Liu P, Lu XD, Zhang SJ, Lu YQ, Tang WX. Efficacy of telbivudine in the treatment of chronic hepatitis b and liver cirrhosis and its effect on immunological responses. J Huazhong Univ Sci Technolog Med Sci. 2015;35:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Chen Y, Li X, Ye B, Yang X, Wu W, Chen B, Pan X, Cao H, Li L. Effect of telbivudine therapy on the cellular immune response in chronic hepatitis B. Antiviral Res. 2011;91:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 92. | Li C, Ji H, Cai Y, Ayana DA, Lv P, Liu M, Jiang Y. Serum interleukin-37 concentrations and HBeAg seroconversion in chronic HBV patients during telbivudine treatment. J Interferon Cytokine Res. 2013;33:612-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Jiang Y, Ma Z, Xin G, Yan H, Li W, Xu H, Hao C, Niu J, Zhao P. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm. 2010;2010:143026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | van der Molen RG, Sprengers D, Biesta PJ, Kusters JG, Janssen HL. Favorable effect of adefovir on the number and functionality of myeloid dendritic cells of patients with chronic HBV. Hepatology. 2006;44:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Zheng Y, Huang Z, Chen X, Tian Y, Tang J, Zhang Y, Zhang X, Zhou J, Mao Q, Ni B. Effects of telbivudine treatment on the circulating CD4+ T-cell subpopulations in chronic hepatitis B patients. Mediators Inflamm. 2012;2012:789859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314. [PubMed] |
| 97. | Larrubia JR, Benito-Martínez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149-7159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 98. | Jaroszewicz J, Ho H, Markova A, Deterding K, Wursthorn K, Schulz S, Bock CT, Tillmann HL, Manns MP, Wedemeyer H. Hepatitis B surface antigen (HBsAg) decrease and serum interferon-inducible protein-10 levels as predictive markers for HBsAg loss during treatment with nucleoside/nucleotide analogues. Antivir Ther. 2011;16:915-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 99. | Papatheodoridis G, Goulis J, Manolakopoulos S, Margariti A, Exarchos X, Kokkonis G, Hadziyiannis E, Papaioannou C, Manesis E, Pectasides D. Changes of HBsAg and interferon-inducible protein 10 serum levels in naive HBeAg-negative chronic hepatitis B patients under 4-year entecavir therapy. J Hepatol. 2014;60:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Fung SK, Wong F, Hussain M, Lok AS. Sustained response after a 2-year course of lamivudine treatment of hepatitis B e antigen-negative chronic hepatitis B. J Viral Hepat. 2004;11:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Enomoto M, Tamori A, Kohmoto MT, Hayashi T, Morikawa H, Jomura H, Sakaguchi H, Habu D, Kawada N, Shiomi S. Optimal duration of additional therapy after biochemical and virological responses to lamivudine in patients with HBeAg-negative chronic hepatitis B: a randomized trial. Hepatol Res. 2008;38:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Yeh CT, Hsu CW, Chen YC, Liaw YF. Withdrawal of lamivudine in HBeAg-positive chronic hepatitis B patients after achieving effective maintained virological suppression. J Clin Virol. 2009;45:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Fung J, Lai CL, Tanaka Y, Mizokami M, Yuen J, Wong DK, Yuen MF. The duration of lamivudine therapy for chronic hepatitis B: cessation vs. continuation of treatment after HBeAg seroconversion. Am J Gastroenterol. 2009;104:1940-1946; quiz 1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Wang L, Liu F, Liu YD, Li XY, Wang JB, Zhang ZH, Wang YZ. Stringent cessation criterion results in better durability of lamivudine treatment: a prospective clinical study in hepatitis B e antigen-positive chronic hepatitis B patients. J Viral Hepat. 2010;17:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Kuo YH, Chen CH, Wang JH, Hung CH, Tseng PL, Lu SN, Changchien CS, Lee CM. Extended lamivudine consolidation therapy in hepatitis B e antigen-positive chronic hepatitis B patients improves sustained hepatitis B e antigen seroconversion. Scand J Gastroenterol. 2010;45:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Cai W, Xie Q, An B, Wang H, Zhou X, Zhao G, Guo Q, Gu R, Bao S. On-treatment serum HBsAg level is predictive of sustained off-treatment virologic response to telbivudine in HBeAg-positive chronic hepatitis B patients. J Clin Virol. 2010;48:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Jung YK, Yeon JE, Lee KG, Jung ES, Kim JH, Kim JH, Seo YS, Yim HJ, Um SH, Ryu HS. Virologic response is not durable after adefovir discontinuation in lamivudine-resistant chronic hepatitis B patients. Korean J Hepatol. 2011;17:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Chaung KT, Ha NB, Trinh HN, Garcia RT, Nguyen HA, Nguyen KK, Garcia G, Ahmed A, Keeffe EB, Nguyen MH. High frequency of recurrent viremia after hepatitis B e antigen seroconversion and consolidation therapy. J Clin Gastroenterol. 2012;46:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Song MJ, Song DS, Kim HY, Yoo SH, Bae SH, Choi JY, Yoon SK, Paik YH, Lee JS, Lee HW. Durability of viral response after off-treatment in HBeAg positive chronic hepatitis B. World J Gastroenterol. 2012;18:6277-6283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 110. | He D, Guo S, Chen W, Chen X, Yan G, Wang J, Li M, Zhu P, Huang H, Wang Y. Long-term outcomes after nucleos(t)ide analogues discontinuation in chronic hepatitis B patients with HBeAg-negative. BMC Infect Dis. 2013;13:458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Kim YJ, Kim K, Hwang SH, Kim SS, Lee D, Cheong JY, Cho SW. Durability after discontinuation of nucleos(t)ide therapy in chronic HBeAg negative hepatitis patients. Clin Mol Hepatol. 2013;19:300-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Kwon JH, Jang JW, Choi JY, Park CH, Yoo SH, Bae SH, Yoon SK. Should lamivudine monotherapy be stopped or continued in patients infected with hepatitis B with favorable responses after more than 5 years of treatment? J Med Virol. 2013;85:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Ridruejo E, Marciano S, Galdame O, Reggiardo MV, Muñoz AE, Adrover R, Cocozzella D, Fernandez N, Estepo C, Mendizábal M. Relapse rates in chronic hepatitis B naïve patients after discontinuation of antiviral therapy with entecavir. J Viral Hepat. 2014;21:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 114. | Sohn HR, Min BY, Song JC, Seong MH, Lee SS, Jang ES, Shin CM, Park YS, Hwang JH, Jeong SH. Off-treatment virologic relapse and outcomes of re-treatment in chronic hepatitis B patients who achieved complete viral suppression with oral nucleos(t)ide analogs. BMC Infect Dis. 2014;14:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 115. | He D, Guo S, Zhu P, Tao S, Li M, Huang H, Wang J, Wang Y, Ding M. Long-term outcomes after nucleos(t)ide analogue discontinuation in HBeAg-positive chronic hepatitis B patients. Clin Microbiol Infect. 2014;20:O687-O693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Jiang JN, Huang ZL, He LX, Huang YH, Su MH, Xie R, Liang YX, Fu WD, Huang XH, Guo WW. Residual amount of HBV DNA in serum is related to relapse in chronic hepatitis B patients after cessation of nucleos(t)ide analogs. J Clin Gastroenterol. 2015;49:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, Yuen MF, Chan HL. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut. 2015;64:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 118. | Huang YH, Wu JC, Chang TT, Sheen IJ, Lee PC, Huo TI, Su CW, Wang YJ, Chang FY, Lee SD. Analysis of clinical, biochemical and viral factors associated with early relapse after lamivudine treatment for hepatitis B e antigen-negative chronic hepatitis B patients in Taiwan. J Viral Hepat. 2003;10:277-284. [PubMed] |
| 119. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 856] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 120. | Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 915] [Cited by in RCA: 908] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 121. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 122. | Paik YH, Kim JK, Kim DY, Park JY, Ahn SH, Han KH, Chon CY, Lee KS. Clinical efficacy of a 24-months course of lamivudine therapy in patients with HBeAg negative chronic hepatitis B: a long-term prospective study. J Korean Med Sci. 2010;25:882-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 123. | Liang Y, Jiang J, Su M, Liu Z, Guo W, Huang X, Xie R, Ge S, Hu J, Jiang Z. Predictors of relapse in chronic hepatitis B after discontinuation of anti-viral therapy. Aliment Pharmacol Ther. 2011;34:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 124. | Jin YJ, Kim KM, Yoo DJ, Shim JH, Lee HC, Chung YH, Lee YS, Suh DJ. Clinical course of chronic hepatitis B patients who were off-treated after lamivudine treatment: analysis of 138 consecutive patients. Virol J. 2012;9:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |