Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1262
Peer-review started: November 7, 2016
First decision: December 19, 2016
Revised: January 2, 2017
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 21, 2017
Processing time: 105 Days and 8.7 Hours
To identify early predictive markers of poor outcomes in patients with acute liver injury from wild mushroom intoxication.
This observational, retrospective record review involved adults aged ≥ 18 years admitted to emergency department with mushroom intoxication from January 2005 to December 2015. The diagnosis of mushroom intoxication was based on the following: (1) a positive history of recent wild mushroom intake (either raw or cooked); (2) the onset of gastrointestinal symptoms, such as watery diarrhea, vomiting, and/or abdominal pain, after ingestion; and (3) the exclusion of other possible causes of acute liver injury. Acute liver injury was defined by a > 5-fold elevation of liver enzymes or moderate coagulopathy [international normalized ratio (INR) > 2.0]. Clinical and laboratory findings were compared in survivors and non-survivors.
Of 93 patients with mushroom intoxication, 23, 11 men (47.8%) and 12 women (52.2%), of median age 61 years, developed acute liver injury. The overall in-hospital mortality rate was 43.5% (10/23). Among the laboratory variables, mean serum alkaline phosphatase (73.38 ± 10.89 mg/dL vs 180.40 ± 65.39 mg/dL, P < 0.01), total bilirubin (2.312 ± 1.16 mg/dL vs 7.16 ± 2.94 mg/dL, P < 0.01) concentrations and indirect/direct bilirubin (2.45 ± 1.39 mg/dL vs 0.99 ± 0.45 mg/dL, P < 0.01) ratio as well as prothrombin time (1.88 ± 0.83 mg/dL vs 10.43 ± 4.81 mg/dL, P < 0.01), and activated partial thromboplastin time (aPTT; 32.48 ± 7.64 s vs 72.58 ± 41.29 s, P = 0.01), were significantly higher in non-survivors than in survivors. Logistic regression analysis showed that total bilirubin concentration (OR = 3.58, 95%CI: 1.25-10.22), indirect/direct bilirubin ratio (OR = 0.14, 95%CI: 0.02-0.94) and aPTT (OR = 1.30, 95%CI: 1.04-1.63) were significantly associated with mortality. All patients with total bilirubin > 5 mg/dL or aPTT > 50 s on day 3 died.
Monitoring of bilirubin concentrations and aPTT may help in predicting clinical outcomes in patients with acute liver injury from wild mushroom intoxication.
Core tip: Wild mushroom-induced acute liver failure is potentially fatal. Many candidates for liver transplantation progress to multi-organ failure resulting in deterioration while awaiting liver transplantation. Identifying early predictive markers of poor outcomes in patients with acute liver injury resulting from wild mushroom intoxication is critical for improving survival rates. Total bilirubin and activated partial thromboplastin time (aPTT) levels were associated with in-hospital mortality in patients with acute liver injury from wild mushroom intoxication. Monitoring total bilirubin and aPTT as predictors of survival outcomes may determines the need for advanced intervention such as liver transplantation.
- Citation: Kim T, Lee D, Lee JH, Lee YS, Oh BJ, Lim KS, Kim WY. Predictors of poor outcomes in patients with wild mushroom-induced acute liver injury. World J Gastroenterol 2017; 23(7): 1262-1267
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1262.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1262
Although mushroom poisoning is associated with high morbidity and mortality rates[1], little is known about the in-hospital prognosis of these patients during treatment. Wild mushroom-induced acute liver failure (ALF) is potentially fatal, with selected patients requiring liver transplantation[2,3]. Unfortunately, many candidates for transplantation progress to multi-organ failure resulting in deterioration while awaiting liver transplantation[3-5]. Early and precise determination of patients who will not survive without liver transplantation is crucial for improving survival rates[1], indicating the importance of identifying suitable candidates as quickly as possible[5]. Despite several proposed sets of criteria for emergency liver transplantation in patients with ALF, the criteria for emergency liver transplantation in patients with wild mushroom-induced ALF have not been clearly determined[3,6]. This study was therefore designed to identify early predictive markers of poor outcomes in patients with acute liver injury resulting from wild mushroom intoxication.
This retrospective single-center study was performed at a 2800-bed, university-affiliated, tertiary referral center in Seoul, South Korea. The electronic charts of all adults, aged ≥ 18 years, with wild mushroom intoxication admitted to the emergency department (ED) from January 2005 to December 2015 were retrospectively reviewed. The diagnosis of mushroom intoxication was based on the following: (1) a positive history of recent intake of wild mushrooms, either raw or cooked; (2) the onset of gastrointestinal symptoms, such as watery diarrhea, vomiting, and/or abdominal pain, after ingestion; and (3) the exclusion of other possible causes of acute liver injury. This study was approved by the ethics committee of our institution, which waived informed consent because of the retrospective design of this study.
Baseline data on patients, including age, gender, medical history, and initial vital signs in the ED, were obtained, as were laboratory data obtained between the time of ED admission (day 1) and hospital day 7. Acute liver injury was defined as a > 5-fold elevation in liver enzymes or moderate coagulopathy (INR > 2.0). The primary outcome was in-hospital mortality, and clinical and laboratory findings were compared in survivors and non-survivors.
Continuous variables are presented as median and interquartile range (IQR), and categorical variables as absolute or relative frequencies. Continuous variables were compared in groups of survivors and non-survivors by Mann-Whitney U tests, and categorical variables by Fisher’s exact tests. Logistic regression analysis was performed to identify factors associated with in-hospital mortality, with the results of logistic regression analyses summarized as odds ratios (ORs) and 95% confidence intervals (CIs). Because total bilirubin concentrations and activated partial thromboplastin time (aPTT) are numerical data, receiver operating characteristic (ROC) curves were constructed and areas under the curves (AUCs) were evaluated. The cutoff values of total bilirubin and aPTT predicting death were estimated by ROC analyses. A two-sided P value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows, version 21.0 (IBM Corp, Armonk, NY, United States).
During the study period, 93 patients with wild mushroom intoxication were admitted to the ED, with 23 of these patients developing acute liver injury. These 23 patients consisted of 11 men (47.8%) and 12 women (52.2%), of median age 61 years (IQR, 51-66 years). The median time between mushroom ingestion and hospital admission was 3.0 d. The overall in-hospital mortality rate was 43.5% (10/23).
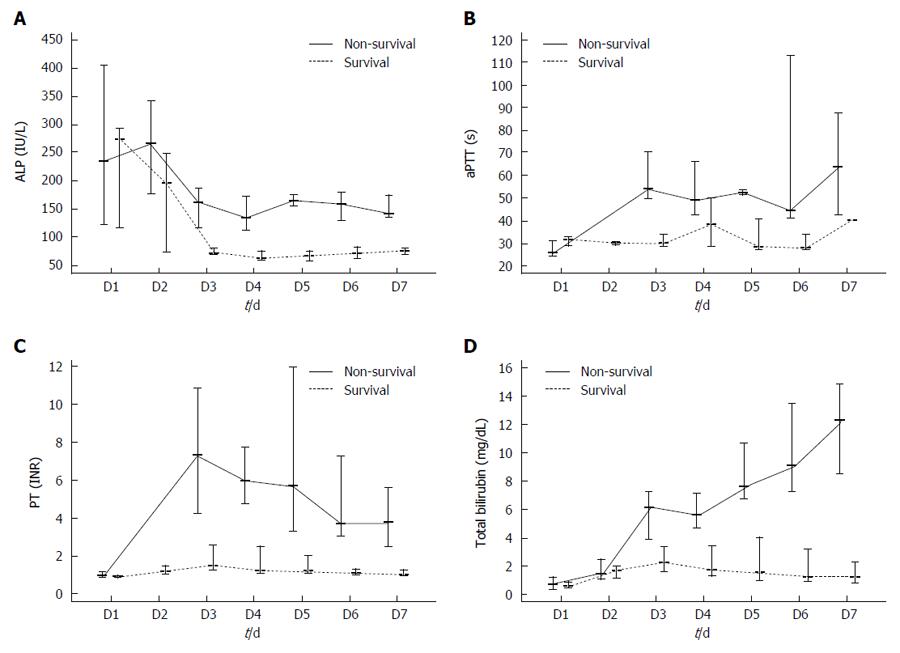
Table 1 summarizes the demographic characteristics, comorbidities, initial vital signs, and laboratory values of the survivor and non-survivor groups. Mean serum alkaline phosphate (ALP; 73.38 ± 10.89 mg/dL vs 180.40 ± 65.39 mg/dL, P < 0.01), total bilirubin (2.31 ± 1.16 mg/dL vs 7.16 ± 2.94 mg/dL, P < 0.01) concentrations and indirect/direct bilirubin (2.45 ± 1.39 vs 0.99 ± 0.45, P < 0.01) ratio as well as prothrombin time (PT)-INR (1.88 ± 0.83 s vs 10.43 ± 4.81 s, P < 0.01) and activated partial thromboplastin time (aPTT; 32.48 ± 7.64 s vs 72.58 ± 41.29 s, P = 0.01), were significantly higher in the non-survivor than in the survivor group. However, other variables, including white blood cell (WBC) and platelet counts and concentrations of hemoglobin (Hb), blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transferase (γ-GT), did not differ significantly in the survivor and non-survivor groups. Logistic regression analysis showed that elevated total bilirubin concentration (OR = 3.579, 95%CI: 1.253-10.221), indirect/direct bilirubin ratio (OR = 0.14, 95%CI: 0.02-0.94) and prolonged aPTT (OR = 1.301, 95%CI: 1.037-1.634) were significantly associated with patient mortality (Table 2). Figure 1 shows static serum total bilirubin, aPTT, PT INR, and ALP levels in survivors and non-survivors. All patients with total bilirubin > 5 mg/dL or aPTT > 50 s on day 3 died in-hospital of ALF.
| Total patients | Survivors | Non-survivors | P value | |
| (n = 23) | (n = 13) | (n = 10) | ||
| Age, yr | 61.0 (51.0-66.0) | 61.0 (53.0-69.0) | 52.0 (38.0-62.0) | 0.74 |
| Male | 11 (48) | 6 (46) | 5 (50) | 0.86 |
| Comorbidities | ||||
| Hypertension | 6 (26) | 5 (38) | 1 (10) | 0.11 |
| Diabetes mellitus | 2 (9) | 2 (17) | 0 (0) | 0.17 |
| Chronic kidney disease | 0 (0) | 0 (0) | 0 (0) | |
| Chronic liver disease | 0 (0) | 0 (0) | 0 (0) | |
| Time from ingestion to hospitalization, d | 3.0 (3.0-3.0) | 3.0 (3.0-3.0) | 3.0 (3.0-3.0) | 0.26 |
| Vital signs, initial | ||||
| SBP, mmHg | 126.0 (112.0-134.0) | 122.0 (116.0-133.0) | 128.5 (112.0-146.0) | 0.47 |
| DBP, mmHg | 71.0 (66.0-89.0) | 78.0 (67.0-90.0) | 69.5 (66.5-77.5) | 0.59 |
| Heart rate, min | 78.0 (65.0-89.0) | 70.0 (63.0-87.0) | 94.5 (78.5-116.2) | 0.03 |
| Respiratory rate, min | 20.0 (18.0-20.0) | 20.0 (18.0-20.0) | 20.0 (20.0-20.0) | 0.24 |
| Temperature, °C | 36.6 (36.1-37.1) | 36.6 (36.2-36.1) | 36.6 (36.1-37.0) | 0.56 |
| SpO2, % | 98.0 (97.0-99.0) | 98.0 (97.0-99.0) | 98.0 (97.0-99.0) | 0.36 |
| Laboratory data | ||||
| WBC, × 103/mL | 7.5 (5.8-12.2) | 7.0 (6.0-8.3) | 11.3 (6.0-15.6) | 0.11 |
| Hemoglobin, g/dL | 14.1 (13.0-15.4) | 14.2 (13.4-15.4) | 12.8 (10.6-15.0) | 0.12 |
| Platelet, × 103/mL | 172.0 (115.0-223.0) | 197.0 (156.0- 223.0) | 125.5 (87.3-181.3) | 0.06 |
| BUN, mg/dL | 21.0 (9.0-33.0) | 15.0 (10.0-29.0) | 23.0 (10.3-31.5) | 0.66 |
| Cr, mg/dL | 0.90 (0.60-1.30) | 0.70 (0.60-0.90) | 1.70 (1.00-1.80) | 0.23 |
| AST, IU/L | 4568.0 (863.0-6933.0) | 4568.0 (702.0-5471.0) | 5650.0 (2065.3-8155.8) | 0.19 |
| ALT, IU/L | 4750.0 (1561.0-5543.0) | 4436.0 (1561.0-5543.0) | 5002.0 (2235.5-6584.3) | 0.31 |
| γ-GT, IU/L | 38.0 (23.0-71.0) | 26.0 (13.0-53.0) | 55.50 (39.0-85.0) | 0.35 |
| ALP, IU/L | 85.0 (71.0-162.0) | 71.0 (69.0-81.0) | 165.0 (151.0-208.0) | < 0.01 |
| Total bilirubin, mg/dL | 3.50 (1.70-6.40) | 1.80 (1.60-3.00) | 6.80 (6.00-8.80) | < 0.01 |
| Indirect/direct bilirubin ratio | 1.79 (0.5-3.08) | 2.45 (1.06-3.84) | 0.99 (0.54-1.44) | < 0.01 |
| PT-INR, s | 2.90 (1.40-8.80) | 1.60 (1.20-2.60) | 9.70 (7.60-14.30) | < 0.01 |
| aPTT, s | 41.4 (28.9-54.3) | 29.1 (28.9-35.3) | 54.6 (52.6-71.4) | 0.01 |
| Management | ||||
| Vasopressor | 7 (30) | 0 (0) | 7 (70) | < 0.01 |
| Mechanical ventilation | 6 (26) | 0 (0) | 6 (60) | < 0.05 |
| CRRT | 5 (22) | 0 (0) | 5 (50) | < 0.01 |
| OR | 95%CI | P value | |
| Alkaline phosphatase | 1.21 | (0.91, 1.60) | 0.20 |
| Total bilirubin | 3.58 | (1.25, 10.22) | 0.02 |
| Indirect/direct bilirubin ratio | 0.13 | (0.02, 0.94) | 0.04 |
| Prothrombin time | 789.00 | - | 0.98 |
| Activated partial thromboplastin time | 1.30 | (1.04, 1.63) | 0.02 |
Poisoning of toxic mushrooms can be divided into seven main categories: amatoxin, gyromitrin, coprine, muscarine, ibotenic acid-muscimol, psilocybin-psilocin and gastrointestinal irritants[7,8]. In South Korea, the vast majority of toxic mushroom ingestions were the amatoxin-containing mushrooms such as Amanita abrupta Peck, Amanita castanopsidis Hongo, Amanita subjunquillea S. Imai, Amanita verna (Bull.) Lam., Amanita virosa (Fr.) Bertill., Conocybe filaris (Fr.) Kühner, Galerina calyptrata P.D. Orton, Galerina helvoliceps (Berk. and M.A. Curtis) Singer[8,9].
This study showed that total bilirubin and aPTT levels were associated with in-hospital mortality in patients with acute liver injury from wild mushroom intoxication. Indeed, all patients with total bilirubin > 5 mg/dL or aPTT > 50 s on day 3 died in-hospital. These results emphasize the critical need for monitoring total bilirubin and aPTT as predictors of survival outcomes and for determining the need for advanced intervention such as liver transplantation.
Although the precise incidence of mushroom poisoning cannot be estimated, the mortality rate in 15 studies involving more than 20 patients from 1980 to 1999 ranges from 4.8% to 34.5%[10]. However, more recent studies report mortality rates of around 10%[11-13]. This is consistent with the results of the present study, which found that 10 (10.8%) of the 93 patients with mushroom poisoning died. These 10 patients constituted 43.5% of the 23 patients with acute liver injury.
Mushroom-induced acute liver injury is potentially fatal, with the definitive treatment being liver transplantation[2,3]. Laboratory studies showed that urea, AST, ALT, LDH, and total bilirubin concentrations, as well as PT and aPTT, were significantly higher in patients who died than in those who survived[11]. Similarly, patients with ALT or AST concentrations > 2000 IU/mL, or PT > 50 s, were at serious risk of death[14]. By contrast, we found that AST and ALT levels at admission were not associated with patient mortality. This was not surprising, as AST and ALT levels were shown not to reflect hepatocellular necrosis and hepatotoxicity in patients with ALF[15,16]. Similarly, although serum Cr concentration has been associated with unfavorable outcome[6], we found that serum Cr concentrations did not differ significantly in our groups of survivors and non-survivors. Moreover, in contrast to findings that PT levels were prognostic in patients with liver dysfunction[17], the present study found that PT was not associated with mortality, perhaps because elevated PT may have been masked by the transfusion of coagulation factors into patients hospitalized for mushroom-induced acute liver injury.
Total bilirubin concentration may be a better indicator of the severity of acute liver injury than ALT concentration[18]. High bilirubin concentrations can not only predict short-term mortality, but can also constitute a biochemical marker to improve triage of patients with acute-on-chronic liver failure, especially selecting patients for emerging interventions, such as extracorporeal liver assist devices and possibly improved early phase pharmacological therapies[19].
aPTT measures coagulation via the intrinsic (i.e., contact-activated) pathway[17]. Recent studies show that aPTT reflects failure of coagulation as a multi-organ dysfunction in critically ill patients with ALF. Stravitz et al[20] noted monitoring the reaction time by thromboelastography or the aPTT might be more appropriate to assess bleeding risks in patients with ALI and ALF than the INR. Although aPTT is often less valuable than INR for diagnosing liver dysfunction, it may be more suitable for monitoring mushroom-induced liver injury[21].
Deciding whether to transplant livers into patients with wild mushroom-induced ALF continues to be challenging. Although liver transplantation was shown to dramatically increase the survival rate of patients with amatoxin-induced ALF[1], the specific criteria and optimal timing of emergency transplantation remain to be determined[3,6]. Traditionally, transplant decisions are based on King’s College[22,23] or Clichy[24] criteria. Other, more specific, criteria have been developed to assess the need for transplantation in patients with amatoxin-induced acute liver injury. Ganzert’s criteria suggest that patients with amatoxin poisoning be listed for urgent LT, regardless of the presence of hepatic encephalopathy, if their prothrombin index is < 25% and their serum Cr is > 106 μmol/L on the third day after ingestion[6]. Escudie’s criteria suggest that urgent LT be considered in patients showing a reduction in prothrombin index below 10% of normal (INR > 6) 4 or more days after ingestion[25]. Our study showed that both total bilirubin concentration and aPTT level were factors associated with in-hospital mortality in patients with acute liver injury arising from wild mushroom intoxication.
This study had several major limitations. First, we enrolled patients from a single institution who ingested wild mushrooms; therefore, caution should be used when applying our results to other populations, including those in other countries. Second, the retrospective observational design of this study suggests that undetected bias may have been present. Third, because kits that measure amatoxin levels in serum and urine are not commercially available in South Korea, serum alpha-amanitin levels were not analyzed in this study.
This study showed that total bilirubin concentration and aPTT were useful in predicting outcomes in patients with acute liver injury caused by wild mushroom intoxication. Moreover, total bilirubin > 5 mg/dL or aPTT > 50 s on day 3 were prognostic of impending death.
Wild mushroom induced acute liver failure (ALF) is rare but fatal, with selected patients requiring liver transplantation. Many candidates for transplantation progress to multi-organ failure resulting in rapid deterioration while awaiting liver transplantation. However, the specific criteria and optimal timing for emergency liver transplantation in patients with wild mushroom-induced ALF are unclear.
Currently, little is known about wild mushroom induced ALF, including its etiology, pathophysiology, clinical outcome, and the in-hospital prognosis in these patients. Few studies have addressed appropriate treatment with wild mushroom induced ALF. Appropriate criteria and precise timing for emergency liver transplantation in patients with wild mushroom-induced ALF are lacking in the medical literature.
Although prior studies have suggested the association of the prothrombin index and mortality, this study showed that both total bilirubin concentration and activated partial thromboplastin time (aPTT) level were associated with in-hospital mortality in patients with acute liver injury arising from wild mushroom intoxication.
This study demonstrates more specific criteria to assess the need for transplantation in patients with amatoxin-induced acute liver injury. Daily monitoring with total bilirubin and aPTT level appears to be of benefit in these patients.
Poisoning of toxic mushrooms in South Korea, the vast majority of toxic mushroom ingestions were the amatoxin-containing mushrooms. Acute liver injury was defined as a > 5-fold elevation in liver enzymes or moderate coagulopathy (INR > 2.0).
In this study, specific criteria and precise timing for emergency liver transplantation in patients with wild mushroom-induced ALF were assessed. Presence of total bilirubin > 5 mg/dL or aPTT > 50 s on day 3 was prognostic of impending death.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Shimizu Y, Villa-Trevino S S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Ahishali E, Boynuegri B, Ozpolat E, Surmeli H, Dolapcioglu C, Dabak R, Bahcebasi ZB, Bayramicli OU. Approach to mushroom intoxication and treatment: can we decrease mortality? Clin Res Hepatol Gastroenterol. 2012;36:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Miloh T, Kerkar N, Parkar S, Emre S, Annunziato R, Mendez C, Arnon R, Suchy F, Rodriguez-Laiz G, Del Rio Martin J. Improved outcomes in pediatric liver transplantation for acute liver failure. Pediatr Transplant. 2010;14:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Santi L, Maggioli C, Mastroroberto M, Tufoni M, Napoli L, Caraceni P. Acute Liver Failure Caused by Amanita phalloides Poisoning. Int J Hepatol. 2012;2012:487480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Pinson CW, Daya MR, Benner KG, Norton RL, Deveney KE, Ascher NL, Roberts JP, Lake JR, Kurkchubasche AG, Ragsdale JW. Liver transplantation for severe Amanita phalloides mushroom poisoning. Am J Surg. 1990;159:493-499. [PubMed] |
| 5. | Simpson KJ, Bates CM, Henderson NC, Wigmore SJ, Garden OJ, Lee A, Pollok A, Masterton G, Hayes PC. The utilization of liver transplantation in the management of acute liver failure: comparison between acetaminophen and non-acetaminophen etiologies. Liver Transpl. 2009;15:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol. 2005;42:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Jo WS, Hossain MA, Park SC. Toxicological profiles of poisonous, edible, and medicinal mushrooms. Mycobiology. 2014;42:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Sohn CH. Type and treatment of toxic mushroom poisoning in Korea. J Korean Med Assoc. 2015;58:818-824. |
| 9. | Seok S, Kim Y, Kim W, Suh J, Jeong M, Lim K, Sohn C, Lee Y. Encyclopedia of poisonous mushrooms. : Encyclopedia 2011; . |
| 10. | Jander S, Bischoff J, Woodcock BG. Plasmapheresis in the treatment of Amanita phalloides poisoning: II. A review and recommendations. Ther Apher. 2000;4:308-312. [PubMed] |
| 11. | Trabulus S, Altiparmak MR. Clinical features and outcome of patients with amatoxin-containing mushroom poisoning. Clin Toxicol (Phila). 2011;49:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Bergis D, Friedrich-Rust M, Zeuzem S, Betz C, Sarrazin C, Bojunga J. Treatment of Amanita phalloides intoxication by fractionated plasma separation and adsorption (Prometheus®). J Gastrointestin Liver Dis. 2012;21:171-176. [PubMed] |
| 13. | Roberts DM, Hall MJ, Falkland MM, Strasser SI, Buckley NA. Amanita phalloides poisoning and treatment with silibinin in the Australian Capital Territory and New South Wales. Med J Aust. 2013;198:43-47. [PubMed] |
| 14. | Fantozzi R, Ledda F, Caramelli L, Moroni F, Blandina P, Masini E, Botti P, Peruzzi S, Zorn M, Mannaioni PF. Clinical findings and follow-up evaluation of an outbreak of mushroom poisoning--survey of Amanita phalloides poisoning. Klin Wochenschr. 1986;64:38-43. [PubMed] |
| 15. | Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 825] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 16. | Contreras-Zentella ML, Hernández-Muñoz R. Is Liver Enzyme Release Really Associated with Cell Necrosis Induced by Oxidant Stress? Oxid Med Cell Longev. 2016;2016:3529149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027-2049. [PubMed] |
| 18. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem. 2000;46:2050-2068. [PubMed] |
| 19. | López-Velázquez JA, Chávez-Tapia NC, Ponciano-Rodríguez G, Sánchez-Valle V, Caldwell SH, Uribe M, Méndez-Sánchez N. Bilirubin alone as a biomarker for short-term mortality in acute-on-chronic liver failure: an important prognostic indicator. Ann Hepatol. 2013;13:98-104. [PubMed] |
| 20. | Stravitz RT, Lisman T, Luketic VA, Sterling RK, Puri P, Fuchs M, Ibrahim A, Lee WM, Sanyal AJ. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol. 2012;56:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Moia M, Martinelli I, Gridelli B, Langer M, Galmarini D, Mannucci PM. Prognostic value of hemostatic parameters after liver transplantation. J Hepatol. 1992;15:125-128. [PubMed] |
| 23. | O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 24. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2069] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 25. | Escudié L, Francoz C, Vinel JP, Moucari R, Cournot M, Paradis V, Sauvanet A, Belghiti J, Valla D, Bernuau J. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol. 2007;46:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |