Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8008
Peer-review started: August 4, 2017
First decision: September 5, 2017
Revised: October 10, 2017
Accepted: October 17, 2017
Article in press: October 17, 2017
Published online: December 7, 2017
Processing time: 124 Days and 15.1 Hours
To evaluate the role of albumin at the time of ulcerative colitis (UC) diagnosis in predicting the clinical course of disease.
Nationwide cohort of patients with newly diagnosed UC in the Veterans Affairs health care system was identified and divided into two categories: hypoalbuminemia (i.e., ≤ 3.5 gm/dL) or normal albumin levels (i.e., > 3.5 gm/dL) at the time of UC diagnosis. The exposure of interest was presence of hypoalbuminemia defined as albumin level ≤ 3.5 g/dL at the time of UC diagnosis. Patients were then followed over time to identify the use of ≥ 2 courses of corticosteroids (CS), thiopurines, anti-TNF medications and requirement of colectomy for UC management.
The eligible study cohort included 802 patients, but 92 (11.4%) patients did not have their albumin levels checked at the time of UC diagnosis, and they were excluded. A total of 710 patients, who had albumin levels checked at time of UC diagnosis, were included in our study. Amongst them, 536 patients had a normal albumin level and 174 patients had hypoalbuminemia. Patients with hypoalbuminemia at diagnosis had a higher likelihood of ≥ 2 courses of CS use (adjusted HR = 1.7, 95%CI: 1.3-2.3), higher likelihood of thiopurine or anti- TNF use (adjusted HR = 1.72, 95%CI: 1.23-2.40) than patients with normal albumin level at diagnosis. There was a trend of higher likelihood of colectomy in hypoalbuminemic patients, but it was not statistically significant (Adjusted HR = 1.7, 95%CI: 0.90-3.25).
Hypoalbuminemia at disease diagnosis can serve as a prognostic marker to predict the clinical course of UC at the time of diagnosis.
Core tip: The current literature states that pancolitis, anemia and steroid use at ulcerative colitis (UC) diagnosis are considered poor prognostic features in UC. Ours is a first community based nationwide multi-center study on this subject evaluating serum albumin at the time of UC diagnosis as a prognostic marker. We identified a new easily measurable prognostic marker for the clinical course of UC, which in conjunction with other prognostic markers would help us identify a subset of UC patients who will eventually develop severe disease. This subset of patients may benefit from closer follow up and early escalation of therapy.
- Citation: Khan N, Patel D, Shah Y, Trivedi C, Yang YX. Albumin as a prognostic marker for ulcerative colitis. World J Gastroenterol 2017; 23(45): 8008-8016
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8008.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8008
Ulcerative colitis (UC) is a chronic inflammatory condition of unknown etiology characterized by relapsing and remitting episodes of inflammation limited to the mucosal layer of the colon. The age and gender-adjusted prevalence is 214 per 100000 persons in the United States[1]. The clinical presentation and course of UC is variable and in severe cases it can lead to colectomy[2,3]. Unlike Crohn’s disease (CD), in which several clinical and epidemiological factors at diagnosis are associated with a poor prognosis, there are limited prognostic indicators available at the time of UC diagnosis to predict the course of disease[4,5].
It would be beneficial for physicians to have predictive markers available at the time of UC diagnosis that can help them identify a subset of patients likely to develop a more severe disease course in the future. These patients may be a potential target for early effective management strategies that can induce steroid-free remission and reduce the need of colectomy. Albumin is an example of a negative acute phase reactant and decreased levels may be found during inflammatory conditions. A chronic inflammatory condition like UC can affect albumin concentration in the body in several ways. Chronic inflammation is associated with a greater fractional catabolic rate (FCR) of albumin and it also increases the transfer of albumin out of the vascular compartment[6]. Also, other conditions associated with UC such as malnutrition and malabsorption can cause low albumin level[7].
We hypothesize lower albumin level is associated with higher inflammatory activities in UC and thus, may be associated with poor clinical outcomes related to higher inflammatory activity, such as higher chances of ≥ 2 courses of CS, ≥ 1 courses of thiopurines/anti-TNF and colectomy. Previous studies have also shown that lower serum albumin level “at the time of UC exacerbation” predicted treatment failure and colectomy[8-11]. However, none of the studies to our knowledge have reported the association of albumin level in newly diagnosed UC patients and disease severity over time. Also, most of the previous studies that have looked at prognostic markers at time of UC diagnosis were done prior to the widespread use of immunomodulators and biologics and primarily looked at colectomy as an outcome, while we looked at escalation of therapy and colectomy both.
Our aim in this study was to evaluate albumin level at the time of UC diagnosis as a predictive marker for long-term outcomes in a population-based nationwide cohort of incident UC cases. To achieve this goal, we evaluated patients from the Department of Veterans Affairs (VA) which is the largest integrated health care system in the United States.
We conducted a retrospective cohort study in a previously validated nationwide inception cohort of UC patients[12,13] from the VA Database. The VA is the largest integrated health care system in the United States serving approximately 8.3 million Veterans per year[14]. National VA-wide data were obtained from the VA Pharmacy Benefits Management and Corporate Data Warehouse databases. This data source allows automated data extractions to collect information regarding patients’ demographics, pharmacy records, medical diagnoses, laboratory findings and procedures. Individual patient records were then evaluated by two reviewers to extract the data. Our validated VA Database represents 48 states plus Puerto Rico and 118 sites across the country.
We used a previously validated cohort of newly diagnosed UC patients to conduct this study[12,13]. Veterans who were seen and followed in the VA health care system from October 1, 2001, to October 1, 2011, were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes for UC (556.xx). We have used a definition that has been validated in previous studies by Khan et al[12,13] to identify incident cases of UC in the VA system to form an inception cohort.
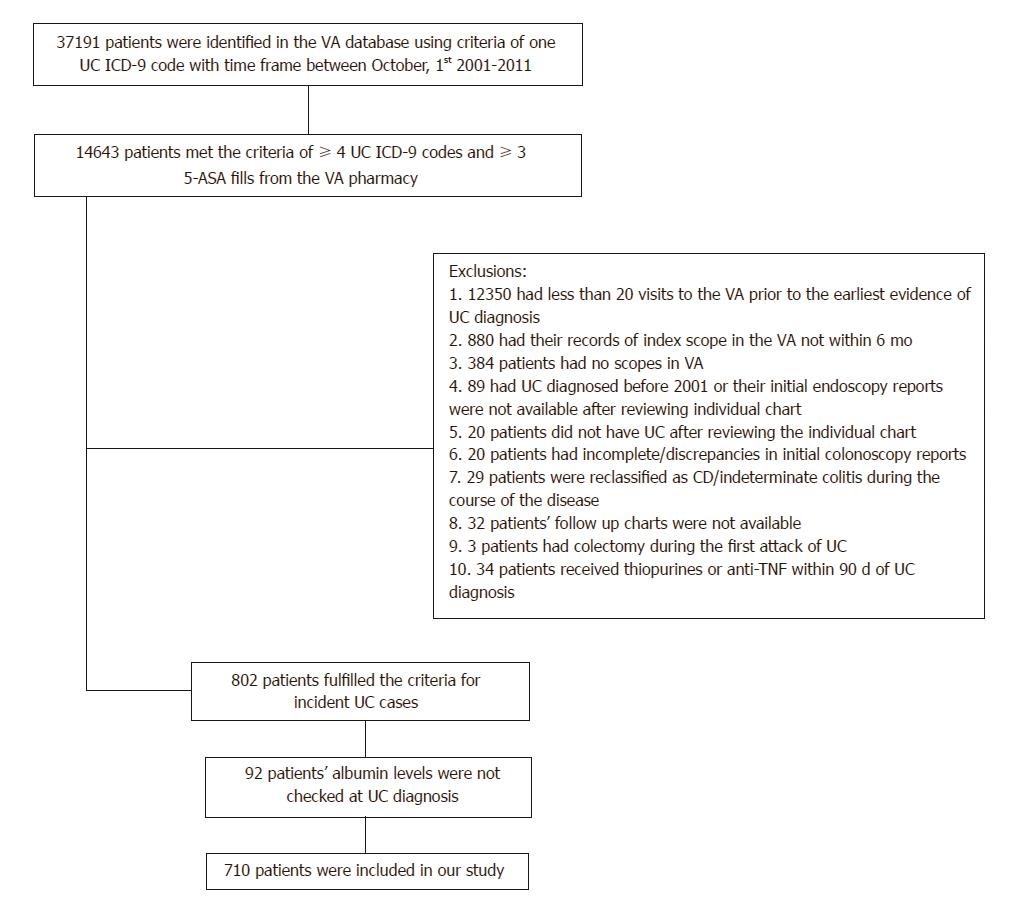
(1) patients have ≥ 4 UC ICD-9 codes in the VA database and ≥ 3 5-ASA prescriptions; (2) patients have at least 20 encounters within the VA prior to the date of the first evidence of UC; (3) UC must be diagnosed after October 2001; (4) patients have their index scope in the VA system within 6 months of the first evidence UC; (5) did not have colectomy during the first attack of UC; (6) did not develop features of CD or inflammatory bowel disease unclassified (IBDU) over the course of the follow up; and (7) did not receive thiopurines or anti-TNF agents within 90 days of initial UC diagnosis. The records of the patients identified in this cohort were then individually reviewed by two investigators to collect the relevant data. (Figure 1)
The purpose behind requirement of > 20 encounters within VA system was to allow enough exposure to the health care provider after which any history of UC should have been recorded; furthermore, this helped ensure good adherence of the patients in seeking health care through the VA system and, subsequently, increased the validity of our outcome detection. The requirement of index colonoscopy within 6 mo of UC diagnosis helped to ensure that the reported UC diagnosis was based on endoscopic findings.
The exposure of interest was presence of hypoalbuminemia defined as albumin level ≤ 3.5 g/dL at the time of UC diagnosis. All study patients were dichotomously categorized as having hypoalbuminemia (i.e., ≤ 3.5 g/dL) or normal albumin levels (i.e., > 3.5 g/dL) based on their laboratory tests results for albumin level at the time of UC diagnosis. For all patients, the follow-up began on the day of UC diagnosis and ended at the first of outcome occurrence, colectomy for UC-related reason or for colorectal cancer, death, date of loss to follow-up (i.e., last documented contact in VA electronic medical records), or the last date of follow-up (i.e., October 2015).
The outcomes were (1) the use of 2 or more courses of oral corticosteroids, separated by a three month time interval between the courses, during the follow-up subsequent to the UC diagnosis; (2) use of thiopurines or anti-TNFs during the follow up course; and (3) colectomy for UC-related reasons during follow-up. Information regarding relevant medication prescriptions, occurrence of colectomy and the indication for colectomy was collected based on manual chart review. Medication data elements that were collected included: dispense dates and stop dates, refill dates. Medications dispensed for reasons other than UC and non-UC related colectomies were not included in the outcome.
Covariate data collected by manual chart review included patients’ demographic at the time of UC diagnosis, age, gender, race (i.e., Caucasian, African American, Hispanics, others, unknown), and extent of UC at index endoscopy based on Montreal classification (E0 = no involvement, E1 = proctitis, E2 = left sided disease till splenic flexure, E3 = right sided disease or pancolitis)[15].
We report crude incidence rates (IRs) for colectomy for both exposure groups. The Kaplan-Meier analysis was conducted to generate the survival curves for each group. Multivariable Cox proportional hazards models including all covariates were used to estimate the adjusted hazard ratios (HR) along with 95%CIs for the risk of the respective outcome associated with baseline hypoalbuminemia. The PH-assumption was tested using Schoenfeld’s residuals for the treatment variable[16] and graphically. All analyses were conducted in STATA, version 14 (STATA Corp., College Station, TX, United States).
The statistical methods of this study were reviewed by Dr. Yu-Xiao Yang who is affiliated with The University of Pennsylvania, Perelman School of Medicine as well as Corporal Michael J. Crescenz VA Medical Center in Philadelphia as an associate professor for medicine and epidemiology and also a senior scholar in the department of epidemiology and biostatistics.
The eligible study cohort included 802 patients. Among them, 209 patients had received ≥ 2 courses of corticosteroid therapy and 178 patients had received at least one course of thiopurine or anti-TNF therapy during follow up duration. In addition, 48 underwent colectomy for UC-related reasons during the follow-up. Baseline albumin level was not checked in 92 (11.4%) patients, leaving 710 patients available for analysis (Table 1). Of 710 patients, 174 patients (24.5%) had hypoalbuminemia (albumin level ≤ 3.5 gm/dL) and 536 patients (75.5%) had normal albumin level (> 3.5 gm/dL) at new diagnosis of UC.
| Normal albumin at UC diagnosis (n = 536) | Hypoalbuminemia at UC diagnosis ( n = 174) | P value | |
| Age at anemia diagnosis (mean, SD, yr) | 55 (13) | 59 (12) | 0.001 |
| Sex | 0.65 | ||
| Male | 500 (93.3) | 164 (94.3) | |
| Female | 36 (6.7) | 10 (5.7) | |
| Race | 0.433 | ||
| Caucasian | 397 (74.1) | 130 (74.7) | |
| AA | 82 (15.3) | 24 (13.8) | |
| Hispanic | 7 (1.3) | 2 (1.2) | |
| Others | 8 (1.5) | 0 (0) | |
| Unknown | 42 (7.8) | 18 (10.3) | |
| Disease extent at baseline | < 0.001 | ||
| E1 | 118 (22.0) | 14 (8.1) | |
| E2 | 263 (49.1) | 84 (48.3) | |
| E3 | 155 (28.9) | 76 (43.7) |
≥ 1 course of thiopurine or anti-TNF therapy
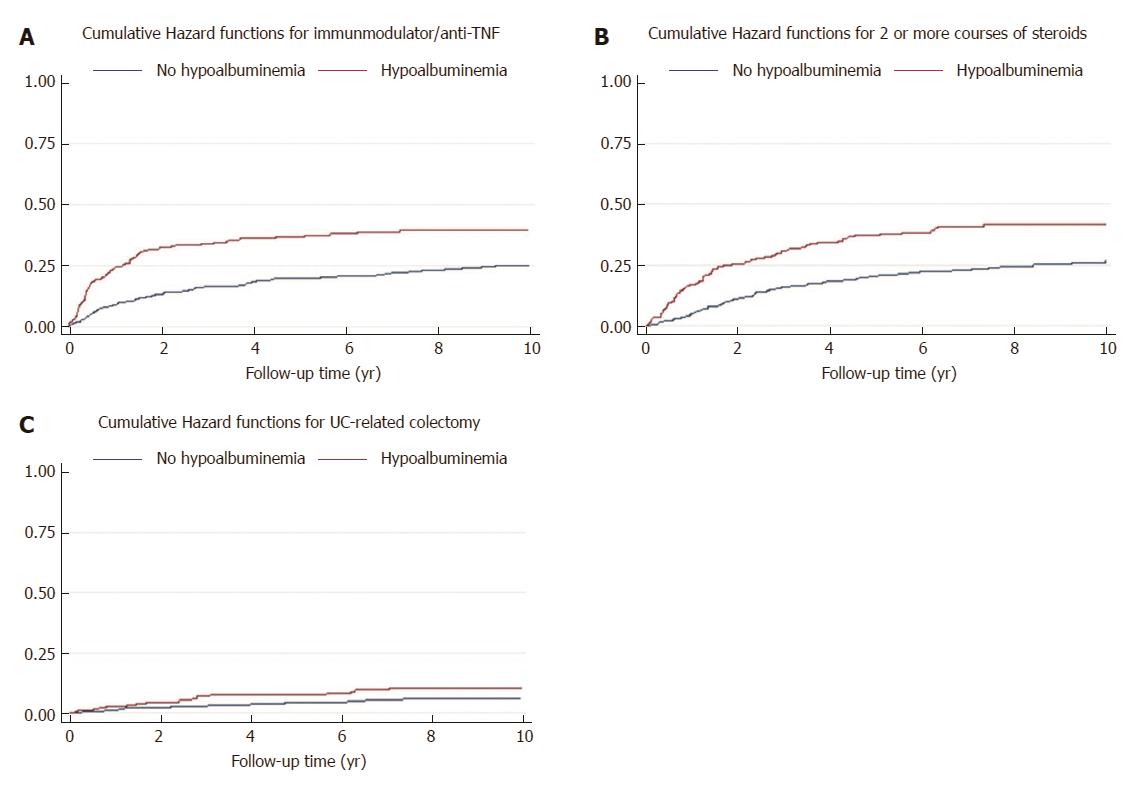
The incidence rate of receiving ≥ 1 course of thiopurine or anti-TNF therapy was 3 per 100 person-year and 6 per 100 person-year among those without and with hypoalbuminemia at UC diagnosis, respectively. In the multiple variable Cox regression analysis, presence of hypoalbuminemia at UC diagnosis was associated with a significantly higher risk of receiving ≥ 1 course of thiopurine or anti-TNF therapy during follow-up (adjusted HR = 1.72, 95%CI: 1.23-2.40) (Figure 2A).
≥ 2 courses of corticosteroid therapy
The incidence rate of receiving ≥ 2 courses of corticosteroid therapy was 3 per 100 person-year and 7 per 100 person-year among those without and with hypoalbuminemia at UC diagnosis, respectively. In the multiple variable Cox regression analysis, presence of hypoalbuminemia at UC diagnosis was associated with a significantly higher risk of receiving ≥ 2 courses of corticosteroid therapy during follow-up (adjusted HR = 1.7, 95%CI: 1.3-2.3) (Figure 2B).
The median time between the use of first and second course of CS in patients with hypoalbuminemia at UC diagnosis was 11 mo compared to 14 mo in patients with normal albumin level at UC diagnosis (P = 0.06).
The incidence rate of UC-related colectomy was 0.7 per 100 person-year and 1.2 per 100 person-year among those without and with hypoalbuminemia at UC diagnosis, respectively. In the multiple variable Cox regression analysis, presence of hypoalbuminemia at UC diagnosis was not statistically associated with a higher risk of UC-related colectomy during follow-up, but showed a higher trend of colectomy in the future (Adjusted HR = 1.7, 95%CI: 0.90-3.25) (Figure 2C).
Of 174 patients who had low albumin level (≤ 3.5 gm/dL) at UC diagnosis, 72 patients (41.4%) used oral formulation of 5-ASA only, 4 patients (2.3%) used rectal formulation of 5-ASA only, and 98 patients (56.3%) used both oral and rectal formulation of 5-ASA during the follow up course.
Utilizing a large nationwide population based cohort of newly diagnosed UC patients, we found that low albumin level (≤ 3.5 gm/dL) in newly diagnosed UC patients is a poor prognostic indicator. It is associated with the higher likelihood of requiring ≥ 2 courses of CS and ≥ 1 course of thiopurines or anti-TNF medications over the course of disease. Also, our result showed a trend of higher chances of colectomy in the future in patients with lower albumin level at disease diagnosis compared to patients with normal albumin level.
Different clinical and epidemiological factors in newly diagnosed UC patients have been studied as prognostic indicators in UC at diagnosis (younger age[17,18], extensive colitis at diagnosis[17], non-smoker[19,20], higher ESR[17], and early use of CS[12]). Our goal was not to replace these established factors but to find an additional factor which is routinely available, easily measurable and a part of initial laboratory work up in newly diagnosed UC patients. Our study is the first study, to our knowledge, to evaluate the prognostic role of albumin level in newly diagnosed UC patients in predicting disease outcome. Additionally, in contrast to most previous studies we have not just looked at colectomy as an endpoint but have also evaluated the use of immunomodulators and biologics which are being increasingly used. In conjunction with other above mentioned factors albumin levels at the time of UC diagnosis could be used to develop a comprehensive prediction model to prognosticate the disease course.
Goals of UC management are induction and maintenance of steroid free remission as well as preventing the development of colorectal cancer or colectomy in the future[21]. Multiple population-based studies focusing on natural history of UC showed that rates of CS use range from 34% to 63% over a variable period of follow up ranging from 1 year to 7 years[12,22-26]. Among patients who require one course of CS therapy, almost half never required further CS therapy and remained in prolonged remission[22,23]. Thus, almost ¾th of UC patients have less aggressive phenotype of disease. It is the remaining group of patients which requires ≥ 2 courses of CS therapy that have a poor prognosis. It is for this reason that we used ≥ 2 courses of CS as one of our outcomes to predict poor prognosis. These patients would frequently require thiopurines as well as biologics and may even require colectomy to control their disease[12,22-24]. It is thus appropriate for physicians to recognize a subset of patients at diagnosis who will have a high propensity for aggressive disease in the near future.
Albumin has never been evaluated as a prognostic marker for long term disease outcomes, but has been evaluated as a marker for response to therapy. Hypoalbuminemia in acute severe UC predicts treatment failure and colectomy[8-11] within the same hospitalization. Furthermore, low serum albumin level during disease course is associated with thiopurine failure[27] and non-responding to anti-TNF therapy in UC patients[28]. Our study is the first study to evaluate the role of low albumin to severe long term disease outcome. Our study showed that hypoalbuminemia defined as ≤ 3.5 gm/dL, at the time of disease diagnosis is a poor prognostic indicator. Multivariate analysis showed patients with hypoalbuminemia at diagnosis are associated with almost two-fold increase in ≥ 2 courses of CS use and almost two-fold increase in thiopurine or anti- TNF use than patients with normal albumin level at diagnosis. Furthermore, low albumin level at diagnosis shows a trend of higher chances of colectomy in the future disease course.
Identifying prognostic factors at the time of diagnosis can assist in disease management. Patients with low albumin levels at UC diagnosis could potentially benefit from higher dose of 5-ASA at disease onset. However, no trials have specifically looked at 5-ASA dosing based upon risk- stratification at the time of UC diagnosis to induce remission and prevent relapses in the future. This is an area of further research. However, in the absence of such data, a meta-analysis from Ford et al[29] on the use of 5-ASA in UC patients showed that higher dose of 5-ASA in quiescent UC appears to reduce the risk of disease relapse than lower dose of 5-ASA. [Relative risk (RR) of relapse = 0.79, 95%CI: 0.64-0.97, P = 0.02].
Additionally, it has been shown that combination therapy with oral and rectal 5-ASA products is more effective than oral alone. A study by d’albasio et al[30] on efficacy of a combination of oral and rectal 5-ASA for the maintenance treatment of ulcerative colitis showed that patients in combined group had lower relapse rate than patients in oral treatment group alone. (39% vs 69%, P = 0.036). Other studies[31,32] showed that combined therapy with oral and rectal formulation of 5-ASA is superior to oral formulation alone for induction of remission and improving endoscopic and histologic severity in extensive mild-to-moderate active UC. In our study, only 56% patients with low albumin level were treated with a combination of oral and rectal 5-ASA therapy. We suggest that the finding of low albumin level should prompt all physicians to prescribe combination therapy with an aim to preventing recurrent steroid use and immunosuppressive agents.
Adherence with medications has been identified as the key factor in maintaining disease remission in UC. A study by Kane et al[33] showed that non-adherence to a prescribed regimen of 5-ASA dramatically increased the risk of symptomatic relapse (61% vs 11%, P = 0.001). 30%-45% of UC patients showed non-adherence to oral medication. A study by Hawthorne et al[34] showed that a combination of educational and behavioral strategies tailored to individual patient optimized 5-ASA adherence in UC patients. We suggest that patients with a low albumin level at disease diagnosis will help guide patient-physician discussion of the disease course, which in turn will lead to improve medication compliance and decrease flare rates in the future. Also, patients with a low albumin level at disease diagnosis may benefit from more frequent monitoring through clinical follow up and evaluation of different biomarkers.
There are certain limitations in our study. In utilizing the VA data base, we had to conduct a retrospective study which has its own inherent limitations. Certain demographic characteristics such as smoking status which may impact disease course are not uniformly recorded. The VA population is predominately composed of males, which could potentially limit the generalizability of the findings to females. However, none of the previous studies has shown that gender plays a role in the prognosis of UC. Because we used the VA pharmacy database to estimate corticosteroid/thiopurine/biologics exposure rates, prescriptions filled outside the VA were not captured by our analysis. We anticipate the magnitude of such misclassification to be negligible as previous reports have shown that veterans have very good adherence in using the VA pharmacy[35-37]. Furthermore, all veterans included in this study had to have more than 20 visits prior to UC to ensure and maximizing the chance that they receive all their medical care within the VA system. Our cohort is comprised of only 802 patients with newly diagnosed UC in nationwide VA database (2.2% of total populations and 5.7% of the population meeting first inclusion criteria). There are various reasons behind this. Approximately half the veterans in VA systems are from Vietnam and Korean War. Thus, they most likely developed their UC prior to 2001. Also, veterans from the first Gulf war who form a large percentage of the remaining block also probably developed UC prior to 2001. A large proportion of veterans are diagnosed with UC while they are still in service and once they retire and join the VA, they already have a UC diagnosis. There is also a group of patients who do not get their clinical care at the VA but only use it to obtain medicines. We required all our patients to have an endoscopy in the VA. We also required all our patients to have > 20 visits in VA prior to UC diagnoses plus ≥ 4 UC ICD-9 codes and ≥ 3 5-ASA prescription, in order to increase the validity of our cohort and to ensure good adherence of the patients in seeking health care through the VA system.
The strength of this study was based on population-representative nation-wide VA data rather than data from tertiary centers, thus minimizing the likelihood of referral bias. We had patients from 118 sites spanning 48 states including Puerto Rico. We had complete records on all the patients including endoscopic and pathological records. To our knowledge this is the largest cohort of UC patients and the first multicenter nationwide study conducted in the US. Patients were followed for the median observation period of 8 years to identify CS use, thiopurine use, anti-TNF use and colectomy after their UC diagnosis.
In conclusion, we observed that low albumin at disease diagnosis was associated with a more severe subsequent disease course of UC. Assessing albumin levels at UC diagnosis could help physicians identify patients who may require escalation of therapy and closer follow up.
Ulcerative colitis (UC) has a variable clinical course ranging from disease remission to a severe exacerbation leading to colectomy. It is very difficult to predict the disease course at the time of UC diagnosis. There are several clinical and epidemiological factors available for Crohn’s disease to predict the disease course, but there are limited prognostic markers available at the time of UC diagnosis. We believe that identifying these predictive markers will help physicians identify a subset of patients likely to develop a more severe disease course in the future and subsequently manage them more effectively at disease onset. Inflammatory activity of UC can lower albumin level by various mechanisms, such as malnutrition, malabsorption, greater fractional catabolic rate of albumin and increase transfer of albumin out of the vascular system. In view of this, we hypothesized that hypoalbuminemia at the time of UC diagnosis can be predictive of a more severe disease course of UC. Previous studies have shown that lower serum albumin level “at the time of UC exacerbation” predicted treatment failure and colectomy but none of these studies have looked at the level of albumin at the time of UC diagnosis and its relationship with the clinical course of the disease.
It is very hard to predict the disease course at the time of UC diagnosis. There are limited numbers of prognostic factors available to determine the course of UC at the time of diagnosis. None of the studies have considered the role of albumin in predicting UC course which is easy to measure on routine blood work by general practitioner as well as gastroenterologist. We thought that by identifying this new prognostic factor that can predict the course of UC at the time of UC diagnosis, it would be helpful for the physicians to identify a subset of patients at high risk of developing severe course and manage them more effectively early on in the disease course. In future studies, the impact of more aggressive therapy at the time of UC diagnosis among patients with poor prognostic factors could be evaluated.
Our main objective was to identify a new prognostic marker which is cheap, reliable and readily available at the time of UC diagnosis that predicts the long-term clinical course of the disease. We hypothesized that hypoalbuminemia at the time of UC diagnosis is associated with a more severe disease course of UC. We were able to show that that low albumin at disease diagnosis was associated with a more severe subsequent disease course of UC. This in conjunction with other prognostic markers would help us identify a subset of UC patients who will eventually develop severe disease. This subset may benefit from closer follow up and early escalation therapy. The impact of early escalation of therapy can be evaluated in future studies.
We conducted a nationwide retrospective cohort study of newly diagnosed UC patients followed in the VA healthcare system. These patients were divided into 2 groups based on their albumin levels at the time of UC diagnosis, i.e., hypoalbuminemic (≤ 3.5 gm/dL) or normal albumin levels (> 3.5 gm/dL). Our outcomes of interest were 1) the use of 2 or more courses of oral corticosteroid, separated by a three month time interval between the courses; (2) use of thiopurines or anti-TNFs; and (3) colectomy for UC-related reasons during the clinical course of their UC. We also included other covariate data like patient demographics as well as the extent of UC at index colonoscopy based on the Montreal classification. We then subjected this data including all the covariates to statistical analysis and evaluation using the multivariate cox proportional hazard models to estimate the adjusted Hazard ratio (HR) along with 95%CI. The Kaplan Meier analysis was also conducted. The pH assumption was tested using Schoenfeld’s residuals. All this analysis was done using the STATA software, version 14 (STATA Corp., College Station, TX, United States).
Our cohort included 710 newly diagnosed UC patients who had their albumin levels checked at the time of diagnosis and amongst them, 174 patients, i.e., 24.5% had hypoalbuminemia. It was observed that there was a higher predilection to ≥ 2 courses of CS use as well as thiopurine or anti-TNF use during the clinical course of disease among hypoalbuminemic patients. The chances of requiring subsequent colectomy were also higher in this group, although it was not statistically significant. Thus, we identified a new factor that is readily available and measurable at the time of UC diagnosis which can prognosticate the disease course. The problem that needs to be resolved is the impact of more aggressive therapy at the time of UC diagnosis among patients with poor prognostic factors on the clinical course of the disease.
Low albumin at disease diagnosis was associated with a more severe subsequent disease course of UC. People with poor prognostic factors may benefit from aggressive therapy at onset of disease. We outlined the clinical and epidemiological factors in newly diagnosed UC patients which have been studied as prognostic indicators in UC at the time of diagnosis. This study identified a new prognostic factor which is readily available to predict the disease course of UC at the time of diagnosis. We hypothesized and confirmed that albumin level at the time of disease diagnosis can predict disease course of UC. Assessing albumin levels at UC diagnosis could help physicians identify patients who may require escalation of therapy and closer follow up.
By using a large nationwide cohort of patients, we were able to identify a new prognostic factor to determine the clinical course of UC. This was made possible by having complete medical records and long duration of follow up. Using other large nationwide cohorts future studies may be able to identify other predictive factors. The future research should address the impact of more aggressive therapy at the time of UC diagnosis among patients with poor prognostic factors on the clinical course of the disease. The methodology that was employed by us can be utilized in future research.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: DayAS, Fierbinteanu-Braticevici C, Naito Y S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Loftus CG, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ 3rd, Sandborn WJ. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 476] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 2. | Meucci G, Vecchi M, Astegiano M, Beretta L, Cesari P, Dizioli P, Ferraris L, Panelli MR, Prada A, Sostegni R. The natural history of ulcerative proctitis: a multicenter, retrospective study. Gruppo di Studio per le Malattie Infiammatorie Intestinali (GSMII). Am J Gastroenterol. 2000;95:469-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Robert JH, Sachar DB, Aufses AH Jr, Greenstein AJ. Management of severe hemorrhage in ulcerative colitis. Am J Surg. 1990;159:550-555. [PubMed] |
| 4. | Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol. 2008;43:948-954. [PubMed] |
| 5. | Beaugerie L, Seksik P, Nion-Larmurier I, Gendre JP, Cosnes J. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 640] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 6. | Don BR, Kaysen G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Proceedings of the Seminars in dialysis; Wiley Online Library. 130:432-437. |
| 7. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 634] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Ghoshal UC, Aggarwal R, Saraswat VA, Choudhuri G. Severe ulcerative colitis: prospective study of parameters determining outcome. J Gastroenterol Hepatol. 2004;19:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Gelbmann CM. Prediction of treatment refractoriness in ulcerative colitis and Crohn’s disease--do we have reliable markers? Inflamm Bowel Dis. 2000;6:123-131. [PubMed] |
| 10. | Ho GT, Mowat C, Goddard CJ, Fennell JM, Shah NB, Prescott RJ, Satsangi J. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Lennard-Jones JE, Ritchie JK, Hilder W, Spicer CC. Assessment of severity in colitis: a preliminary study. Gut. 1975;16:579-584. [PubMed] |
| 12. | Khan N, Abbas A, Williamson A, Balart L. Prevalence of corticosteroids use and disease course after initial steroid exposure in ulcerative colitis. Dig Dis Sci. 2013;58:2963-2969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Khan NH, Almukhtar RM, Cole EB, Abbas AM. Early corticosteroids requirement after the diagnosis of ulcerative colitis diagnosis can predict a more severe long-term course of the disease - a nationwide study of 1035 patients. Aliment Pharmacol Ther. 2014;40:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | The National Center for VeteransAnalysis and Statistics. Trends in the Utilization of VAPrograms and Services, Washington, DC. 2012;. |
| 15. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 16. | Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239-241. |
| 17. | Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009;44:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 18. | Höie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E, Stockbrügger R, Vatn M, Moum B; European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD). Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Fraga XF, Vergara M, Medina C, Casellas F, Bermejo B, Malagelada JR. Effects of smoking on the presentation and clinical course of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:683-687. [PubMed] |
| 20. | Lakatos PL, Vegh Z, Lovasz BD, David G, Pandur T, Erdelyi Z, Szita I, Mester G, Balogh M, Szipocs I. Is current smoking still an important environmental factor in inflammatory bowel diseases? Results from a population-based incident cohort. Inflamm Bowel Dis. 2013;19:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 942] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 22. | Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260. [PubMed] |
| 24. | Garcia-Planella E, Mañosa M, Van Domselaar M, Gordillo J, Zabana Y, Cabré E, López San Román A, Domènech E. Long-term outcome of ulcerative colitis in patients who achieve clinical remission with a first course of corticosteroids. Dig Liver Dis. 2012;44:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Henriksen M, Jahnsen J, Lygren I, Sauar J, Kjellevold Ø, Schulz T, Vatn MH, Moum B; IBSEN Study Group. Ulcerative colitis and clinical course: results of a 5-year population-based follow-up study (the IBSEN study). Inflamm Bowel Dis. 2006;12:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Shapiro JM, Hagin SE, Shah SA, Bright R, Law M, Moniz H, Giacalone J, Jackvony T, Taleban S, Samad Z. Corticosteroid Use in a Prospective, Community-Based Cohort of Newly Diagnosed Inflammatory Bowel Disease Patients. Dig Dis Sci. 2016;61:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Thapa SD, Hadid H, Usman M, Imam W, Hassan A, Schairer J, Jafri SM, Kaur N. Predictors of Thiopurine Treatment Failure in Biologic-Naïve Ulcerative Colitis Patients. Dig Dis Sci. 2016;61:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Morita Y, Bamba S, Takahashi K, Imaeda H, Nishida A, Inatomi O, Sasaki M, Tsujikawa T, Sugimoto M, Andoh A. Prediction of clinical and endoscopic responses to anti-tumor necrosis factor-α antibodies in ulcerative colitis. Scand J Gastroenterol. 2016;51:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Ford AC, Achkar JP, Khan KJ, Kane SV, Talley NJ, Marshall JK, Moayyedi P. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:601-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | d’Albasio G, Pacini F, Camarri E, Messori A, Trallori G, Bonanomi AG, Bardazzi G, Milla M, Ferrero S, Biagini M. Combined therapy with 5-aminosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: a randomized double-blind study. Am J Gastroenterol. 1997;92:1143-1147. [PubMed] |
| 31. | Probert CS, Dignass AU, Lindgren S, Oudkerk Pool M, Marteau P. Combined oral and rectal mesalazine for the treatment of mild-to-moderately active ulcerative colitis: rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014;8:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Marteau P, Probert CS, Lindgren S, Gassul M, Tan TG, Dignass A, Befrits R, Midhagen G, Rademaker J, Foldager M. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 231] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114:39-43. [PubMed] |
| 34. | Hawthorne AB, Rubin G, Ghosh S. Review article: medication non-adherence in ulcerative colitis--strategies to improve adherence with mesalazine and other maintenance therapies. Aliment Pharmacol Ther. 2008;27:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Klein RE. Data on the Socioeconomic Status of Veterans and on VA Program Usage. Office of the Actuary, Veterans Health Administration. 2013;. |
| 36. | Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | US Government Accountability Office Report VHC. Use of VA Services by Medicare-Eligible Veterans, Washington, DC. 2013;. |