Published online Nov 21, 2017. doi: 10.3748/wjg.v23.i43.7776
Peer-review started: June 10, 2017
First decision: July 13, 2017
Revised: August 3, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: November 21, 2017
Processing time: 164 Days and 18 Hours
To examine the medical status of children with biliary atresia (BA) surviving with native livers.
In this cross-sectional review, data collected included complications of chronic liver disease (CLD) (cholangitis in the preceding 12 mo, portal hypertension, variceal bleeding, fractures, hepatopulmonary syndrome, portopulmonary hypertension) and laboratory indices (white cell and platelet counts, total bilirubin, albumin, international normalized ratio, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase). Ideal medical outcome was defined as absence of clinical evidence of CLD or abnormal laboratory indices.
Fifty-two children [females = 32, 62%; median age 7.4 years, n = 35 (67%) older than 5 years] with BA (median age at surgery 60 d, range of 30 to 148 d) survived with native liver. Common complications of CLD noted were portal hypertension (40%, n = 21; 2 younger than 5 years), cholangitis (36%) and bleeding varices (25%, n = 13; 1 younger than 5 years). Fifteen (29%) had no clinical complications of CLD and three (6%) had normal laboratory indices. Ideal medical outcome was only seen in 1 patient (2%).
Clinical or laboratory evidence of CLD are present in 98% of children with BA living with native livers after hepatoportoenterostomy. Portal hypertension and variceal bleeding may be seen in children younger than 5 years of age, underscoring the importance of medical surveillance for complications of BA starting at a young age.
Core tip: Previous study showed that more than 90% of children with biliary atresia (BA) surviving with native livers have clinical and laboratory evidence of chronic liver disease (CLD). In the present cohort, we found that 71% of patients with BA living with native livers had no clinical complications of CLD and 90% had normal liver synthetic function, only 2% had ideal medical outcome. Common medical complications encountered were cholangitis, portal hypertension and bleeding oesophageal varices. Portal hypertension and bleeding oesophageal varices were seen in 12% and 6% of children younger than 5 years of age. Medical surveillance in children with BA after Kasai surgery for medical complications should start even before 5 years of age.
- Citation: Lee WS, Ong SY, Foo HW, Wong SY, Kong CX, Seah RB, Ng RT. Chronic liver disease is universal in children with biliary atresia living with native liver. World J Gastroenterol 2017; 23(43): 7776-7784
- URL: https://www.wjgnet.com/1007-9327/full/v23/i43/7776.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i43.7776
Biliary atresia (BA) is a progressive fibro-obliterative cholangiopathy presenting only in the first 3 mo of life[1,2]. Without surgery, BA is a fatal disease with children rarely surviving beyond 3 years of age[3]. Worldwide, the reported prevalence of BA ranges from 1 in 5000 to 18000 new-borns[4]. Since the introduction of Kasai hepatoportoenterostomy (HPE), long term survival of children with BA has been possible[1,2]. However, the surgery can only provide temporary relief to the biliary obstruction and has been considered as a bridge to eventual liver transplantation (LT)[3,5,6].
In individuals surviving with native livers after HPE, long term follow up is necessary to ascertain the health status and to detect complications of biliary cirrhosis[7-9]. Malnutrition[10,11], cholangitis[12,13], and fractures are common medical complications[14,15]. Ng et al[7] reported that cholangitis and bone fractures are the two major complications in long-term survivors of children with BA living with native livers. More importantly, the majority of these children have clinical or biochemical evidence of chronic liver disease (CLD)[7]. Most of the studies addressing the medical complications of children with BA surviving with native livers were in a setting where LT is readily available[7-9]. Children who needed LT were transplanted when indicated. Those children who survived with native livers were probably still in “optimal health”[7].
We have previously reported that the short-to-medium term outcome of Malaysian children with BA were favourable[16]. However, like in other developing countries, LT is not widely available in Malaysia[16]. Many children with BA who had unsuccessful surgery died within the first few years of life due to a lack of timely LT[4,16]. Nevertheless, in those who survived with their native livers after initial successful surgery, the quality of life was comparable to healthy children[17]. However, the prevalence and types of medical complications were not addressed[16,17].
The aim of the present study was to describe the medical status of children with BA living with their native livers in a setting where LT is not common. The present study is unique as most of the current studies on the long-term outcome and medical status of BA were conducted in countries where LT is readily available.
This was a cross-sectional study conducted among children with BA attending the Paediatric Gastroenterology Unit of University Malaya Medical Centre (UMMC), Malaysia. Patients with BA diagnosed between January 1993 and December 2015 were identified. The medical record, latest clinic review and laboratory data were reviewed. The present study was approved by the institutional ethic review committee (MEC reference: 902.15).
Only children who had surgery performed at UMMC were included. The diagnosis of BA was confirmed via an operative cholangiogram and a histology compatible with BA. The following children were excluded: (1) patients who had surgery performed at other centres but were subsequently followed up at UMMC; (2) patients younger than 6 mo of age at the last clinic review; (3) patients with inadequate data; and (4) patients who had LT.
After HPE, prophylactic oral antibiotics were given for 3 mo. All episodes of cholangitis were treated with 10-14 d of intravenous antibiotics. Children with persistent abnormal liver indices were given ursodeoxycholic acid. All children received fat-soluble vitamins, including vitamin D supplementation.
Demographic, clinical and laboratory data were collected from chart review. Data collected included age, sex, ethnicity, date of birth, age at surgery, latest age at review, physical examination, growth status, size of liver and spleen, and signs of chronic liver disease. Laboratory data obtained were part of routine laboratory assessment performed on patients, and included the following: indices of hypersplenism [white cell (WBC) and platelet counts], synthetic [international normalized ratio (INR), albumin] and excretory (total bilirubin) functions of liver, and indices of hepatic inflammation [aspartate aminotransaminase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (γGT)].
Age at Kasai surgery was considered as ‘early’ if the surgery was performed ≤ 60 d of age, and ‘late’ if > 60 d of life.
For children aged ≤ 5 years old, the World Health Organization (WHO) age- and sex-specific growth charts were used[18]. For children aged > 5 years, the Centers for Disease Control and Prevention (CDC) age- and sex-specific growth charts were used[19]. Failure to thrive (FTT) was characterized as the weight-for-age z-score < -2.0, while short stature was defined as height-for-age z-score of < -2.0.
Medical complications of CLD included portal hypertension (PHT), bleeding oesophageal varices, cholangitis in the preceding 12 mo, hepatopulmonary syndrome (HPS), portopulmonary hypertension (POPT), and fracture.
Criteria for the diagnosis of PHT were the presence of at least one of the following: (1) complication of PHT (such as ascites); (2) splenomegaly (≥ 2 cm palpable below the costal margin) and thrombocytopenia (platelet count ≤ 150 × 109/L); or (3) endoscopic evidence of esophageal varies, esophageal variceal bleeding, or hypertensive gastropathy[7].
As the yield of a positive blood culture in cholangitis is low, a positive blood culture was not required. Its diagnosis was based on the presence of fever of > 38 °C without other obvious source of infection, abdominal pain and new onset of acholic stools, and an elevation of conjugated bilirubin and/or (γGT) from the previous baseline[7].
The presence of HPS required evidence of reduced partial pressure of oxygen < 80 mmHg under room air and evidence of intrapulmonary shunting by contrast echocardiography with agitated saline[20]. POPH was defined as the presence of echocardiographic evidence of raised pulmonary arterial pressure (≥ 25 mmHg)[20,21].
Laboratory evidence of hypersplenism included the presence of WBC < 4.0 × 109/L and/or platelet count < 150 × 109/L in the absence of other identifiable causes, such as virus infection. Indices of CLD included: (1) impaired excretory function [total serum bilirubin ≥ 17 μmol/L]; (2) impaired synthetic function [albumin level < 35 g/L or INR ≥ 1.3]; and (3) presence of hepatitis (ALT ≥ 40 IU/L, or AST ≥ 40 IU/L, or γGT ≥ 55 IU/L).
The definition of an ideal medical status in survivors of BA with native livers was modified from that of Ng et al[7] and included: (1) absence of clinical complications of CLD; (2) absence of cholangitis in the preceding 12 mo; and (3) normal liver laboratory indices.
Data were entered by using SPSS 21.0 (SPSS Inc., Chicago, IL, United States) for Windows XP (Microsoft, Seattle, WA, United States). Data are quoted as medians and range. χ2 tests were used for categorical data, while one-sample t-test was used for comparison of numerical data. Independent samples t-test was used when two different groups of continuous variables were compared.
During the study period between 1993 and 2015, 140 children were diagnosed with BA at UMMC, Kuala Lumpur (Figure 1). Of these, 112 (80%) had Kasai surgery. Twenty of these 112 patients developed liver cirrhosis and had LT. Seven of these 20 patients died after LT. Forty of the remaining 92 patients who had Kasai surgery died of liver cirrhosis without LT. Six of the 28 children who did not have Kasai surgery had LT as primary treatment, while the remaining 22 patients died without LT. Fifty two patients (37% of the original cohort) survive with their native livers and were included in the present analysis. The overall survival rate (native livers and LT) was 51%.
The median age of the 52 patients [females = 32, (62%); Table 1] at review was 7.4 years (range of 10 mo to 22 years). Two-thirds (n = 35, 67%) were aged 5 years or older. The median age at Kasai surgery was 60 d (range of 30 d to 148 d). Twenty nine (59%) of the patients had HPE performed before 60 d of age. Three (6%) patients had associated congenital anomalies: one each for aortic stenosis, atrial septal defect and craniosynostosis. Another 3 patients developed unrelated medical conditions: asthma, Sjogren’s syndrome and polycystic ovarian syndrome. None of the patients in the present cohort had BA splenic malformation (BASM) syndrome.
| Characteristic | n (%) | |
| Sex | Male | 20 (38.4) |
| Female | 32 (61.6) | |
| Ethnicity | Chinese | 32 (61.5) |
| Malays | 15 (28.8) | |
| Indians | 5 (9.6) | |
| Age at Kasai surgery in d | mean ± SD | 65.5 ± 26.3 |
| Median | 60 | |
| Range | 30-148 | |
| Kasai surgery ≤ 60 d | Yes | 30 (57.7) |
| No | 22 (42.3) | |
| Age at latest follow-up in yr | mean ± SD | 8.3 ± 6.1 |
| Median | 7.4 | |
| Medical conditions present | No | 46 (88.5) |
| Yes | 6 (11.5) | |
| Congenital anomalies | 3 (5.7) | |
| Presence of failure to thrive | ||
| All, n = 52 | Yes | 14 (26.9) |
| No | 38 (73.1) | |
| < 5 yr old, n = 17 | Yes | 3 (17.6) |
| No | 14 (82.4) | |
| ≥ 5 yr old, n = 35 | Yes | 11 (31.4) |
| No | 24 (68.6) | |
| Short stature | ||
| All, n = 51 | Yes | 10 (19.6) |
| No | 41 (80.4) | |
| < 5 yr old, n = 16 | Yes | 1 (5.9) |
| No | 16 (94.1) | |
| ≥ 5 yr old, n = 32 | Yes | 9 (26.5) |
| No | 25 (73.5) | |
| Laboratory indices at review | White cell count, × 109/L | 6.2 (4.8) |
| Median (IQR) | Platelet count, × 109/L | 131 (169) |
| Total bilirubin, μmol/L | 18 (39) | |
| Serum albumin, g/L | 41 (8) | |
| International normalised ratio | 1.1 (0.1) | |
| Alanine transferase, IU/L | 54 (64) | |
| Aspartate transferase, IU/L | 70 (80) | |
| Gamma glutamyl-transpeptidase, IU/L | 109 (174) |
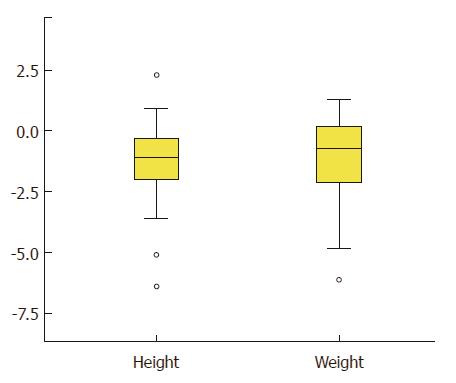
Presence of FTT and short stature are shown in Table 1, while the distribution of weight and height-adjusted z-score are shown in Figure 1. Overall, approximately one-quarter (n = 14; 27%) had a weight-for-age z-score < - 2.0, while 10 children (20%) had a height-for-age z-score < -2.0. Data on parental height were not available for comparison. FTT was present in 17.6% of children younger than 5 years of age, and in 31.4% of children older than 5 years (P = 0.29). There was no difference between the proportion of children with short stature in those younger (6.3%) and older than 5 years of age (27%; < 5 years old vs ≥ 5 years old; P = 0.089).
PHT (n = 21, 40%), the most common complication encountered, was seen in 4 out of every 10 patients, but bleeding oesophageal varices only occurred in one-quarter of the patients (n = 13, 25%). Another indicator of PHT, ascites was uncommon (n = 3, 6%). Cholangitis was common and was diagnosed in approximately one-third (n = 19, 36%) of the patients in the preceding 12 mo. No patients with bone fracture were noted in the present cohort. No patients were found to have HPS. However, 2 patients (aged 19 and 20 years, respectively) were diagnosed to have POPH. One was asymptomatic and the hepatopulmonary hypertension was diagnosed during pre-LT assessment, while the second patient presented with chest symptoms. Both patients are receiving appropriate therapy while being assessed for LT (Table 2).
| Medical variable | Data available, n | All, n = 52 | < 5 yr old, n = 17 | ≥ 5 yr old, n = 35 | P value, |
| < 5 yr old vs ≥ 5 yr old | |||||
| Failure to thrive, weight-for-age z-score < 2 SD | 52 | 14 (26.9) | 3 (17.6) | 11 (31.4) | 0.29 |
| Short stature, height-for-age z-score < 2 SD | 51 | 10 (19.6) | 1 (5.9) | 9 (26.5) | 0.089 |
| Medical complications | |||||
| Portal hypertension | 52 | 21 (40.4) | 2 (11.8) | 19 (54.3) | 0.034 |
| Variceal bleeding | 52 | 13 (25.0) | 1 (5.8) | 12 (34.3) | 0.026 |
| Ascites | 52 | 3 (5.8) | 2 (11.8) | 1 (2.9) | 0.20 |
| Cholangitis | 52 | 19 (36.3) | 8 (47.1) | 11 (31.4) | 0.27 |
| Portopulmonary hypertension | 52 | 2 (3.8) | 0 (0) | 2 (5.7) | 0.31 |
| Hepatopulmonary syndrome | 52 | 0 (0) | 0 (0) | 0 (0) | - |
| Bone fracture | 52 | 0 (0) | 0 (0) | 0 (0) | - |
| Laboratory indices | |||||
| White cell count, < 4 × 109/L | 49A | 13 (25.0) | 1 (5.9) | 13 (40.6) | 0.017 |
| Platelet count, < 150 × 109/L | 50A | 28 (53.8) | 8 (47.1) | 22 (66.7) | 0.28 |
| Total bilirubin, ≥ 17 μmol/L | 52 | 26 (50.0) | 6 (35.3) | 21 (60.0) | 0.09 |
| Albumin, < 35 g/L | 52 | 6 (11.5) | 1 (5.8) | 5 (14.3) | 0.37 |
| International normalized ratio, ≥ 1.3 | 52 | 12 (23.1) | 5 (29.4) | 7 (20.0) | 0.70 |
| Alanine transferase, ≥ 40 IU/L | 52 | 35 (67.3) | 13 (76.5) | 24 (68.6) | 0.56 |
| Aspartate transferase, ≥ 40 IU/L | 52 | 38 (73.1) | 15 (88.2) | 25 (71.4) | 0.18 |
| Gamma glutamyl-transpeptidase, ≥ 55 IU/L | 52 | 36 (69.2) | 11 (64.7) | 27 (77.1) | 0.34 |
| Absence of any medical complications | 52 | 15 (28.8) | 6 (35.3) | 9 (25.7) | 0.98 |
| Absence of abnormal laboratory indices | 49 | 3 (6.1) | 1 (5.9) | 2 (6.3) | 0.47 |
| Ideal medical outcome | 49 | 1 (2.0) | 0 (0) | 1 (2.9) |
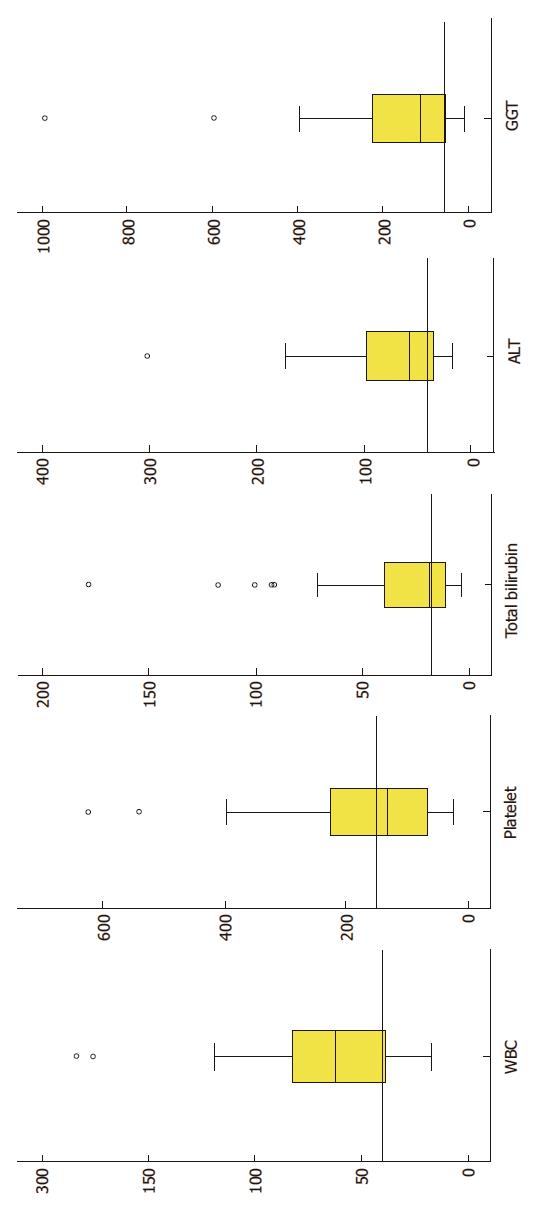
The distribution of various laboratory indices is shown in Figure 2. Leukopenia and thrombocytopenia were noted in 25% and 54% of the patients, respectively. Half (n = 26, 50%) of the patients had raised total bilirubin level. Elevated liver enzymes were also common (ALT in 67%, AST in 73% and γGT in 69%; Figure 2). A great majority of patients (n = 46, 90%) had normal serum albumin level, while more than half (n = 27 of 39, 59.2%) had a normal INR value.
Generally, as compared to patients younger than 5 years of age, patients older than 5 years of age were more likely to develop complications of liver cirrhosis, i.e., portal hypertension (P = 0.034), bleeding oesophageal varices (P = 0.026) and leukopenia (P = 0.017; Table 2).
Fifteen patients (29%) had no complications associated with CLD noted (Table 2). These included 6 of the 17 (36%) patients younger than 5 years old and 9 of the 35 (26%) older than 5 years of age. Three patients had normal laboratory indices. Of these, only 1 patient (2% of the entire cohort) had an ideal outcome, i.e. absence of any clinical complications and normal laboratory indices. The remaining 2 patients who had normal laboratory indices had an episode of cholangitis in the preceding 12 mo. Ninety eight percent of the patients in the present study had either presence of medical complications or abnormal laboratory indices.
The present study confirmed that the vast majority of patients with BA surviving with native livers after HPE had either clinical or laboratory evidence of CLD[7]. Although 71% of the patients had no clinical complications of CLD and 90% had normal liver synthetic function, only 1 of the 49 patients (2%) in the present study fulfilled the criteria for an ‘ideal’ outcome after HPE. This is consistent with the finding from Childhood Liver Disease Research and Education Network (CHiLDREN), where 98% of children with BA either had clinical complications of CLD or biochemical abnormalities[7]. Hadzić et al[22], using slightly different criteria, also noted that only 11% of children with BA living with native livers had absence of surgical complications and normal laboratory indices.
In the present study, although clinical complications of CLD were absent in 30% of the children living with native livers after HPE, most had raised liver enzymes indicating persistent hepatitis. Normal liver biochemistries were only seen in 3 children in the present cohort, 2 of whom had cholangitis in the preceding 12 mo. This underscores the importance of continuing surveillance in children with BA after HPE as well as regular laboratory assessment, even in the absence of clinical complications of CLD.
There are major similarities and differences in the findings between the present study and the CHiLDREN study[7]. In both studies, PHT and cholangitis were the two most common complications noted. Bleeding oesophageal varices and cholangitis were noted in 19% and 17%, respectively, in the CHiLDREN cohort and 13% and 19%, respectively, in the present cohort[7].
However, fracture of the long bone, which was common in the CHiLDREN cohort (27%), was not seen in the present study. Hepatic osteodystrophy is a well-known complication in children with cholestatic CLD[23]. Radiological change of long bone has been reported to be as high as 76% in children with BA[23]. The reported incidence of bone fractures in children with BA before LT was between 8% and 35%[24,25]. The reason for the discrepancies in the incidence of fractures observed in the CHiLDREN as well as other studies and present cohort is unknown. We previously reported that 28% of children with CLD had vitamin D non-sufficiency despite being on vitamin D supplementation in a tropical climate setting[26]. Thus, it is unlikely that adequate exposure to sunlight in the tropical climate among the patients in the present study is a satisfactory reason.
Malnutrition in patients with BA after HPE, a well-known complication[10], is associated with LT or death by 24 mo of age[10]. It is also an independent risk factor for pre- and post-LT mortality, and even graft failure[4]. Careful monitoring of the nutritional intake, early nutritional supplementation, regular monitoring of growth parameters including triceps skinfold thickness and mid-upper arm circumference, are important[27]. When adequate nutrition has been provided and malnutrition persisted, LT should be considered[28].
In the present study, the prevalence of weight and height z-score of < -2.0 were 28% and 20%, respectively. This is much higher as compared to the findings in the CHiLDREN cohort, where only 0.5% and 3.2% of the patients had a weight and height z-score of < -2.0[7]. These discrepancies can be partly explained by a much higher prevalence of both underweight (13.0%, 95%CI: 11.7, 14.5) and stunting (13.4%, 95%CI: 15.1, 21.5) in Malaysian children under 18 years of age[29]. Nevertheless, underweight and stunting in children with BA post-HPE in the present cohort was common. Intensive efforts in improving the nutritional status of these children is important. In the present study, we did not include growth parameters in the analysis of ideal medical status. Besides the high prevalence of underweight and stunting in Malaysian children, the growth status of the parents of these children were not available. Thus, we were unable to exclude familial short stature.
PHT is a common occurrence in BA, commonly presenting as splenomegaly and thrombocytopenia[30]. In the present study, 40% of the patients had PHT while 25% had bleeding varices. This is consistent with another study on PHT from the CHiLDREN cohort, where 49% and 15% of the 211 patients had PHT and bleeding varices, respectively[30]. Ascites, another complication of PHT, was uncommon, being observed in only 6% of the patients. More importantly, 12% and 6% of the patients in the present study who were younger than 5 years old had PHT and bleeding oesophageal varices. This underscores the importance of monitoring of PHT and its complications even in younger patient with BA[30].
In the present study, 36% of the patients experienced at least one episode of cholangitis. This included 31% of the patients older than 5 years of age. A total of 8 children aged older than 10 years experienced cholangitis. Thus, the risk of cholangitis after HPE continues throughout early childhood to the adolescent period and even into early adulthood[7].
POPH and HPS are reportedly uncommon complications of BA[21]. In the present study, POPH was diagnosed in 2 young adults (3.8%).
As compared to the CHiLDREN cohort, the strength of the current study was that it was conducted in a single unit, where consistent post-surgical clinical care was practiced throughout the study period. The variability in clinical care as seen in the CHiLDREN cohort was not seen in our study[7]. Other practice, such as routine administration of fat-soluble vitamins in BA after HPE, could also explain the low incidence of bone fracture seen in the present study.
However, there are several weaknesses in the present study. Firstly, it was a single-centre study and the number of patients studied was relatively small. Thus, children younger than 5 years of age were included in this review. Nevertheless, even in young children, PHT and bleeding oesophageal varices were seen in 12% and 6% of patients, respectively.
Health-related quality of life survey was not included in the present study but was studied separately[17]. The health-related quality of life in children with BA was similar to that of healthy children, and was not adversely affected by the presence of complications of CLD, such as PHT, cholangitis or impaired synthetic function of the liver[17].
The most important factor adversely affecting the overall outcome of children with BA in Malaysia was a lack of timely LT in those with unsuccessful surgery[16]. The median age for HPE in the present study was 60 d, which was comparable to figures from other centres[31], ranging from 54 days in England and Wales[32] to 68 d in Switzerland[33].
The survival rate of patients with BA living with native livers in the present study was 37%. This was comparable to the survival rates in the review by Verkade et al[31], where the survival rates with native livers ranged from as low as 20% in Germany[34] to 60% in Japan[6]. The overall survival rate of the present study at 51%, however, is much lower that similar figures of 73% in the Netherlands[35] to 92% in Switzerland[33].
Thus, the lack of timely LT in those with unsuccessful surgery is the biggest unmet medical need in children with BA in Malaysia. Factors contributing to a lack of LT included a low deceased organ donation rate and the relative high cost of LT surgery[36]. It is unlikely that the lack of deceased organ donation in Malaysia will be overcome in the near future.
We have previously shown that early referral for surgery significantly affected the outcome of surgery[16]. Children with BA who were operated before 60 d of age had a 65% chance of surviving with native liver at two years of age as compared to those who had surgery at or after 60 d of age. The median age of survival for those with unsuccessful surgery was 14 mo (range of 3 mo to 25 mo)[16]. Thus, in a country such as Malaysia where LT remains limited, early referral for successful surgery remains the most important opportunity to avoid early LT.
Thus, clinicians in Malaysia caring for children need to familiarize themselves with signs of an unsuccessful surgery to facilitate early referral for assessment of LT. We have shown that children with unsuccessful surgery were more likely to have a significantly bigger liver and spleen size, a more deranged coagulation profile, a significantly lower serum albumin level, and a significantly higher serum γGT level, as compared to children who had successful surgery. Thus, monitoring of children with BA after surgery should include regular measurement of liver and spleen size, assessment of liver synthetic function, such as coagulation and albumin level, and determination of liver enzymes.
As stated earlier, the opportunity to improve the overall survival rate of BA in Malaysia includes strategies to enhance early referral and surgery[31]. The provision of stool colour cards to parents of new-borns for identification of acholic stools has been practiced in Taiwan and Switzerland[37,38]. In Taiwan, 5 years after starting the stool colour card screening, the rate of HPE performed at < 60 d increased from 49% to 66% while the 5-year survival rate with native liver improved from 27% to 64%[37]. This strategy should be adopted in countries where LT is limited, such as Malaysia.
In conclusion, about 98% of children with BA living with native livers after HPE had clinical or laboratory evidence of CLD, underscoring the importance of continuing medical surveillance. PHT and variceal bleeding may be seen in children younger than 5 years of age. We recommend that surveillance for the complications of CLD in children with BA living with native livers should be started before 5 years of age. The major unmet medical need for children with BA living with their native livers is a lack of timely LT in those with unsuccessful surgery. Opportunities to improve the overall survival rate include universal new-born screening in all infants to improve early referral rate and the surgical outcome of HPE.
In individuals with biliary atresia (BA) surviving with native livers after hepatoportoenterostomy, biliary cirrhosis is an inevitable long-term sequelae. Malnutrition, cholangitis and fractures are common medical complications encountered in these children, arising as a result of biliary cirrhosis. Previous study has shown that cholangitis and bone fractures are the two major complications in long-term survivors of children with BA living with native livers.
It is important to determine the prevalence of medical complications in children with BA surviving with their native livers, particularly in a setting where liver transplantation (LT) is limited.
The present study shows that clinical or laboratory evidence of chronic liver disease are present in 98% of children with BA living with native livers after surgery. Portal hypertension and variceal bleeding may be seen in children younger than 5 years of age, underscoring the importance of medical surveillance for complications of BA starting at a young age.
The authors recommend that in all children with BA who survive with their native livers, systemic surveillance to ascertain medical complications be started at an age younger than 5 years. Ascending cholangitis, portal hypertension and variceal bleeding are important medical complications encountered.
This manuscript describes the current situation of treatment of BA in Malaysia. In Malaysia, LT is not common. Therefore, most patients with BA must live with their native livers after Kasai’s hepatic portoenterostomy. This manuscript reemphasized the devastating nature of BA and essentialness of LT for patients with BA.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Malaysia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hashimoto D, Neri V S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y
| 1. | Chardot C. Biliary atresia. Orphanet J Rare Dis. 2006;1:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Schreiber RA, Kleinman RE. Biliary atresia. J Pediatr Gastroenterol Nutr. 2002;35 Suppl 1:S11-S16. [PubMed] |
| 3. | Liu MB, Huong TB, Hoang X, Doan L, Trinh S, Anh Nguyen HP, Thanh Le H, Holterman AX. Biliary atresia in Vietnam: Management and the burden of disease. Surgery. 2017;161:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Utterson EC, Shepherd RW, Sokol RJ, Bucuvalas J, Magee JC, McDiarmid SV, Anand R; Split Research Group. Biliary atresia: clinical profiles, risk factors, and outcomes of 755 patients listed for liver transplantation. J Pediatr. 2005;147:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Shneider BL, Mazariegos GV. Biliary atresia: a transplant perspective. Liver Transpl. 2007;13:1482-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Nio M, Ohi R, Miyano T, Saeki M, Shiraki K, Tanaka K; Japanese Biliary Atresia Registry. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38:997-1000. [PubMed] |
| 7. | Ng VL, Haber BH, Magee JC, Miethke A, Murray KF, Michail S, Karpen SJ, Kerkar N, Molleston JP, Romero R, Rosenthal P, Schwarz KB, Shneider BL, Turmelle YP, Alonso EM, Sherker AH, Sokol RJ; Childhood Liver Disease Research and Education Network (CHiLDREN). Medical status of 219 children with biliary atresia surviving long-term with their native livers: results from a North American multicenter consortium. J Pediatr. 2014;165:539-546.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Kumagi T, Drenth JP, Guttman O, Ng V, Lilly L, Therapondos G, Hiasa Y, Michitaka K, Onji M, Watanabe Y. Biliary atresia and survival into adulthood without transplantation: a collaborative multicentre clinic review. Liver Int. 2012;32:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Shinkai M, Ohhama Y, Take H, Kitagawa N, Kudo H, Mochizuki K, Hatata T. Long-term outcome of children with biliary atresia who were not transplanted after the Kasai operation: >20-year experience at a children's hospital. J Pediatr Gastroenterol Nutr. 2009;48:443-450. [PubMed] |
| 10. | DeRusso PA, Ye W, Shepherd R, Haber BA, Shneider BL, Whitington PF, Schwarz KB, Bezerra JA, Rosenthal P, Karpen S. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology. 2007;46:1632-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Shepherd RW, Chin SE, Cleghorn GJ, Patrick M, Ong TH, Lynch SV, Balderson G, Strong R. Malnutrition in children with chronic liver disease accepted for liver transplantation: clinical profile and effect on outcome. J Paediatr Child Health. 1991;27:295-299. [PubMed] |
| 12. | Lünzmann K, Schweizer P. The influence of cholangitis on the prognosis of extrahepatic biliary atresia. Eur J Pediatr Surg. 1999;9:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Wu ET, Chen HL, Ni YH, Lee PI, Hsu HY, Lai HS, Chang MH. Bacterial cholangitis in patients with biliary atresia: impact on short-term outcome. Pediatr Surg Int. 2001;17:390-395. [PubMed] |
| 14. | Okada T, Honda S, Miyagi H, Minato M, Taketomi A. Hepatic osteodystrophy complicated with bone fracture in early infants with biliary atresia. World J Hepatol. 2012;4:284-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Högler W, Baumann U, Kelly D. Endocrine and bone metabolic complications in chronic liver disease and after liver transplantation in children. J Pediatr Gastroenterol Nutr. 2012;54:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Lee WS, Chai PF, Lim KS, Lim LH, Looi LM, Ramanujam TM. Outcome of biliary atresia in Malaysia: a single-centre study. J Paediatr Child Health. 2009;45:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lee WS, Ong SY. Health-Related Quality of Life in Children with Biliary Atresia Living with Native Livers. Ann Acad Med Singapore. 2016;45:61-68. [PubMed] |
| 18. | Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee. World Health Organization, 1995. . |
| 19. | Growth Charts. Atlanta: Centers for Disease Control and Prevention, 2010. . |
| 20. | Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 21. | Ecochard-Dugelay E, Lambert V, Schleich JM, Duché M, Jacquemin E, Bernard O. Portopulmonary Hypertension in Liver Disease Presenting in Childhood. J Pediatr Gastroenterol Nutr. 2015;61:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Hadzić N, Davenport M, Tizzard S, Singer J, Howard ER, Mieli-Vergani G. Long-term survival following Kasai portoenterostomy: is chronic liver disease inevitable? J Pediatr Gastroenterol Nutr. 2003;37:430-433. [PubMed] |
| 23. | Hirano A, Katayama H, Shirakata A. [Bone changes in congenital biliary atresia--review of 42 cases after surgery]. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50:29-39. [PubMed] |
| 24. | DeRusso PA, Spevak MR, Schwarz KB. Fractures in biliary atresia misinterpreted as child abuse. Pediatrics. 2003;112:185-188. [PubMed] |
| 25. | Guichelaar MM, Schmoll J, Malinchoc M, Hay JE. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatology. 2007;46:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Lee WS, Siow YY, Wong SY, Ong SY, Foo HW, Ng RT. Vitamin D insufficiency is common in children with chronic inflammatory bowel disease and chronic liver disease in a tropical country. World Congress of Paediatric Gastroenterology, Hepatology and Nutrition; October 5-8. 2016;Montreal. |
| 27. | Squires RH, Ng V, Romero R, Ekong U, Hardikar W, Emre S, Mazariegos GV. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology. 2014;60:362-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 28. | Sundaram SS, Mack CL, Feldman AG, Sokol RJ. Biliary atresia: Indications and timing of liver transplantation and optimization of pretransplant care. Liver Transpl. 2017;23:96-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 29. | National Health and Morbidity Survey. Institute of Public Health, Ministry of Health Malaysia, 2015. . |
| 30. | Shneider BL, Abel B, Haber B, Karpen SJ, Magee JC, Romero R, Schwarz K, Bass LM, Kerkar N, Miethke AG. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr. 2012;55:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Verkade HJ, Bezerra JA, Davenport M, Schreiber RA, Mieli-Vergani G, Hulscher JB, Sokol RJ, Kelly DA, Ure B, Whitington PF. Biliary atresia and other cholestatic childhood diseases: Advances and future challenges. J Hepatol. 2016;65:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Davenport M, Ong E, Sharif K, Alizai N, McClean P, Hadzic N, Kelly DA. Biliary atresia in England and Wales: results of centralization and new benchmark. J Pediatr Surg. 2011;46:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 33. | Wildhaber BE, Majno P, Mayr J, Zachariou Z, Hohlfeld J, Schwoebel M, Kistler W, Meuli M, Le Coultre C, Mentha G. Biliary atresia: Swiss national study, 1994-2004. J Pediatr Gastroenterol Nutr. 2008;46:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Leonhardt J, Kuebler JF, Leute PJ, Turowski C, Becker T, Pfister ED, Ure B, Petersen C. Biliary atresia: lessons learned from the voluntary German registry. Eur J Pediatr Surg. 2011;21:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | de Vries W, de Langen ZJ, Groen H, Scheenstra R, Peeters PM, Hulscher JB, Verkade HJ; Netherlands Study Group of Biliary Atresia and Registry (NeSBAR). Biliary atresia in the Netherlands: outcome of patients diagnosed between 1987 and 2008. J Pediatr. 2012;160:638-644.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Ghods AJ. Current status of organ transplant in Islamic countries. Exp Clin Transplant. 2015;13 Suppl 1:13-17. [PubMed] |
| 37. | Lien TH, Chang MH, Wu JF, Chen HL, Lee HC, Chen AC, Tiao MM, Wu TC, Yang YJ, Lin CC. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology. 2011;53:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Wildhaber BE. Screening for biliary atresia: Swiss stool color card. Hepatology. 2011;54:367-368; author reply 369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |