Published online Nov 14, 2017. doi: 10.3748/wjg.v23.i42.7609
Peer-review started: August 31, 2017
First decision: September 20, 2017
Revised: October 2, 2017
Accepted: October 26, 2017
Article in press: October 26, 2017
Published online: November 14, 2017
Processing time: 73 Days and 12.2 Hours
To investigate the post-colonoscopy colorectal cancer (PCCRC) rate for high-definition (HD) colonoscopy compared with that for standard-definition colonoscopy reported previously.
Using medical records at Sano Hospital (SH) and Dokkyo Medical University Koshigaya Hospital (DMUKH), we retrospectively obtained data on consecutive patients diagnosed as having CRC between January 2010 and December 2015. The definition of PCCRC was diagnosis of CRC between 7 and 36 mo after initial high-definition colonoscopy that had detected no cancer, and patients were divided into a PCCRC group and a non-PCCRC group. The primary outcome was the rate of PCCRC for HD colonoscopy. The secondary outcomes were factors associated with PCCRC and possible reason for occurrence of early and advanced PCCRC.
Among 892 CRC patients, 11 were diagnosed as having PCCRC and 881 had non-PCCRC. The PCCRC rate was 1.7% (8/471) at SH and 0.7% (3/421) at DMUKH. In comparison with the non-PCCRC group, the PCCRC group had a significantly higher preponderance of smaller tumors (39 mm vs 19 mm, P = 0.002), a shallower invasion depth (T1 rate, 25.4% vs 63.6%, P = 0.01), a non-polypoid macroscopic appearance (39.0% vs 85.7%, P = 0.02) and an earlier stage (59.7% vs 90.9%, P = 0.03). Possible reasons for PCCRC were “missed or new” in 9 patients (82%), “incomplete resection” in 1 (9%), and “inadequate examination’” in 1 (9%). Among 9 “missed or new” PCCRC, the leading cause was non-polypoid shape for early PCCRC and blinded location for advanced PCCRC.
The PCCRC rate for HD colonoscopy was 0.7%-1.7%, being lower than that for standard-definition colonoscopy (1.8%-9.0%) reported previously employing the same methodology.
Core tip: Technological advance from standard-definition to high-definition colonoscopy has the potential to reduce the incidence of post-colonoscopy colorectal cancer (PCCRC). We demonstrated the lower PCCRC rate for high-definition colonoscopy compared for standard-definition colonoscopy reported previously (0.7%-1.7% vs 1.8%-9.0%). Our data might help to set a benchmark for the quality of colonoscopy in Asian countries, where data on PCCRC are scarce. We firstly analyzed the possible reasons for both early and advanced “missed or new” PCCRC cases and found differences between the two groups. The leading cause was non-polypoid shape for early PCCRC and blinded location for advanced PCCRC.
- Citation: Iwatate M, Kitagawa T, Katayama Y, Tokutomi N, Ban S, Hattori S, Hasuike N, Sano W, Sano Y, Tamano M. Post-colonoscopy colorectal cancer rate in the era of high-definition colonoscopy. World J Gastroenterol 2017; 23(42): 7609-7617
- URL: https://www.wjgnet.com/1007-9327/full/v23/i42/7609.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i42.7609
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in females and the third most common in males worldwide[1]. Colonoscopy can reduce the likelihood of CRC-related death by resecting precursor lesions and detecting CRC at an early stage[2-4]. Unfortunately, the quality of colonoscopy is insufficient to prevent all interval CRCs, and some patients still develop CRC before the next recommended surveillance date, an event known as post-colonoscopy CRC (PCCRC).
A better understanding of the factors associated with PCCRC may help to reduce its incidence. Previous reports have suggested that in comparison with non-PCCRC, PCCRC is associated with various clinical factors (e.g. older age, female gender, location in the proximal colon, and presence of diverticula) and also endoscopist-related factors (those with less experience at adenoma detection, or non-specialists in gastroenterology)[5-14]. Around 70% of PCCRCs appear to result from lesions that have been missed or incompletely resected at initial colonoscopy, and could theoretically have been avoidable[12]. Therefore, the PCCRC rate has been proposed as a key indicator of the quality of colonoscopy, and a meta-analysis has shown that this varies from 1.8% to 9.0%[13].
High-definition (HD) colonoscopy yields markedly clearer images and has the clinical benefit of increasing the adenoma detection rate in comparison with standard-definition (SD) colonoscopy[15]. Theoretically, HD colonoscopy has the potential to reduce the incidence of PCCRC, but clinical data related to this issue are still insufficient.
We therefore conducted a retrospective observational study at two academic centers to investigate the PCCRC rate for HD colonoscopy in Japan.
By reference to the medical records at Sano Hospital (SH) and Dokkyo Medical University Koshigaya Hospital (DMUKH), we included in this study consecutive individuals diagnosed as having CRC between January 2010 and December 2015. Exclusion criteria were as follows: (1) patients with IBD or hereditary disease; (2) those with a previous diagnosis of CRC; (3) those for which data related to CRC (tumor size, shape, site, and histopathology) were insufficient; (4) those with a CRC histopathology other than adenocarcinoma; and (5) those that did not comply with the Japanese clinical guidelines for the management of colorectal polyps at initial colonoscopy[16]. Patients who met the eligibility criteria were divided into a PCCRC group and a non-PCCRC group according to the definition of PCCRC given below. HD colonoscopy with a LUCERA-SPECTRUM or ELITE video processor and HD monitors (Olympus, Japan) had been used for all patients since 2006 at both hospitals. The study protocol was approved by the institutional review boards of both hospitals.
Based on a previous research method, we defined PCCRC as CRC that had been diagnosed 7 to 36 mo after initial HD colonoscopy, when no cancer had been detected[13]. CRC diagnosed within 6 mo of HD colonoscopy yielding negative findings was considered to have been a cancer confirmed after follow-up of a suspicious lesion, and was classified as non-PCCRC. CRC was defined as tumors that have penetrated through the muscularis mucosae into submucosa according to the classification of the World Health Organization.
Primary outcome: The primary outcome of interest was the PCCRC rate for HD colonoscopy, calculated as the number of PCCRC events divided by the total number of CRCs examined during the study period.
Secondary outcome: (1) Factors associated with PCCRC: We collected data on patients (age, sex) and tumors (size, location, shape, depth of invasion, UICC stage) for comparison between the PCCRC and non-PCCRC groups; and (2) possible reason for occurrence of early and advanced PCCRC: We assigned each PCCRC case into one of three categories: “incomplete resection” defined as CRC detected on the scar where an advanced polyp had been incompletely resected at the time of colonoscopy, “inadequate examination” defined as failure to intubate the colon to the cecum or poor bowel preparation, and “missed or new” as “others”. Differentiation of “missed” CRC from “new” CRC is challenging. In fact, most CRCs categorized as “missed or new” were thought to have been “missed”, in view of the fact that le Clercq had defined “new” CRC as CRC detected > 36 mo after the index colonoscopy[14]. Therefore, we additionally classified the “missed or new” category into four subcategories to determine which factor was most closely associated with “missed” CRC (multiple choice): (1) tumor morphology: Polypoid or non-polypoid; (2) tumor size: Small (< 10 mm) or not; (3) tumor location: In a blind area (e.g., behind a fold or close to the ileocecal valve/junction) or not; and (4) the endoscopist’s observational skill: Multiple (n ≥ 3) polyps evident at initial colonoscopy or not. We assumed that if an endoscopist took a long time to examine a patient with multiple polyps, this would prove exhausting and lead to loss of concentration in detecting polyps. We divided ‘missed or new’ PCCRC into early PCCRC (T1 stage) and advanced PCCRC (T2-4 stage) to clarify how the factors associated with PCCRC differed between the two groups.
Categorical variables were compared using the χ2 test or Mid-P exact test, normally distributed continuous variables were compared using t-test, and non-normally distributed continuous variables were compared using the Wilcoxon rank sum test. A two-sided P value of < 0.05 was considered statistically significant.
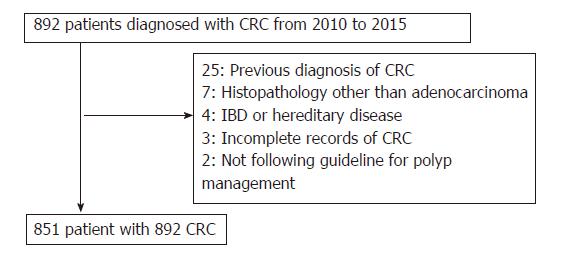
A total of 892 patients with CRC were identified from the records of both hospitals during the period January 2010 to December 2015. On the basis of the exclusion criteria, 41 patients were discarded and 851 patients (444 at SH, and 407 at DMUKH) with 892 CRCs were analyzed retrospectively (Figure 1). All of the CRCs were detected by gastroenterologists with more than 3 years of colonoscopy experience.
Among the 892 CRCs (471 at SH, and 421 at DMUKH), 2 (1 at each at SH and DMUKH) were diagnosed within 6 mo after initial colonoscopy and 11 (8 in SH, and 3 in DMUKH) between 7 and 36 mo after initial colonoscopy. The PCCRC rate was 1.7% (8/471) at SH, 0.7% (3/421) at DMUKH, and 1.2 % (11/892) for both hospitals.
Baseline variables in the PCCRC and non-PCCRC groups are listed in Table 1. Among patient-related variables, gender and mean age showed no significant inter-group difference. Among tumor-related variables, there were significant differences in size, depth, morphology and UICC stage between the two groups. In comparison with non-PCCRC patients, those with PCCRC were more likely to have small tumors (mean size, 39 mm vs 19 mm respectively, P = 0.002), a shallow tumor depth (T1 rate, 25.4% vs 63.6%, P = 0.01), early CRCs with a non-polypoid macroscopic appearance (39.0% vs 85.7%, P = 0.02), and an early UICC stage (stage I or II, 59.7% vs 90.9%, P = 0.03).
| PCCRC | Non-PCCRC | P value | |
| Patients | 11 | 840 | |
| Gender | NS | ||
| Male | 6 (54.5) | 485 (57.7) | |
| Female | 5 (45.5) | 355 (42.3) | |
| Age (yr) | NS | ||
| mean ± SD | 70 ± 10 | 68 ± 11 | |
| Range | 53-82 | 29-92 | |
| Tumors Size (mm) | 11 | 881 | 0.002 |
| Mean ± SD | 19 ± 13 | 39 ± 20 | |
| Range | 4-50 | 4-110 | |
| Location | NS | ||
| Proximal | 6 (54.5) | 283 (32.1) | |
| Distal | 5 (45.5) | 598 (67.9) | |
| Depth | 0.010 | ||
| T1 | 7 (63.6) | 224 (25.4) | |
| T2-4 | 4 (36.4) | 657 (74.6) | |
| Shape1 | 0.020 | ||
| Polypoid | 1 (14.3) | 136 (61.0) | |
| Non-polypoid | 6 (85.7) | 87 (39.0) | |
| UICC stage | 0.033 | ||
| Stage I,II | 10 (90.9) | 526 (59.7) | |
| Stage III, IV | 1 (9.1) | 355 (40.3) |
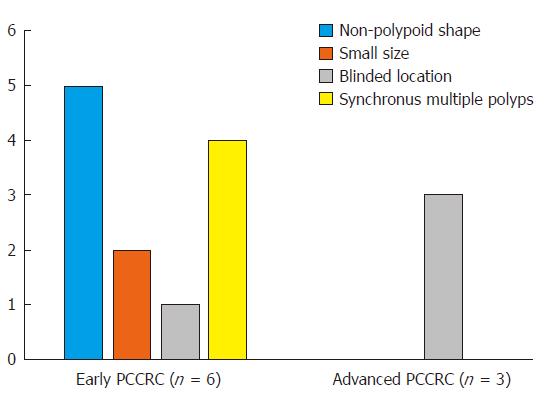
Details of the 11 patients with PCCRC are shown in Table 2. The possible reasons for PCCRC were “missed or new” in 9 cases (82%), “incomplete resection” in 1 (9%), and “inadequate examination” in 1 (9%). Possible explanations for the 9 “missed or new” cases (6 early and 3 advanced PCCRC) are summarized in Figure 2. The 6 early ‘missed or new’ PCCRC cases could have been due to a non-polypoid shape in 5 (83%), presence of synchronous multiple polyps at initial colonoscopy in 4 (67%), a small tumor size (< 10 mm) in 2 (33%), and location at a blind spot in 1 (17%). The 3 advanced “missed or new” PCCRC cases were likely due to their location at a blind spot (100%). Some representative PCCRC cases are presented in detail in Figures 3-7.
| No | Sex | Age(yr) | Interval (mo) | Tumor shape | Size (mm) | Depth | Location | Initial CS | Possible reason |
| 1 | M | 79 | 7 | IIc | 5 | T1a | T | Multiple polyps | Missed/new |
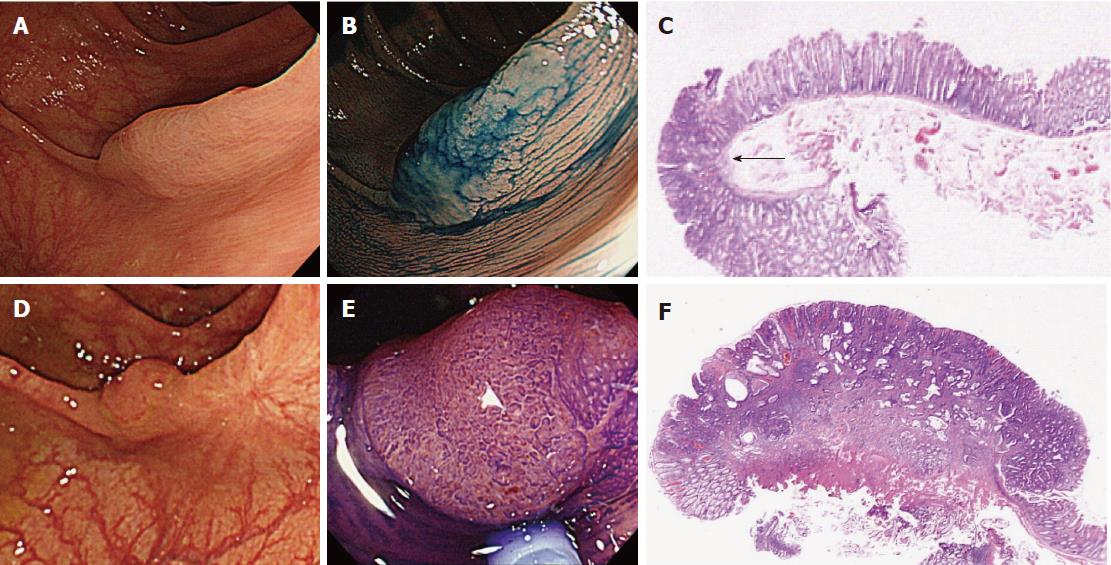
| 2 | M | 76 | 14 | IIa (LST-NG) | 15 | T1a | S | Multiple polyps | Missed/new |
| 3 | M | 82 | 17 | IIa (LST-NG) | 25 | T1a | T | No polyps | Missed/new |
| 4 | F | 65 | 22 | IIa (LST-NG) | 20 | T1a | A | Multiple polyps | Missed/new |
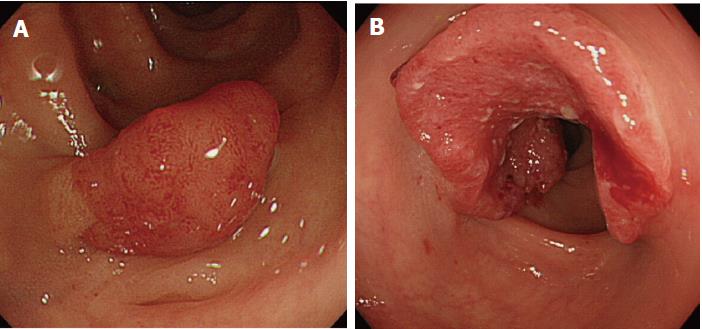
| 5 | M | 59 | 26 | Is | 12 | T1a | R | Two polyps | Missed/new |
| 6 | F | 73 | 11 | IIa | 10 | T1b | T | Piecemeal EMR | Incomplete resection |
| 7 | M | 79 | 15 | Is + IIc | 4 | T1b | S | Multiple polyps | Missed/new |
| 8 | F | 70 | 9 | Type 2 | 30 | T3 | C | No polyps | Inadequate examination |
| 9 | F | 53 | 12 | Type 2 | 17 | T3 | S (SDJ) | No polyps | Missed/new |
| 10 | F | 77 | 12 | Type 2 | 50 | T3 | RS | One polyp | Missed/new |
| 11 | M | 66 | 10 | Type 2 | 20 | T4 | C | Two polyps | Missed/new |
In this study, we investigated the PCCRC rate in cases examined by HD colonoscopy. It was anticipated that our data might help to set a benchmark for the quality of colonoscopy in Asian countries, where data on PCCRC are scarce. We analyzed the possible reasons for both early and advanced “missed or new” PCCRC cases and found differences between the two groups.
The PCCRC rate in the present study was 0.7%-1.7%, and lower than that in previous reports from Western countries (1.8%-9.0%) calculated using the same methodology[6-9,13]. There are several possible reasons for this difference. First, as we performed HD colonoscopy in all cases, we might have detected a larger number of pre-malignant polyps or CRC at the time of initial examination. Second, all colonoscopies were performed by experienced gastroenterologists. A population-based study in Manitoba reported that colonoscopy performed by general physicians was associated with a 60% higher risk of missed CRC in comparison with that performed by specialist gastroenterologists[7]. Third, racial differences in the incidence of CRC between Asian and Western countries. Fourth, the rate of recurrence (9.1% for all incompletely resected lesions including sessile serrated polyps, 0% for adenomas) in this study was low in comparison with previous studies (8.8%-36.8% for adenoma) performed in Western countries[12,14,17]. The difference in the recurrence rate for large colorectal tumors between Asian and Western countries is thought to be attributable to the treatment strategy employed, i.e., whether or not endoscopic submucosal dissection (ESD) is available. The ESD technique, originally developed in Japan for large colorectal (≥ 20 mm) tumors, has resulted in higher rates of en bloc resection and lower rates of local recurrence in comparison with conventional endoscopic mucosal resection (EMR) that is generally performed worldwide[18,19]. The ESD technique has not been popular in Western countries because of its technical difficulty, but it is now becoming increasingly available and employed successfully as practitioners gain experience[20,21]. The criteria employed to define PCCRC significantly affects the PCCRC rate[22]. Therefore, we followed the definition of PCCRC adopted in the majority of population-based studies and a recent meta-analysis[6-9,13].
In this study, we were able to identify several tumor-related factors associated with PCCRC. Such cases were significantly associated with a smaller tumor size, a shallower tumor depth, a non-polypoid shape and an earlier UICC stage, which were features characteristic of missed lesions. Our data support previous studies that have investigated tumor-related risk factors for PCCRC, except for tumor location. Although it has been suggested previously that PCCRC is more likely to arise in the proximal colon rather than the distal colon, we did not find any significant difference in the incidence of PCCRC between these two colon regions. This difference in results may have been attributable to the proportion of incomplete examinations, which can potentially lead to an increase in the rate of proximal colon PCCRC. The rate of complete examination in this study was 99%, as compared with 87%-92% for population-based studies in the United States[11,23]. Although there was a tendency for the PCCRC group to include older patients and a higher proportion of women than the non-PCCRC group, consistent with other reports, the differences between the two groups were not significant[6-11,13]. Other possible explanations may have been an insufficient sample size or the racial composition of the population.
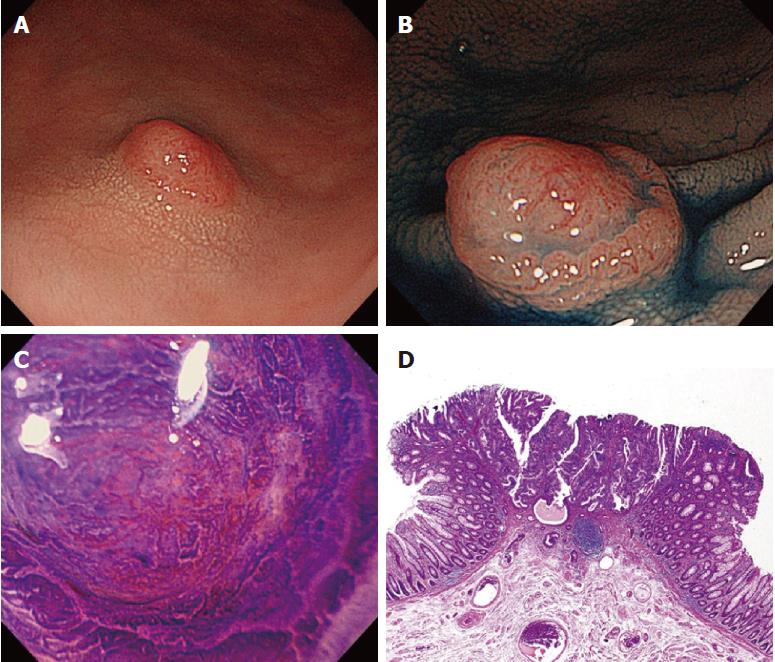
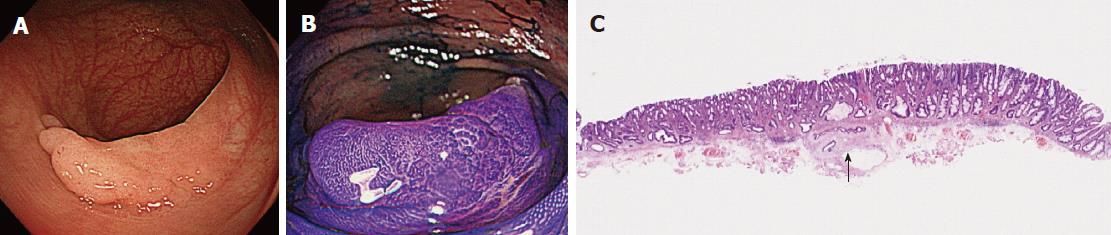
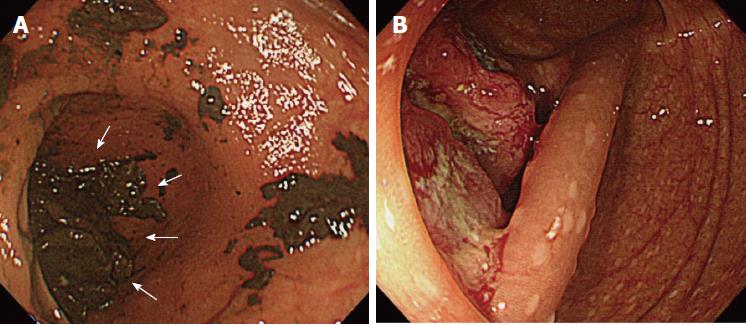
Of the three possible reasons for PCCRC, the majority (82%) of such cases were categorized as “missed or new”, consistent with previous reports[12,14]. We classified “missed or new” PCCRC into early and advanced cases. The major possible explanations for early “missed or new” PCCRC were a non-polypoid shape (83%) and the presence of synchronous multiple polyps at initial colonoscopy (67%). Among non-polypoid lesions, the mean size of depressed lesions was 4.5 mm and that of flat lesions including LST-NG (laterally spreading tumor, non-granular type) was 17.5 mm (Figures 3 and 4). As non-polypoid lesions are less conspicuous than polypoid lesions, they are often missed even if they are large. Endoscopists should pay closer attention to subtle changes in the mucosa, including red areas, loss of vessel visibility, and deformation of the colonic folds, in order to detect flat or depressed lesions[24-26]. We found that the presence of synchronous multiple polyps at initial colonoscopy was a factor associated with around 70% of early “missed or new” PCCRC cases, and was unrelated to advanced cases. We speculated that a long time spent examining a patient with multiple polyps might lead to a decrease in the concentration of the endoscopist, thus increasing the likelihood that small early CRCs (mean size: 13.5 mm), but not large advanced ones (mean size: 39.0 mm), would be overlooked. On the other hand, one possible explanation for advanced “missed or new” PCCRC cases was thought to be the location of lesions at blind spots, such as the junctions of the recto-sigmoid and sigmoid-descending colon and the ileocecal valve (Figure 5). Endoscopists should be aware that even large advanced CRCs can be easily overlooked during colonoscopy. The development of accessory devices and new modalities is expected to improve observation in “blind” areas of the colon[27-29]. One technique for improving the visual field in blind areas where the colon is sharply angled might be to actively push the colonoscope in order to straighten the colon. Among the possible reasons for PCCRC, “incomplete resection” and “inadequate examination” were considered. We experienced a case of PCCRC after piecemeal EMR for a 20-mm sessile serrated adenoma/polyp (SSA/P) in the transverse colon (Figure 6). Although histopathological examination revealed high-grade dysplasia with a negative margin and no lymphovascular involvement, the lesion recurred as a submucosal deeply invasive cancer at 11 mo after the treatment. We speculate that histopathological assessment of the tumor margin for this type of divided specimen may not have been accurate, and that some high-grade dysplasia may have remained in situ after initial colonoscopy. Unclear margin of SSA/P may result in incomplete resection. Pohl et al[30] reported incomplete resection rate for SSAP was higher than for conventional adenoma (31.0% vs 7.2%). Moreover, Zhu et al[31] found that for colorectal serrated polyps, a large size (≥ 10 mm) and histologic subtype (SSA/P and conventional serrated adenoma) were significantly associated with synchronous CRC. SSA/P should be resected en bloc especially when it exceeds 10 mm in size. Finally, one advanced PCCRC case that arose in the cecum after 9 mo was probably attributable to poor preparation at initial colonoscopy (Figure 7). This case serves to illustrate that residual stools at colonoscopy can hide not only small polyps but also large advanced CRCs. Early repeat colposcopy is therefore recommended for patients who have undergone colonoscopy after low-quality bowel preparation[32].
Our study had several limitations. First, the total number of PCCRC cases at the two hospitals was small (n = 11) during short study period from 2010 to 2015, and insufficient for investigating the factors associated with PCCRC using a multivariate logistic regression model. This is because HD colonoscopy has been available since 2006 at the both hospitals and patients with PCCRC diagnosed within 36 mo after initial HD colonoscopy began to be recruited in 2010. A further study including a larger number of PCCRC cases in an Asian setting will be necessary. Second, we did not have any information about the indications for colonoscopy, use of prophylactic medicines (e.g., aspirin) and family history of CRC, which could potentially affect the incidence of PCCRC. Third, the data on the PCCRC rate with SD colonoscopy in our hospitals were not available before HD colonoscopy was introduced. It would be better to compare the PCCRC rate using HD colonoscopy with that using SD colonoscopy in the same hospitals. Finally, as all of the examinations were performed by experienced gastroenterologists, our data cannot be generalized to non-gastroenterologists or inexperienced colonoscopists.
In conclusion, we have shown that the PCCRC rate with HD colonoscopy in our present series was 0.7%-1.7%, being lower than that for SD colonoscopy in previous studies using the same methodology. Further advances in technology may help to reduce the PCCRC rate in the future.
Post-colonoscopy colorectal cancers (PCCRC) has been recognized as a key quality indicator for colonoscopy. The data of PCCRC has been reported from Western counties, however that from Asian countries is lacking. Theoretically, HD colonoscopy has the potential to reduce the incidence of PCCRC, but clinical data related to this issue are still insufficient.
The PCCRC rate at two academic centers might help to set a benchmark for the quality of colonoscopy in Asian countries, where data on PCCRC are scarce.
To investigate the PCCRC rate for HD colonoscopy compared with that for standard-definition colonoscopy reported previously.
We retrospectively examined the medical records of consecutive adult patients with CRC between 2010 and 2015 at Sano Hospital (SH) and Dokkyo Medical University Koshigaya Hospital (DMUKH) in Japan. Patients with CRC diagnosed within 6 to 36 mo of HD colonoscopy were classified as a PCCCRC group, and the others as a non-PCCRC group. The primary outcome was the PCCRC rate with HD colonoscopy. The secondary outcomes were factors associated with PCCRC and possible reason for occurrence of early and advanced PCCRC.
We analyzed 851 patients with 892 CRCs including 11 of PCCRC and 881 of non-PCCRC. The PCCRC rate was 1.7% (8/471) at SH and 0.7% (3/421) at DMUKH. Factors significantly associated with PCCRC were smaller size, a shallower invasion depth, a non-polypoid macroscopic appearance, and an earlier stage. The leading possible reason was non-polypoid shape for early PCCRC and blinded location for advanced PCCRC.
We demonstrated the lower PCCRC rate for high-definition colonoscopy compared for standard-definition colonoscopy reported previously (0.7%-1.7% vs 1.8%-9.0%). Technological advance from standard-definition to high-definition colonoscopy has the potential to reduce the incidence of PCCRC.
Early PCCRC may be missed by inconspicuous macroscopic type, and advanced PCCRC by the position in blinded location. Endoscopists should be aware that even large advanced CRC can be easily missed during colonoscopy. We should learn the reason why we missed CRC during colonoscopy and prevent the PCCRC in the future. The development of accessory devices and new modalities are expected to improve observation in “blind” areas of the colon and decrease the PCCRC.
We wish to thank Sachiyo Komai, Takahiro Utsumi, Akira Teramoto, and Daizen Hirata for their help in obtaining and analyzing data of this study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Langner C, Vujasinovic M S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25541] [Article Influence: 1824.4] [Reference Citation Analysis (7)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3127] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 3. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2286] [Article Influence: 175.8] [Reference Citation Analysis (2)] |
| 4. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1158] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 5. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1468] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 6. | Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 449] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 7. | Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 404] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 9. | Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044-3052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 10. | Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012;61:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Samadder NJ, Curtin K, Tuohy TM, Pappas L, Boucher K, Provenzale D, Rowe KG, Mineau GP, Smith K, Pimentel R. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 12. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 13. | Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 14. | le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Tanaka S, Saitoh Y, Matsuda T, Igarashi M, Matsumoto T, Iwao Y, Suzuki Y, Nishida H, Watanabe T, Sugai T. Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 2015;50:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, Burke CA, Snover DC, Bresalier RS, McKeown-Eyssen G. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34-41. [PubMed] |
| 18. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 19. | Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 20. | Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: The narrowing divide between East and West. Dig Endosc. 2016;28:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 22. | Morris EJ, Rutter MD, Finan PJ, Thomas JD, Valori R. Post-colonoscopy colorectal cancer (PCCRC) rates vary considerably depending on the method used to calculate them: a retrospective observational population-based study of PCCRC in the English National Health Service. Gut. 2015;64:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 23. | Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology. 2007;132:2297-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 267] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 556] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 25. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, Tanaka S, Watanabe H, Sung JJ, Feld AD, Inadomi JM, O’Brien MJ, Lieberman DA, Ransohoff DF, Soetikno RM, Triadafilopoulos G, Zauber A, Teixeira CR, Rey JF, Jaramillo E, Rubio CA, Van Gossum A, Jung M, Vieth M, Jass JR, Hurlstone PD. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-S47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 26. | Matsuda T, Saito Y, Hotta K, Sano Y, Fujii T. Prevalence and clinicopathological features of nonpolypoid colorectal neoplasms: should we pay more attention to identifying flat and depressed lesions? Dig Endosc. 2010;22 Suppl 1:S57-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Westwood DA, Alexakis N, Connor SJ. Transparent cap-assisted colonoscopy versus standard adult colonoscopy: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Biecker E, Floer M, Heinecke A, Ströbel P, Böhme R, Schepke M, Meister T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Gralnek IM. Emerging technological advancements in colonoscopy: Third Eye® Retroscope® and Third Eye® Panoramic(TM), Fuse® Full Spectrum Endoscopy® colonoscopy platform, Extra-Wide-Angle-View colonoscope, and NaviAid(TM) G-EYE(TM) balloon colonoscope. Dig Endosc. 2015;27:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 31. | Zhu H, Zhang G, Yi X, Zhu X, Wu Y, Liang J, Zhang S, Zeng Y, Fan D, Yu X. Histology subtypes and polyp size are associated with synchronous colorectal carcinoma of colorectal serrated polyps: a study of 499 serrated polyps. Am J Cancer Res. 2014;5:363-374. [PubMed] |
| 32. | Clark BT, Rustagi T, Laine L. What level of bowel prep quality requires early repeat colonoscopy: systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am J Gastroenterol. 2014;109:1714-1723; quiz 1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |