Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7407
Peer-review started: July 13, 2017
First decision: August 10, 2017
Revised: August 24, 2017
Accepted: September 6, 2017
Article in press: September 5, 2017
Published online: November 7, 2017
Processing time: 114 Days and 20.1 Hours
To investigate human epidermal growth factor 2 (HER2) overexpression and validate its prognostic effect in stage II-III gastric cancer.
We reviewed the data of patients who were diagnosed with gastric cancer between March 2008 and October 2013 at the Yonsei University Medical Center. Among these patients, 384 patients who met the inclusion criteria were analyzed retrospectively.
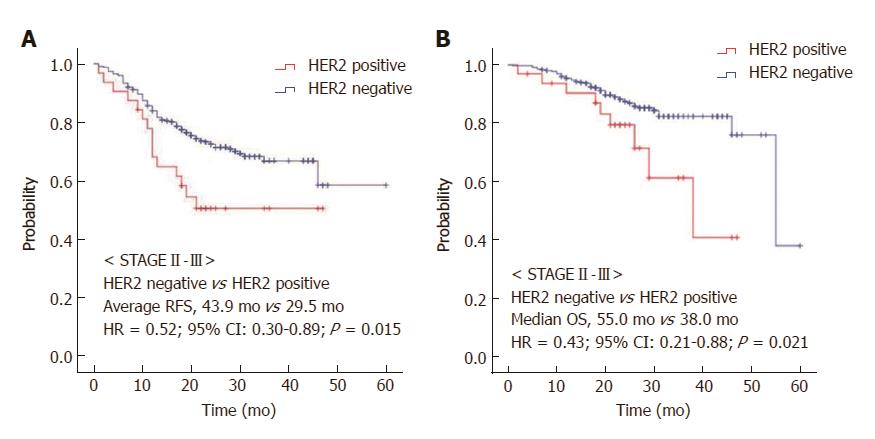
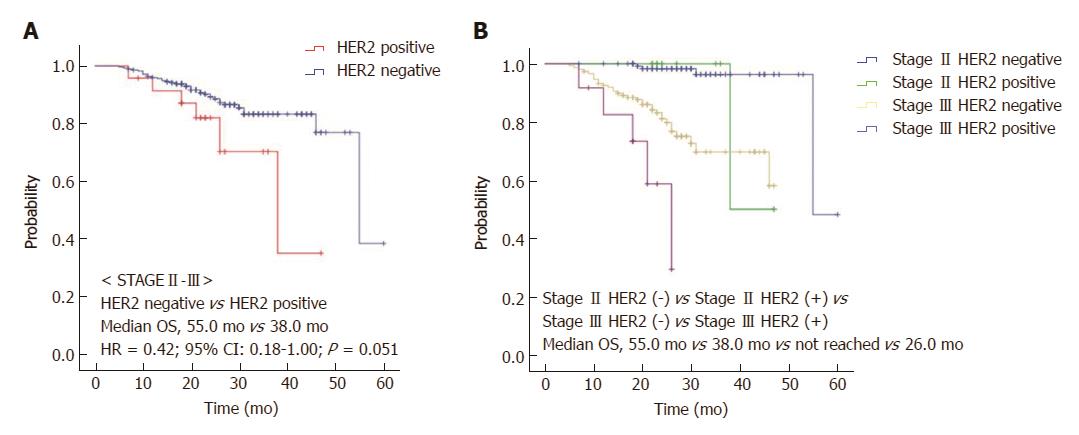
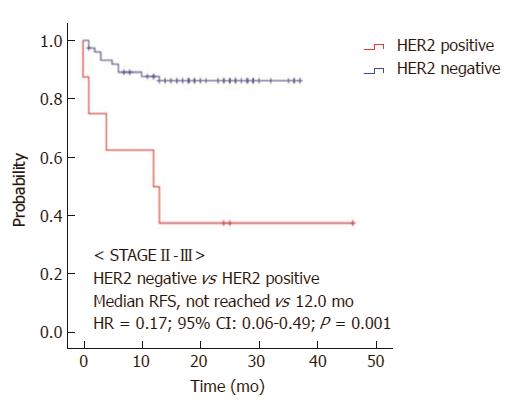
Thirty-two (8.3%) of the 384 stage II-III gastric cancer patients exhibited HER2 overexpression. The median follow-up duration was 26.0 mo. HER2-negative patients had superior recurrence-free survival (RFS) compared to HER2-positive patients (HR = 0.52, 95%CI: 0.30-0.89; P = 0.015). The median overall survival (OS) was significantly prolonged in the HER2-negative group compared with the HER2-positive group (55.0 mo vs 38.0 mo, HR = 0.43, 95%CI: 0.21-0.88, P = 0.021). OS was also prolonged in HER2-negative patients who received adjuvant chemotherapy compared to HER2-positive patients (55.0 vs 38.0 mo, HR = 0.42, 95%CI: 0.18-1.00, P = 0.051). In patients who did not receive adjuvant chemotherapy, the median RFS was prolonged in the HER2-negative group compared to the HER2-positive group (not reached vs 12.0 mo, HR = 0.17, 95%CI: 0.06-0.49, P = 0.001). In a multivariate analysis, HER2 status (HR = 0.421, 95%CI: 0.206-0.861, P = 0.018) and Eastern Cooperative Oncology Group performance status (HR = 2.002, 95%CI: 1.530-2.618, P < 0.001) were independent predictors of OS.
Our findings showed that HER2-positive patients had inferior OS and RFS. Stage II-III HER2-positive patients might be potential candidates for targeted therapies involving trastuzumab.
Core tip: Our study aimed to investigate human epidermal growth factor 2 (HER2) overexpression and validate its prognostic effect in stage II-III gastric cancer. Clinical data from 384 patients were analyzed. HER2-positive patients had inferior overall survival and recurrence-free survival. Stage II-III HER2-positive patients might be potential candidates for targeted therapies involving trastuzumab.
- Citation: Cho JH, Lim JY, Cho JY. Survival analysis based on human epidermal growth factor 2 status in stage II-III gastric cancer. World J Gastroenterol 2017; 23(41): 7407-7414
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7407.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7407
Gastric cancer is the fifth most common type of cancer, and half of all cases worldwide occur in East Asia[1]. In addition, gastric cancer is the third leading cause of cancer-related deaths worldwide[1,2]. In South Korea, gastric cancer is the second most commonly diagnosed cancer after thyroid cancer, and remains the third-leading cause of cancer-related mortality after lung cancer and hepatocellular carcinoma. More than 30000 patients are diagnosed annually in South Korea, and more than 10000 patients die annually[3]. Curative resection is the gold standard of treatment, and systemic chemotherapy prolongs the survival of patients with recurrent or metastatic gastric cancer[4]. However, the 5-year survival rates for advanced or metastatic gastric cancer are only approximately 5%-20%, with a median overall survival duration of < 1 year. Classically, only cytotoxic agents have been administered for malignant cancer. Recently, however, molecular pathologic research has been widely conducted, and molecular targeted agents have been applied for cancer treatment, leading to personalized treatment. The identification of biomarkers predictive of prognosis and drug responses is an important issue.
One well-established target is human epidermal growth factor receptor 2 (HER2, ERBB2), one of a family of receptors associated with tumor cell proliferation, apoptosis, adhesion, migration, and differentiation. Increasing evidence suggests that HER2 is an important biomarker and key driver of tumorigenesis in gastric cancer, with studies reporting amplification or overexpression in 7%-34% of tumors[5-7]. Despite conflicting results[8,9], some studies have suggested that HER2 positivity is associated with poor outcomes and an aggressive profile in gastric cancer.
Trastuzumab (Herceptin), a monoclonal antibody that targets HER2, induces antibody-dependent cellular cytotoxicity, inhibits HER2-mediated signaling, and prevents cleavage of the extracellular domain of HER2[10]. In HER2-positive breast cancers, trastuzumab has yielded survival advantages in patients with early and metastatic disease and is now the standard of care. In patients with metastatic breast cancer, high levels of HER2 protein expression and amplification indicate better outcomes with trastuzumab[11-14].
The antitumor activity of trastuzumab, as observed in human gastric cancer xenograft models, warrants the consideration of its use in clinical treatment regimens for gastric cancer patients in combination with various chemotherapeutic agents[15]. In 2010, the Trastuzumab for Gastric Cancer (ToGA) trial, an international phase 3, open-label, randomized controlled trial, demonstrated a significant survival benefit of trastuzumab plus chemotherapy in patients with advanced gastric or gastro-esophageal junction (GEJ) cancer whose tumors were found to overexpress the HER2 protein via immunohistochemistry or gene via fluorescence in-situ hybridization (FISH). This trial revealed significant increases in overall survival (OS, 13.8 mo vs 11.1 mo), progression-free survival (PFS, 6.7 mo vs 5.5 mo), and overall response rates (47% vs 35%). Furthermore, an exploratory analysis identified that patients with strong HER2 protein expression [immunohistochemistry [(IHC) 3(+) or IHC 2(+)/FISH (+)] were more likely to exhibit improved OS with the addition of trastuzumab (16.0 mo vs 11.8 mo)[16]. Subsequently, trastuzumab combined with chemotherapy was established as a standard treatment for HER2-positive gastric cancer patients.
Efforts to improve survival among Korean gastric cancer patients begin with an understanding of the clinical practice patterns actually used in Korean hospitals. This study aimed to investigate the frequency of HER2 overexpression among gastric cancer patients and evaluate the relationship between HER2 overexpression and prognosis.
We reviewed the data of 4680 patients who were diagnosed with gastric cancer between March 2008 and October 2013 at the Yonsei University Medical Center in South Korea. Among these patients, the inclusion criteria were as follows: (1) histologically confirmed gastric adenocarcinoma; (2) diagnosis between 2006 and 2013; and (3) HER2 expression status evaluation in a primary gastric tumor. Patients with a second primary tumor within 5 years were excluded. A total of 384 patients met the eligibility criteria. We analyzed the data of patients who were initially diagnosed stage II or III on the pathology report (according to the American Joint Committee on Cancer TNM staging, 7th edition). Approximately 15% of the total patient cohort was expected to be HER2-positive based on the proportions and prognostic power of HER2 status[5-7]. The study was approved by the Yonsei University Health System Institutional Review Board (IRB #3-2013-0188).
Clinicopathologic parameters were collected from outpatient clinical or admission records, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, diagnosis date, curative resection date, adjuvant chemotherapy (regimen and duration), recurrence date, last follow-up date, date of death, histologic subtype, TNM stage, HER2 IHC, and HER2 FISH; information regarding patient survival was obtained from the Korean National Statistics Registry Database.
HER2 overexpression and gene amplification were examined with semiquantitative standardized IHC staining using the DAKO-HercepTestTM, silver in situ hybridization, and FISH. HER2 IHC results were classified as 0/1+/2+/3+. HER2 positivity was defined as (1) IHC of 3+ or (2) IHC of 2+ with HER2-FISH amplification. HER2 negativity was defined as (1) IHC of 0/1+ or (2) IHC of 2+ with no HER2-FISH amplification. OS was defined as the duration from gastrectomy to gastric cancer-specific death or the last follow-up. RFS was measured from gastrectomy to recurrence in patients diagnosed with stage II or III gastric cancer.
All statistical analyses were performed using SPSS version 21.0 (IBM Co., Armonk, NY, United States). For continuous variables, two-tailed Student’s t-tests were used to compare the demographic and clinical characteristics. The χ2 was used for discrete variables. Survival rates and 95%CI were calculated using the Kaplan-Meier method. The influences of covariates on survival length between the two treatment arms were assessed using the log-rank test. A P < 0.05 was considered significant. Significant variables in the univariate analysis were entered in the multivariate analysis using the Cox proportional hazards model. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors version 1.1.
A total of 384 patients with HER2 status data were analyzed in this study. The patients’ baseline characteristics are described in Table 1. The median patient age was 61.7 years (range: 28-90 years), and 238 patients (62.0%) were men. Nearly all patients (99.7%) had an ECOG performance status of 0 or 1. Pathologic differentiation was confirmed as well differentiated, moderately differentiated, or poorly differentiated tubular adenocarcinoma, signet ring cell carcinoma, and other in 10 (2.6%), 98 (25.5%), 207 (53.9%), 60 (15.6%), and 9 cases (2.3%), respectively.
| Characteristic | Stage II (n = 193, II A: 97, II B: 96) | Stage III (n = 191, III A: 55, III B: 63, III C: 73) |
| Median age (range, yr) | 61.27 ± 12.75 (28-90) | 62.21 ± 13.20 (33-86) |
| Gender | ||
| Male | 124 (64.2) | 114 (59.7) |
| Female | 69 (35.8) | 77 (40.3) |
| ECOG PS | ||
| 0 | 119 (61.7) | 76 (39.8) |
| 1 | 74 (38.3) | 114 (59.7) |
| 2 | 0 | 1 (0.5) |
| HER2 status | ||
| HER2 positive | ||
| IHC 3 (+) | 15 (7.8) | 13 (6.8) |
| IHC 2 (+)/FISH (+) | 2 (1.0) | 2 (1.0) |
| HER2 negative | ||
| IHC 2 (+)/FISH (-) | 6 (3.1) | 4 (2.1) |
| IHC 1 (+) or 0 | 168 (87.1) | 169 (88.5) |
| IHC not done/FISH (-) | 2 (1.0) | 3 (1.6) |
| Pathologic differentiation | ||
| WD TA | 7 (3.6) | 3 (1.6) |
| MD TA | 54 (28.0) | 44 (23.0) |
| PD TA | 96 (49.7) | 111 (58.1) |
| Signet ring cell carcinoma | 33 (17.1) | 27 (14.1) |
| Others | 3 (1.6) | 6 (3.2) |
| T stage | ||
| T1 | 17 (88.1) | 0 |
| T2 | 48 (24.9) | 4 (2.1) |
| T3 | 90 (46.6) | 51 (26.7) |
| T4 | 38 (19.7) | 136 (71.2) |
| N stage | ||
| N0 | 97 (50.3) | 1 (0.5) |
| N1 | 60 (31.0) | 30 (15.7) |
| N2 | 33 (17.1) | 52 (27.3) |
| N3 | 3 (1.6) | 108 (56.5) |
| Received adjuvant chemotherapy | ||
| Yes | 141 (73.1) | 161 (84.3) |
| No | 52 (26.9) | 30 (15.7) |
HER2 overexpression status was confirmed based on an IHC result of 3+ in 28 patients (7.3%) and based on an IHC of 2+/FISH (+) in 4 patients (1.0%). The HER2 status was confirmed by FISH alone in 5 patients (1.3%).
As shown in Table 1, 32 (8.3%) of the 384 gastric adenocarcinoma patients with stage II to III disease exhibited HER2 overexpression. Seventeen (8.8%) stage II patients and 15 (7.8%) stage III patients had HER2 overexpression.
A high correlation between HER2 expression and the intestinal histologic type has been confirmed in recent studies[5]. Our study showed a higher rate of HER2 overexpression in the intestinal type than in the diffuse type (17% vs 2.5%, P < 0.001; Table 2). In the ToGA trial, HER2 positivity differed significantly by histological subtype (intestinal 34%, diffuse 6%, and mixed 20%)[16]. Moreover, numerous studies have reported that HER2 expression is more common in GEJ cancers than in gastric cancers. Our study found 20% and 9.5% HER2 expression in GEJ and gastric cancers, respectively (P = 0.008; Table 2). These results are similar to those of the ToGA study, in which HER2 positivity was found in 32% and 18% of GEJ and gastric cancers, respectively[16].
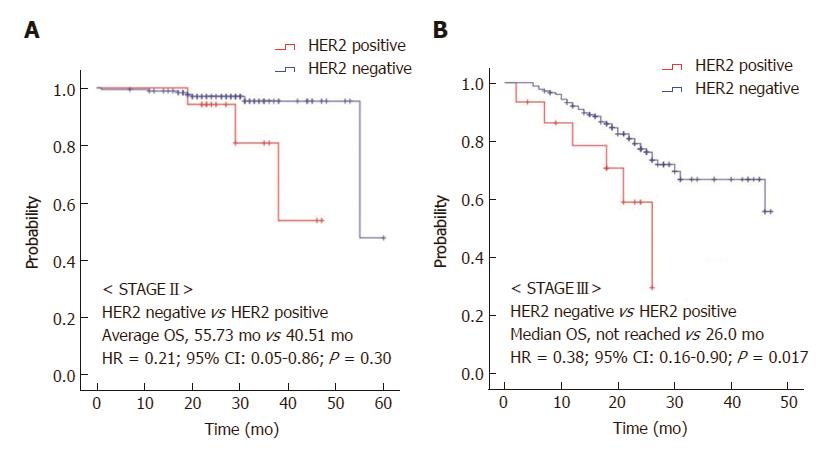
The median follow-up duration was 26.0 mo (95%CI: 24.9-27.1 mo). In stage II and III gastric cancer patients, HER2-negative patients had improved RFS compared with HER2-positive patients (HR = 0.52, 95%CI: 0.30-0.89, P = 0.015, Figure 1A). The median OS was significantly prolonged in the HER2-negative group compared with the HER2-positive group (55.0 mo vs 38.0 mo, HR = 0.43, 95%CI: 0.21-0.88, P = 0.021, Figure 1B). We also found that in each stage, HER2-negative patients had a significantly improved OS relative to HER2-positive patients. Stage II HER2-negative patients had improved OS compared to stage II HER2-positive patients, although the difference was not statistically significant (HR = 0.21, 95%CI: 0.05-0.86, P = 0.30, Figure 2A). In stage III, the median OS was not reached in the HER2-negative group, as compared to 26.0 mo (95%CI: 18.6-33.4 mo) in the HER2-positive group (HR = 0.38, 95%CI: 0.16-0.90, P = 0.017, Figure 2B).
We also analyzed survival based on HER2 expression stratifying by the administration of adjuvant chemotherapy. OS was prolonged in the HER2-negative group in stage II-III patients who received adjuvant chemotherapy relative to the HER2-positive group (55.0 vs 38.0 mo, HR = 0.42, 95%CI: 0.18-1.00, P = 0.051; Figure 3A and B). In stage II-III patients who did not receive adjuvant chemotherapy, the median RFS was prolonged in the HER2-negative group relative to the HER2-positive group (not reached vs 12.0 mo, HR = 0.17, 95%CI: 0.06-0.49, P = 0.001; Figure 4).
A univariate analysis of all patients identified HER2 status and ECOG performance status as prognostic factors associated with survival. After adjusting for covariates in a multivariate analysis, HER2 status (HR = 0.421, 95%CI: 0.206-0.861, P = 0.018) and ECOG performance status (HR = 2.002, 95%CI: 1.530-2.618, P < 0.001) remained independent predictors of OS (Table 3).
| Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| HER2 status | 0.424 (0.208-0.865) | 0.018 | 0.421 (0.206-0.861) | 0.018 |
| WD-MD vs PD-SRC | 0.694 (0.374-1.290) | 0.248 | ||
| Sex | 1.376 (0.821-2.307) | 0.226 | ||
| ECOG PS | 2.149 (1.648-2.803) | < 0.001 | 2.002 (1.530-2.618) | < 0.001 |
| Age (< 65) | 1.408 (0.837-2.369) | 0.197 | ||
| Adjuvant chemotherapy | 1.750 (0.978-3.128) | 0.059 | ||
We showed that HER2-positive patients had worse prognosis compared with HER2-negative patients in stage II and III gastric cancer patients and that HER2 status was independent predictor of OS in multivariate analysis. We also found that only 8.3% of the study patients with stage II and III gastric cancer exhibited HER2 overexpression.
There is no standard post-operative adjuvant chemotherapy in stage II-III gastric cancer patients. To the best of our knowledge, there are few studies about targeted therapies in the adjuvant setting. Although many studies have reported on the outcomes of patients with unresectable or metastatic gastric cancer receiving single or combination chemotherapy[4], it is very hard to improve patient outcomes with chemotherapy alone. For some cancers, targeted therapies have been developed, and several targeted agents have been evaluated for gastric cancer[17-19]; however, most did not improve survival relative to standard chemotherapy. In contrast, trastuzumab, a recombinant humanized anti-HER2 monoclonal antibody, has been proven effective in the ToGA trial[16]. This agent is now a standard first-line treatment for HER2-positive metastatic gastric cancer in combination with chemotherapy. In addition, predictive biomarkers of trastuzumab response are known. Recently, ramucirumab yielded impactful results in refractory gastric cancer patients[20,21]; however, predictive markers for this agent have not been validated.
This study aimed to investigate prognosis based on HER2 status in stage II-III gastric cancer patients. Our study showed that evaluation of the predictive biomarker HER2 was too infrequent among gastric cancer patients. We reviewed medical records from 4680 patients but only found 834 patients with HER2 status information. In other words, only approximately 18% of patients underwent HER2 status testing. Most stage IV gastric cancer patients were tested, but most stage II and III patients (i.e., those with early or operable gastric cancer) were not tested. Generally, HER2 status is only tested if, following complete resection, gastric cancer recurs. This leads to a significant loss of time. If cancer pathology samples are no longer available, rebiopsy of the recurrent lesion is needed. Therefore, all gastric cancer patients should undergo early HER2 status testing, irrespective of the initial stage.
The carcinogenic role of HER2 in gastric cancer has been investigated. Although the results of the studies have been conflicting, many studies demonstrated that HER2 protein overexpression negatively affects survival in gastric cancer patients[5-7]. Our study also showed that HER2 positivity was an independent factor predictive of OS in a multivariate analysis. We observed a 57% reduction in the risk of death in this study in HER2-positive patients.
The current study analyzed survival according to HER2 status. In stage II-III disease, regardless of stage, the median OS was significantly better among HER2-negative patients than among HER2-positive patients. The RFS was also improved among HER2-negative patients with stage II-III disease than among corresponding HER2-positive patients. Most patients initially diagnosed with stage II-III disease undergo gastrectomy and are treated with adjuvant chemotherapy to reduce the risk of recurrence. However, the benefit of adjuvant chemotherapy is not convincing. Each patient treated with adjuvant chemotherapy incurs different effects. Trastuzumab was first developed as a targeted agent for HER2-positive breast cancer. Many of the clinical trials that demonstrated clinical efficacy and safety were conducted in a HER2-positive breast cancer adjuvant setting. Accordingly, trastuzumab is currently a component of a standard adjuvant therapy regimen for HER2-positive breast cancer that also includes chemotherapy. We suggest that trastuzumab or other humanized monoclonal antibodies might play a similar role in an adjuvant setting in patients with stage II-III HER2-positive gastric cancer. Although some experimental studies have been conducted[22], and further studies are required.
In our study of Korean patients with gastric adenocarcinoma, the frequency of HER2 protein overexpression was similar to that reported in other articles. Our findings show that HER2-positive gastric cancer patients have an inferior OS and RFS compared to HER2-negative patients. We suggest that stage II-III patients exhibiting HER-2/neu amplification might be potential candidates for new adjuvant therapies involving the use of humanized monoclonal antibodies. These results will provide essential data for evidence-based strategies of gastric cancer control in South Korea.
One well-established target is human epidermal growth factor receptor 2 (HER2, ERBB2), one of a family of receptors associated with tumor cell proliferation, apoptosis, adhesion, migration, and differentiation. Increasing evidence suggests that HER2 is an important biomarker and key driver of tumorigenesis in gastric cancer, with studies reporting amplification or overexpression in 7%-34% of tumors. Despite conflicting results, some studies have suggested that HER2 positivity is associated with poor outcomes and an aggressive profile in gastric cancer.
In 2010, the Trastuzumab for Gastric Cancer (ToGA) trial, an international phase 3, open-label, randomized controlled trial, demonstrated a significant survival benefit of trastuzumab plus chemotherapy in patients with advanced gastric or gastro-esophageal junction cancer whose tumors were found to overexpress the HER2 protein via immunohistochemistry or gene via fluorescence in-situ hybridization (FISH). This trial revealed significant increases in overall survival, progression-free survival, and overall response rates. Efforts to improve survival among Korean gastric cancer patients begin with an understanding of the clinical practice patterns actually used in Korean hospitals. This study aimed to investigate the frequency of HER2 overexpression among gastric cancer patients and evaluate the relationship between HER2 overexpression and prognosis.
The authors showed that HER2-positive patients had worse prognosis compared with HER2-negative patients in stage II and III gastric cancer patients and that HER2 status was independent predictor of OS in multivariate analysis. OS was also prolonged in HER2-negative patients who received adjuvant chemotherapy compared to HER2-positive patients. In patients who did not receive adjuvant chemotherapy, the median RFS was prolonged in the HER2-negative group compared to the HER2-positive group. In a multivariate analysis, HER2 status and Eastern Cooperative Oncology Group performance status were independent predictors of OS. The study also showed that evaluation of the predictive biomarker HER2 was too infrequent among gastric cancer patients.
The findings show that HER2-positive gastric cancer patients have an inferior OS and RFS compared to HER2-negative patients. The authors suggest that stage II-III patients exhibiting HER-2/neu amplification might be potential candidates for new adjuvant therapies involving the use of humanized monoclonal antibodies. The authors suggest that trastuzumab or other humanized monoclonal antibodies might play a similar role in an adjuvant setting in patients with stage II-III HER2-positive gastric cancer. These results will provide essential data for evidence-based strategies of gastric cancer control in South Korea.
This is a well-written manuscript showing survival analysis based on human epidermal growth factor 2 status in stage II-III gastric cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Giordano A, Li W, Nagahara H S- Editor: Wei LJ L- Editor: A E- Editor: Huang Y
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20491] [Article Influence: 2049.1] [Reference Citation Analysis (20)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25535] [Article Influence: 1823.9] [Reference Citation Analysis (7)] |
| 3. | National Cancer Information Center. Cancer registry. Goyang: National Cancer Information Center, c2012 [cited 2012; July 22] Available from: http://www. cancer.go.kr/cms/statics/ incidence/index.html. |
| 4. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 894] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 5. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 869] [Article Influence: 51.1] [Reference Citation Analysis (2)] |
| 6. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 523] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 7. | Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Tateishi M, Toda T, Minamisono Y, Nagasaki S. Clinicopathological significance of c-erbB-2 protein expression in human gastric carcinoma. J Surg Oncol. 1992;49:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Sasano H, Date F, Imatani A, Asaki S, Nagura H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993;24:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1924] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 11. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3742] [Cited by in RCA: 3678] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 12. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4010] [Cited by in RCA: 3920] [Article Influence: 196.0] [Reference Citation Analysis (0)] |
| 13. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8122] [Article Influence: 338.4] [Reference Citation Analysis (0)] |
| 14. | Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1058] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 15. | Fujimoto-Ouchi K, Sekiguchi F, Yasuno H, Moriya Y, Mori K, Tanaka Y. Antitumor activity of trastuzumab in combination with chemotherapy in human gastric cancer xenograft models. Cancer Chemother Pharmacol. 2007;59:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5296] [Article Influence: 353.1] [Reference Citation Analysis (3)] |
| 17. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 18. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 679] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 19. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 20. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1761] [Article Influence: 160.1] [Reference Citation Analysis (0)] |
| 21. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1569] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 22. | Li W, Zhao H, Qian W, Li H, Zhang L, Ye Z, Zhang G, Xia M, Li J, Gao J. Chemotherapy for gastric cancer by finely tailoring anti-Her2 anchored dual targeting immunomicelles. Biomaterials. 2012;33:5349-5362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Gravalos C, Márquez A, Colomer R, García-Garbonero R, Sastre J, Rivera F, Saenz Cusi A, Velasco A, Guzman C, Jimeno A. Correlation between HER2/neu overexpression/amplification and clinicopathological parameters in advanced gastric cancer patients: a prospective study 2007. Presented at: Gastrointestinal Cancers Symposium 130: abstract 89. Available from: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=45&abstractID=10315. |
| 24. | Lordick F, Bang YJ, Kang YK, Otero Reyes D, Manikhas GM, Shen L, Kulikov E, Stoss O, Jordan BWM, Van Cutsem E. HER2-positive advanced gastric cancer: similar HER2-positivity levels to breast cancer. Eur J Cancer. 2007;5:271. |