Published online Apr 7, 2018. doi: 10.3748/wjg.v24.i13.1398
Peer-review started: January 28, 2018
First decision: February 10, 2018
Revised: February 12, 2018
Accepted: March 3, 2018
Article in press: March 3, 2018
Published online: April 7, 2018
Processing time: 67 Days and 9.3 Hours
To investigate the protective effects of Ampelopsis grossedentata (AMP) on dextran sulfate sodium (DSS)-induced colitis in mice based on systems pharmacology approach.
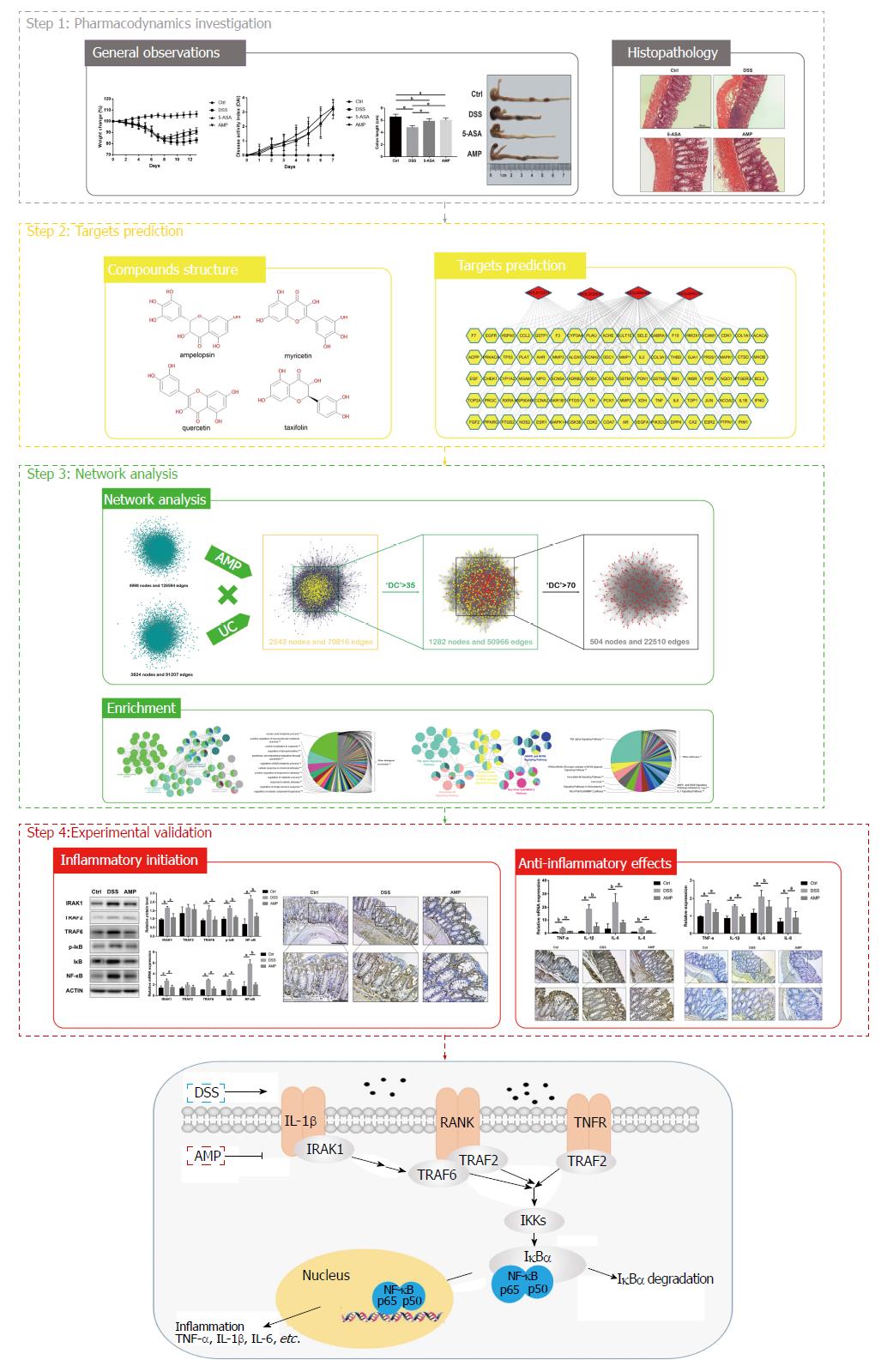
Systems pharmacology approach was used to predict the active ingredients, candidate targets and the efficacy of AMP on ulcerative colitis (UC) using a holistic process of active compound screening, target fishing, network construction and analysis. A DSS-induced colitis model in C57BL/6 mice (n = 10/group) was constructed and treated with 5-aminosalicylic acid (100 mg/kg/d) and AMP (400 mg/kg/d) to confirm the underlying mechanisms and effects of AMP on UC with western blot analyses, polymerase chain reaction, histological staining and immunohistochemistry.
The therapeutic effects of AMP against DSS-induced colitis were determined in the beginning, and the results showed that AMP significantly improved the disease in general observations and histopathology analysis. Subsequent systems pharmacology predicted 89 corresponding targets for the four candidate compounds of AMP, as well as 123 candidate targets of UC, and protein-protein interaction networks were constructed for the interaction of putative targets of AMP against UC. Enrichment analyses on TNF-α and RANKL/RANK, a receptor activator of NF-κB signaling pathways, were then carried out. Experimental validation revealed that inflammation-related signaling pathways were activated in the DSS group, and AMP significantly suppressed DSS-induced high expression of IRAK1, TRAF6, IκB and NF-κB, and inhibited the elevated expression levels of TNF-α, IL-1β, IL-6 and IL-8.
AMP could exert protective effects on UC via suppressing the IRAK1/TRAF6/NF-κB-mediated inflammatory signaling pathways.
Core tip: Ulcerative colitis (UC), as one of the major forms of inflammatory bowel disease, could lead to various intestinal and extra-intestinal manifestations, which brings a huge challenge to the health care system. Given studies have confirmed that the flavonoid bioactive compounds contained in Ampelopsis grossedentata (AMP) possess strong antiinflammatory activity, we examined the potential therapeutic effects of AMP on UC based on systems pharmacology. Results showed that AMP could suppress the inflammation-related signaling pathways in dextran sulfate sodium-induced colitis, indicating protective effects on UC, which might provide an effective natural therapy for the treatment and prevention of UC.
- Citation: Chen YL, Zhang YL, Dai YC, Tang ZP. Systems pharmacology approach reveals the antiinflammatory effects of Ampelopsis grossedentata on dextran sodium sulfate-induced colitis. World J Gastroenterol 2018; 24(13): 1398-1409
- URL: https://www.wjgnet.com/1007-9327/full/v24/i13/1398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i13.1398
Ulcerative colitis (UC), which is primarily characterized by recurrent abdominal pain, diarrhea and bloody purulent stools[1], is a chronic inflammatory condition of the intestine, with mucosal inflammation beginning in the rectum and extending continuously to part of or the entire colon. Inflammation in UC can lead to the occurrence of multifocal ulcers on the wall of the large intestine, causing nausea, cramps, diarrhea, pus, bleeding and fatigue. In general, patients with UC may develop varying degrees of extra-intestinal manifestations, which are attributed to the inflammatory cascade in the colorectum, including skin, mucosal, joint, ocular, hepatic and pulmonary disorders[2,3]. Meanwhile, the increasing prevalence of UC brings a considerable challenge to health care systems worldwide[4].
Given that UC is a kind of long-term disease with uncertain etiology, the aim of therapy is to induce and maintain clinical remission, defined as control of symptoms, endoscopic mucosal healing and avoidance of complications[1,5]. In addition to dietary control, the available pharmacologic treatments include 5-aminosalicylates, steroids, thiopurines and biological agents[6]. However, the routine medical treatments for UC are not fully curative, and investigations have shown that compared with standard care, the elevated cost-utility ratios of biologics reached up to $456979 (in United States’ dollars)[7]. In addition, as UC mostly affects young people and takes a lifelong treatment, as well as has a low mortality[8,9] that is not different from that in the healthy population, the disease poses an enormous economic burden on individuals, families and society. Thus, promising and novel therapeutic tactics are urgently needed to be explored and developed for UC.
Complementary and alternative medicine, especially Chinese herbal medicine, is widely used among UC patients. Recent investigations revealed that many natural compounds have significant protective efficacies in UC patients[10]. Ampelopsis grossedentata (AMP), which contains a large amount of flavonoid active ingredients, has been widely consumed as a functional beverage and may be used consecutively as a supplementary option to the current standard treatment of UC. Dihydromyricetin, a major compound of AMP, has been reported to be highly distributed in the intestinal tract[11], and has hepatoprotective[12], insulin resistant[13], antioxidation[14], anticancer[15] and antiinflammatory activities, which are produced by suppression of nuclear factor kappa-B (NF-κB) activation[16,17]. However, few researchers have investigated the underlying mechanisms of the potential protective effects of AMP against UC, although AMP contains much more flavonoids and deserves further study.
Considering the complex combination and multitarget interactions of Chinese herbal plants, it is quite difficult to conduct a systematic study of the effects of AMP on diseases using conventional methods. Whereas, systems pharmacology, an emerging systems-oriented approach which has been reported to reveal the mechanism of a disease and link it to the chemical network of a drug[18,19], provides new perspectives to predict the active ingredients and candidate targets through a holistic process of active compound screening, target fishing, network construction and analysis[20,21]. To further investigate the potential mechanisms and effects of AMP on UC, a systems pharmacology analysis and animal experiments were conducted in this study.
The active compounds and their corresponding putative targets of AMP were identified by the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database. Known UC-related targets were obtained from the Genetic Association database, Therapeutic Target database and Online Mendelian Inheritance in Man database. Protein-protein interaction (PPI) networks were constructed for the interaction of putative targets of AMP against UC based on BisoGenet, and the degree centrality (DC) value, which represents the topological importance of a node in the intersection network, was used to filter the candidate targets. Finally, enrichment analysis was conducted using ClueGO, a Cytoscape plugin.
Male C57BL/6 mice aged 6 weeks were obtained from the SLAC Animal Laboratories (Shanghai, China). All mice were raised under standard conditions (room temperature, 24 ± 2 °C; humidity, 50%-60%; light, 12-h light/12-h dark cycle).
Experimental colitis was induced by administration of 3.5% (w/v) dextran sulfate sodium (DSS, 36-50 kDa; MP Biomedicals, United States) in drinking water provided ad libitum for 7 d. The DSS solution was changed every day. For each experiment, the mice were randomly divided into four groups (n = 10/group): the control group was given normal water only; the DSS group, 5-aminosalicylic acid (5-ASA) group and AMP group received a 3.5% DSS solution for 7 d, respectively. For treatment experiment, the control group and DSS group were administered normal saline intragastrically daily. The 5-ASA group was given 5-ASA solution (100 mg/kg/d) and the AMP group were administered AMP solution (400 mg/kg/d) intragastrically. The severity of colitis was calculated daily using the disease activity index[22]; the disease activity index scores are shown in Supplementary Table 1. After treatment, blood was extracted by cardiac puncture under anesthesia. Intestinal tissues were fixed in 4% buffered formalin for hematoxylin & eosin/immunohistochemistry and the other tissues were snap-frozen for western blot/RT-PCR.
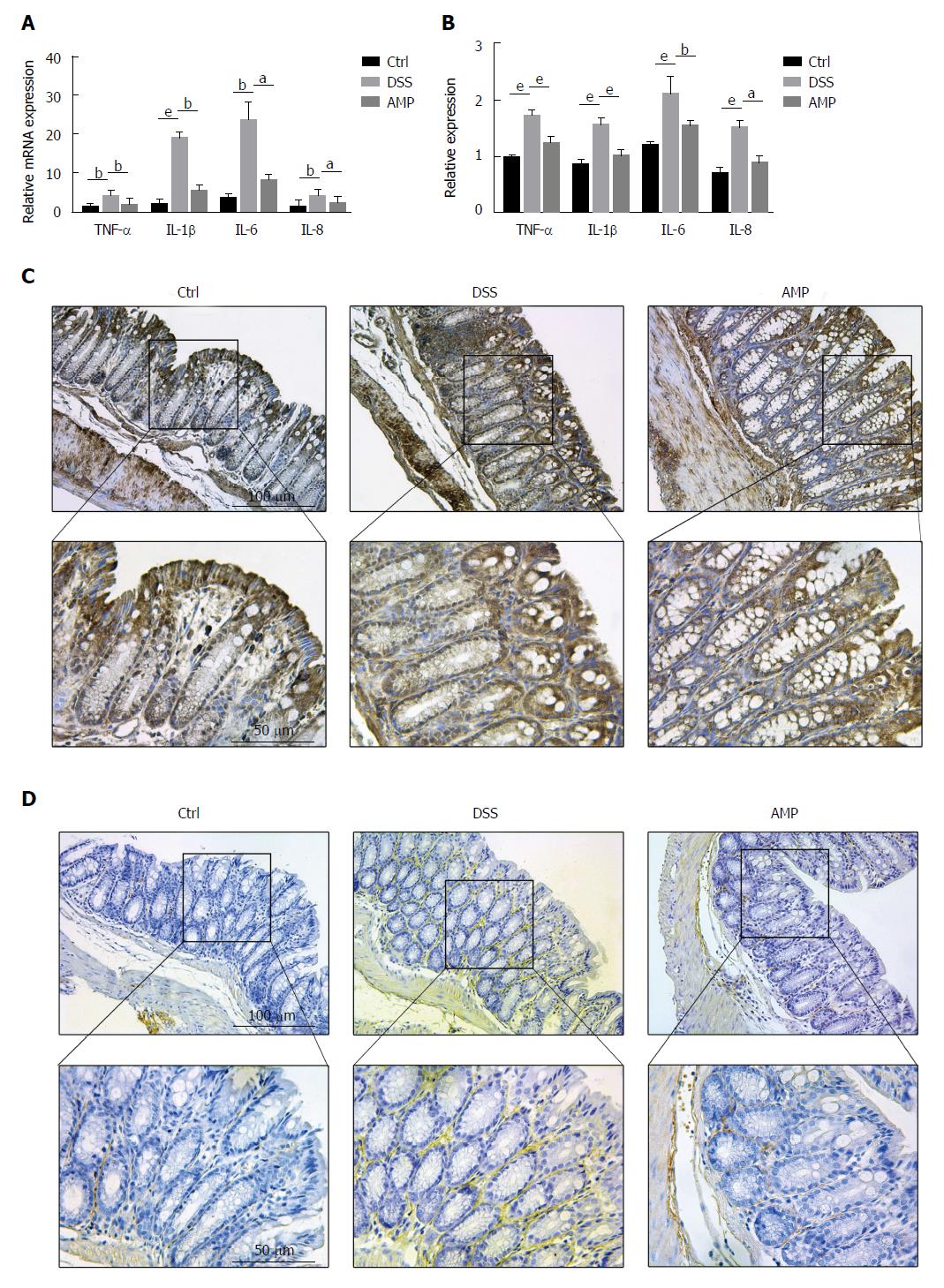
Paraffin-embedded colon samples were stained with HE for histological evaluation, as well as were immunostained with anti-NF-κB, anti-tumor necrosis factor-alpha (TNF-α) and anti-interleukin-1 beta (IL-1β) for protein expression in intestinal tissues after being cut into 4 μm sections.
Protein extracts were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes (Millipore, Germany). Protein expression was detected by western blot analysis. Primary antibodies used were rabbit monoclonal anti-NF-κB, anti-inhibitor of NF-κB (IκB), anti-p-IκB, anti-IL-1 receptor associated kinase (IRAK1), anti-TNF receptor-associated factor 2 (TRAF2) and anti-TNF receptor-associated factor 6 (TRAF6). All antibodies were purchased from Cell Signaling Technology (United States).
Total RNA was extracted using TRIzol reagent (Invitrogen, United States). RT-PCR was carried out using SYBR Green PCR Master Mix (Toyobo, Japan) in an Eppendorf PCR system. The data were analyzed by the ΔΔCt method and samples were normalized to β-actin. Primer sequences are shown in Supplementary Table 2.
The levels of inflammation-related cytokines (TNF-α, IL-1β, IL-6 and IL-8) were detected using mouse-specific ELISA kits (Dakewe Bio-engineering Co. Ltd., China), following the manufacturer’s instructions.
All data are presented as the mean ± SD. Statistical analyses were performed using the Student’s t-test or one-way ANOVA for group comparisons, and P < 0.05 was considered statistically significant.
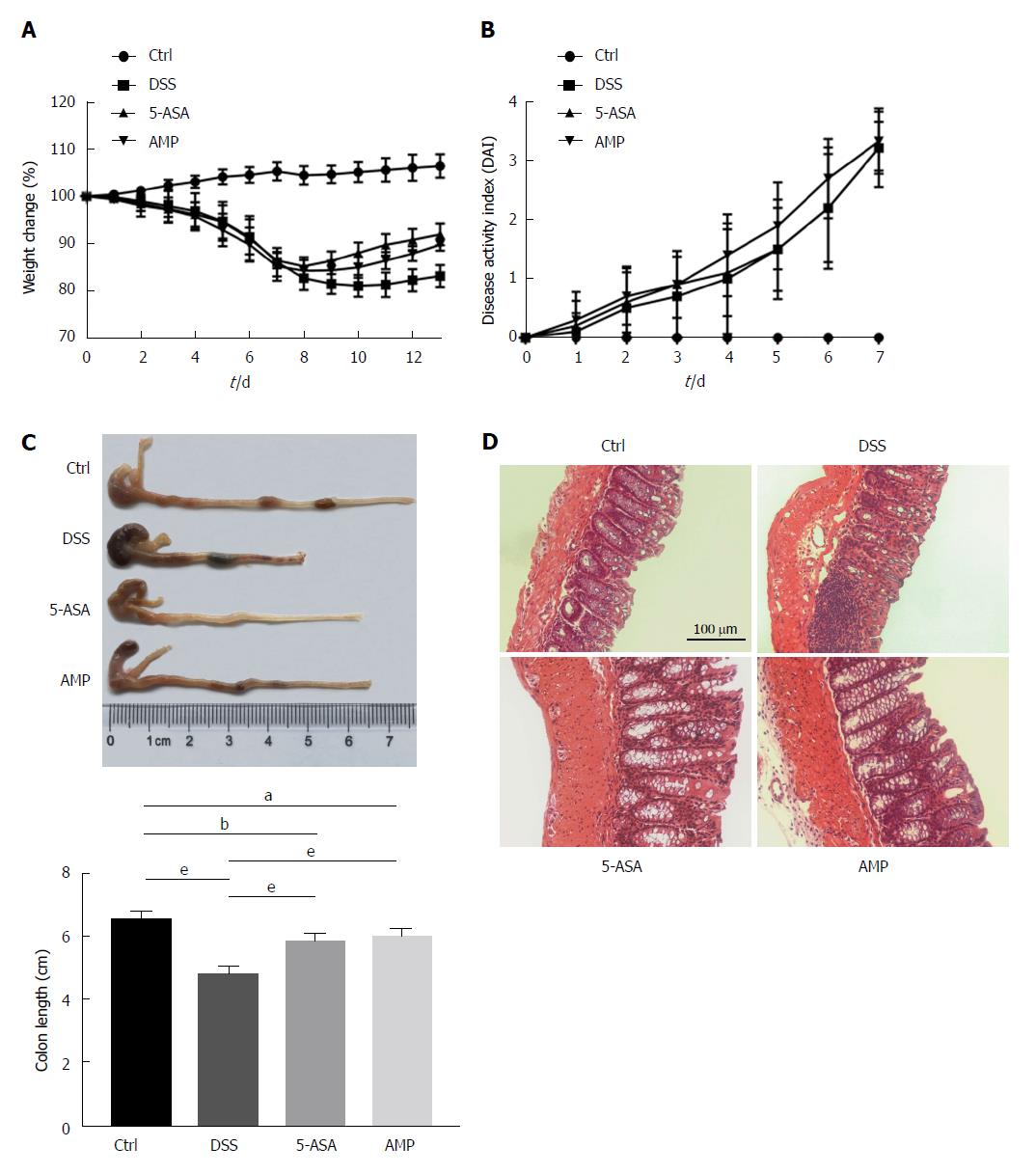
As the flavonoid bioactive compound extracted from AMP could inhibit inflammatory response in vitro and in vivo[16], the protective effects of AMP in DSS-induced colitis were examined in this study. Results presented a reduction of body weight together with an elevation of disease activity index scores under DSS stimulation (Figure 1A and B); meanwhile, treatment with 5-ASA and AMP prominently improved the body weight as well as the length of colon, with significant difference (Figure 1A and C). Additionally, histological analyses revealed more neutrophil infiltration, increased ulceration and crypt loss in the colon in the DSS group compared with the control and treatment groups (Figure 1D), indicating a protective effect of AMP on experimental colitis.
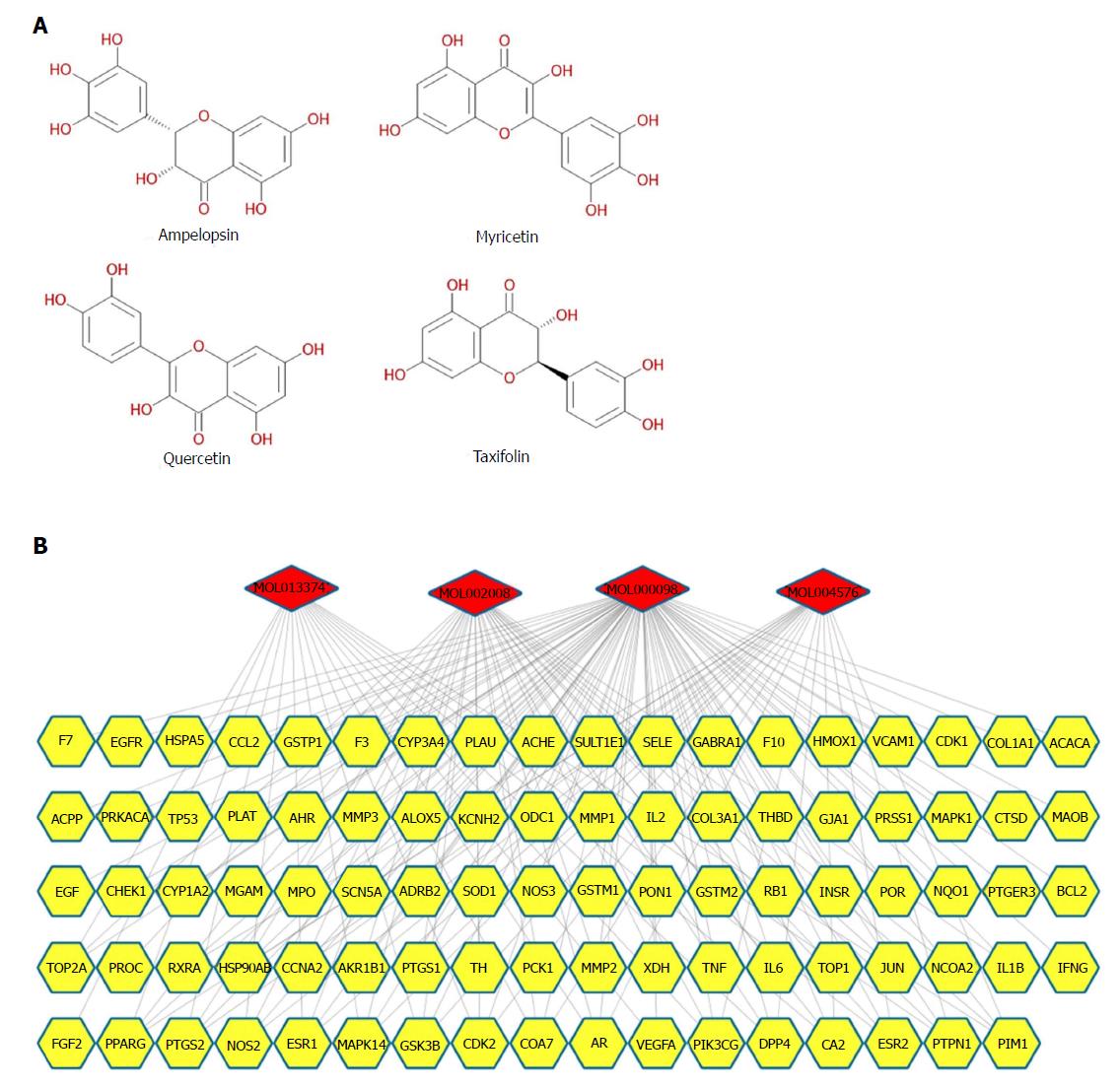
Candidate compound screening and putative target prediction for AMP: Four candidate compounds of AMP were obtained from the TCMSP database, including dihydromyricetin, myricetin, quercetin and taxifolin (Figure 2A and Supplementary Table 3). Since recent research studies have demonstrated strong antiinflammatory activities for all the selected compounds[16,23-25], which may be crucially involved in the improvement of UC, putative target prediction was subsequently performed to confirm this hypothesis. Given that the comprehensive biological and pharmacological effects of Chinese herbal plants rely upon their complex compounds and multitarget interactions, a compound-target network was built based on massive open-source initiatives and free web-based tools. A total of 156 potential targets were collected from TCMSP, and 89 targets were ultimately included after filtering (Figure 2B and Supplementary Tables 4 and 5). Moreover, 67 targets overlapped in the 4 compounds, which indicated that most of the compounds of AMP hit multitargets to exert multifarious therapeutic effects.
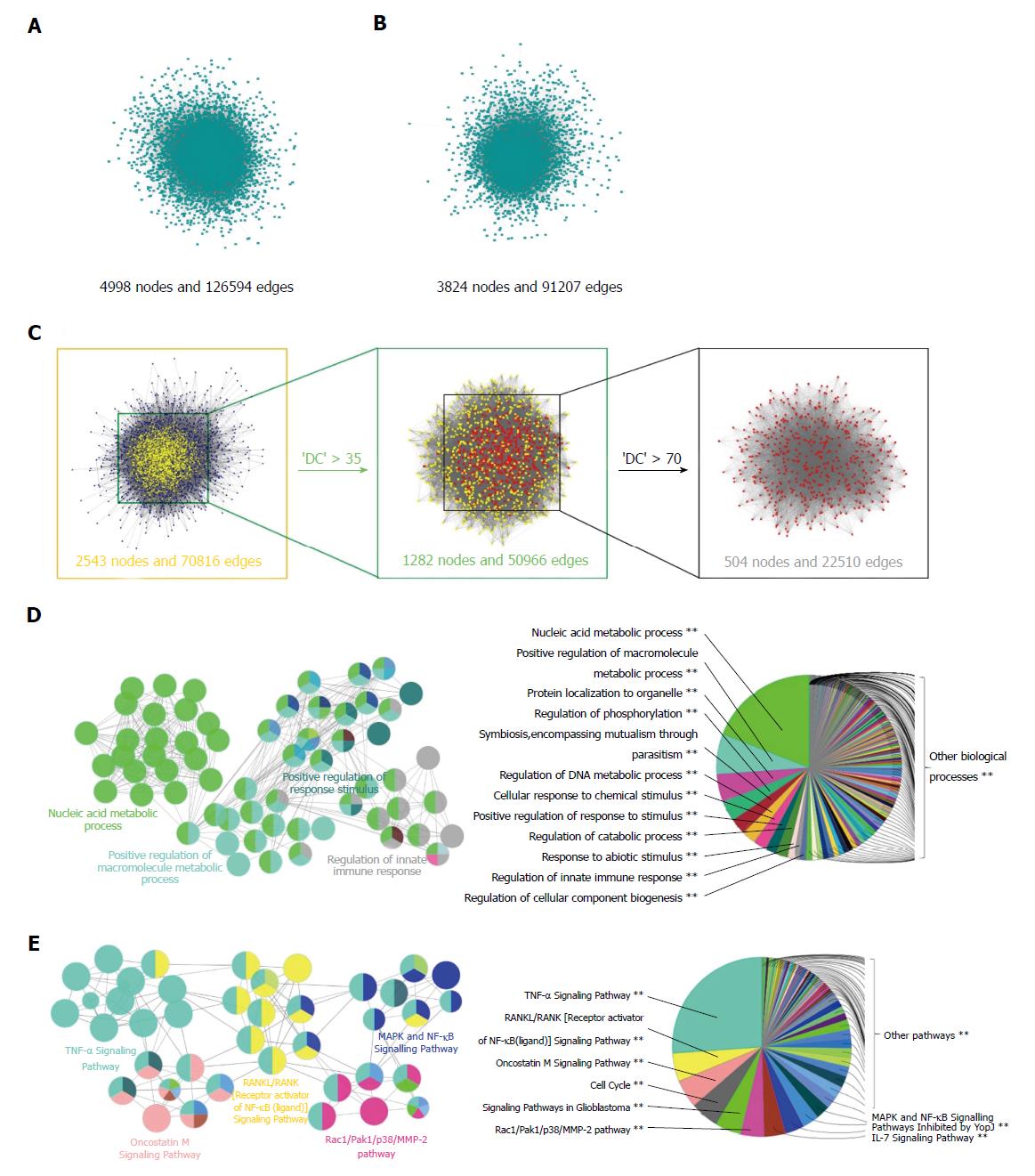
PPI network constitution and identification of candidate targets for AMP against UC: To better understand the mechanisms of AMP in the treatment of UC, 123 candidate targets of UC were obtained from Genetic Association database, Therapeutic Target database and Online Mendelian Inheritance in Man database (Supplementary Table 6). As PPI maps represented physical interactions on a molecular level[19], a putative-target network (4998 nodes and 126594 edges) and a known UC-related target network (3824 nodes and 91207 edges) were constructed based on PPI database (Figure 3A and B). Subsequently, to confirm the candidate targets of AMP against UC, an intersection of the above networks was conducted, which consisted of 2543 nodes and 70816 edges, and the DC values of 35 and 70 were computed by CytoNCA to identify the putative targets during the process (Figure 3C).
In order to further elucidate the possible effects of AMP on UC, the biological processes and signaling pathways were determined through Cytoscape software. The results showed that the biological processes were largely related to the nucleic acid metabolic process, positive regulation of macromolecule metabolic process, positive regulation of response to stimulus and regulation of the innate immune response (Figure 3D), and the signaling pathways were mainly involved with the TNF-αsignaling pathway, RANKL/RANK (receptor activator of NF-κB) signaling pathway, oncostatin M pathway, Rac1/Pak1/p38/MMP-2 pathway and MAPK/NF-κB signaling pathway (Figure 3E). Based on these data, we proposed a hypothesis that the protective mechanisms of AMP on UC were possibly related to inflammatory signaling pathways.
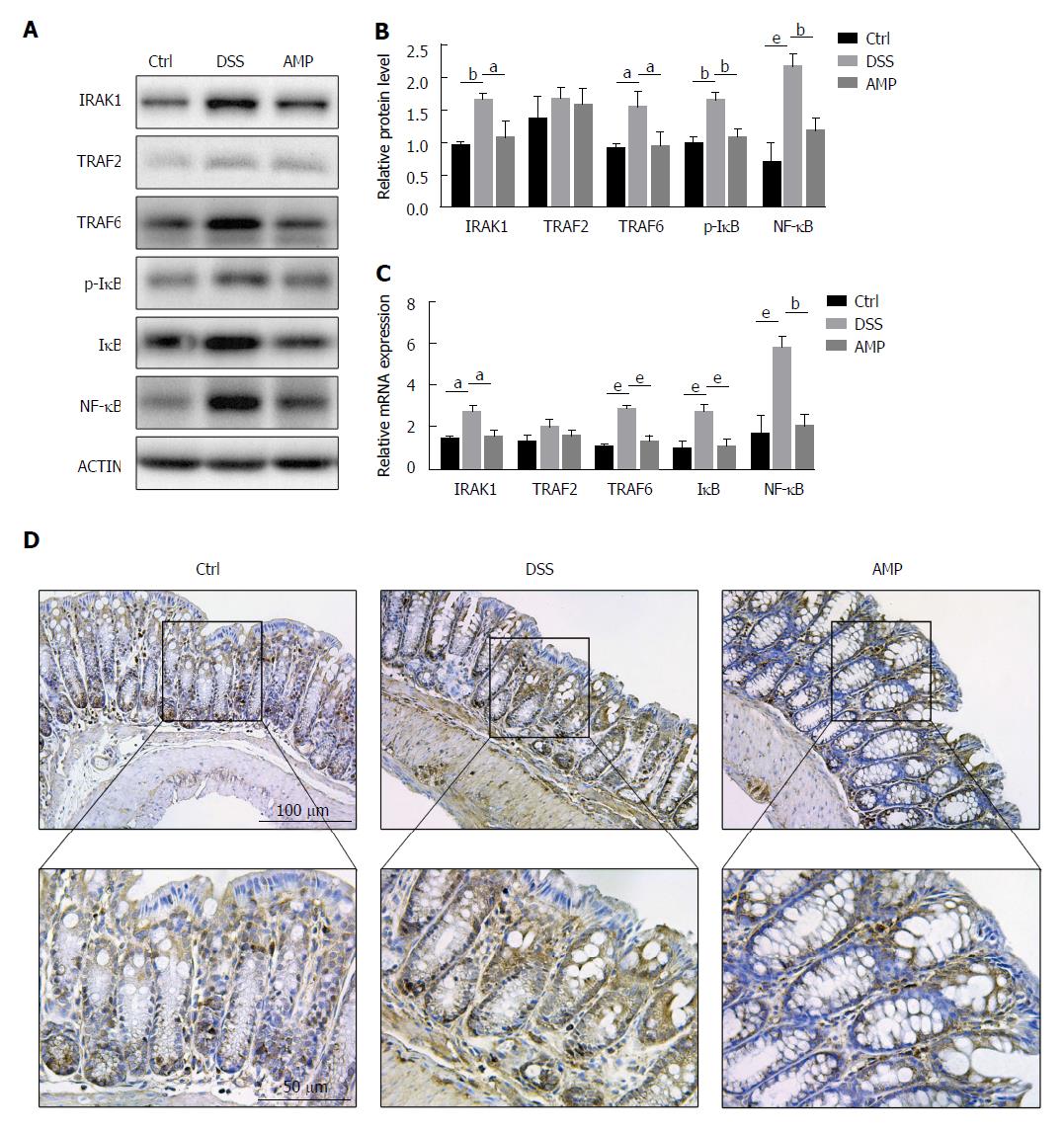
AMP could suppress the inflammation-related signaling pathways in DSS-induced colitis: Since systems pharmacology demonstrated that TNF-α and RANKL/RANK (receptor activator of NF-κB) signaling pathways were involved in the therapeutic effects of AMP on UC, western blot analyses were performed to evaluate TRAF2 and TRAF6, the downstream events of TNFR and RANK. Interestingly, outcomes presented a significant increase of TRAF6 expression in the DSS group as well as a mild increase of TRAF2 expression, compared with the control group, and AMP could improve this condition (Figure 4A and B). Thus, the upstream indicator of TRAF6, IRAK1 was determined next to investigate the further mechanisms in the TRAF6-mediated inflammatory signaling pathway, and results showed a marked elevation in the DSS group and that AMP could alleviate this situation, suggesting that IL-1-mediated proinflammatory signaling might be involved in the antiinflammatory action of AMP in UC. Accordingly, the mRNA expression of TRAF6 and IRAK1 was enhanced, whereas the expression of TRAF2 was not (Figure 4C), indicating that AMP might exert antiinflammatory effects via IRAK1/TRAF6-mediated signaling pathway.
Given IκB was the shared core downstream event of TRAF2/6 and IRAK1, and phosphorylation of IκB could release NF-κB into the nucleus thus initiating the gene transcription of relevant proinflammatory signals to result in inflammatory responses in intestine, we determined the levels of p-IκB and NF-κB consequently. Western blot analyses manifested significant differences in p-IκB/IκB and NF-κB between the AMP group and the DSS group, and these changes were also significant compared with the control group (Figure 4A and B). Meanwhile, parallel changes were seen with the PCR analyses (Figure 4C), suggesting that NF-κB was activated in the DSS-induced colitis and was inhibited by AMP. Additionally, immunohistochemical estimation revealed that the protein expression of NF-κB in inflamed colon tissue was significantly increased in the DSS group and was prevented in the AMP group (Figure 4D), confirming the previous findings.
AMP has protective effects on UC by inhibiting inflammation: As NF-κB was tightly associated with a great deal of inflammation-related genes, which could release a series of proinflammatory cytokines, including TNF-α and IL-1β, thus activating the whole inflammatory feedback cycle process[26,27], we measured the relative expression levels of TNF-α, IL-1β, IL-6 and IL-8 with PCR and ELISA. Results presented that all the indicators were distinctly elevated under DSS stimulation, being alleviated with AMP treatment (Figure 5A and B). Furthermore, immunohistochemical estimation showed that the expression of secretory proteins TNF-α and IL-1β were both prominently increased in the DSS group and were improved in the AMP group (Figure 5C and D). Taken together, all the findings indicated that AMP might exert protective effects on UC via suppressing the IRAK1/TRAF6/NF-κB-mediated inflammatory signaling pathway.
UC, as one of the major forms of inflammatory bowel disease, represents an increased risk for progressing colorectal cancer[28]. Recent investigations have confirmed that flavonoid bioactive compounds isolated from the edible herb AMP have strong antiinflammatory activities in macrophages[16], and may provide effective natural therapies for the treatment and prevention of UC. Thus, a systems pharmacology approach was conducted in this study to explore the underlying pharmacological mechanisms of AMP on UC. The schematic diagram of the research methodology and the proposed model of AMP acting on UC was shown in Figure 6.
A DSS-induced experimental colitis model was constructed to verify the therapeutic effects, and results showed that AMP could significantly improve the general observations and histopathology analysis. Thus, systems pharmacology was performed subsequently to identify the active compounds and their corresponding targets, and results showed that four candidate compounds obtained from the TCMSP database were ultimately included in this study. Meanwhile, another four compounds, including ambrein, apiin, ampeloptin and myricomplanoside, were obtained from the National Scientific Data Sharing Platform database and Traditional Chinese Medicine Database @Taiwan, but were excluded due to rare corresponding targets. Finally, a compound-target network was constructed for the four selected compounds and their corresponding targets, indicating synergistic as well as multifarious effects for each compound of AMP.
PPI maps were next constructed for the functional analysis, on the basis of putative-target networks of AMP and UC. Enrichment analyses presented a series of biological processes and signaling pathways, and a large number of those were supposed to be tightly associated with the inflammatory response, including the nucleic acid metabolic process, positive regulation of macromolecule metabolic process, positive regulation of response to stimulus and the regulation of innate immune response, together with the TNF-αsignaling pathway, RANKL/RANK (receptor activator of NF-κB) signaling pathway, oncostatin M pathway, Rac1/Pak1/p38/MMP-2 pathway and MAPK/NF-κB signaling pathway. Thus, we proposed that the NF-κB-related inflammatory signaling pathway, which was highly involved in all the above findings, might be a pivotal target in the treatment of AMP on UC.
NF-κB, as a central transcription factor of inflammatory mediators and a key participant in innate and adaptive immune responses involved in the perpetuation of inflammatory cascade[29], has been considered as the central molecular pathway for UC incidence[27]. Recent investigations revealed that the activation of proinflammatory cytokines, such as TNF-α and IL-1β, could induce the translocation of NF-κB from the cytoplasm to the nucleus, leading to the expression of a variety of inflammatory genes[30]. Given that inflammation was a major factor for the progression of UC[31], we performed the experimental validation to verify the above assumption.
The TNF-α signaling pathway and RANKL/RANK (receptor activator of NF-κB) signaling pathway were the most important parts in the signaling pathways obtained from enrichment analyses. TRAF2 and TRAF6, the downstream events of TNFR and RANK, were detected firstly, and results presented a significant response on TRAF6 but an insignificant response on TRAF2, suggesting the activation of TRAF6 in DSS-induced colitis. IRAK1, a protein kinase which is partially responsible for IL1-induced up-regulation of NF-κB by combining with TRAF6 and was also detected in previous enrichment analyses[32], was determined next to further explore the potential antiinflammatory mechanisms of AMP on UC. Outcomes presented a high expression of IRAK1 under DSS stimulation, indicating that IRAK1/TRAF6-mediated proinflammatory signaling might be participated in the antiinflammatory action of AMP on UC.
Thus, we validated the downstream proinflammatory signaling and the therapeutic effects, and results showed the activation of NF-κB under DSS stimulation as well as the protective effects of AMP against UC in mice. Interestingly, in the DSS group compared with the control group, there was an increased expression of proinflammatory signaling pathways and also of the levels of IκB; however, the increased expression of IκB detected by PCR was in accordance with the western blot analyses and the p-IκB/IκB ratio was elevated ultimately, which might be due to the feedback regulation of activated NF-κB. Together, all the findings revealed that AMP could exert protective effects on UC via suppressing the IRAK1/TRAF6/NF-κB-mediated inflammatory signaling pathway.
Limitations should be acknowledged in regards to the fact that research studies on AMP have been rare, resulting in a lack of retrieved compounds, so that the active compounds of AMP might not be fully predicted. Furthermore, some selected compounds were also rejected for the absence of efficient corresponding targets, which might be due to the relevant databases still not being well-developed. Besides, since UC is a chronic inflammatory condition of the intestine combined with a great deal of other biological processes, which have also been detected in this study, further studies are necessary to explore the systematic effects of AMP on UC.
Ulcerative colitis (UC), as a recurrent chronic inflammatory disease, greatly affects the quality of life of patients, which brings an enormous challenge for both individuals and society worldwide. However, the etiology of UC is still unknown and conventional medical treatments for UC are not fully curative. Thus, promising and novel therapeutic strategies are imperatively needed and should be explored for UC.
Inflammation is a major factor for the progression of UC, and studies have confirmed that a great deal of natural medicines are intended for inhibition of various chronic inflammation-associated diseases, such as Ampelopsis grossedentata (AMP). Thus, we explored the mechanisms of therapeutic effects of AMP on UC, and provided a valid complementary treatment to the standard therapy.
To investigate the underlying mechanisms of protective effects of AMP on dextran sulfate sodium (DSS)-induced colitis.
As an emerging approach, systems pharmacology was performed in this study to explore the systematic effects of AMP on DSS-induced colitis by active compound screening, target fishing, network construction and analysis.
The study revealed an antiinflammatory effect of AMP against DSS-induced colitis based on systems pharmacology and animal experiments.
AMP could exert beneficial effects on DSS-induced colitis via suppressing inflammation-related signaling pathways.
Since UC is an inflammatory condition combined with a large amount of other biological processes, we determined the inflammatory-related signaling pathways in this study. Other biological processes and signaling pathways as well as the systematic effects of AMP on UC were still unknown and need to be explored in further studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Zouiten-Mekki L S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 2. | Olpin JD, Sjoberg BP, Stilwill SE, Jensen LE, Rezvani M, Shaaban AM. Beyond the Bowel: Extraintestinal Manifestations of Inflammatory Bowel Disease. Radiographics. 2017;37:1135-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 4. | M’Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013;6:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 5. | Reinisch W, Van Assche G, Befrits R, Connell W, D’Haens G, Ghosh S, Michetti P, Ochsenkühn T, Panaccione R, Schreiber S. Recommendations for the treatment of ulcerative colitis with infliximab: a gastroenterology expert group consensus. J Crohns Colitis. 2012;6:248-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH, Tse F, Feagan B; Toronto Ulcerative Colitis Consensus Group. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035-1058.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | Stawowczyk E, Kawalec P. A Systematic Review of the Cost-Effectiveness of Biologics for Ulcerative Colitis. Pharmacoeconomics. 2017;36:419-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV Jr; Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013;19:2001-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1547] [Article Influence: 119.0] [Reference Citation Analysis (5)] |
| 10. | Zhao L, Zhang S, He P. Mechanistic Understanding of Herbal Therapy in Inflammatory Bowel Disease. Curr Pharm Des. 2017;23:5173-5179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Fan L, Tong Q, Dong W, Yang G, Hou X, Xiong W, Shi C, Fang J, Wang W. Tissue Distribution, Excretion, and Metabolic Profile of Dihydromyricetin, a Flavonoid from Vine Tea (Ampelopsis grossedentata) after Oral Administration in Rats. J Agric Food Chem. 2017;65:4597-4604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Zeng X, Yang J, Hu O, Huang J, Ran L, Chen M, Zhang Y, Zhou X, Zhu J, Zhang Q. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid Redox Signal. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Le L, Jiang B, Wan W, Zhai W, Xu L, Hu K, Xiao P. Metabolomics reveals the protective of Dihydromyricetin on glucose homeostasis by enhancing insulin sensitivity. Sci Rep. 2016;6:36184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Wang W, Qiu E. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J Biol Sci. 2017;24:837-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Wang Z, Sun X, Feng Y, Liu X, Zhou L, Sui H, Ji Q, E Q, Chen J, Wu L. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anticancer Drugs. 2017;28:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Wang R, Pi J, Su X, Liu J, Zeng X, Wong I, Huang L, Zhou H, Cai J, Li T. Dihydromyricetin suppresses inflammatory responses in vitro and in vivo through inhibition of IKKβ activity in macrophages. Scanning. 2016;38:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Tang N, Ma J, Wang KS, Mi C, Lv Y, Piao LX, Xu GH, Li X, Lee JJ, Jin X. Dihydromyricetin suppresses TNF-α-induced NF-κB activation and target gene expression. Mol Cell Biochem. 2016;422:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Fotis C, Antoranz A, Hatziavramidis D, Sakellaropoulos T, Alexopoulos LG. Network-based technologies for early drug discovery. Drug Discov Today. 2017;23:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Bloomingdale P, Nguyen VA, Niu J, Mager DE. Boolean network modeling in systems pharmacology. J Pharmacokinet Pharmacodyn. 2018;45:159-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Pei F, Li H, Henderson MJ, Titus SA, Jadhav A, Simeonov A, Cobanoglu MC, Mousavi SH, Shun T, McDermott L. Connecting Neuronal Cell Protective Pathways and Drug Combinations in a Huntington’s Disease Model through the Application of Quantitative Systems Pharmacology. Sci Rep. 2017;7:17803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Suh SY, An WG. Systems Pharmacological Approach to the Effect of Bulsu-san Promoting Parturition. Evid Based Complement Alternat Med. 2017;2017:7236436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Chaudhary G, Mahajan UB, Goyal SN, Ojha S, Patil CR, Subramanya SB. Protective effect of Lagerstroemia speciosa against dextran sulfate sodium induced ulcerative colitis in C57BL/6 mice. Am J Transl Res. 2017;9:1792-1800. [PubMed] |
| 23. | Zhang MJ, Su H, Yan JY, Li N, Song ZY, Wang HJ, Huo LG, Wang F, Ji WS, Qu XJ. Chemopreventive effect of Myricetin, a natural occurring compound, on colonic chronic inflammation and inflammation-driven tumorigenesis in mice. Biomed Pharmacother. 2018;97:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Zhu M, Zhou X, Zhao J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-κB and STAT3 signaling pathway. Exp Ther Med. 2017;14:6169-6175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Kim A, Nam YJ, Lee CS. Taxifolin reduces the cholesterol oxidation product-induced neuronal apoptosis by suppressing the Akt and NF-κB activation-mediated cell death. Brain Res Bull. 2017;134:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Vlantis K, Wullaert A, Polykratis A, Kondylis V, Dannappel M, Schwarzer R, Welz P, Corona T, Walczak H, Weih F. NEMO Prevents RIP Kinase 1-Mediated Epithelial Cell Death and Chronic Intestinal Inflammation by NF-κB-Dependent and -Independent Functions. Immunity. 2016;44:553-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 27. | Eissa N, Hussein H, Kermarrec L, Elgazzar O, Metz-Boutigue MH, Bernstein CN, Ghia JE. Chromofungin (CHR: CHGA47-66) is downregulated in persons with active ulcerative colitis and suppresses pro-inflammatory macrophage function through the inhibition of NF-κB signaling. Biochem Pharmacol. 2017;145:102-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 29. | DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1231] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 30. | Matsuhisa K, Watari A, Iwamoto K, Kondoh M, Yagi K. Lignosulfonic acid attenuates NF-κB activation and intestinal epithelial barrier dysfunction induced by TNF-α/IFN-γ in Caco-2 cells. J Nat Med. 2017;72:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, Kumar SS. Role of Antioxidants and Natural Products in Inflammation. Oxid Med Cell Longev. 2016;2016:5276130. [PubMed] |
| 32. | Hui B, Zhang L, Zhou Q, Hui L. Pristimerin Inhibits LPS-Triggered Neurotoxicity in BV-2 Microglia Cells Through Modulating IRAK1/TRAF6/TAK1-Mediated NF-κB and AP-1 Signaling Pathways In Vitro. Neurotox Res. 2018;33:268-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |