Published online Dec 14, 2017. doi: 10.3748/wjg.v23.i46.8227
Peer-review started: October 23, 2017
First decision: October 31, 2017
Revised: November 10, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: December 14, 2017
Processing time: 53 Days and 0.8 Hours
To assess the efficacy and safety of balloon dilatation for the treatment of hepatic venous outflow obstruction (HVOO) following pediatric liver transplantation.
A total of 246 pediatric patients underwent liver transplantation at our hospital between June 2013 and September 2016. Among these patients, five were ultimately diagnosed with HVOO. Seven procedures (two patients underwent two balloon dilatation procedures) were included in this analysis. The demographic data, types of donor and liver transplant, interventional examination and therapeutic outcomes of these five children were analyzed. The median interval time between pediatric liver transplantation and balloon dilatation procedures was 9.8 mo (range: 1-32).
Five children with HVOO were successfully treated by balloon angioplasty without stent placement, with seven procedures performed for six stenotic lesions. All children underwent successful percutaneous intervention. Among these five patients, four were treated by single balloon angioplasty, and these patients did not develop recurrent stenosis. In seven episodes of balloon angioplasty across the stenosis, the pressure gradient was 12.0 ± 8.8 mmHg before balloon dilatation and 1.1 ± 1.5 mmHg after the procedures, which revealed a statistically significant reduction (P < 0.05). The overall technical success rate among these seven procedures was 100% (7/7), and clinical success was achieved in all five patients (100%). The patients were followed for 4-33 mo (median: 15 mo). No significant procedural complications or procedure-related deaths occurred.
Balloon dilatation is an effective and safe therapeutic option for HVOO in children undergoing pediatric liver transplantation. Venous angioplasty is also recommended in cases with recurrent HVOO.
Core tip: Hepatic venous outflow obstruction (HVOO) is a rare and severe complication following pediatric liver transplantation that leads to graft loss in the majority of patients. However, it remains controversial whether stent placement or balloon angioplasty is required for patients with HVOO. This study reported our experiences with using balloon dilatation as part of the treatment for HVOO in five children subjected to pediatric liver transplantation, providing valuable information for the successful treatment of such patients. Balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation, and re-venoplasty is recommended even for patients with recurrent HVOO.
- Citation: Zhang ZY, Jin L, Chen G, Su TH, Zhu ZJ, Sun LY, Wang ZC, Xiao GW. Balloon dilatation for treatment of hepatic venous outflow obstruction following pediatric liver transplantation. World J Gastroenterol 2017; 23(46): 8227-8234
- URL: https://www.wjgnet.com/1007-9327/full/v23/i46/8227.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i46.8227
Liver transplantation is the most effective treatment for end-stage liver disease. In recent years, significant progress has been achieved in surgical techniques, immunosuppressants and operative care, which has led to the optimization of post-transplant outcomes[1]. In an attempt to solve the shortage of donor organs and diminish the high mortality rate after re-transplantation, efforts have been made to develop liver transplantation techniques with reduced-size, split and living donor organs[2]. Nevertheless, vascular complications are still the most significant determinant factor for graft loss, especially for those undergoing partial liver transplantation with living donor grafts, during which the use of short vascular pedicles complicates the surgical procedure. In particular, hepatic venous outflow obstruction (HVOO) is a profound complication of pediatric liver transplantation; it has a high incidence (4%-9%) owing to the smaller anastomosis diameter and size mismatch of the hepatic vessels between the donor and recipient[3]. The treatments for HVOO in children after liver transplantation include surgical reconstruction, re-transplantation and endovascular angioplasty[4]. Endovascular angioplasty, either via balloon angioplasty or stent placement, is a less-invasive therapeutic option compared with surgical approaches for relieving HVOO and has become the first-line treatment of choice in pediatric transplant recipients[3,5]. However, it remains unclear whether stent placement or balloon angioplasty is required for patients with HVOO.
Therefore, this retrospective study was performed to evaluate data from pediatric patients undergoing endovascular angioplasty for HVOO after liver transplantation at our hospital from June 2013 to September 2016. This paper presents our successful treatment of HVOO in a series of five children using balloon angioplasty.
Between June 2013 and September 2016, a total of 246 pediatric patients (< 18 years old) underwent liver transplantation at our hospital, including 149 living-donor and 97 deceased-donor transplants. Among these patients, five consecutive patients (four transplanted at our hospital and one transplanted at another hospital) underwent venography and manometric measurements due to suspicion of HVOO. Patients were suspected of having HVOO when they showed typical clinical signs or symptoms, including ascites, pleural effusion, or hepatomegaly. Enhanced computed tomography (CT) and/or Doppler ultrasound (US) were used to further detect hepatic venous outflow abnormalities. Abnormal Doppler US images included an undetectable flow signal, a persistent monophasic waveform, a slow flow of < 10 cm/s, or a reversed flow direction. Patients highly suspected of hepatic venous outflow abnormalities through Doppler US examination were subjected to a CT scan. The hepatic venous outflow abnormality was confirmed when the CT scan revealed non-opacification of the hepatic veins, greater than 70% focal luminal narrowing relative to the adjacent normal hepatic vessel in diameter, or a geographic area of low attenuation in the liver, along with clinical symptoms of hepatic congestion (ascites, pleural effusion, elevated liver enzymes, or abnormal findings) on the immediate postoperative Doppler US examination[3]. The hepatic venous outflow abnormality was further diagnosed by hepatic venography and through pressure measurements in the hepatic vein, inferior vena cava (IVC) and right atrium. Significant hepatic venous outflow stenosis was defined as a pressure gradient > 3 mmHg.
All five patients with hepatic venous outflow abnormalities underwent endovascular angioplasty. The median length of the period from the liver transplantation to the initial procedure was 9.8 mo (range: 1-32 mo). The baseline characteristics of these patients are summarized in Table 1.
| Case No. | Gender | Age | Original disease | Type of transplant | Graft type | Onset after transplantation (mo) |
| 1 | F | 10 mo | Biliary atresia | LDLT | Left lateral lobe | 4 |
| 2 | F | 3 yr | Biliary atresia | LDLT | Left lateral lobe | 32 |
| 3 | F | 9 yr | OTCD | Cross-auxiliary double-domino donor liver transplantation | Right lobe | 1 |
| 4 | M | 7 mo | Biliary atresia | LDLT | Left lateral lobe | 9 |
| 5 | F | 10 mo | Biliary atresia | LDLT | Left lateral lobe | 3 |
Each patient or their legal guardian provided written informed consent. The present study was approved by our Institutional Ethics Committee (No. 2017-P2-029-01).
Three authors (Jin L, Chen G, and Su TH., with 22, 21 and 10 years of experience in interventional radiology, respectively) performed these procedures. Percutaneous interventions were performed under general anesthesia in all patients (n = 5). Selective hepatic venography and balloon angioplasty were performed via the right internal jugular vein. A 0.035-inch hydrophilic guide wire (Terumo, Tokyo, Japan) and 5F cobra catheter (Cook, Bloomington, Indiana) were used to gain entry into the hepatic vein. After hepatic venography, the anastomotic pressure gradient was obtained. After the catheter passed through the desired site, the narrow pressure gradient (right atrium and left hepatic vein) was calculated by recording the intravenous pressure on both sides of the narrowing. Patients with a pressure gradient of > 3 mmHg were considered to have a significant outflow obstruction and were candidates for venoplasty, in which a percutaneous transluminal angioplasty catheter (Powerflex Plus, Cordis) with a 6- to 8-mm-diameter and 20- to 40-mm-long balloon was utilized. The selection of the balloon was based on the contrast-enhanced CT and venography findings and was matched in size to the vein on the hepatic side of the stenosis. The balloon was laid across the stenosis and was inflated to widen the vein for 60 s at a pressure of 10 atm. Balloon dilation was performed three times, and the effectiveness of the venoplasty was examined by repeated venography and manometric measurements. Hemostasis was achieved by manual compression, without transhepatic track embolization. Patients did not receive heparinization during the whole procedure. Hence, the international normalized ratio was maintained between 1.5-2.0.
The patients’ vital signs were monitored in the intensive care unit or general ward every 4-8 h. US was performed one day after the procedure to examine the hepatic venous outflow and to detect post-procedural complications. CT was performed in selected patients within 1 wk after the procedure, according to the physician’s need to investigate the hepatic vein and extrahepatic variceal flow and determine any post-procedural complications. Doppler US was performed bimonthly on an outpatient basis, and venography and manometry were performed when recurrent HVOO was suspected.
The following data were retrospectively collected: technical and clinical success, pre- and post-operative pressure gradients across the stenosis, major complications, and patency of the hepatic venous outflow. Technical success was defined as the achievement of a pressure gradient across a stenosis of < 3 mmHg on postprocedural manometry, or a stenosis of < 30% on postprocedural venography. Clinical success was defined as ameliorated signs and symptoms of HVOO, and an improvement in the preprocedural status evaluated by Doppler US[6]. The definition of a major complication was an event that required extended hospitalization or an advanced level of care[7].
Changes between pre- and post-procedural pressure gradients across the hepatic vein stenosis were analyzed by paired Student’s t-test. The analyses were performed using SPSS 21.0 statistical software (IBM, Chicago, IL, United States). A P-value < 0.05 was considered statistically significant.
Four liver transplant recipients (one male and three females; age range: seven months to three years old) and one crossed-auxiliary double-domino donor liver transplantation patient (female, nine years old) were diagnosed with HVOO following liver transplantation in the present study (Table 1). The preliminary diagnosis of HVOO was initially assessed by Doppler US and confirmed by subsequent abdominal CT angiography and venography in all five patients. The hepatic vein stenosis rate was 1.62%. The onset of hepatic vein stenosis ranged from 1-32 mo (mean: 9.80 mo) after liver transplantation.
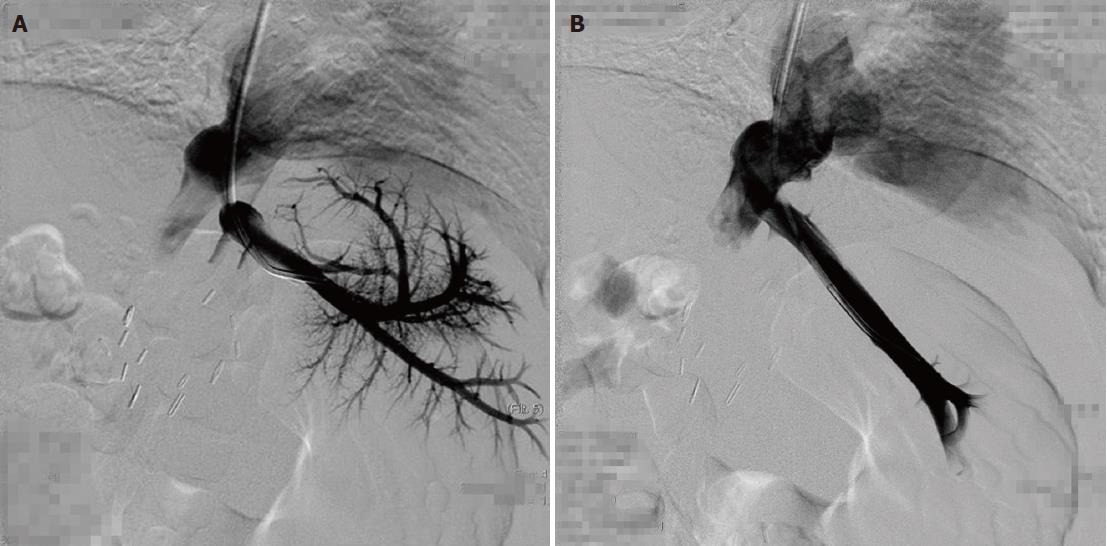
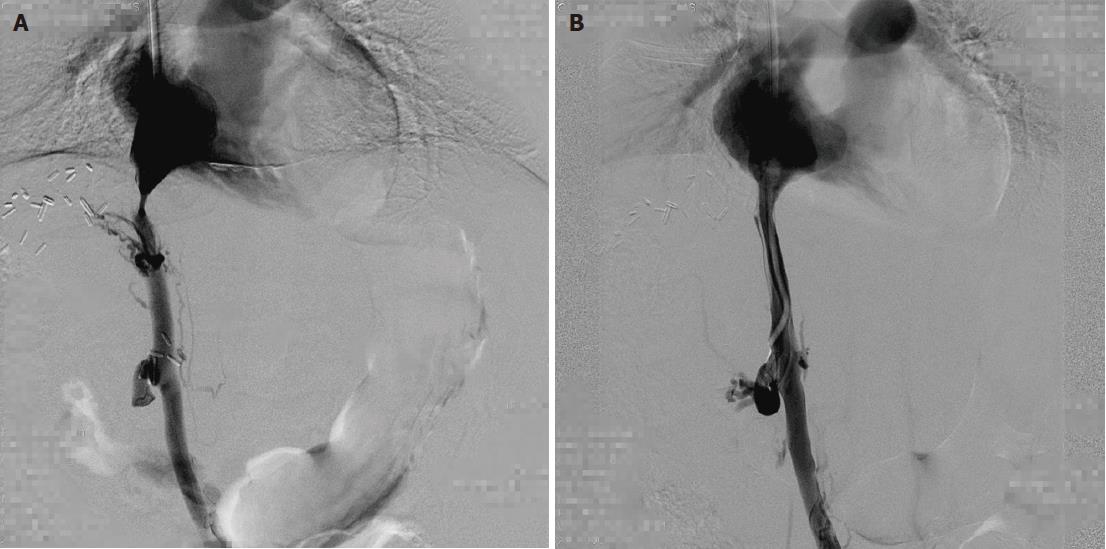
A total of seven procedures were performed for six stenotic lesions in five patients (there were two anastomoses in one patient, Table 2). The overall technical success rate among these seven procedures was 100% (7/7). A patient with HVOO underwent a balloon angioplasty procedure twice at 15 d after liver transplantation and at one month after liver transplantation due to recurrence. The pressure gradient across the stenotic lesions at the anastomoses (n = 7) was 12.0 ± 8.8 mmHg (range: 4-26 mmHg) before balloon dilatation, and this value decreased significantly to 1.1 ± 1.5 mmHg (range: 0-3 mmHg) immediately after the procedure (P < 0.05).
| Case No. | Vessel | Procedure | Size | Pre-pressure (mmHg) | Post-pressure (mmHg) | Follow-up (mo) |
| 1 | HV | PTA | 6 mm-4 cm | 34/8 | 13/9 | 16 |
| 2 | HV | PTA | 8 mm-4 cm | 29/7 | 18/18 | 16 |
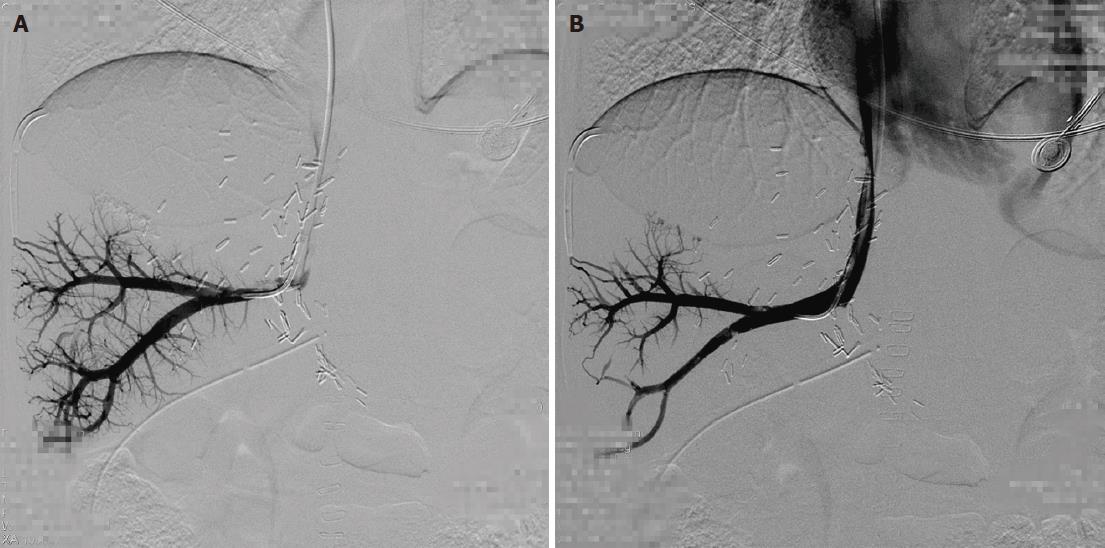
| 3 | RHV | PTA | 8 mm-4 cm | 17/10 | 10/10 | 1, recurrence |
| RHV | PTA | 8 mm-4 cm | 22/14 | 15/15 | 10 | |
| 4 | HV | PTA | 8 mm-4 cm | 30/17 | 22/19 | 33 |
| 5 | HV | PTA | 6 mm-2 cm | 17/13 | 17/15 | 4 |
| IVC | PTA | 6 mm-2 cm | 17/13 | 14/14 | 4 |
Clinical success was achieved in all five patients (100%), as manifested by improved ascites and pleural effusion.
The patients were followed for 4-33 mo (median: 15 mo). The outcomes are shown in Table 2. Among these five patients, four patients were treated by single balloon angioplasty (Figures 1 and 2), and these patients did not develop recurrent stenosis. The patient who underwent two sessions of balloon angioplasty showed no recurrent HVOO for 10 mo during the subsequent follow-up period (Figure 3). Furthermore, no major procedural complications during any of the seven balloon angioplasty procedures, procedure-related deaths or permanent adverse sequelae occurred.
Few cases of HVOO have been reported in pediatric patients following liver transplantation; there have been fewer than ten cases within the past five years. This study reported our experiences with using balloon dilatation as part of the treatment for HVOO in five children who underwent pediatric liver transplantation, providing valuable reference for the successful treatment of such patients.
HVOO is an infrequent but life-threatening complication following liver transplantation[8]. Living-donor or auxiliary liver transplantation has been accepted as an effective method to expand the pool of organ donors. At present, technical challenges remain, including HVOO[8,9]. In the present study, four cases who underwent transplantation at our hospital and one patient who underwent transplantation at another hospital were diagnosed with HVOO. In our center, the incidence of HVOO was 1.62% (4/246), which was lower than the incidences reported at other centers[10-12]. It has been reported that with the evolution and improvement of surgical techniques, the overall incidence of long-term hepatic vein stenosis has significantly decreased between the eras of 1988 to 1994 and 1995 to 2002[2]. In the present study, the time to onset of hepatic vein stenosis was 0.5-32 mo (mean: 9.70 mo) after liver transplantation, which was consistent with other studies[10-12].
At our institution, patients with clinical signs and symptoms of HVOO routinely underwent some forms of noninvasive imaging. Color Doppler US was the most frequently used imaging technique in the present study, providing information on flow direction and velocity. Its results can be made immediately available without the use of ionizing radiation, and it can be conveniently performed at the bedside. Magnetic resonance (MR) venography and CT are excellent alternatives but require scanner availability and sedation in the pediatric population[13]. Although noninvasive evaluation can aid diagnosis, IVC venography with direct pressure gradient measurement across the suspected lesion is the gold standard for HVOO diagnosis[4]. In our cases, color Doppler US and enhanced CT findings were supported by the results of venography.
The quantification of the pressure gradient across the stenosis may help verify the significance of the lesion. At present, conflicting opinions exist for the deterministic diagnosis of HVOO through a pressure gradient across the stenotic region between the right atrium and hepatic vein of > 3 mmHg or > 5 mmHg. We recommend the consideration of the diagnosis of HVOO in pediatric recipients with clinical presentations of HVOO, even if their pressure gradients between the right atrium and hepatic vein are < 3 mmHg. A pressure gradient that can sufficiently lead to clinical HVOO may be misleadingly reduced when the patient is ambulatory or lying in the supine position during an operation, leading to a missed or faulty diagnosis. In addition, pressure gradients are typically measured in children under general anesthesia, which affects hepatic and portal vein flow velocities, resulting in the misdiagnosis of HVOO[11]. In the present study, we recommend measuring the pressure gradient repeatedly at the same location and as far as possible from the vessel wall. In addition, a diagnostic standard of > 3 mmHg for the pressure gradient is recommended.
At present, endovascular treatment, including percutaneous balloon angioplasty and stent placement, is the preferred treatment for HVOO after liver transplantation[14]. However, it remains unclear whether balloon angioplasty or stent placement is preferable[10,11,15]. Kubo et al[16] performed endovascular treatments in 20 patients with HVOO after liver transplantation. A technically successful balloon venoplasty procedure was initially achieved in all cases. However, 11 (55%) patients had recurrent obstruction, which is suggestive of a high rate of reintervention. Most patients (n = 10) were treated by balloon angioplasty, and one patient was rescued by stent placement. The patency rate of the hepatic venous outflow was 1.00 during a follow-up period of 60 mo. Another study reported 10 pediatric patients with HVOO who were treated with stents; after 42 months of follow-up, all stent lumens remained patent. Moreover, the retained stents did not cause profound adverse effects that were harmful to the growth of blood vessels12]. Ko et al[17] performed primary stent placement for early post-transplant HVOO and achieved favorable long-term patency. Overall, the 1-, 3- and 5-year primary patency rates were 82.3%, 75.0% and 72.4%, respectively.
However, many centers hesitate to perform stent placement in children for several reasons[18]. First, stents are prone to the development of neointimal hyperplasia, leading to recurrent stenosis. Second, a stenotic lesion would be developed when the child matures, since the stent placed has a fixed diameter. Third, the location of the internal stent in children is technically problematic if re-transplantation is required. Finally, the long-term patency of stents remains unknown. The present study demonstrated excellent results for both technical and clinical success (100.0% and 100.0%, respectively) with a low recurrence rate (20%). During the follow-up period, severe complications such as dissection, vascular rupture, thrombosis or death did not occur. More importantly, repeated balloon dilation could achieve satisfactory or even curative outcomes, as shown by our results[11]. Therefore, we propose that repeated balloon angioplasty can be a primary treatment modality for HVOO in pediatric patients following liver transplantation, even for those with recurrent HVOO.
The individualized selection of balloons (type and size) as well as careful deployment is vital for achieving a desirable outcome. In the present study, the selection of the balloon was based on the experience and skill of the interventional radiologist. We used short balloons (diameter range: 6-8 mm; length: ≤ 4 cm) for all patients. Although the impact of the type and size of the balloon on long-term patency has not been clearly demonstrated, we speculate that a balloon with an appropriate diameter and a short length ensures better long-term patency for treating HVOO in pediatric liver transplant recipients.
Although insufficient evidence has been obtained to support the benefits of stent placement for HVOO, novel stenting devices, such as drug-eluting and biodegradable stents, may be promising for the management of HVOO. Averin et al[15] reported the use of a customized endovascular stent for the treatment of inferior vena cava obstruction following pediatric liver transplantation, in an attempt to relieve the risk of hepatic venous vein egress. However, the long-term efficacy of endovascular stent placement remains unproven.
In spite of the technical success and satisfactory clinical outcomes in these five children, the present study had certain limitations, including the retrospective nature of the study, a relatively small sample size, and a relatively short follow-up period. Further studies with a larger sample size that could identify relevant risk factors for HVOO development following transplantation and HVOO recurrence after balloon angioplasty are needed.
In conclusion, balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation, and re-venoplasty is recommended even for patients with recurrent HVOO.
Liver transplantation is the most effective treatment for end-stage liver disease. Hepatic venous outflow obstruction (HVOO) is a severe complication of pediatric liver transplantation, which has a high incidence of 4%-9% owing to the smaller anastomosis diameter and size mismatch of the hepatic vessels between the donor and recipient. Endovascular angioplasty is a less-invasive therapeutic option that has become the first-line treatment option in pediatric transplant recipients. However, it remains controversial whether stent placement or balloon angioplasty is required for patients with HVOO. Rare cases of HVOO have been reported in pediatric patients following liver transplantation. This study reported our experiences with using balloon dilatation as part of the treatment for HVOO in five children subjected to pediatric liver transplantation, providing valuable data for the successful treatment of such patients.
HVOO is a rare and severe complication following pediatric liver transplantation that leads to graft loss in the majority of patients. However, it remains controversial whether stent placement or balloon angioplasty is required for patients with HVOO. This study reported our experiences with using balloon dilatation as part of the treatment for HVOO in five children subjected to pediatric liver transplantation, providing valuable information for the successful treatment of such patients. Balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation, and re-venoplasty is recommended even for patients with recurrent HVOO.
Balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation, and re-venoplasty is recommended even for patients with recurrent HVOO.
The authors enrolled a total of 246 pediatric patients who underwent liver transplantation between June 2013 and September 2016. Among these patients, five were ultimately diagnosed with HVOO. Percutaneous interventions were performed under general anesthesia in all patients (n = 5). The demographic data, types of donor and liver transplant, interventional examination and therapeutic outcomes of these five children were collected and analyzed with SPSS version 21.0 software. Changes between pre- and post-procedural pressure gradients across the hepatic vein stenosis were analyzed by paired Student’s t-test.
The authors found that balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation. The hepatic vein stenosis rate was 1.62%. The time to onset of hepatic vein stenosis ranged from 1-32 mo (mean: 9.80 mo) after liver transplantation. The pressure gradient across the stenotic lesions at the anastomoses before balloon dilatation decreased significantly after the procedure (P < 0.05). Sustained follow-up did not reveal significant procedural complications or procedure-related deaths. Further studies with a larger sample size that could identify relevant risk factors for HVOO development following transplantation and HVOO recurrence after balloon angioplasty are needed.
This study investigated the efficacy and safety of balloon dilatation for the treatment of hepatic venous outflow obstruction following pediatric liver transplantation. HVOO is a rare and severe complication following pediatric liver transplantation that leads to graft loss in the majority of patients. However, it remains unclear whether stent placement or balloon angioplasty is required for patients with HVOO. This study reported our experiences with using balloon dilatation as part of the treatment for HVOO in five children subjected to pediatric liver transplantation, providing valuable information regarding the successful treatment of such patients. Balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation, and re-venoplasty is recommended even for patients with recurrent HVOO. In spite of the technical success and satisfactory clinical outcomes in these five children, the present study had certain limitations, including the retrospective nature of the study, a relatively small sample size, and a short follow-up period. Further studies with a large sample size that could identify risk factors for HVOO development following transplantation and HVOO recurrence after balloon angioplasty are needed.
This study reported our experiences with using balloon dilatation as part of the treatment for HVOO in five children subjected to pediatric liver transplantation, providing valuable information for the successful treatment of such patients. Balloon dilatation is an effective and safe treatment for HVOO in pediatric patients following liver transplantation, and re-venoplasty is recommended even for patients with recurrent HVOO. In spite of the technical success and satisfactory clinical outcomes in these five children, the present study had certain limitations, including the retrospective nature of the study, a relatively small sample size, and a short follow-up period. Further studies with a large sample size that could identify risk factors for HVOO development following transplantation and HVOO recurrence after balloon angioplasty are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Haggar M, Quak SH, Rolle U S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Cheng YF, Chen CL, Huang TL, Chen TY, Chen YS, Wang CC, Tsang LL, Sun PL, Chiu KW, Eng HL. Angioplasty treatment of hepatic vein stenosis in pediatric liver transplants: long-term results. Transpl Int. 2005;18:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Buell JF, Funaki B, Cronin DC, Yoshida A, Perlman MK, Lorenz J, Kelly S, Brady L, Leef JA, Millis JM. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Choi JW, Jae HJ, Kim HC, Yi NJ, Lee KW, Suh KS, Chung JW. Long-term outcome of endovascular intervention in hepatic venous outflow obstruction following pediatric liver transplantation. Liver Transpl. 2015;21:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Lee BB, Villavicencio L, Kim YW, Do YS, Koh KC, Lim HK, Lim JH, Ahn KW. Primary Budd-Chiari syndrome: outcome of endovascular management for suprahepatic venous obstruction. J Vasc Surg. 2006;43:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Fujimori M, Yamakado K, Takaki H, Nakatsuka A, Uraki J, Yamanaka T, Hasegawa T, Sugino Y, Nakajima K, Matsushita N. Long-Term Results of Stent Placement in Patients with Outflow Block After Living-Donor-Liver Transplantation. Cardiovasc Intervent Radiol. 2016;39:566-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Ohm JY, Ko GY, Sung KB, Gwon DI, Ko HK. Safety and efficacy of transhepatic and transsplenic access for endovascular management of portal vein complications after liver transplantation. Liver Transpl. 2017;23:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, Rholl KS, Meranze SG, Lewis CA. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2002;13:879-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Chu HH, Yi NJ, Kim HC, Lee KW, Suh KS, Jae HJ, Chung JW. Longterm outcomes of stent placement for hepatic venous outflow obstruction in adult liver transplantation recipients. Liver Transpl. 2016;22:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Qu W, Zhu ZJ, Wei L, Sun LY, Liu Y, Zeng ZG. Reconstruction of the Outflow Tract in Cross-Auxiliary Double-Domino Donor Liver Transplantation. Transplant Proc. 2016;48:2738-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Yabuta M, Shibata T, Shibata T, Shinozuka K, Isoda H, Okamoto S, Uemoto S, Togashi K. Long-term outcome of percutaneous interventions for hepatic venous outflow obstruction after pediatric living donor liver transplantation: experience from a single institute. J Vasc Interv Radiol. 2013;24:1673-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Rao W, Sun LY, Zhu ZJ, Chen G, Sun XY, Gao W, Shi R. Successful percutaneous transluminal balloon dilatation for hepatic venous outflow obstruction after pediatric liver transplantation: A series of cases. Hepatol Res. 2013;43:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Carnevale FC, Machado AT, Moreira AM, De Gregorio MA, Suzuki L, Tannuri U, Gibelli N, Maksoud JG, Cerri GG. Midterm and long-term results of percutaneous endovascular treatment of venous outflow obstruction after pediatric liver transplantation. J Vasc Interv Radiol. 2008;19:1439-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Lorenz JM, Van Ha T, Funaki B, Millis M, Leef JA, Bennett A, Rosenblum J. Percutaneous treatment of venous outflow obstruction in pediatric liver transplants. J Vasc Interv Radiol. 2006;17:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Zhang ZY, Jin L, Chen G, Su TH, Zhu ZJ, Wei L, Xiao GW. Endovascular interventional therapy of portal vein stenosis after pediatric liver transplantation. Zhongguo Jieru Yingxiang Yu Zhiliaoxue. 2017;14:210-213. [DOI] [Full Text] |
| 15. | Averin K, Bucuvalas J, Alonso MH, Kohli R, Heubi JE, Johnson ND, Goldstein BH. Treatment of Inferior Vena Cava Obstruction Following Pediatric Liver Transplantation: Novel Use of a Customized Endovascular Stent. J Pediatr. 2017;180:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Kubo T, Shibata T, Itoh K, Maetani Y, Isoda H, Hiraoka M, Egawa H, Tanaka K, Togashi K. Outcome of percutaneous transhepatic venoplasty for hepatic venous outflow obstruction after living donor liver transplantation. Radiology. 2006;239:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Ko GY, Sung KB, Yoon HK, Kim JH, Song HY, Seo TS, Lee SG. Endovascular treatment of hepatic venous outflow obstruction after living-donor liver transplantation. J Vasc Interv Radiol. 2002;13:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Sakamoto S, Egawa H, Kanazawa H, Shibata T, Miyagawa-Hayashino A, Haga H, Ogura Y, Kasahara M, Tanaka K, Uemoto S. Hepatic venous outflow obstruction in pediatric living donor liver transplantation using left-sided lobe grafts: Kyoto University experience. Liver Transpl. 2010;16:1207-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |