Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7303
Peer-review started: July 11, 2017
First decision: July 27, 2017
Revised: August 15, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: October 28, 2017
Processing time: 123 Days and 22 Hours
To investigate whether the short-term prognosis of hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF) could be improved by using a modified model for end-stage liver disease (MELD) including serum lactate.
This clinical study was conducted at the First Affiliated Hospital, Fujian Medicine University, China. From 2009 to 2015, 236 patients diagnosed with HBV-related ACLF at our center were recruited for this 3-month follow-up study. Demographic data and serum lactate levels were collected from the patients. The MELD scores with or without serum lactate levels from survival and non-survival groups were recorded and compared.
Two hundred and thirty-six patients with HBV-ACLF were divided into two groups: survival group (S) and non-survival group (NS). Compared with the NS group, the patients in survival the S group had a significantly lower level of serum lactate (3.11 ± 1.98 vs 4.67 ± 2.43, t = 5.43, P < 0.001) and MELD score (23.33 ± 5.42 vs 30.37 ± 6.58, t = 9.01, P = 0.023). Furthermore, serum lactate level was positively correlated with MELD score (r = 0.315, P < 0.001). Therefore, a modified MELD including serum lactate was developed by logistic regression analysis (0.314 × lactate + 0.172 × MELD - 5.923). In predicting 3-month mortality using the MELD-LAC model, the patients from the S group had significantly lower baseline scores (-0.930 ± 1.34) when compared with those from the NS group (0.771 ± 1.32, t = 9.735, P < 0.001). The area under the receiver operating characteristic curve (AUROC) was 0.859 calculated by using the MELD-LAC model, which was significantly higher than that calculated by using the lactate level (0.790) or MELD alone (0.818). When the cutoff value was set at -0.4741, the sensitivity, specificity, positive predictive value and negative predictive value for predicting short-term mortality were 91.5%, 80.10%, 94.34% and 74.62%, respectively. When the MELD-LAC scores at baseline level were set at -0.5561 and 0.6879, the corresponding mortality rates within three months were 75% and 90%, respectively.
The short-term prognosis of HBV-related ACLF was improved by using a modified MELD including serum lactate from the present 6-year clinical study.
Core tip: This is a retrospective study to evaluate the short-term prognosis of hepatitis B virus (HBV)-acute-on-chronic liver failure (ACLF). Two hundred and thirty-six patients with HBV-ACLF were divided into two groups: survival group (S) and non-survival group (NS). In predicting 3-mo mortality using the model for MELD-LAC model, patients from the S group had significantly lower baseline scores compared with those from NS group using model for end-stage liver disease (MELD)-LAC model. AUROC was 0.859 calculated by using the MELD-LAC model, which was significantly higher than those calculated by using the lactate level (0.790) or MELD alone (0.818). The short-term prognosis of HBV-ACLF was improved by using a modified MELD including serum lactate from the present 6-year clinical study.
- Citation: Chen W, You J, Chen J, Zheng Q, Jiang JJ, Zhu YY. Modified model for end-stage liver disease improves short-term prognosis of hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol 2017; 23(40): 7303-7309
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7303.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7303
Hepatitis B virus (HBV) infection is a severe public health problem all over the world. The prevalence of hepatitis B is particularly high in China. HBV-related acute-on-chronic liver failure (ACLF) is a rapidly progressive disease with a high mortality rate up to 60%-75%[1]. The accurate assessment of the disease severity is critical before clinicians can make decisions on potential treatments like medication or liver transplantation (LT). The model for end-stage liver disease (MELD)[2,3] is a well-accepted model for assessing the feasibility of LT; however, the accuracy of its prediction is still unsatisfying[4]. A more objective and quantitative model with higher repeatability to predict the short-term prognosis of HBV-related ACLF is urgently needed.
Lactate (LAC) is mainly metabolized in the liver and is widely used as an important indicator of organ failure or serious bacterial infection. Hyperlactatemia normally reflects both increased production and impaired clearance in patients with liver dysfunction[5], and a higher level of LAC always indicates a worse prognosis[6]. Although several potential hypoxic and non-hypoxic mechanisms have been implicated on the persistent hyperlactatemia and the high level of LAC, the exact role of these parameters has not been specifically addressed in clinical studies[7,8]. In this study, we investigated the serum LAC level in patients with HBV-related ACLF, and developed a modified MELD including serum lactate (MELD-LAC model) to predict the short-term prognosis (three months) of HBV-related ACLF.
Three hundred and ninety-three patients, who were diagnosed with HBV-related ACLF and admitted in our center, were included in the present clinical study between September 2009 and October 2015. The subjects were aged from 18 to 65 years. The diagnosis of ACLF was conducted according to the American Association for the Study of Liver Failure: Update 2011[9]. Three months after diagnosis, these patients were followed by telephone. The patients were divided into two groups: survival group (S group) and non-survival group (NS group), based on whether they were surviving or not three months after admission. The clinical data were collected within the first 24 h after admission, including hepatic, renal, and coagulation functions, as well as the LAC level. The onset time of hepatic encephalopathy (HE) was also noted if it happened. MELD scores were calculated as follows: MELD = 3.8 × Ln(Tbil [mg/dL]) + 11.2 × Ln(INR) + 9.6 × Ln (Cr [mg/dL]) + 6.4 × cause. Since the ACLF was due to HBV infection, the cause was counted as 1 in this study.
On admission, each patient’s baseline values of serum LAC level, total bilirubin, creatinine level and prothrombin time-international normalized ratio were measured. The serum LAC level was measured using a commercial test kit (Johnson & Johnson Inc., United States) with a normal value of 0.9-2.0 mmol/L. Total bilirubin and creatinine levels were tested using an Olympus Chemistry Analyzer (AU2700). All reagents for bilirubin and creatinine tests were purchased from Olympus Corporation (Japan). PT test and INR calculation were performed using a Stago STA Compact coagulation analyzer (Diagnotica Stago, French).
After HBV-related ACLF was diagnosed, all the patients were treated with standard protocols, including nutritional support, hepatocyte proliferation, prevention of infection, correction of anomalies in the coagulation system and improvement of cerebral edema. In addition, plasmapheresis was also performed when it was necessary. The survival data were collected after three months.
Data analyses were performed using SPSS 17.0 software. The significance of data was tested by the Student’s test or the χ2 test. The correlation between groups was analyzed by the Pearson's product-moment correlation coefficient test. P-values less than 0.05 were considered statistically significant. Logistic regression analysis was used to establish the prognosis model. The accuracies of the newly developed prognosis model (MELD-LAC model), LAC and MELD scores in predicting the short-term prognosis of HBV-related ACLF patients were assessed by the area under the receiver operating characteristic (AUROC) curve.
A total of 393 serum samples from patient with HBV-related ACLF were collected, 157 of which were excluded from the present study due to the complications of hepatocellular carcinoma, kidney injury, diabetes or alcoholic liver diseases. According to the follow-up results in the 236 enrolled cases, 130 (110 males and 20 females) were recruited into the S group, and 106 (87 males and 19 females) were recruited into the NS group. The two groups had no statistical difference in gender or age, but had significant differences in TBIL, Cr, INR, LAC levels and MELD scores (P < 0.05 for all) (Table 1). There were 68 patients in the S group and 57 patients in the NS group who had received plasmapheresis as an additional enhanced therapy, but it did not provide any positive outcome (P = 0.896). No patient in this study had received LT.
| Group | S (n = 130) | NS (n = 106) | t or t’ | P value |
| Age (yr) | 43.51 ± 14.13 | 45.97 ± 10.69 | 1.480 | 0.140 |
| Male/Female (n) | 110/20 | 87/19 | 0.273 | 0.363 |
| Liver cirrhosis, n (%) | 63 (48.46) | 68 (64.15) | 5.820 | 0.011 |
| TBil (µmol/L) | 344.85 ± 160.82 | 456.63 ± 180.43 | 5.040 | < 0.001 |
| ALT | 92.62 ± 82.80 | 101.84 ± 83.71 | 0.847 | 0.389 |
| AST | 92.81 ± 91.84 | 103.86 ± 79.20 | 0.978 | 0.329 |
| GLO | 27.72 ± 8.95 | 27.84 ± 8.85 | 0.100 | 0.920 |
| WBC | 8.27 ± 4.12 | 8.21 ± 4.29 | -0.117 | 0.907 |
| PCT | 0.53 ± 0.29 | 0.58 ± 0.54 | 0.886 | 0.377 |
| Ammonia | 67.29 ± 35.55 | 69.03 ± 47.57 | -0.312 | 0.755 |
| INR | 2.46 ± 0.97 | 3.11 ± 1.63 | 3.733 | < 0.001 |
| Cr (µmol/L) | 79.31 ± 45.25 | 99.80 ± 88.74 | 2.293 | < 0.001 |
| CHE (U/L) | 3956.30 ± 1377.54 | 3529.00 ± 1509.89 | 0.170 | 0.859 |
| Alb (g/L) | 31.22 ± 3.98 | 30.99 ± 3.96 | -0.428 | 0.398 |
| DNA | 594275 ± 230160 | 425886 ± 110810 | -0.691 | 0.491 |
| LAC (mmol/L) | 3.11 ± 1.98 | 4.67 ± 2.43 | 5.430 | < 0.001 |
| < 2.68 | 76 | 14 | 50.685 | 0 |
| 2.68-4 | 37 | 29 | 0.035 | 0.484 |
| ≥ 4 | 17 | 63 | 55.999 | < 0.001 |
| CTP | 10.54 ± 1.63 | 10.74 ± 1.73 | 0.892 | 0.373 |
| MELD score | 23.33 ± 5.42 | 30.37 ± 6.58 | 9.010 | 0.023 |
| Plasmapheresis | 68 | 57 | 0.05 | 0.896 |
Approximately 82.3% (107 of 130) of patients in the S group and 95.2% (101 of 106) of patients in the NS group were found to have elevated LAC level of more than 2 mmol/L. A significant difference was found in the above percentages between the two groups (χ2 = 9.40, P = 0.002). Furthermore, 13.1% of patients (17 of 130) in the S group and 59.4% (63 of 106) in NS group were found to have elevated LAC level of more than 4 mmol/L. A significant difference between the percentages of the S group and the NS group was also found (χ2 = 55.999, P < 0.001). The mean LAC level in the S group (3.11 mmol/L ± 1.98 mmol/L) was significantly lower than that in the NS group (4.67 mmol/L ± 2.43 mmol/L) (P < 0.001). No significant difference was shown in the CTP scores between the S and NS groups (P = 0.373), but a significant difference was shown in the MELD scores between the two groups (P = 0.023).
Using the Spearman analysis method, the correlations between MELD score/serum LAC levels and the mortality in three months were analyzed (Table 2). The results from Pearson’s analysis also showed that both serum LAC level and MELD score had a statistically significant correlation with the short-term prognosis of HBV-related ACLF (r = 0.315, P < 0.001).
| Factor | Correlation index | P value |
| MELD | 0.548 | < 0.001 |
| LAC | 0.499 | < 0.001 |
| TBiL | 0.308 | < 0.001 |
| INR | 0.289 | < 0.001 |
| Cr | 0.014 | 0.828 |
| Alb | 0.071 | 0.273 |
| CHE | 0.058 | 0.373 |
Based on single factor analysis, both MELD score and LAC level were correlated with the 3-mo mortality of HBV-related ACLF. The forward logistic regression method was used to establish the MELD-LAC model: 0.314 × LAC + 0.172 × MELD score - 5.923 (Table 3). The patients from the S group had significantly lower baseline MELD-LAC scores (-0.930 ± 1.34) compared with those from the NS group (0.771 ± 1.32, t = 9.735, P < 0.001).
| Index | β | SE | Wald | df | P value | Exp(β) |
| MELD | 0.172 | 0.028 | 37.198 | 1 | 0.000 | 1.188 |
| LAC | 0.314 | 0.098 | 10.195 | 1 | 0.001 | 1.368 |
| Constant | -5.923 | 0.824 | 51.724 | 1 | 0.000 | 0.003 |
The short-term prognosis of HBV-related ACLF was improved by using the modified MELD including serum lactate from the present 6-year clinical study.
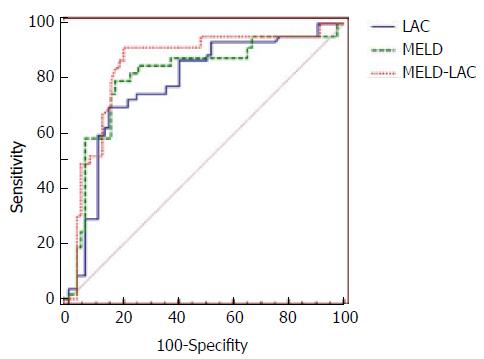
To predict the 3-month mortality, the LAC model alone had a very similar positive predictive value (PPV), negative predictive value (NPV), sensitivity and specificity to MELD. Interestingly, the new MELD-LAC model by combining these two independent factors had a better predicting score than these two models alone. When the cutoff value of the MELD-LAC model was set at -0.4741, based on the best Yoden index, an AUROC curve of 0.859 (Table 4) was obtained when this equation was applied to evaluate the prognosis, with a sensitivity of 91.5% and a specificity of 80.10%, which were much greater than the other two models. Comparing the AUROCs obtained from the above three models, the prognosis performances were listed as follows: MELD-LAC > MELD > LAC (Figure 1). From our analysis, the patients with an MELD-LAC score of -0.5561, -0.4741 and 0.6879 had the three-month mortality rate of 75%, 78.86% and 90%, respectively. The PPV and NPV of MELD-LAC were 94.34% and 74.62%, which were significantly improved compared with the scores obtained from the MELD and LAC model alone.
| model | AUROC | 95%CI | Cut-offvalue | Sensitivity | Specificity | PPV | NPV |
| MELD | 0.818 | 0.759-0.877 | 24.5 | 87.70 % | 63.80% | 85.85% | 64.62% |
| LAC | 0.79 | 0.730-0.850 | 2.68 | 86.80% | 62.10% | 86.79% | 58.46% |
| MELD-LAC | 0.859 | 0.807-0.911 | -0.4741 | 91.50% | 80.10% | 94.34% | 74.62% |
The patients were divided into two groups, when the cutoff value of the MELD-LAC model was set at -0.4741. The first group (MELD-LAC ≥ -0.4741) included 123 patients, and 78.86% were dead after three months. The second group (MELD-LAC < -0.4741) included 113 patients, and only 11.50% died after three months. A statistically significant difference was observed between these two groups (χ2 = 107.35, P < 0.001). When compared with MELD with a cutoff value of 25, the MELD-LAC model with a cutoff value of -0.4741 apparently achieved better prediction of short-term prognosis.
ACLF is a severe health problem with a high mortality rate[10-12]. In clinical practice, predicting the progression of this disease is the biggest challenge for clinicians. Currently, three scoring systems, namely, King’s College Hospital criteria, Child-Turcotte-Pugh (CTP) score and MELD score[4,13] systems, are commonly implemented to predict the prognosis of ACLF patients; CTP score has been used traditionally to assess the prognosis of cirrhosis instead of ACLF; the MELD scoring system has been developed to assess various liver diseases, including assessing the severity of ACLF of all causes to determine the ideal timing of liver transplantation, as well as providing direct information to support medical decision making[14]. The MELD scoring system is widely considered to be better than the CTP score; however, it does have several limitations, for example, the diagnostic sensitivity and specificity of MELD are not high enough.
In the MELD scoring system, several variables associated with poor prognosis of ACLF are not considered, including HE, infections, and hemorrhage. Therefore, modification for MELD is needed to improve its outcome in clinical practice. Addition of Na+ into the MELD (MELD-Na) has been proposed and has been successfully evaluated as a better score in patients with ascites, but only for those patients with sodium levels below normal. However, such a condition is only presented in 30% of patients with decompensated cirrhosis[15].
The elevation of lactate level in ACLF situation has been reported and might be attributed to several suggested mechanisms[16,17]. First, the liver is the organ primarily responsible for LAC clearance, and the LAC clearance may be impaired in the presence of severe liver dysfunction. Second, some ACLF-related complications, e.g., bacterial infection, may also raise the LAC level. Last but not least, hypovolemia-related hypoperfusion is also very likely to induce an elevation of LAC level during the early pre-resuscitative phase. In fact, some previous studies have shown that the acutely injured liver may act as a source of evaluating LAC18]. Taken together, we suggest that the LAC level could be used as a useful marker for assessing the severity of ACLF. As a simple measure widely available in current hospital system, early measurement of serum LAC can provide important prognostic information in patients with acute variceal hemorrhage requiring ICU admission[19-21]. But to the best of our knowledge, the LAC level had never been considered as a critical biomarker for prognosis prediction of HBV-related ACLF.
Our results showed that LAC could be used as a useful tool to assess the situation of HBV-related ACLF. The evaluated LAC level was found in 82.3% of patients in the S group, but 95.2% of those in the NS group (χ2 = 9.40, P = 0.002). The mean LAC level in the S group (3.11 mmol/L ± 1.98 mmol/L) was lower than that in the NS group (4.67 mmol/L ± 2.43 mmol/L) (P < 0.001). Further analysis showed that the baseline LAC level was related with patients’ prognosis with a rational sensitivity (86.80%). The AUROC for predicting the 3 mo mortality based on the LAC model was relatively lower than that from MELD (0.79 vs 0.818), which is consistent with previous studies[21].
In our study, a positive correlation between the LAC and MELD scores was demonstrated, suggesting that a combination of LAC and MELD scores was very likely to increase prediction accuracy for ACLF prognosis. Therefore, we developed a modified MELD including serum lactate level to improve the short-term prognosis prediction of HBV-related ACLF by combining two parameters together (LAC and MELD). By adopting the forward logistic regression method, LAC and MELD scores were subjected to the equation, to yield a new MELD-LAC model.
The new model showed a better (AUROC = 0.859) prediction score for the prognosis of HBV-related ACLF when compared with either LAC (0.790) or MELD (0.818) alone. When the cutoff value was set at -0.4741, the MELD-LAC model had a sensitivity of 91.50% and a specificity of 80.10% for predicting the mortality within three months. The patients were further divided into two groups according to the above cutoff value. The mortality was 78.86% in the higher score group (MELD-LAC ≥ -0.4741) and 7.96% in the lower score group (MELD-LAC < -0.4741), showing a significant difference between these two groups (χ2 = 107.35, P = 0.000). Under such a cutoff value setting (-0.4741), the MELD-LAC model seemed to provide a much more accurate prediction than normal MELD with a cutoff value of 25 (Table 5). According to our data, the three-month mortality rate of the patients was 75%, and 90% could be precisely predicted by the scores of MELD-LAC (-0.5561 or 0.6879) at baseline. Our results also showed no difference between the S group and NS group in CTP scores (Table 1), suggesting that CTP system is not a good tool in evaluating the outcome of HBV-related ACLF.
| Groups | Non-survival | Survival | χ2, P value | |
| MELD | MELD < 25 | 16 | 83 | 56.98, < 0.001 |
| MELD≥ 25 | 90 | 47 | ||
| MELD-LAC | MELD-LAC < -0.4741 | 13 | 100 | 107.35, < 0.001 |
| MELD-LAC ≥ -0.4741 | 97 | 26 | ||
There are some limitations of this study. Our study indicated, for the first time, that a modified MELD including serum lactate was a better prediction model for the prognosis of HBV-related ACLF, with higher PPV and NPV. Further research is required to combine this model into the classic model or develop a more accurate prognostic model. Another limitation of this study is that the patient population came merely from a single center in this study and it was a retrospective study; further research and verification need to be done in larger multi-center studies.
Serum LAC test is a simple and mature clinical measurement in modern hospitals, and it reflects both direct liver injury and other organ dysfunction. Dynamic monitoring of LAC was able to provide a new opportunity for clinicians with more accurate disease assessment, prognosis predication and treatment guidance at the early stage of the liver disease. We concluded that the modified MELD including serum lactate is a better prediction model for the prognosis of HBV-related ACLF, with higher PPV and NPV.
Hepatitis B virus (HBV)-related acute-on-chronic liver failure (ACLF) is a rapidly progressive disease with a high mortality rate up to 60%-75%. The accurate assessment of disease severity is critically needed, before clinicians make decisions on potential treatments such as medication or liver transplantation (LT). The model for end-stage liver disease (MELD) score model is a well-accepted model for assessing the feasibility of LT; however, the accuracy of prediction is still unsatisfying. A more objective and quantitative model with higher repeatability to predict the short-term prognosis of HBV-related ACLF is urgently needed.
In the MELD scoring system, several variables associated with poor prognosis of ACLF are not considered, including hepatic encephalopathy (HE), infections, and hemorrhage. Therefore, modification of MELD is needed to improve its outcome in clinical practice. Addition of Na+ into MELD (MELD-Na) has been proposed and has been successfully evaluated as a better score in patients with ascites, but only for those patients with sodium levels below normal. However, such a condition is only presented in 30% of patients with decompensated cirrhosis.
Lactate (LAC) is mainly metabolized in the liver and is widely used as an important indicator in organ failure or serious bacterial infection. Hyperlactatemia normally reflects both increased production and impaired clearance in patients with liver dysfunction, and a higher level of LAC always indicates a worse prognosis. Although several potential hypoxic and non-hypoxic mechanisms have been implicated on the persistent hyperlactatemia and the high level of LAC, the exact role of these parameters has not been specifically addressed in clinical studies. In the present study, they investigated the serum LAC level in patients with HBV-related ACLF, and developed a modified MELD including serum lactate (MELD-LAC model) to predict the short-term prognosis (three months) of HBV-related ACLF.
This study developed a modified MELD model including serum lactate level to improve the short-term prognosis prediction of HBV-related ACLF by combining the two parameters together (LAC and MELD).
The authors of this paper found that the efficacy of the short-term prognosis of HBV-related ACLF was improved by using a modified MELD including serum lactate from the present 6-year clinical study. The new model showed a better prediction score on the prognosis of HBV-related ACLF, when compared with either LAC or MELD alone.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonzalez-Reimers E, Tornesello MLL, Waheed Y
S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma YJ
| 1. | Chamuleau RA. Bioartificial liver support anno 2001. Metab Brain Dis. 2002;17:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, Yap P, Fevery J, Roskams T, Pirenne J. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 3. | Du WB, Pan XP, Li LJ. Prognostic models for acute liver failure. Hepatobiliary Pancreat Dis Int. 2010;9:122-128. [PubMed] |
| 4. | Polson J. Assessment of prognosis in acute liver failure. Semin Liver Dis. 2008;28:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf). 2010;199:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013;73:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Hernandez G, Bruhn A, Castro R, Regueira T. The holistic view on perfusion monitoring in septic shock. Curr Opin Crit Care. 2012;18:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Levy B, Perez P, Gibot S, Gerard A. Increased muscle-to-serum lactate gradient predicts progression towards septic shock in septic patients. Intensive Care Med. 2010;36:1703-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | William M, Lee M, Larson AM, Stravitz RT. AASLD Position Paper, The management of acute liver failure: update 2011. Hepatology. 2011;55:1-22. |
| 10. | Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, Sangfelt P, Danielsson A, Sandberg-Gertzén H, Lööf L. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Liou IW, Larson AM. Role of liver transplantation in acute liver failure. Semin Liver Dis. 2008;28:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Taylor RM, Tujios S, Jinjuvadia K, Davern T, Shaikh OS, Han S, Chung RT, Lee WM, Fontana RJ. Short and long-term outcomes in patients with acute liver failure due to ischemic hepatitis. Dig Dis Sci. 2012;57:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim RW. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3674] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 14. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 15. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1050] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 16. | Oria M, Jalan R. Brain lactate in hepatic encephalopathy: friend or foe? J Hepatol. 2014;60:476-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Bosoi CR, Zwingmann C, Marin H, Parent-Robitaille C, Huynh J, Tremblay M, Rose CF. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol. 2014;60:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Murphy ND, Kodakat SK, Wendon JA, Jooste CA, Muiesan P, Rela M, Heaton ND. Liver and intestinal lactate metabolism in patients with acute hepatic failure undergoing liver transplantation. Crit Care Med. 2001;29:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, Shaw S, Burroughs AK. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006;23:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Hadem J, Stiefel P, Bahr MJ, Tillmann HL, Rifai K, Klempnauer J, Wedemeyer H, Manns MP, Schneider AS. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol. 2008;6:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |