Published online Dec 14, 2017. doi: 10.3748/wjg.v23.i46.8200
Peer-review started: October 6, 2017
First decision: October 18, 2017
Revised: October 30, 2017
Accepted: November 14, 2017
Article in press: November 14, 2017
Published online: December 14, 2017
Processing time: 68 Days and 1.3 Hours
To assess cancer-testis antigens (CTAs) expression in gastric cancer patients and examined their associations with clinicopathological factors.
Eighty-three gastric cancer patients were evaluated in this study. Gastric cancer specimens were evaluated for the gene expression of CTAs, Kitakyushu lung cancer antigen-1 (KK-LC-1), melanoma antigen (MAGE)-A1, MAGE-A3 and New York esophageal cancer-1 (NY-ESO-1), by reverse transcription PCR. Clinicopathological background information, such as gender, age, tumor size, macroscopic type, tumor histology, depth of invasion, lymph node metastasis, lymphatic invasion, venous invasion, and pathological stage, was obtained. Statistical comparisons between the expression of each CTA and each clinicopathological background were performed using the χ2 test.
The expression rates of KK-LC-1, MAGE-A1, MAGE-A3, and NY-ESO-1 were 79.5%, 32.5%, 39.8%, and 15.7%, respectively. In early stage gastric cancer specimens, the expression of KK-LC-1 was 79.4%, which is comparable to the 79.6% observed in advanced stage specimens. The expression of KK-LC-1 was not significantly associated with clinicopathological factors, while there were considerable differences in the expression rates of MAGE-A1 and MAGE-A3 with vs without lymphatic invasion (MAGE-A1, 39.3% vs 13.6%, P = 0.034; MAGE-A3, 47.5% vs 18.2%, P = 0.022) and/or vascular invasion (MAGE-A1, 41.5% vs 16.7%, P = 0.028; MAGE-A3, 49.1% vs 23.3%, P = 0.035) and, particularly, MAGE-A3, in patients with early vs advanced stage (36.5% vs 49.0%, P = 0.044), respectively. Patients expressing MAGE-A3 and NY-ESO-1 were older than those not expressing MAGE-A3 and NY-ESO-1 (MAGE-A3, 73.7 ± 7.1 vs 67.4 ± 12.3, P = 0.009; NY-ESO-1, 75.5 ± 7.2 vs 68.8 ± 11.2, P = 0.042).
The KK-LC-1 expression rate was high even in patients with stage I cancer, suggesting that KK-LC-1 is a useful biomarker for early diagnosis of gastric cancer.
Core tip: The Kitakyushu lung cancer antigen-1 (KK-LC-1) is a relatively later cancer-testis antigen and has received interest because it was reported that KK-LC-1 is a predominant antigen for cancer immunotherapy. We found that the expression rate of KK-LC-1 in gastric cancer was 79.5%, which was higher than that of other cancer-testis antigens and the same as that in both early- and late-stage patients. KK-LC-1 is a potential biomarker for early detection of gastric cancer and identification of patients at high risk for gastric cancer. Furthermore, KK-LC-1 is a potential target for immunotherapy in gastric cancer.
- Citation: Futawatari N, Fukuyama T, Yamamura R, Shida A, Takahashi Y, Nishi Y, Ichiki Y, Kobayashi N, Yamazaki H, Watanabe M. Early gastric cancer frequently has high expression of KK-LC-1, a cancer-testis antigen. World J Gastroenterol 2017; 23(46): 8200-8206
- URL: https://www.wjgnet.com/1007-9327/full/v23/i46/8200.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i46.8200
Gastric cancer is the third leading cause of cancer-related death worldwide, after lung and liver cancers[1]. Endoscopic therapy, surgical resection, and chemotherapy are established treatments for gastric cancer; however, the mortality rate of gastric cancer patients remains high and thus new treatment strategies must be developed.
Cancer-testis antigens (CTAs) are a group of tumor antigens expressed in a wide variety of cancer tissues but not in normal tissue except for testicular germ cells[2]. Because of their tumor specificity, CTAs are considered potential targets for new treatment strategies including immunotherapy[3,4], and the expression of each CTA has potential significance for the prognosis of several types of cancer[5]. While studies have reported the expression patterns of CTAs in various cancer types, fewer studies have focused on those in gastric cancer[5-7].
Kita-Kyushu lung cancer antigen-1 (KK-LC-1) was first identified in patients with lung cancer[8] and is a CTA recognized by cytotoxic T lymphocytes (CTL). When CTL against KK-LC-1 accumulate predominantly among tumor-infiltrating lymphocytes (TILs), adaptive immunotherapy using TILs leads to a good response[9]. The expression rate of KK-LC-1 in non-small cell cancer was reported to be 32.6%[10] and has also been reported in other types of cancer. In a study of triple-negative breast cancer, the expression rate of KK-LC-1 was reported to be 75%[11]. In our previous study of CTAs in gastric cancer, the expression rate of KK-LC-1 was shown to be as high as 81.6%, which was higher than the rates of other CTAs[6]. There are no reports of tumor-associated antigens being expressed as highly as KK-LC-1 in gastric cancer, indicating that KK-LC-1 is an ideal therapeutic target. In terms of diagnostic applications, tumor-associated antigens that are highly expressed in early stage cancers are considered useful targets. Here, the expression patterns of CTAs including KK-LC-1 were assessed in gastric cancer patients and the usefulness of these CTAs for diagnosis and determining the appropriate treatment strategy were examined.
The study protocol was approved by the Human Ethics Review Committee of Kitasato University Medical Center, Japan, and signed informed consent was obtained from each patient prior to collecting the tissue samples used in this study.
A total of 134 patients underwent surgical resection of gastric cancer at the Department of Surgery, Kitasato University Medical Center, Kitamoto, Japan between June 2011 and March 2014, and 83 specimens were successfully obtained from 52 male patients and 31 female patients. The mean age of the patients was 69.8 years (range, 30-86 years).
Clinicopathological findings were classified according to the Japanese Classification of Gastric Carcinoma (14th edition)[12]. In the present study, based on macroscopic data, tumor types 0, 1, and 2 were reclassified as localized tumors and 3, 4, and 5 as infiltrated tumors. Based on histological findings, papillary adenocarcinoma and tubular adenocarcinoma were reclassified as differentiated tumors, while poorly differentiated adenocarcinoma, signet ring cell adenocarcinoma, and mucinous carcinoma were classified as undifferentiated tumors. The relationships between CTAs and each of the nine clinicopathological factors (gender, age, tumor size, macroscopic type, tumor histology, depth, lymphatic invasion, and venous invasion) were assessed in this study.
The tumor tissue samples obtained from the 83 patients with gastric cancer were immediately preserved in RNAlater® (Life Technologies, Carlsbad, CA, United States). The specimens were incubated at 4 °C overnight to allow for penetration of RNAlater and then stored at -80 °C until use.
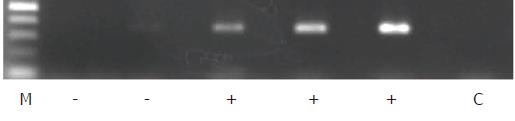
Total RNA was isolated from the tumor specimens using the BioRobot® EZ1™ and EZ1 RNA Tissue Mini Kits (48) (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and then converted to cDNA using oligo-p(dN)6 random primers and Superscript™ II reverse transcriptase (Life Technologies). β-Actin was used as an internal standard to assess the quality of the isolated RNA in which the expression of the CTAs, including KK-LC-1, was evaluated. The expression levels of ACTB (β-actin), MAGE-A1, MAGE-A3, and NY-ESO-1 were measured with TaqMan® Gene Expression Assays, ID numbers Hs99999903_m1, Hs00607097_m1, H200366532_m1, and Hs00265824_m1, respectively, using a 7900HT Fast Real-Time PCR system (Life Technologies). For cDNAs for which expression [represented by threshold cycle number (Ct)] of the ACTB gene yielded a Ct of < 28, the expression of KK-LC-1 was examined using endpoint reverse transcription PCR (RT-PCR) rather than a probe-based assay, as an appropriate probe for detecting KK-LC-1 mRNA has not been established. For RT-PCR of KK-LC-1, the oligonucleotides 5’-ATGAACTTCTATTTACTCCTAGCGAGC-3’ and 5’-TTAGGTGGATTTCCGGTGAGG-3’ were used as specific primers, and annealing was performed at 67 °C for 40 cycles, yielding a 342-base pair product. The intensity of KK-LC-1 positive- or negative-amplicon was shown in Figure 1.
Statistical comparisons between the expression levels of the four CTAs and between the nine clinicopathological factors were performed using the χ2 test with the level of significance set at P < 0.05. All statistical analyses were conducted using EZR (Saitama Medical Centre, Jichi Medical University; Kanda, 2012)[13].
The expression rates of KK-LC-1, MAGE-A1, MAGE-A3, and NY-ESO-1 in gastric cancer were 79.5%, 32.5%, 39.8%, and 15.7%, respectively. Among them, KK-LC-1 had the highest expression rate (Table 1).
| CTA | Positive | Negative | Proportion (%) |
| KK-LC-1 | 66 | 17 | 79.5 |
| MAGE-A1 | 27 | 56 | 32.5 |
| MAGE-A3 | 33 | 50 | 39.7 |
| NY-ESO-1 | 13 | 70 | 15.7 |
Table 2 shows the expression of CTAs that exhibited significant associations with any of the measured clinicopathological factors including age, sex, tumor size, macroscopic type, tumor histology, invasion depth, lymph node metastasis, lymphatic invasion, venous invasion, and disease stage. The expression rate of MAGE-A1 was significantly higher in patients with lymphatic and/or venous invasion than in those without (P = 0.034 and 0.028, respectively). The age of patients expressing MAGE-A3 was significantly higher than that of patients not expressing MAGE-A3 (P = 0.009). The expression rate of MAGE-A3 was higher in patients with advanced stage (P = 0.044), lymphatic (P = 0.022), and/or venous (P = 0.035) invasion than in those without. The age of patients expressing NY-ESO-1 was significantly higher than that of patients not expressing NY-ESO-1 (P = 0.042).
| Characteristics | The expression number of each CTA | |||
| KK-LC-1 | MAGE-A1 | MAGE-A3 | NY-ESO-1 | |
| Age (yr) (mean ± SD) | P = 0.944 | P = 0.104 | P = 0.009 | P = 0.042 |
| Positive | 69.8 ± 11.4 (n = 66) | 72.7 ± 7.9 (n = 27) | 73.7 ± 7.1 (n = 33) | 75.5 ± 7.2 (n = 13) |
| Negative | 70.1 ± 9.5 (n = 17) | 68.5 ± 12.0 (n = 56) | 67.4 ± 12.3 (n = 50) | 68.8 ± 11.2 (n = 70) |
| Tumor size (mm) (mean ± SD) | P = 0.5202 | P = 0.4752 | P = 0.862 | P = 0.831 |
| Positive | 59.9 ± 4.2 (n = 65) | 62.5 ± 6.6 (n = 27) | 57.9 ± 5.9 (n = 33) | 60.5 ± 9.5 (n = 13) |
| Negative | 53.8 ± 8.5 (n = 16) | 56.8 ± 4.6 (n = 54) | 59.2 ± 4.9 (n = 48) | 58.3 ± 4.1 (n = 68) |
| Gender | P = 0.406 | P = 1 | P = 0.645 | P = 0.758 |
| Male (n = 52) | 43 (82.7) | 17 (32.7) | 22 (42.3) | 9 (17.3) |
| Female (n = 31) | 23 (74.2) | 10 (32.3) | 11 (35.5) | 4 (12.9) |
| Macroscopic type | P = 0.591 | P = 1 | P = 0.649 | P = 1 |
| Localized (n = 49) | 40 (81.6) | 16 (32.7) | 18 (36.7) | 8 (16.3) |
| Infiltrated (n = 34) | 26 (76.5) | 11 (32.4) | 15 (44.1) | 5 (14.7) |
| Histological type | P = 0.599 | P = 0.246 | P = 1 | P = 1 |
| Differentiated (n = 44) | 36 (81.8) | 17 (38.6) | 18 (40.9) | 7 (15.9) |
| Undefferentiated (n = 39) | 30 (76.9) | 10 (25.6) | 15 (38.5) | 6 (15.4) |
| Depth of invasion | P = 0.269 | P = 0.089 | P = 0.243 | P = 0.761 |
| T1 (n = 30) | 26 (86.7) | 6 (20.0) | 9 (30.0) | 4 (13.3) |
| Intramucosal (n = 11) | 11 (100) | 0 (0) | 2 (18.2) | 1 (9.1) |
| Submucosal 1 (n = 5) | 4 (80.0) | 2 (40.0) | 3 (60.0) | 1 (20.0) |
| Submucosal 2 (n = 14) | 11 (78.6) | 4 (28.6) | 4 (28.6) | 2 (14.3) |
| T2-T4 (n = 53) | 40 (75.5) | 21 (39.6) | 24 (45.3) | 9 (17.0) |
| Lymph node metastasis | P = 1 | P = 0.17 | P = 0.823 | P = 1 |
| Negative (n = 40) | 32 (80.0) | 10 (25.0) | 15 (37.5) | 6 (15.0) |
| Positive (n = 43) | 34 (79.1) | 17 (39.5) | 18 (41.9) | 7 (16.3) |
| Lymphatic invasion | P = 1 | P = 0.034 | P = 0.022 | P = 0.498 |
| Negative (n = 22) | 18 (81.8) | 3 (13.6) | 4 (18.2) | 2 (9.1) |
| Positive (n = 61) | 48 (78.7) | 24 (39.3) | 29 (47.5) | 11 (18.0) |
| Venous invasion | P = 0.583 | P = 0.028 | P = 0.035 | P = 0.121 |
| Negative (n = 30) | 25 (83.3) | 5 (16.7) | 7 (23.3) | 2 (6.7) |
| Positive (n = 53) | 41 (77.4) | 22 (41.5) | 26 (49.1) | 11 (20.8) |
| Stage | P = 1 | P = 0.162 | P = 0.044 | P = 0.222 |
| Early (I) (n = 34) | 27 (79.4) | 8 (23.5) | 9 (26.5) | 3 (8.8) |
| Advanced (II-IV) (n = 49) | 39 (79.6) | 19 (38.8) | 24 (49.0) | 10 (20.4) |
| II (n = 21) | 16 (76.2) | 7 (33.3) | 11 (52.4) | 3 (14.3) |
| III (n = 15) | 11 (73.3) | 7 (46.7) | 7 (46.7) | 3 (20.0) |
| IV (n = 13) | 12 (92.3) | 5 (38.5) | 6 (46.2) | 4 (30.8) |
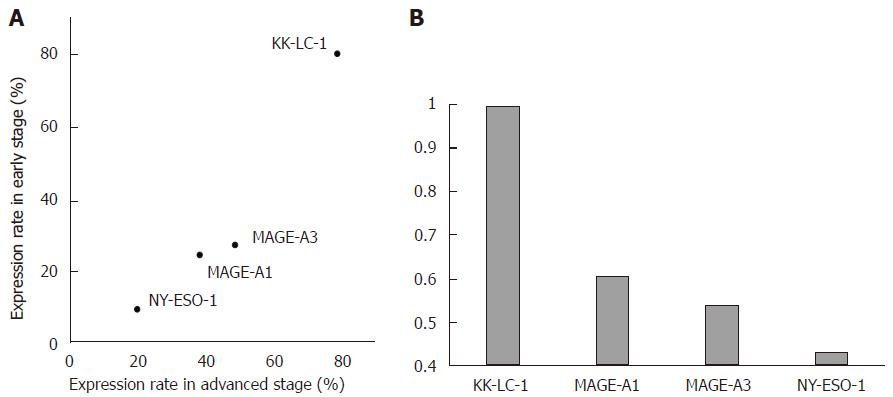
Although KK-LC-1 was not significantly associated with any of the nine clinicopathological factors, the expression rate of KK-LC-1 (79.4%) in early stage samples was markedly higher than that of MAGE-A1 (23.5%), MAGE-A3 (26.5%), and NY-ESO-1 (8.8%) in early stage samples and was comparable to the KK-LC-1 expression rate in advanced stage cancer (79.6%; Figure 2A). In contrast, the expression rates of the other CTAs were not comparable to those of KK-LC-1 in advanced stage samples (38.8% for MAGE-A1, 49.0% for MAGE-A3, and 16.0% for NY-ESO-1). The ratios of the expression rates in early/advanced stage (E/A ratio) were estimated for each CTA (Figure 2B) and higher ratios (closer to 1; i.e., the CTA concerned was expressed at similar levels in early stage compared to advanced stage) were associated with CTAs that are more useful in early stage diagnosis and targeted immunotherapy. The E/A ratio of KK-LC-1 was the highest among them (KK-LC-1: 1.00, MAGE-A1: 0.61, MAGE-A3: 0.54, and NY-ESO-1: 0.43).
In the present study, the potential associations between KK-LC-1 expression and various clinicopathological factors were examined. The usefulness of KK-LC-1 as a target for cancer immunotherapy and a biomarker for early diagnosis was also evaluated.
The expression rate of KK-LC-1 in the present study (79.5%) was shown to be markedly higher than those of other CTAs measured. We assessed KK-LC-1 expression as visibility of PCR amplicon, and have not experienced contamination of other sequences in the 342-bp of KK-LC-1 amplicon, then there would be few pseudo-positive cases. On the other hand, the specimens included much normal cells, such as normal stomach cells, fibroblasts and immune cells, the expression of KK-LC-1 would not detect even if tumor cells produced it. The specimens used in this study were not evaluated the rate of inclusion tumor cells, then the pseudo-negative specimens might be included.
The expression of KK-LC-1 has been assessed in various cancer types[10,11,14]. The basal form of breast cancer, also known as triple-negative breast cancer, exhibited high expression rates of KK-LC-1 compared with other CTAs; however, KK-LC-1 expression in whole breast cancer was low. In this study, high KK-LC-1 expression rates were detected in whole gastric cancer, where detecting KK-LC-1 in stomach tissue is considered more advantageous for mining tumors than in breast tissue.
The expression rates of other CTAs in gastric cancer have been reported in several studies: Wang et al[15] reported the expression rates of MAGE-A1, MAGE-A3, and NY-ESO-1 as 23.8%, 41.6%, and 11.9%, respectively; Ogata et al[5] reported the expression rate of MAGE-A1 to be 32.6%; and Mashino et al[16] reported the expression rate of NY-ESO-1 as 7.8%. In our study, the expression rates of MAGE-A1, MAGE-A3, and NY-ESO-1 were 32.5%, 39.8%, and 15.7%, respectively, which agree with previously reported rates.
Assessing CTAs in relation to the clinicopathological factors in this study revealed that the expression of KK-LC-1 was not significantly associated with any of the measured clinicopathological factors, indicating that KK-LC-1 was highly expressed in gastric cancer patients regardless of the presence of lymph node metastasis, lymphatic invasion, or disease stage. This suggests that KK-LC-1 is a useful target for cancer immunotherapy, regardless of disease stage.
Regarding tumor-associated antigens including tumor markers and CTAs (shown in part in Figure 1), it is known that the detection rate in early stage cancer is lower than that in advanced stage cancer[17]. The expression rates of MAGE-A1 and MAGE-A3 were higher in patients with lymphatic and venous invasion than in those without. The ages of groups expressing MAGE-A3 or NY-ESO-1 were higher than those not expressing these genes. Examination of the influence of the disease stage revealed a significant difference in the expression rates of MAGE-A3 between disease stages (from stage I to stage IV). Ogata et al[5] reported that MAGE-A1 expression is significantly associated with age, macroscopic type, and venous invasion, but not with disease stage, invasion depth, or lymph node metastasis. In another study, however, the expression rates of CTAs were reported to be higher in gastric cancer patients with advanced stages or in those with greater invasion depth[7], which is inconsistent with the above findings.
Recently, a KK-LC-1 peptide restricted by HLA-B62 and HLA-A2 was discovered[8,10]. The frequencies of HLA-B62 and HLA-A2 expression among Japanese gastric cancer patients were reported to be 15% and 45%, respectively[18] and the frequency of patients expressing at least one of these two HLA proteins was approximated at 55%. Given that the expression rate of KK-LC-1 in gastric cancer was found to be 79.5% in this study, approximately 44% of patients with gastric cancer are potential candidates for immunotherapy targeting KK-LC-1. By the same reasoning and using the peptide database of shared tumor-specific antigens from Cancer Immunity (http://cancerimmunity.org/peptide/tumor-specific/) as well as findings published by Ikeda et al[18], the KK-LC-1 expression rates in patients considered potential candidates for immunotherapy targeting MAGE-A1 and MAGE-A3 were approximated at 27% and 38%, respectively. Although 10 epitope peptides of MAGE-A3 bind to HLA and are recognized by CTL, the covering rate of patients for MAGE-A3 immunotherapy would be lower than that of KK-LC-1 immunotherapy, as KK-LC-1 contains only two epitope peptides.
Fukuyama et al[19] further demonstrated that Helicobacter pylori infection induced the expression of murine Mage-A3. After KK-LC-1, MAGE-A3 was the most highly expressed CTA among those tested in the present study. Notably, the 79.5% KK-LC-1 expression frequency is in accordance with the > 80% of patients who had gastric cancer caused by H. pylori infection[20]. In the study by Fukuyama, the potential correlation between murine Kk-lc-1 and H. pylori infection could not be evaluated because there is no sequence in the murine genome comparable to the human KK-LC-1 gene sequence. Considering that KK-LC-1 is expressed in tumor tissues regardless of invasion depth and disease stage, it is possible that H. pylori induces CTA expression[21].
Given that KK-LC-1 is tumor-specific and can be frequently detected even in early stages, the assessment of CTAs in gastric mucosa infected with H. pylori may be useful for identifying patients with a high risk of gastric cancer. Overall, immunotherapy targeting KK-LC-1 may represent a new treatment strategy for gastric cancer.
One CTA’s expression rate was high in gastric cancer. The expression rate of CTAs is commonly higher at advanced stages than early stage of cancers of any organs. We hypothesized that KK-LC-1 expression rate would be higher in advanced stage than in early stage.
One CTA was not analyzed the relation of clinicopathological factors and other CTAs were little known the relation of them. Now, tumor immunotherapy has been remarked because of PD-1 therapy, then CTAs, targets for immunotherapy, will be also remarked.
We search the new approach of CTAs for therapy or diagnosis of cancer. For the objective, we performed their characteristics. Especially, the high-rate expressing CTAs should be mined and characterized for cancer therapy/diagnosis.
The process was very simple. We collected fresh tumor tissues of stomach resected from gastric cancer patients and evaluated the expression of four CTAs using RT-PCR method. Also we collected the clinicopathological background in their clinical records. We calculated the proportion of each CTA expression and evaluated the difference of clinicopathological background between the patients with expression of each CTA and without them. We performed statistical analysis of χ2 test using EZR.
The expression rate of KK-LC-1 in early stage was as high as in advanced stages. The expression rate of KK-LC-1 was about 80% high at any statement of clinicopathological background. And we supplied the knowledges about the correlation between expression of each CTA and clinicopathological background.
Our results of High-rate expression of KK-LC-1 not only in advanced stage but also in early stage indicated that it would be an attractive target for immunotherapy and early diagnosis in gastric cancer.
We need to evaluate that KK-LC-1 is correlate to infection of H. pylori in gastric cancer and that KK-LC-1 is detected at the less invasive methods than biopsy.
The authors thank Ms. Etsuko Terayama, and Ms. Maki Kobayashi for providing technical assistance.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aoyagi K, Luo HS S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Stewart BW, Wild CP, editors . World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer 2014; . |
| 2. | van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643-1647. [PubMed] |
| 3. | Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6:15772-15787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Ghafouri-Fard S, Modarressi MH. Cancer-testis antigens: potential targets for cancer immunotherapy. Arch Iran Med. 2009;12:395-404. [PubMed] |
| 5. | Ogata K, Aihara R, Mochiki E, Ogawa A, Yanai M, Toyomasu Y, Ando H, Ohno T, Asao T, Kuwano H. Clinical significance of melanoma antigen-encoding gene-1 (MAGE-1) expression and its correlation with poor prognosis in differentiated advanced gastric cancer. Ann Surg Oncol. 2011;18:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Shida A, Futawatari N, Fukuyama T, Ichiki Y, Takahashi Y, Nishi Y, Kobayashi N, Yamazaki H, Watanabe M. Frequent High Expression of Kita-Kyushu Lung Cancer Antigen-1 (KK-LC-1) in Gastric Cancer. Anticancer Res. 2015;35:3575-3579. [PubMed] |
| 7. | Honda T, Tamura G, Waki T, Kawata S, Terashima M, Nishizuka S, Motoyama T. Demethylation of MAGE promoters during gastric cancer progression. Br J Cancer. 2004;90:838-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Fukuyama T, Hanagiri T, Takenoyama M, Ichiki Y, Mizukami M, So T, Sugaya M, So T, Sugio K, Yasumoto K. Identification of a new cancer/germline gene, KK-LC-1, encoding an antigen recognized by autologous CTL induced on human lung adenocarcinoma. Cancer Res. 2006;66:4922-4928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Stevanović S, Pasetto A, Helman SR, Gartner JJ, Prickett TD, Howie B, Robins HS, Robbins PF, Klebanoff CA, Rosenberg SA. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science. 2017;356:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 10. | Shigematsu Y, Hanagiri T, Shiota H, Kuroda K, Baba T, Mizukami M, So T, Ichiki Y, Yasuda M, So T. Clinical significance of cancer/testis antigens expression in patients with non-small cell lung cancer. Lung Cancer. 2010;68:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Paret C, Simon P, Vormbrock K, Bender C, Kölsch A, Breitkreuz A, Yildiz Ö, Omokoko T, Hubich-Rau S, Hartmann C, Häcker S, Wagner M, Roldan DB, Selmi A, Türeci Ö, Sahin U. CXorf61 is a target for T cell based immunotherapy of triple-negative breast cancer. Oncotarget. 2015;6:25356-25367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association: Japanese Classification of Gastric Carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. |
| 13. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13321] [Article Influence: 1110.1] [Reference Citation Analysis (0)] |
| 14. | Yao J, Caballero OL, Yung WK, Weinstein JN, Riggins GJ, Strausberg RL, Zhao Q. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res. 2014;2:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Wu XJ, Zhao AL, Yuan YH, Chen YT, Jungbluth AA, Gnjatic S, Santiago D, Ritter G, Chen WF. Cancer/testis antigen expression and autologous humoral immunity to NY-ESO-1 in gastric cancer. Cancer Immun. 2004;4:11. [PubMed] |
| 16. | Mashino K, Sadanaga N, Tanaka F, Yamaguchi H, Nagashima H, Inoue H, Sugimachi K, Mori M. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br J Cancer. 2001;85:713-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Wang W, Chen XL, Zhao SY, Xu YH, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget. 2016;7:35423-35436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Ikeda N, Kojima H, Nishikawa M, Hayashi K, Futagami T, Tsujino T, Kusunoki Y, Fujii N, Suegami S, Miyazaki Y. Determination of HLA-A, -C, -B, -DRB1 allele and haplotype frequency in Japanese population based on family study. Tissue Antigens. 2015;85:252-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 19. | Fukuyama T, Yamazaki T, Fujita T, Uematsu T, Ichiki Y, Kaneko H, Suzuki T, Kobayashi N. Helicobacter pylori, a carcinogen, induces the expression of melanoma antigen-encoding gene (Mage)-A3, a cancer/testis antigen. Tumour Biol. 2012;33:1881-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |