Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8035
Peer-review started: June 1, 2017
First decision: June 22, 2017
Revised: August 15, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: December 7, 2017
Processing time: 186 Days and 22.1 Hours
To introduce a two-step method for creating a gastric tube during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy and assess its clinical application.
One hundred and twenty-two patients with middle or lower esophageal cancer who underwent laparoscopic-thoracoscopic Ivor-Lewis esophagectomy at Liaoning Cancer Hospital and Institute from March 2014 to March 2016 were included in this study, and divided into two groups based on the procedure used for creating a gastric tube. One group used a two-step method for creating a gastric tube, and the other group used the conventional method. The two groups were compared regarding the operating time, surgical complications, and number of stapler cartridges used.
The mean operating time was significantly shorter in the two-step method group than in the conventional method group [238 (179-293) min vs 272 (189-347) min, P < 0.01]. No postoperative death occurred in either group. There was no significant difference in the rate of complications [14 (21.9%) vs 13 (22.4%), P = 0.55] or mean number of stapler cartridges used [5 (4-6) vs 5.2 (5-6), P = 0.007] between the two groups.
The two-step method for creating a gastric tube during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy has the advantages of simple operation, minimal damage to the tubular stomach, and reduced use of stapler cartridges.
Core tip: The two-step method accomplishes totally laparoscopic-thoracoscopic Ivor-Lewis esophagectomy, by avoiding an additional abdominal incision and conducting operations via the operating port to simplify the complicated operation steps, thus greatly reducing the difficulty in creating the gastric tube after anastomosis, and shortening the operating time.
- Citation: Liu Y, Li JJ, Zu P, Liu HX, Yu ZW, Ren Y. Two-step method for creating a gastric tube during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy. World J Gastroenterol 2017; 23(45): 8035-8043
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8035
Esophageal cancer (EC) is a common malignancy of the upper digestive tract in China. Statistics show that EC ranks fifth in incidence among all malignancies and is the fourth leading cause of cancer death in China[1]. Surgery is the first-choice treatment for EC. Even for patients with locally advanced EC, surgery remains a very important component of multi-modality therapy. Currently, many procedures for esophageal resection and reconstruction are available, and Ivor-Lewis esophagectomy combined with two-field lymphadenectomy has become the standard procedure for middle and lower EC[2-6]. Compared with open Ivor-Lewis esophagectomy, minimally invasive laparoscopic-thoracoscopic Ivor-Lewis esophagectomy has an obvious advantage of reducing the incidence of perioperative complications while having similar therapeutic outcomes[7-16].
After esophagectomy, the stomach is the most commonly used substitute for the esophagus. Because of its sufficient length, the gastric tube has the advantages of decreasing anastomotic tension, reducing the pressure on the lungs and heart by thoracic stomach expansion, reducing gastric retention, and not reducing the blood supply to the anastomosis site and surgical site. Therefore, it has been advocated by many surgeons in both China and other countries, and it has been applied clinically[17-22]. However, the creation of a gastric tube is time consuming, requires multiple gastric incisions, and increases patients’ economic burden as many medical devices are required. To overcome these shortcomings, we proposed a two-step method for creating a gastric tube and assessed its feasibility during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy.
One hundred and twenty-two patients with middle or lower EC treated at the Department of Thoracic Surgery, Cancer Hospital of China Medical University/Liaoning Cancer Hospital and Institute from March 2014 to March 2016 were included in this study. All patients underwent upper gastrointestinal radiography, fiber gastroscopic examination (including Lugol’s iodine staining), and pathological biopsy to establish a definitive diagnosis. The inclusion criteria were as follows: (1) patients who had accurate preoperative staging following endoscopic ultrasonography (some patients did not undergo it due to severe obstruction), contrast-enhanced computed tomography (CT) scans of the chest and upper abdomen, color Doppler ultrasound of the neck, contrast-enhanced magnetic resonance imaging (MRI) of the head, and bone emission CT scans to exclude metastases to the head, lungs, bone, liver, gallbladder, spleen, kidneys, and adrenal glands as well as physical examination to exclude enlargement of the neck, supraclavicular and retroperitoneal lymph nodes; (2) Patients who had undergone heart, lung, liver and renal function examinations to exclude potential surgical contraindications; (3) patients who had no prior history of malignancy or gastrointestinal surgery; (4) patients who consented to the study protocol and signed the informed consent form; and (5) patients who had complete follow-up data. The exclusion criteria were: (1) Patients who had incomplete clinical data; and (2) patients who had poor compliance and withdrew from the study.
After admission, patients were divided into two groups based on the procedure used for creating the gastric tube. In one group of 64 patients (50 males and 14 females) with a mean age of 62 years, a two-step method for creating a gastric tube was adopted (two-step method group); and in the other group of 58 patients (46 males and 12 females) with a mean age of 61 years, the conventional method for creating a gastric tube was used (conventional method group). Both groups underwent laparoscopic-thoracoscopic Ivor-Lewis esophagectomy. The clinical data about the patients of the two groups are shown in Table 1.
| Variable | Two-step method | Conventional method | t/χ2-value | P value |
| Age (yr) | 62.4 ± 7.6 | 61.4 ± 7.1 | 0.717 | 0.48 |
| Gender (male/female) | 50/14 | 46/12 | 0.873 | 0.53 |
| Tumor location (middle/lower) | 35/29 | 32/26 | 0.957 | 0.55 |
| TNM stage (I/II/III) | 9/41/14 | 10/32/16 | 1.003 | 0.60 |
The patients were placed in the supine position. A Veress needle was introduced below the umbilicus for CO2 insufflation to maintain the pressure at 12-13 mm Hg. A 10-mm trocar was inserted below the umbilicus to create a camera port. A trocar was inserted 2 cm below the right anterior axillary line to create a main operating port. Two tool ports were made in the right and left midclavicular lines 2 cm above the umbilicus, respectively. An additional tool port was created below the xiphoid process to expose the liver. The stomach was mobilized with an ultrasonic scalpel along the greater curvature of the stomach with the right gastroepiploic vascular arch protected and short gastric artery branch severed. After mobilizing the lesser omentum, 16-19 lymph nodes were dissected. The left gastric artery and gastric coronary vein were severed with an ultrasonic scalpel after clipping three times with Hem-o-lok clips. For the conventional method, gastric tube creation was performed outside the abdominal cavity, while for the two-step method, gastric tube creation was performed inside the abdominal cavity. At this time, the abdominal phase was completed.
In the thoracic phase, the patient was placed in the left lateral decubitus position with elevation of the upper right arms. An artificial pneumothorax was then established by CO2 insufflation (CO2 pressure = 8 mmHg). A 10-mm trocar was inserted in the 7th intercostal space in the mid-axillary line to create a camera port. Two additional 5-mm ports were made in the 6th intercostal space outside the inferior angle of the scapula and 8th intercostal space in the posterior axillary line. The 3-cm main operating port was placed at the 4th intercostal space in the anterior axillary line, respectively. After the mediastinal pleura was longitudinally cut open along the esophagus, an ultrasonic scalpel was used to mobilize the middle and lower segments of the esophagus by separating and removing all the fat tissue around the lower segment of the esophagus. An electric hook and ultrasonic scalpel were alternately used to separate the tissues. After the arch of the azygous vein was fully exposed and severed, the upper segment of the esophagus was mobilized to the top of the right pleura, which was followed by the dissection of stations 4R and 2R lymph nodes and bilateral para-recurrent laryngeal lymph nodes. The esophagus was then severed below the top of the right pleura, and end-to-side anastomosis was performed between the esophagus and the gastric tube. The detailed procedure of anastomosis is described below.
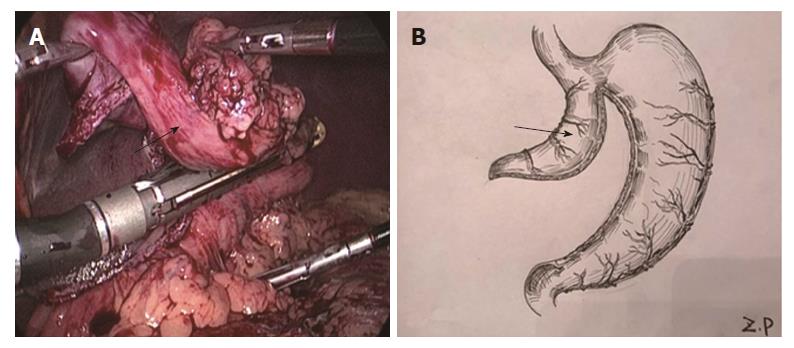
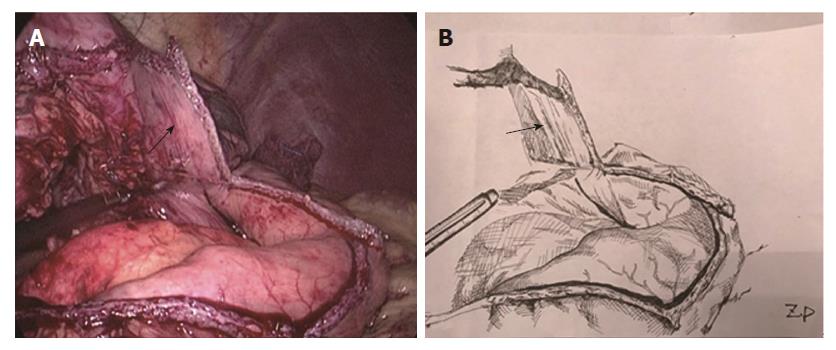
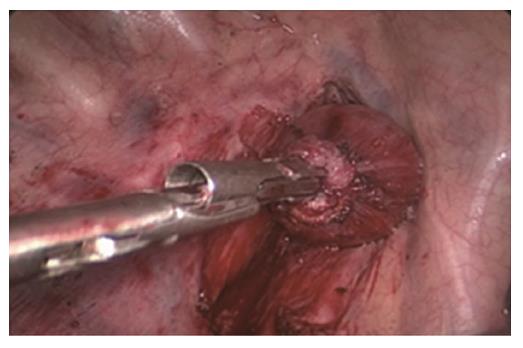
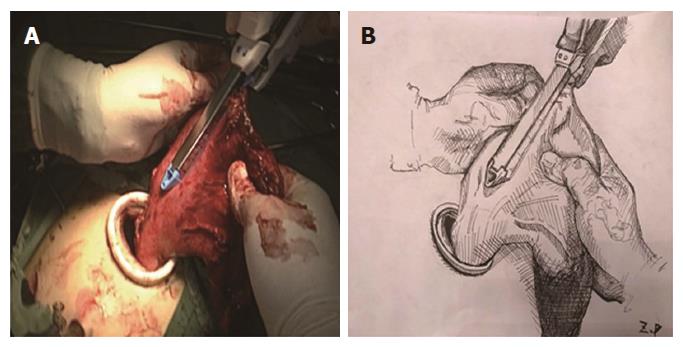
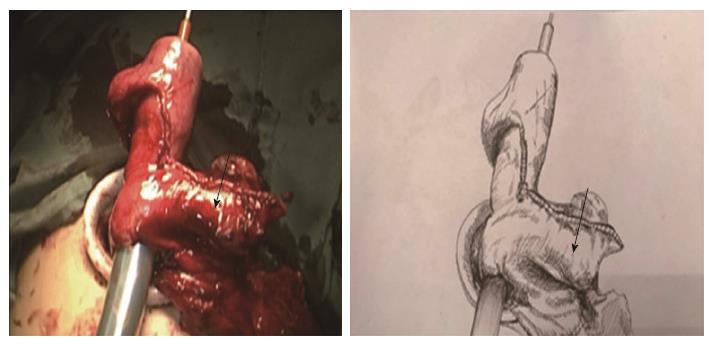
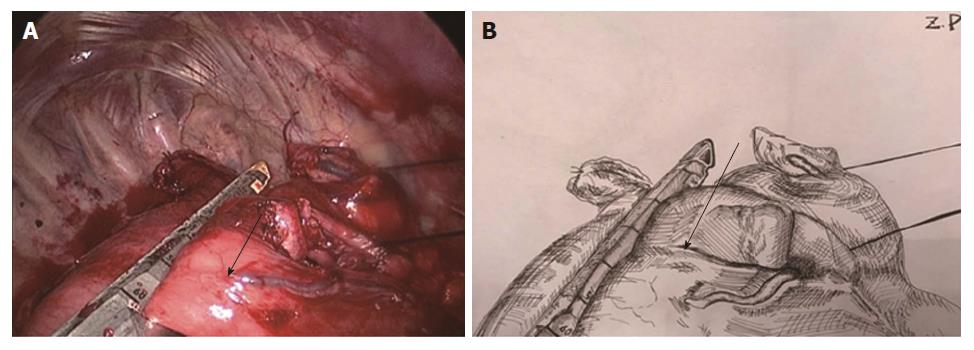
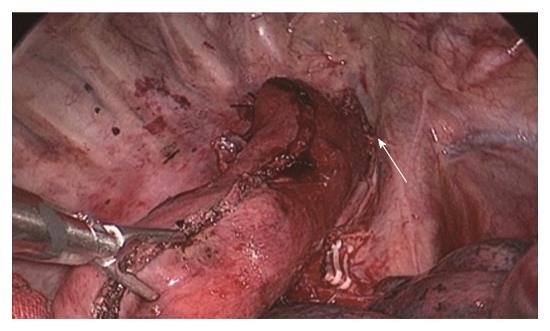
Two-step method: In the abdominal phase, after the stomach was mobilized and lymphadenectomy was performed, a laparoscopic cutting stapler was used to create a 3-4 cm wide gastric tube along the direction from the severed site of the right gastric artery branch on the upper lateral side of the lesser curvature of the stomach to the gastric cardia. The joint area between the gastric cardia and stomach preserved, creating a gastric pouch, as shown in Figures 1 and 2[23]. At this time, the first step was completed. In the thoracic phase, after the large portions of the esophagus and lymph nodes were dissected, the esophagus was resected and the resection edge away from the upper border of tumor was more than 5 cm, in the region from the superior border of the azygos vein to about 3 cm below the thoracic inlet. Subsequently, the esophageal stump was ligated and sterilized, and the proximal esophageal stump was applied with a purse-string suture and placed in the anvil of an anastomat. After the purse-string suture was tightened and tied to fix the anvil (Figure 3), the distal esophagus and gastric pouch were pulled to lift the gastric tube out of the thoracic cavity via the main operating port. A cutting stapler was used to resect a portion of the fundus of the stomach in a retrograde manner (Figure 4A and B), with a connection region, whose width permitted an anastomat pass, between the gastric pouch and gastric tube preserved. A 2-cm incision was then made in the anterior wall of the gastric pouch 3-4 cm away from the connection region to place an anastomat, which was subsequently allowed to pass the connection region and travel up through the highest point of the fundus of the gastric tube (Figure 5A and B). Afterwards, the distal esophagus and gastric pouch were returned to the thoracic cavity to perform the anastomosis. The anastomat was then removed, and 8-character-pattern suturing was performed to close the incision in the anterior wall of the gastric pouch. A cutting stapler was used to resect the connection region, and the resected esophageal and cardiac tissues and the gastric pouch were removed (Figures 6 and 7). Thus, the second step was completed.
Conventional method: In the abdominal phase, after stomach mobilization and lymph node dissection, a laparoscopic cutting stapler was used to transect the stomach and gastric cardia in a tumor-free cardia region. A 4-5 cm incision was made in the midline below the xiphoid process from which a suture was introduced to ligate the lower segment of the esophagus. The stomach was then lifted out of the abdominal cavity, and a gastric tube was created from the fundus along the greater curvature. The lesser curvature was transected at the first or second branch of the right gastric artery, which was followed by suturing with a stapler. The fundus was sutured with 3-0 silk suture as a fixation suture to tightly tie with the suture, ligating the lower segment of the esophagus. The created gastric tube was then returned to the abdominal cavity, and the abdominal incision was closed. In the thoracic phase, after mobilization of the esophagus and resection of lymph nodes, the esophageal resection and anvil fixation were the same as those described in the two-step method. After tissue retrieval, an incision was made in the anterior wall of the gastric tube to place an anastomat. The procedure of anastomosis was the same as that for the two-step method. Finally, a laparoscopic cutting stapler was used to resect the fundus tissue containing the incision in the gastric wall.
The outcome measures included operating time, intraoperative material consumption, intraoperative blood loss, and postoperative complications.
All statistical analyses were performed using SPSS 19.0 statistical software. Numerical data are expressed as the mean ± SD. Differences between two groups were compared using t tests. P < 0.05 was considered statistically significant.
Minimally invasive Ivor-Lewis esophagectomy was successfully performed in all patients in the two groups, and there was no conversion to open thoracotomy. The mean operating time was 238 min (range, 179-293 min) for the two-step method, and 272 min (range, 189-347 min) for the conventional method. There was a significant difference between the two groups (P < 0.01).
In the two-step method group, 14 (21.9%) cases had surgical complications, including anastomotic leakage (n = 1), para-recurrent laryngeal nerve injury (n = 3), pneumonia (n = 8, including one case with concurrent para-recurrent laryngeal nerve injury and intrapulmonary infection secondary to anastomotic leakage), and arrhythmia (n = 4). In the conventional method group, 13 (22.4%) cases had surgical complications, including anastomotic leakage (n = 1), para-recurrent laryngeal nerve injury (n = 2), pneumonia (n = 6, including one case with concurrent anastomotic leakage), and arrhythmia (n = 5). No perioperative death occurred. Table 2 shows the surgical complications that occurred in the two groups.
Regarding intraoperative material consumption, the mean number of stapler cartridges used in the procedure was 4.1 (range, 4-5) for the two-step method group and 4.7 (range, 4-6) for the conventional method group, and there was a significant difference between the two groups (P < 0.01) (Table 3).
| Parameter | Two-step method | Conventional method | P value |
| Operative time (min) | 238 (179-293) | 272 (189-347) | < 0.001 |
| Rate of complications (%) | 21.9 | 22.4 | 0.560 |
| Number of stapler cartridges used | 5.0 (4-6) | 5.2 (5-6) | 0.007 |
Ivor-Lewis esophagectomy can better expose the esophagus, which is beneficial to the dissection of abdominal and thoracic lymph nodes, especially bilateral para-recurrent laryngeal lymph nodes. A prospective randomized study comparing Ivor-Lewis and Sweet esophagectomy for middle and lower EC demonstrated that the former is superior to the latter with respect to the extent of lymph node dissection and incidence of postoperative complications[6]. Ivor-Lewis esophagectomy has currently become the standard surgical procedure for middle and lower EC. With constant improvement of the minimally invasive technique, laparoscopic-thoracoscopic Ivor-Lewis esophagectomy has exhibited more advantages over open or hybrid Ivor-Lewis esophagectomy in terms of the perioperative complications, postoperative pain, length of hospital stay, and survival benefit[24-29]. However, gastric tube creation and esophageal-gastric anastomosis during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy still have significant technical difficulties. The two-step method for creating gastric tubes that we present here combine gastric tube creation and esophageal-gastric anastomosis, simplifying the surgical procedure, reducing the operating time, and achieving totally laparoscopic-thoracoscopic Ivor-Lewis esophagectomy.
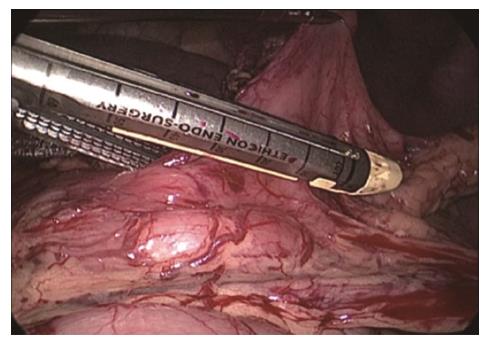
During conventional laparoscopic-thoracoscopic Ivor-Lewis esophagectomy, an additional abdominal incision is required, and the gastric tube is created outside the abdominal cavity. Since the surgical assistant needs to lift the area on the greater curvature side and the area to be anastomosed in the fundus of the stomach to flatten the gastric tissue, this inevitably damages the upper part of the gastric tube, destroying the microvascular blood supply to the region and increasing the risk of poor blood supply to the anastomotic site. During the operation using the two-step method, the gastric tube is created in the thoracic cavity. Due to space limitations, the stomach cannot be flattened, increasing the operative difficulty. The stapling angle and length of the “first firing” are particularly important. According to our experience, the stapling should be performed in the gastric angle with an upward angle of approximately 40 ° with the lessor curvature of the stomach, and the stapling length should be approximately 40 cm (Figure 8). After the first firing, a gastric pouch is formed. By pulling the gastric pouch to adjust the cutting angle and range, a distal gastric tube is created. Since this procedure does not pull the gastric tissue in the anastomotic area to destroy the microvascular network, it is theoretically associated with a lower risk of poor blood supply to the anastomotic site. The creation of a gastric tube in the abdominal cavity requires fewer operative steps and can thus reduce the operating time once surgeons are skilled in the procedure.
During conventional laparoscopic-thoracoscopic Ivor-Lewis esophagectomy, the stomach and esophagus are transected and then connected by suturing. When the slender gastric tube is lifted behind the thoracic cavity, it may be twisted[30]. In contrast, the stomach and esophagus are not transected after the creation of a gastric tube by the two-step method, which makes the tension produced during the lifting process distribute uniformly, avoiding the angulation and twisting of the gastric tube. Moreover, the direction for lifting the gastric tube to the thoracic cavity is easily fixed, reducing the operative difficulty.
For anastomosis in the thoracic phase, the conventional method requires an additional incision in the wall of the gastric tube. After placing the anastomat, the fundus of the stomach is pulled anteroinferiorly. An anastomotic region is then selected in the posterior wall of the stomach from which the link rod of the anastomat goes through and enters the stapler cartridge. The fundus tissue that contains the incision in the gastric wall is excised. This anastomotic procedure will inevitably result in mechanical damage to the upper portion of the gastric tube. In addition, to reduce the risk of poor anastomotic healing, the mechanically damaged gastric fundus region is also resected, increasing the length of the resected gastric tube and the anastomotic tension. When anastomosis is performed in the posterior wall of the stomach, anastomotic tension in the posterior wall is greater than that in the anterior wall, leading to non-uniform tension. In the two-step method, these operations are performed via the operating port, and the gastric pouch and proximal end of the gastric wall can be lifted out of the thoracic cavity, reducing the operative difficulty of the remaining steps in the thoracic cavity, such as clipping of the gastric tube and creation of an incision in the gastric pouch to place the anastomat. The head-end gastric tube is created in a retrograde manner in vitro, and only the connection region is resected and sutured in the thoracic cavity, avoiding significant difficulty in clipping the remaining gastric tube due to the space limitation of the pleural top after high-level anastomosis as well as the anastomotic tension caused by pulling the gastric pouch. Since the majority of operations via the operating port by the two-step method are operated outside the abdominal cavity by the conventional method, this greatly simplifies the operating steps, reducing the operating time and saving the energy consumption of the surgeon and surgical assistants.
Compared to the conventional method, because of the lack of damage to the fundus of the stomach, the two-step method can better protect the microvascular network within the gastric wall, providing a better arterial blood supply and venous return. Moreover, the two-step method can, to the greatest extent, reduce anastomotic tension and make it uniformly distributed. In addition, the two-step method can decrease the number of stapler cartridges used (about 1), reducing the economic burden for patients.
In conclusion, the two-step method realize stotal laparoscopic-thoracoscopic Ivor-Lewis esophagectomy by avoiding an additional abdominal incision and conducting operations via the operating port to simplify the complicated operation steps, which greatly reduces the difficulty in creating the gastric tube after anastomosis and shortens the operating time. This newer approach is easy for surgeons to learn and can reduce the economic burden for patients and the energy consumption of the surgeons and surgical assistants. The temporary reservation of the gastric pouch that will be excised later makes all damage confined in the region to be excised, ensuring no damage to the gastric tube in the whole surgical process and reducing the anastomotic tension and making it more uniform. However, because this method only involves embedding suture of the cutting border and poorly stapled areas and fails to conduct full-length embedding suture of the gastric tube, the safety of the surgical border for the gastric tube remains to be observed.
Esophageal cancer is a common malignancy of the upper digestive tract in China. Ivor-Lewis esophagectomy combined with two-field lymphadenectomy has become the standard procedure for middle and lower esophageal cancer. The stomach is the most commonly used substitute for the esophagus, with advantages in creating gastric tube. However, the process is time consuming, requires multiple gastric incisions, and increases patients’ economic burden as many medical devices are required. To overcome these shortcomings, this study proposed a two-step method for creating a gastric tube and assessed its feasibility during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy.
Currently, many procedures for esophageal resection and reconstruction are available, and Ivor-Lewis esophagectomy combined with two-field lymphadenectomy has become the standard procedure for middle and lower esophageal cancer. Compared with open Ivor-Lewis esophagectomy, minimally invasive laparoscopic-thoracoscopic Ivor-Lewis esophagectomy has an obvious advantage in reducing the incidence of perioperative complications while having similar therapeutic outcomes.
The two-step method realizes totally laparoscopic-thoracoscopic Ivor-Lewis esophagectomy by avoiding an additional abdominal incision and conducting operations via the operating port to simplify the complicated operation steps, thus greatly reducing the difficulty in creating the gastric tube after anastomosis and shortening the operating time. It is easy for surgeons to learn and can reduce the economic burden of patients and the energy consumption of the surgeon and surgical assistants.
The two-step method will benefit the patients for minimally invasive Ivor-Lewis esophagectomy.
In this study, the authors introduced a two-step method for creating a gastric tube during laparoscopic-thoracoscopic Ivor-Lewis esophagectomy and assess its clinical application. The results are interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chadokufa S, Toyonaga T S- Editor: Wei LJ L- Editor: Ma JY E- Editor: Huang Y
| 1. | He J, Shao K. The epidemiology, current status of management, challenge and future strategy for esophageal cancer in China. China Oncology. 2011;7 501-504. |
| 2. | Mitchell RL. Abdominal and right thoracotomy approach as standard procedure for esophagogastrectomy with low morbidity. J Thorac Cardiovasc Surg. 1987;93:205-211. [PubMed] |
| 3. | Ott K, Bader FG, Lordick F, Feith M, Bartels H, Siewert JR. Surgical factors influence the outcome after Ivor-Lewis esophagectomy with intrathoracic anastomosis for adenocarcinoma of the esophagogastric junction: a consecutive series of 240 patients at an experienced center. Ann Surg Oncol. 2009;16:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Xie MR, Liu CQ, Guo MF, Mei XY, Sun XH, Xu MQ. Short-term outcomes of minimally invasive Ivor-Lewis esophagectomy for esophageal cancer. Ann Thorac Surg. 2014;97:1721-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 5. | Zhang H, Wang J, Wang W, Zhou L, Chen J, Yang B, Xia Y, Jiang T. [A meta-analysis of esophagectomy: the comparative study of Ivor-Lewis operation and Sweet operation]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:892-897. [PubMed] |
| 6. | Li B, Xiang J, Zhang Y, Li H, Zhang J, Sun Y, Hu H, Miao L, Ma L, Luo X. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2015;150:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Boone J, Livestro DP, Elias SG, Borel Rinkes IH, van Hillegersberg R. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus. 2009;22:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Ben-David K, Sarosi GA, Cendan JC, Howard D, Rossidis G, Hochwald SN. Decreasing morbidity and mortality in 100 consecutive minimally invasive esophagectomies. Surg Endosc. 2012;26:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Burdall OC, Boddy AP, Fullick J, Blazeby J, Krysztopik R, Streets C, Hollowood A, Barham CP, Titcomb D. A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc. 2015;29:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. |
Nostrand DV.
Festina Lente and the âCrab and the butterflyâ. |
| 11. | Rodham P, Batty JA, McElnay PJ, Immanuel A. Does minimally invasive oesophagectomy provide a benefit in hospital length of stay when compared with open oesophagectomy? Interact Cardiovasc Thorac Surg. 2016;22:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Luketich JD, Alvelo-Rivera M, Buenaventura PO, Christie NA, McCaughan JS, Litle VR, Schauer PR, Close JM, Fernando HC. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 640] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 13. | Palanivelu C, Prakash A, Senthilkumar R, Senthilnathan P, Parthasarathi R, Rajan PS, Venkatachlam S. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg. 2006;203:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, Christie NA, Weksler B, Landreneau RJ, Abbas G. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 621] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 15. | Yamane S, Katada C, Tanabe S, Azuma M, Ishido K, Yano T, Wada T, Watanabe A, Kawanishi N, Furue Y. Clinical Outcomes in Patients with Cancer of Unknown Primary Site Treated By Gastrointestinal Oncologists. J Transl Int Med. 2017;5:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Guan QM, Du JJ, Meng L, Chen JH. [A prospective longitudinal study examining the impact on short term quality of life of hand video-assisted thoracoscopic surgical esophagectomy in patients with esophageal cancer]. Zhonghua Wai Ke Za Zhi. 2007;45:688-691. [PubMed] |
| 17. | Liebermann-Meffert DM, Meier R, Siewert JR. Vascular anatomy of the gastric tube used for esophageal reconstruction. Ann Thorac Surg. 1992;54:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 186] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Shen C, Yang H, Zhang B, Chen H, Chen Z, Chen J. Improved quality of life in patients with adenocarcinoma of esophagogastric junction after gastric tube reconstruction. Hepatogastroenterology. 2013;60:1985-1989. [PubMed] |
| 19. | Zhang M, Wu QC, Li Q, Jiang YJ, Zhang C, Chen D. Comparison of the health-related quality of life in patients with narrow gastric tube and whole stomach reconstruction after oncologic esophagectomy: a prospective randomized study. Scand J Surg. 2013;102:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today. 2016;46:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Yamamoto S, Makuuchi H, Shimada H, Chino O, Nishi T, Kise Y, Kenmochi T, Hara T. Clinical analysis of reflux esophagitis following esophagectomy with gastric tube reconstruction. J Gastroenterol. 2007;42:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Sakai M, Sohda M, Miyazaki T, Yoshida T, Kumakura Y, Honjo H, Hara K, Yokobori T, Kuwano H. Impact of the Level of Anastomosis on Reflux Esophagitis Following Esophagectomy with Gastric Tube Reconstruction. World J Surg. 2017;41:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Dong YN, Zhang L, Sun N, Liu DG, Li JJ, Tong Z, Liu YY. Novel T-shaped linear-stapled intrathoracic esophagogastric anastomosis for minimally invasive Ivor Lewis esophagectomy. Ann Thorac Surg. 2015;99:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1205] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 25. | Dantoc M, Cox MR, Eslick GD. Evidence to support the use of minimally invasive esophagectomy for esophageal cancer: a meta-analysis. Arch Surg. 2012;147:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 26. | Nagpal K, Ahmed K, Vats A, Yakoub D, James D, Ashrafian H, Darzi A, Moorthy K, Athanasiou T. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc. 2010;24:1621-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Ben-David K, Rossidis G, Zlotecki RA, Grobmyer SR, Cendan JC, Sarosi GA, Hochwald SN. Minimally invasive esophagectomy is safe and effective following neoadjuvant chemoradiation therapy. Ann Surg Oncol. 2011;18:3324-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Spengler CM, Verges S, Walder B. Minimally invasive versus open oesophagectomy for oesophageal cancer. Lancet. 2012;380:885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Dantoc MM, Cox MR, Eslick GD. Does minimally invasive esophagectomy (MIE) provide for comparable oncologic outcomes to open techniques? A systematic review. J Gastrointest Surg. 2012;16:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Levy RM, Trivedi D, Luketich JD. Minimally invasive esophagectomy. Surg Clin North Am. 2012;92:1265-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |