Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8062
Peer-review started: September 14, 2017
First decision: October 10, 2017
Revised: October 25, 2017
Accepted: November 8, 2017
Article in press: November 8, 2017
Published online: December 7, 2017
Processing time: 81 Days and 21.8 Hours
To assess the effects of a combination therapy with natriuretic and aquaretic drugs in cirrhotic ascites patients.
A two-center, randomized, open-label, prospective study was conducted. Japanese patients who met the criteria were randomized to trial group and the combination diuretic group (received 7.5 mg of tolvaptan) or the conventional diuretic group (received 40 mg of furosemide) for 7 d in addition to the natriuretic drug which was used prior to enrolment in this study. The primary endpoint was the change in body weight from the baseline. Vital signs, fluid intake, and laboratory and urinary data were assessed to determine the pharmacological effects after administration of aquaretic and natriuretic drugs.
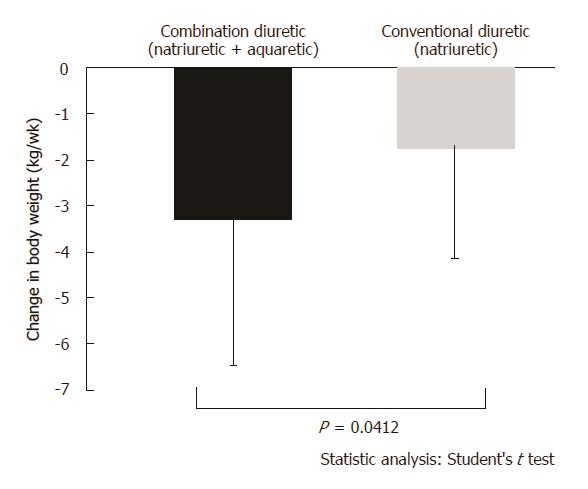
A total of 56 patients were randomized to receive either tolvaptan (n = 28) or furosemide (n = 28). In the combination and conventional diuretic groups, the average decrease in body weight from the baseline was 3.21 ± 3.17 kg (P < 0.0001) and 1.75 ± 2.36 kg (P = 0.0006), respectively, when measured on the final dosing day. Following 1 wk of treatment, a significantly greater reduction in body weight was observed in the combination diuretic group compared to that in the conventional diuretic group (P = 0.0412).
Compared to a conventional diuretic therapy with only a natriuretic drug, a combination diuretic therapy with natriuretic and aquaretic drugs is more effective for patients with cirrhotic ascites.
Core tip: Whether a combination therapy with natriuretic and aquaretic drugs is more effective than conventional therapy with natriuretic drugs only for liver cirrhosis patients with ascites remains unclear. To clarify this, we compared the pharmacological effects of combination therapy with conventional therapy in cirrhotic ascites patients. Compared to a conventional therapy with only natriuretic drugs, a combination therapy with natriuretic and aquaretic drugs is more effective for patients with cirrhotic ascites.
- Citation: Uojima H, Hidaka H, Nakayama T, Sung JH, Ichita C, Tokoro S, Masuda S, Sasaki A, Koizumi K, Egashira H, Kako M. Efficacy of combination therapy with natriuretic and aquaretic drugs in cirrhotic ascites patients: A randomized study. World J Gastroenterol 2017; 23(45): 8062-8072
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8062.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8062
Cirrhosis leads to portal hypertension and end-stage liver disease, with complications[1,2]. Pathophysiology of ascites, one of the most common complications in liver cirrhosis, involves a decrease in effective arterial blood volume due to splanchnic arterial vasodilatation leading to activation of potent vasoconstriction systems, such as the renin-angiotensin-aldosterone system (RAAS), the sympathetic nervous system, and vasopressin release, which in turn results in retention of sodium and free water to restore blood homeostasis[3-6].
Progression of liver diseases is characterized by a large decrease in the excretion of urinary sodium, and accumulation of retained fluid within the abdominal cavity. For liver cirrhosis patients with ascites, current guidelines recommend the administration of a diuretic drug if the efficacy of sodium intake restriction is inadequate[7]. Conventional diuretics are natriuretic drugs that block sodium reabsorption in the nephrons, increasing renal sodium excretion to achieve a negative sodium balance[6,8]. Although ascites in the majority of patients can be controlled by restriction of sodium intake and administration of a natriuretic medication, 5%-10% of patients with ascites develop resistance to conventional therapy as refractory ascites. Refractory ascites is composed of diuretic-resistant ascites and diuretic-intractable ascites. Diuretic-resistant cannot be mobilized or the early recurrence because of a lack of response to dietary sodium restriction and conventional diuretics. For the treatment of diuretic-intractable ascites an effective diuretic dosage has not yet been determined because of the development of severe diuretic-related side effects[9]. The strategy of ascites refractory to diuretic therapy has still not been established.
Recently, several studies have evaluated the effects of aquaretic drugs such as tolvaptan for treating ascites resistant to conventional diuretics[10,11]. Tolvaptan, which blocks arginine vasopressin (AVP) from binding to V2 receptors in the distal nephrons and thus restricts water reabsorption, is an ideal aquaretic drug for the treatment of delusional hyponatraemia in conditions associated with increased circulating levels of antidiuretic hormone such as decompensated liver cirrhosis[12]. However, whether a combination therapy with natriuretic and aquaretic is more effective than conventional therapy with natriuretic only for liver cirrhosis patients with ascites remains unclear. To clarify this, we compared the pharmacological effects of combination diuretics therapy with conventional diuretics therapy in cirrhotic ascites patients.
This study was approved by the Institutional Review Board Ethics Committee of the Tokushukai Medical Group (license number: TGE00406-024). Informed consent was obtained from patients or their families before study participation commenced. This study was registered at the UMIN Clinical Trials Registry as UMIN000015218. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Tokushukai Medical Group’s human research committee.
A two-center, randomized, open-label, prospective study was conducted at Shonan Kamakura General Hospital and Shonan Atsugi Hospital in Kanagawa, Japan. This study consisted of a 2-d pretreatment observation period followed by a 7-d treatment period and 4-wk posttreatment observation period. Patients who met the criteria were randomized to receive aquaretic (7.5 mg of tolvaptan) or natriuretic (40 mg of furosemide) in a 1:1 ratio. Permuted block randomization was used for the creation of the randomization list prepared by an investigator with no clinical involvement in the trial, and a randomization code was pre-assigned to each trial drug and used during drug administration. Patients received oral tolvaptan or furosemide for 7 d in addition to the standard therapy identical to that administered prior to enrolment in this study, which included sodium intake restrictions (< 6 g/d), ad libitum fluid intake, and natriuretic therapy[8]. According to the standard diuretic regimen in Japan, high-dose diuretics such as furosemide at 160 mg/d or spironolactone at 400 mg/d is not recommended. All enrolled patients were admitted to the hospital, and the trial drug was administered orally between 07:00 and 08:00 after breakfast. The dosages of the natriuretic drug used prior to enrolment in this study were not changed, and therapeutic abdominal paracentesis procedures were not performed until the completion of the final assessment on the day following the final administration of the trial drug.
Patients were required to meet the following inclusion criteria: Age > 20 years, liver cirrhosis with ascites even after undergoing a natriuretic with a loop diuretic and an anti-aldosterone agent for at least 7 d, and a daily dose of ≥ 20 mg furosemide and ≥ 25 mg spironolactone, a conventional diuretics regimen in Japan[13]. Additionally, the diuretic dosages should not have been changed for at least 7 d prior to initiating the trial, and an ineffective response was one in which body weight was not reduced in spite of administration of intensive diuretic therapy for the 7 d. A difference in dosage of diuretics was observed among patients because different doctors referred them for treatment. Diagnosis of liver cirrhosis was based on laboratory results and imaging tests (ultrasonography and computed tomography), revealing a hepatic cirrhotic appearance, splenomegaly, esophageal varices, and/or ascites[8]. Patients with hepatic encephalopathy (coma scale ≥ II), poorly controlled hepatocellular carcinoma, and patients receiving blood products including albumin for 7 d or less before initiating the trial drug treatment were excluded.
Baseline characteristics collected included demographic parameters, concomitant medication, ascites volume, mean 24-h urine volume, and baseline laboratory and urinary data obtained immediately preceding the start of the trial drug administration. All the patients exhibited ascites volumes of ≥ 1000 mL as calculated by computed tomography[14]. Physical examination including measurement of body weight, supine blood pressure, and pulse rate was performed daily. Urine volume was measured over a 24-h period from 06:00. Mean differences in daily urine volume and body weight between the two groups were calculated. Cumulative 24-h urine samples were collected before drug administration each day from the day before initiating tolvaptan until the end of the posttreatment period. Laboratory and urinary data were obtained at 06:00 before drug administration and after drug administration on day 7.
Blood parameters measured included levels of hemoglobin, platelets, serum albumin, alanine aminotransferase (ALT), serum blood urea nitrogen (BUN), serum creatinine, serum total bilirubin, plasma brain natriuretic peptide, human atrial natriuretic peptide, serum sodium, serum potassium, serum osmolality (OSM), serum aldosterone, serum renin, ammonia, and plasma AVP. Urinary parameters measured included OSM, sodium, and potassium. The value for 24-h creatinine clearance (CCr) was calculated as urinary creatinine × urinary volume ÷ serum creatinine × 1440[15].
The primary endpoint of this trial was the change from baseline in body weight through the duration of the study. The day a patient completed or discontinued the treatment was defined as the final dosing day. We also studied the responder rate for the trial drug. According to a previous report, patients who lost ≥ 2 kg and < 2 kg body weight after 1 wk of drug administration were defined as responders and non-responders, respectively[16]. Primary endpoint was the change in body weight measured 1 wk after administration of the trial drugs.
Secondary endpoints included changes in urine volume, ascites volume, and improvement of ascites-related symptoms. To determine the pharmacological effects of tolvaptan and furosemide, vital signs, fluid intake, and laboratory data on blood and urinary biomarkers were assessed.
To evaluate drug safety during the treatment period, we assessed the adverse events following the trial drug administration. Data relating to all the adverse events were collected from the start of the trial drug administration until the final dosing day. The adverse events were classified using the Medical Dictionary for Regulatory Activities, version 17.0, and their severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Our estimation of clinical effects using change in body weight after a combination diuretic therapy for a wk was based on a previous study[11]. The change of body weight after 1 wk was 1.95 kg in the combination diuretic group and 0.44 kg in the conventional diuretic group; and therefore, we suspect a difference of at least 1.5 kg/wk between the two groups (the standard deviation of the two groups was 1.8 kg). In order to detect a difference of this magnitude that is significant with a 95% confidence interval and a power of 80%, there was a minimum of 24 patients required in each group. Assuming that 20% of the patients would drop out of the study, the required sample size for this trial was, therefore, estimated to be approximately 60 patients (30 patients each in the furosemide and tolvaptan groups).
Analyses were based on the per-protocol analysis and the full analysis set. The full analysis set included all randomized patients who received the trial drugs at least once. Missing data at the final evaluation day were imputed by the last data obtained after the start of the study. Safety analyses were conducted on all patients who received at least one dose of either of the trial drugs. No interim analyses for this study’s data were done.
Data were analyzed using the statistical software JMP 11.0.1 (SAS Institute) and expressed as mean ± SD. Continuous variables of the conventional diuretic groups and combination diuretic groups were compared using the Student’s t test or the Mann-Whitney U test, whereas the paired t test or the Wilcoxon signed-rank test were used for paired data. Differences with a P value < 0.05 were considered significant. Statistical analyses were performed by the SATT Corporation, Tokyo, Japan.
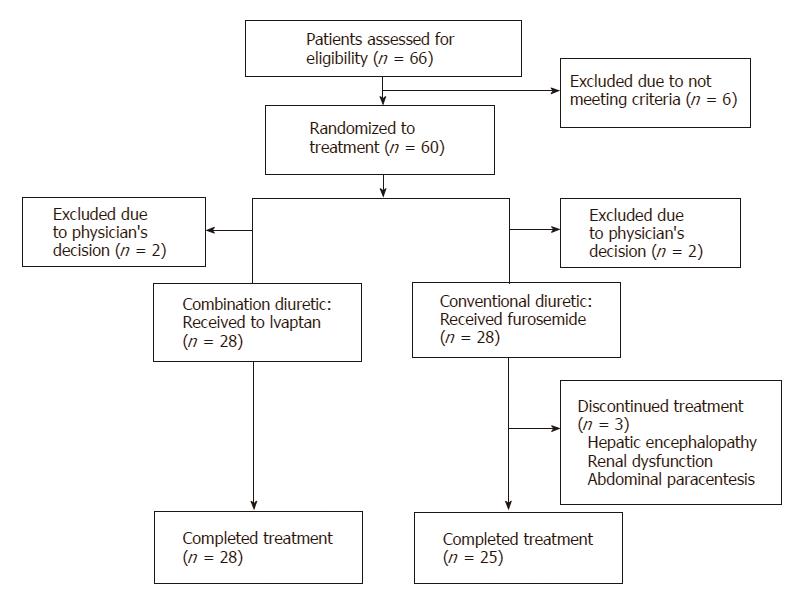
A total of 66 patients were assessed in the present study from July 2014 through August 2016 (Figure 1). Six patients dropped out of the study during the run-in period for failing to meet the criteria for commencing treatment. The remaining 60 patients were randomized to receive either tolvaptan (n = 30) or furosemide (n = 30). However, four patients with unstable vital signs or acute renal failure were withdrawn from the study by their physicians before the drugs were administered (2 patients in each group). Three patients discontinued treatment, two owing to adverse events (hepatic encephalopathy and renal dysfunction, respectively), whereas the third required intervention following administration of standard diuretics therapy in the form of therapeutic abdominal paracentesis for uncontrollable ascites due to a bacterial infection.
Patient demographics and baseline characteristics are summarized in Tables 1 and 2. No significant differences were found in sex, age, cause of liver cirrhosis, or the amount of diuretics used between the two groups. Hemoglobin levels were higher in the conventional diuretic group (P = 0.0481), and platelet counts were slightly elevated (P = 0.0598). No significant differences were found between the groups prior to trial initiation in terms of serum albumin, serum ALT, serum BUN, serum creatinine, serum total bilirubin, serum sodium, serum potassium, serum OSM, serum aldosterone, serum renin, plasma AVP, urinary OSM, urinary sodium, potassium, or 24-h CCr.
| Combination diuretic (n = 28) | Conventional diuretic (n = 28) | P value | |
| Age, yr | 69.3 ± 11.8 | 69.4 ± 12.6 | 0.9739 |
| Gender: Male | 17 (60.7) | 15 (53.6) | 0.7875 |
| Weight, kg | 61.7 ± 13.8 | 58.0 ± 13.5 | 0.3082 |
| Body mass index, kg/m2 | 24.2 ± 4.74 | 22.4 ± 3.48 | 0.1044 |
| Etiology: HBV/HCV/alcoholic/other | 2/12/9/5 | 2/10/9/7 | 0.9341 |
| Child-Pugh score | 10.4 ± 1.2 | 10.3 ± 1.4 | 0.7653 |
| Child-Pugh stage: A/B/C | 0/16/12 | 0/15/13 | 0.7881 |
| Liver cancern | 7 (25.0) | 8 (28.6) | 1.0000 |
| Varicose veins | 23 (82.1) | 25 (89.3) | 0.7049 |
| Liver encephalopathy: Grade 0/I | 24/4 | 24/4 | 1.0000 |
| Concomitant medication: | |||
| Spironolactone, mg/d | 50.9 ± 29.3 | 50.0 ± 22.6 | 0.8987 |
| Furosemide, mg/d | 38.6 ± 18.4 | 37.7 ± 20.3 | 0.8639 |
| BCAA | 21 (75.0) | 19 (67.8) | 0.7674 |
| Non-absorbable disaccharides | 8 (28.5) | 10 (35.7) | 0.7748 |
| ARB or ACE inhibitor | 3 (10.7) | 4 (14.3) | 1.0000 |
| Duration of diuretics therapy, d | 25.9 ± 28.3 | 22.8 ± 20.1 | 0.6318 |
| Pretreatment urine volume, mL/d | 1356 ± 651 | 1517 ± 939 | 0.4213 |
| Ascites volume, mL | 2204 ± 1384 | 2104 ± 1084 | 0.6400 |
| Grade of ascites: Mild/moderate/severe | 5/15/8 | 6/13/9 | 0.8901 |
| Amount of water drunk, mL/d | 1032 ± 491.2 | 1071 ± 870.0 | 0.9710 |
| Pulse rate, /min | 78 ± 10.1 | 78 ± 11.2 | 0.8514 |
| Systolic blood pressure, mmHg | 121 ± 21.6 | 116 ± 12.9 | 0.3130 |
| Diastolic blood pressure, mmHg | 65 ± 13.5 | 72 ± 21.5 | 0.2115 |
| Combination diuretic (n = 28) | Conventional diuretic (n = 28) | P value | |
| Hemoglobin, g/dL | 9.43 ± 1.30 | 10.3 ± 1.62 | 0.0481 |
| Platelets, × 103/μL | 10.1 ± 7.96 | 12.3 ± 6.04 | 0.0598 |
| Prothrombin time, s | 12.5 ± 1.5 | 12.3 ± 1.1 | 0.4672 |
| Serum albumin, g/dL | 2.38 ± 0.49 | 2.36 ± 0.55 | 0.9934 |
| BUN, g/dL | 24.7 ± 14.6 | 27.1 ± 24.7 | 0.8058 |
| Serum creatinine, mg/dL | 1.14 ± 0.52 | 1.03 ± 0.42 | 0.4031 |
| ALT, IU/L | 31.3 ± 29.6 | 25.5 ± 12.2 | 0.7306 |
| Total bilirubin, g/dL | 3.20 ± 3.78 | 2.86 ± 2.96 | 0.7121 |
| BNP, pg/mL | 164 ± 310 | 97.1 ± 76.6 | 0.8957 |
| HANP, pg/mL | 79.5 ± 80.4 | 60.2 ± 33.6 | 0.8831 |
| Ammonia, μg/dL | 82.4 ± 48.5 | 75.6 ± 45.1 | 0.6314 |
| Plasma-aldosterone, pg/mL | 163 ± 178 | 185 ± 267 | 0.5221 |
| Plasma-renin, pg/mL | 9.04 ± 10.6 | 8.45 ± 14.3 | 0.8377 |
| Plasma AVP, pg/mL | 2.42 ± 1.52 | 2.35 ± 1.70 | 0.5007 |
| Serum sodium, mEq/L | 135 ± 5.4 | 135 ± 4.2 | 0.5869 |
| Serum potassium, mEq/L | 3.83 ± 0.52 | 3.99 ± 0.53 | 0.2301 |
| Urinary OSM, mOSM/L | 426 ± 163 | 413 ± 172 | 0.7742 |
| Urinary sodium, mEq/d | 88.8 ± 63.3 | 93.4 ± 65.3 | 0.5869 |
| Urinary potassium, mEq/d | 25.3 ± 13.8 | 26.8 ± 13.4 | 0.5497 |
| 24-h CCr, mL/min | 57.3 ± 27.9 | 62.6 ± 38.4 | 0.9217 |
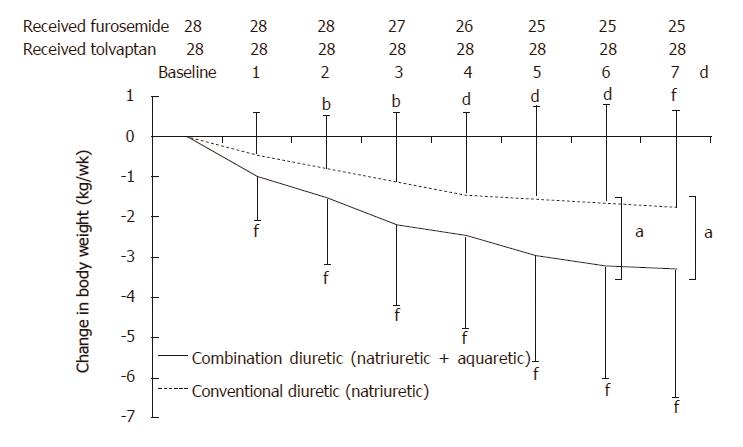
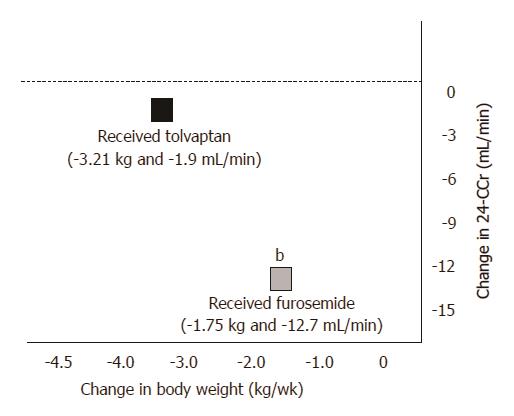
Change in body weight measured 1 wk after administration of the trial drugs is shown in Figures 2 and 3. In the combination diuretic group, the average decrease in body weight from baseline was -3.21 ± 3.17 kg (P < 0.0001) as measured on the final dosing day, with 18 (64.3%) responders. In the conventional diuretic therapy, the average decrease in body weight from baseline was -1.75 ± 2.36 kg (P = 0.0006) on the final dosing day, with 13 (46.4%) responders. Following 1 wk of treatment, a significantly greater reduction in body weight was observed in the combination diuretic group compared to that in the conventional diuretic group (P = 0.0412).
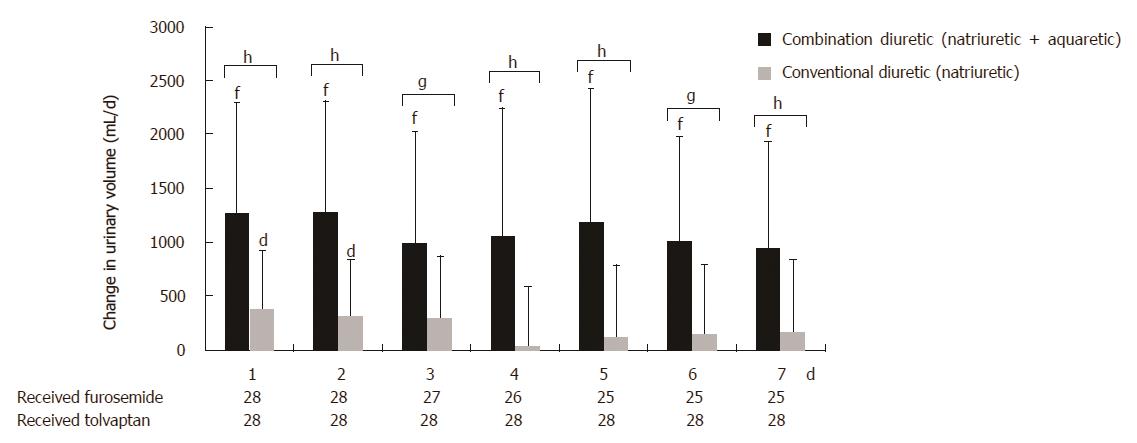
Change in urinary volume 1 wk after administration of the trial drugs is shown in Figure 4. Following the administration of tolvaptan, a significant increase in mean urine excretion volume from 1356 ± 651 mL pretreatment to 2439 ± 1179 mL posttreatment was observed (P < 0.0001). Following the administration of furosemide, a slight increase in mean urine excretion volume was observed, with a pretreatment value of 1517 ± 939 mL and a posttreatment value of 1759 ± 888 mL (P = 0.0253).
Following 1 wk of treatment, a significantly greater increase in urine volume was observed in the combination diuretic group compared to that in the conventional diuretic group (P < 0.0001). In the combination diuretic group, body weight consistently decreased, whereas urine volume consistently increased. In the conventional diuretic group, body weight consistently decreased, whereas urine volume remained constant.
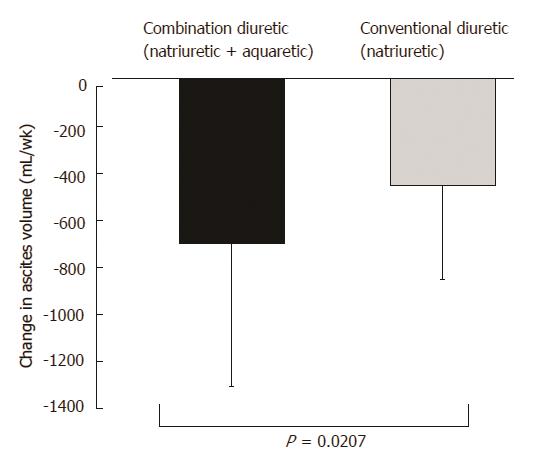
After the administration of tolvaptan, a significant decrease in the ascites volume from 2204 ± 1384 mL pretreatment to 1501 ± 669 mL posttreatment was observed (P < 0.0001). Following the administration of furosemide, a significant decrease in the ascites volume from 2104 ± 1084 mL pretreatment to 1649 ± 401 mL posttreatment was observed (P < 0.0001). A significantly greater decrease in ascites volume was observed in the combination diuretic group compared to that in the conventional diuretic group (P = 0.0207) (Figure 5).
Improvement in ascites-related symptoms after administration of the tolvaptan or furosemide was 71.4% (20/28) and 57.1% (16/28), respectively. There were no significant differences between the groups.
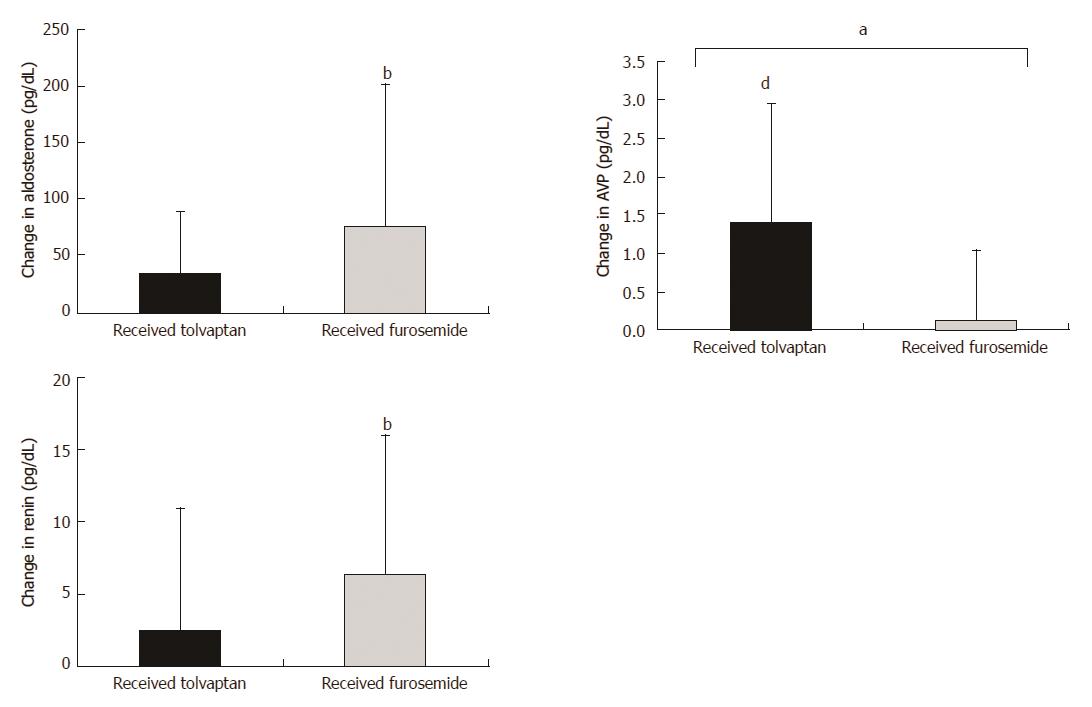
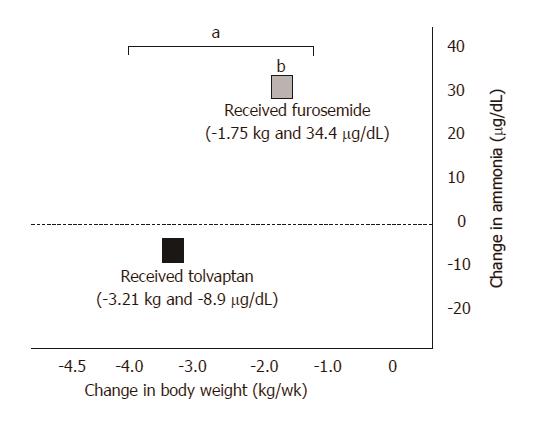
After the administration of tolvaptan, plasma AVP was higher on the final dosing day compared to that at baseline (2.42 ± 1.52 vs 3.87 ± 1.99, P < 0.0001), whereas urinary OSM was lower (426.2 ± 163.3 vs 318.4 ± 118.0, P = 0.0106) (Figure 6). Renal function represented by 24-h CCr was indistinguishable between that at the baseline and the final dosing day (57.2 ± 27.9 vs 55.3 ± 34.1, P = 0.9980), as were ammonia levels (82.4 ± 48.5 vs 73.5 ± 42.2, P = 0.0854) (Figures 7 and 8).
After the administration of furosemide, plasma renin was higher on the final dosing day compared to that at baseline (8.45 ± 14.3 vs 15.0 ± 20.2, P = 0.0128), whereas renal function represented by 24-h CCr was lower (62.6 ± 38.4 vs 49.9 ± 40.4, P = 0.0231). Ammonia levels were higher on the final dosing day compared to those at baseline (75.6 ± 45.1 vs 110.8 ± 66.0, P = 0.0012).
Posttreatment serum ammonia levels were higher in the conventional diuretic group compared to those in the combination diuretic group (P = 0.0008), whereas plasma AVP and urinary OSM were higher in the combination diuretic group (P = 0.0003; P = 0.0296, respectively). No significant differences were observed between the groups in platelet count, serum albumin, serum bilirubin, serum creatinine, serum sodium, plasma renin, plasma aldosterone, urinary sodium, and 24-h CCr after administration of the trial drugs for 1 wk.
No deaths occurred during the treatment period. Adverse events were observed in 13 (46.4%) of the patients receiving tolvaptan and 16 (57.1%) of the patients receiving furosemide (Table 3). Severe adverse events were reported in two patients in the conventional therapy (hepatic encephalopathy and a bacterial infection) during treatment and led to their withdrawal from the study. One patient with acute kidney injury was removed from the study at the request of the physician. Hepatic encephalopathy and acute kidney injury were more frequent in the conventional therapy. Dry mouth and frequent urination were more common in the combination diuretic group. There were no significant differences between the groups in heart rate and blood pressure. Hypokalemia was seen in three patients in the conventional diuretic group, and potassium supplements were given when serum potassium dropped to < 3.0 mEq/L during the study.
| Combination diuretic: (n = 13) (%) | Conventional diuretic: (n = 16) (%) | |||
| Any grade | ≥ Grade 3 | Any grade | ≥ Grade 3 | |
| Encephalopathy | 1 (3.5) | 3 (10.7) | 1 (3.5) | |
| Abdominal infection | 1 (3.5) | |||
| Dry mouth | 6 (21.4) | 2 (7.1) | ||
| Urinary frequency | 4 (14.3) | 2 (7.1) | ||
| Hypopotassemia | 0 | 3 (10.7) | ||
| Acute kidney injury | 2 (7.1) | 4 (14.3) | ||
After the treatment period, we conducted an observation for 1 mo. After the administration of tolvaptan, according to efficacy and tolerability, dose of tolvaptan was changed. Fifteen patients were kept on 7.5 mg, 8 patients were decreased to 3.75 mg; however, for 5 patients the therapy was discontinued because of inefficacy. The average decrease in body weight from baseline was -4.01 ± 3.97 and -4.31 ± 4.07 kg (2 w, 4 w; respectively).
After the administration of furosemide, tolvaptan was administered or the furosemide dose was changed. Twelve patients were treated with tolvaptan. The furosemide doses were decreased for 9 patients. No changes were made in the doses for 5 patients, and only 2 patients received an increased dose of furosemide. Except for the patients who received tolvaptan, the average decrease in body weight from baseline was -3.18 ± 3.15 and -3.61 ± 3.37 kg (2 w, 4 w; respectively).
A combination diuretic therapy including aquaretic resulted in a greater decrease in body weight compared to conventional diuretic with natriuretic only for cirrhotic ascites patients. Moreover, compared to conventional diuretic therapy, combination diuretic therapy reduced the occurrence of diuretic-related severe side effects.
For cirrhotic ascites patients, the developed resistance to the standard medical therapy with sodium intake restrictions and natriuretic therapy was one of the most challenging problems. Negative sodium balance is commonly pursued for managing liver cirrhosis with ascites[3]. Although natriuretic drugs are generally useful, only a minority of ascites patients respond to this clinical treatment[17]. Diuretic-resistant ascites is possibly because of the reduction in the glomerular filtration rate and the loss of nephrons with natriuretic potential[18,19]. Another common reason is the so-called “ceiling effect.” Natriuretic drugs increase renal sodium excretion, and water excretion follows naturally[20-23], until a limit is reached. Ceiling doses of loop diuretics produce a maximal increase in fractional sodium excretion[24].
In the present study, the administration of furosemide in patients undergoing therapy with moderate doses of natriuretic was not change in urine excretion volume. Conventional diuretic therapy with a natriuretic drug only, which blocks sodium reabsorption, may be insufficient to restrict water reabsorption in cases of severe liver cirrhosis with increased circulating levels of AVP and RAAS components. In conditions associated with a lack of response to natriuretic drugs because of insufficient water excretion, concomitant use of aquaretic drugs may provide the ideal treatment. Consequently, combination therapy that involves the blocking of sodium and water reabsorption may be more effective in treating liver cirrhosis with ascites than conventional diuretic therapy, which only blocks sodium reabsorption[25].
The present study does not prove that an aquaretic drug alone is more effective than a natriuretic drug for patients with cirrhotic ascites. The basic therapy used for induction of a negative sodium balance in cases of liver cirrhosis with ascites consists of restricting salt intake and increasing renal sodium excretion[4]. Our previous report shows that administering tolvaptan is beneficial in cases with sufficient sodium excretion induced by a natriuretic drug, and that body weight decreased in proportion to levels of urine sodium excretion as a result of the combination therapy[16]. Therefore, we recommend measuring sodium excretion for a quick and easy determination of the diuretic regimen required.
It is noteworthy that this combination therapy was useful for diuretic-intractable ascites. Addition of tolvaptan had only a slight influence on ammonia levels, and that the incidence of hepatic encephalopathy was reduced in the tolvaptan group, compared to furosemide. Hepatic encephalopathy is related to impaired blood circulation, which decreases renal clearance of ammonia[26,27]. In contrast to furosemide, tolvaptan increases urine output without decreasing renal blood flow, leading to indistinguishable ammonia levels in the tolvaptan group between the baseline and the final dosing day.
This study has shown that addition of furosemide influenced 24-h CCr and plasma renin activity, with renal dysfunction more frequent in the furosemide group than that in the tolvaptan group. A diuretic agent preferably should not activate the sympathetic nervous system or the RAAS, because the pathophysiology of ascites formation in liver cirrhosis is associated with the activation of the RAAS, and occurs to help restore blood homeostasis[28-30]. Although concomitant medications such as branched-chain amino acids, nonabsorbable disaccharides, angiotensin II receptor blockers, or angiotensin converting enzyme inhibitors affect RAAS activation and ammonia levels, combination therapy including aquaretic and natriuretic drugs reduces the incidence of diuretic-related severe side effects. In contrast, increasing the dosage of furosemide above the ceiling dose increases the frequency of severe side effects.
Furthermore, hyponatremia and hypokalemia were not seen in the combination diuretic group. To avoid an electrolyte disturbance, combination diuretic therapy should be evaluated and may resolve hyponatremia with long-term treatment[7]. Hyponatremia, which is characterized by excessive renal retention of water increased release of AVP, is associated with increased mortality and numerous complications in cirrhotic patients. A combination therapy, including a vasopressin V2-receptor antagonist, which increases the serum sodium concentration, has the potential to improve outcomes in liver cirrhosis patients with ascites[7].
Our final objective was to establish the strategy of ascites refractory to diuretic therapy. However, the present study was not suitable for the management of patients with refractory ascites in two points. First, refractory ascites is defined as immobilization of free fluid from the peritoneum despite 160 mg/d of furosemide and 400 mg/d of spironolactone. Therefore, doses of naturetic drugs were not matched, which is different from the standard Japanese guidelines[15]. Second, many patients with refractory ascites develop a chronic renal dysfunction with the progression of cirrhosis. Patients enrolled in this study had a relatively good renal function and good sodium excretion. If they had presented with mild or severe renal dysfunction, which would have affected the response to diuretics, the results would likely have been markedly different. A previous study reported that changes in body weight after administration of tolvaptan were dependent on the level of creatinine or eGFR (estimated glomerular filtration rate)[25].
The present study has a few limitations. First, the study was not a multicenter study. Hence, a relatively small number of patients were enrolled, resulting in patient selection bias. Second, current understanding of the influence of tolvaptan on circulating blood volume and renal clearance of ammonia is insufficient, warranting further study. Finally, it is not clear whether or not the combination therapy improved the long-term prognosis of these patients. Further detailed research of prognosis under combination therapy is warranted.
In summary, to our knowledge, this study is the first study that examines whether the addition of an aquaretic drug is a more effective therapy than only increasing the dose of a natriuretic drug for liver cirrhosis patients with ascites.
Recently, several studies have evaluated the effects of aquaretic drugs such as tolvaptan for treating ascites resistant to conventional therapies.
Whether a combination therapy with natriuretic and aquaretic drugs is more effective than conventional therapy with natriuretic for liver cirrhosis patients with ascites remains unclear.
We assessed the effects of a combination therapy with natriuretic and aquaretic drugs in cirrhotic ascites patients.
A two-center, randomized, open-label, prospective study was conducted. Japanese patients who met the criteria were randomized to trial groups and they received an aquaretic drug (7.5 mg of tolvaptan) or a natriuretic drug (40 mg of furosemide) for 7 d in addition to the natriuretic drug which was used prior to enrolment in this study. The primary endpoint was the change in body weight from the baseline. Vital signs, fluid intake, and laboratory and urinary data were assessed to determine the pharmacological effects of tolvaptan and furosemide.
A total of 56 patients were randomized to receive either tolvaptan (n = 28) or furosemide (n = 28). In the tolvaptan and furosemide groups, the average decrease in body weight from the baseline was 3.21 ± 3.17 kg (P < 0.0001) and 1.75 ± 2.36 kg (P = 0.0006), respectively, when measured on the final dosing day. Following 1 wk of treatment, a significantly greater reduction in body weight was observed in the tolvaptan group compared to that in the furosemide group (P = 0.0412).
Compared to a conventional therapy with only natriuretic drugs, a combination therapy with natriuretic and aquaretic drugs is more effective for patients with cirrhotic ascites. Following 1 wk of treatment, a significantly greater reduction in body weight was observed in the combination therapy compared to that in the conventional therapy. Combination therapy that involves the blocking of sodium and water reabsorption may be more effective in treating liver cirrhosis with ascites than conventional therapy, which only blocks sodium reabsorption. Combination therapy including tolvaptan and conventional diuretics reduced the occurrence of diuretic-related severe side effects. A two-center, randomized, open-label, prospective study was conducted. The criteria were randomized to a trial group and the combination diuretic group (natriuretic and aquaretic drugs) or the conventional diuretic group (a natriuretic drug only). A combination diuretic therapy including an aquaretic drug resulted in a greater decrease in body weight compared to conventional diuretic with only a natriuretic drug for cirrhotic ascites patients. Moreover, compared to conventional diuretic therapy, combination diuretic therapy reduced the occurrence of diuretic-related severe side effects. Compared to a conventional diuretic therapy with only a natriuretic drug, a combination diuretic therapy with natriuretic and aquaretic drugs proved to be more effective for patients with cirrhotic ascites. Combination therapy may establish a strategy to treat ascites refractory to the standard diuretic therapy.
The study compared to a conventional diuretic therapy with only a natriuretic drug, a combination diuretic therapy with natriuretic and aquaretic drugs is more effective for patients with cirrhotic ascites. It is not clear whether or not the combination therapy improved the long-term prognosis of these patients. Therefore, future long-term observational studies are warranted. The future research should assess the influence of renal function after the administration of tolvaptan and furosemide in liver cirrhosis patients with ascites for at least 24 wk.
We thank the SATT Corporation for assistance with the statistical analyses and Robert E. Brandt, Founder, CEO, and CME, of MedEd Japan, for editing and formatting the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cao WK, Gencdal G, Naqvi IH, Wu B, Zhu YY S- Editor: Chen K L- Editor: A E- Editor: Lu YJ
| 1. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1563] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 2. | Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 481] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 3. | Angeli P, Wong F, Watson H, Ginès P; CAPPS Investigators. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Runyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 613] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 5. | Wong F. Management of ascites in cirrhosis. J Gastroenterol Hepatol. 2012;27:11-20. [PubMed] |
| 6. | Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, Garcia-Tsao G, Lee SS. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099-2112. [RCA] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 833] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 8. | Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [RCA] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 518] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 9. | Kashani A, Landaverde C, Medici V, Rossaro L. Fluid retention in cirrhosis: pathophysiology and management. QJM. 2008;101:71-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Okita K, Kawazoe S, Hasebe C, Kajimura K, Kaneko A, Okada M, Sakaida I; ASCITES Dose-Finding Trial Group. Dose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: A randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:83-91. |
| 11. | Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K; ASCITES-DOUBLEBLIND Study Group. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73-82. |
| 12. | Jaber BL, Almarzouqi L, Borgi L, Seabra VF, Balk EM, Madias NE. Short-term efficacy and safety of vasopressin receptor antagonists for treatment of hyponatremia. Am J Med. 2011;124:977.e1-977.e9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 14. | Oriuchi N, Nakajima T, Mochiki E, Takeyoshi I, Kanuma T, Endo K, Sakamoto J. A new, accurate and conventional five-point method for quantitative evaluation of ascites using plain computed tomography in cancer patients. Jpn J Clin Oncol. 2005;35:386-390. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31-41. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10672] [Cited by in RCA: 11000] [Article Influence: 224.5] [Reference Citation Analysis (1)] |
| 16. | Uojima H, Kinbara T, Hidaka H, Sung JH, Ichida M, Tokoro S, Masuda S, Takizawa S, Sasaki A, Koizumi K. Close correlation between urinary sodium excretion and response to tolvaptan in liver cirrhosis patients with ascites. Hepatol Res. 2017;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1359] [Cited by in RCA: 1221] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 18. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38:S69-S89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Hartleb M, Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol. 2012;18:3035-3049. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 20. | Moller S, Henriksen JH, Bendtsen F. Pathogenetic background for treatment of ascites and hepatorenal syndrome. Hepatol Int. 2008;2:416-428. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Misra VL, Vuppalanchi R, Jones D, Hamman M, Kwo PY, Kahi C, Chalasani N. The effects of midodrine on the natriuretic response to furosemide in cirrhotics with ascites. Aliment Pharmacol Ther. 2010;32:1044-1050. [DOI] [Full Text] |
| 22. | Kalambokis G, Tsianos EV. Refractory ascites: can it be defined only by the response to furosemide and spironolactone? Liver Int. 2010;30:1394-1396. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Wilcox CS. New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol. 2002;13:798-805. |
| 24. | Licata G, Tuttolomondo A, Licata A, Parrinello G, Di Raimondo D, Di Sciacca R, Cammà C, Craxì A, Paterna S, Pinto A. Clinical Trial: High-dose furosemide plus small-volume hypertonic saline solutions vs. repeated paracentesis as treatment of refractory ascites. Aliment Pharmacol Ther. 2009;30:227-235. [DOI] [Full Text] |
| 25. | Sakaida I, Terai S, Kurosaki M, Yasuda M, Okada M, Bando K, Fukuta Y. Effectiveness and safety of tolvaptan in liver cirrhosis patients with edema: Interim results of post-marketing surveillance of tolvaptan in liver cirrhosis (START study). Hepatol Res. 2017;47:1137-1146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Assy N, Kayal M, Mejirisky Y Gorenberg M, Hussein O, Schlesinger S. The changes in renal function after a single dose of intravenous furosemide in patients with compensated liver cirrhosis. BMC Gastroenterol. 2006;6:39. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Toniutto P, Pirisi M, Fabris C, Apollonio L, Sereti K, Bartoli EG. The significance of the furosemide test for predicting ascites control by diuretics in cirrhotics: a comparison with volume expansion and octreotide infusion. Dig Dis Sci. 2006;51:1992-1997. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Lenaerts A, Codden T, Meunier JC, Henry JP, Ligny G. Effects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous system. Hepatology. 2006;44:844-849. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Cho HS, Park GT, Kim YH, Shim SG, Kim JB, Lee OY, Choi HS, Hahm JS, Lee MH. [The significance of urine sodium measurement after furosemide administration in diuretics-unresponsive patients with liver cirrhosis]. Taehan Kan Hakhoe Chi. 2003;9:324-331. [PubMed] |
| 30. | Lenaerts A, Codden T, Henry JP, Van Cauter J, Meunier JC, Ligny G. [Biological factors influencing response to diuretics in patients with cirrhosis and ascites]. Gastroenterol Clin Biol. 2001;25:268-272. |