Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7242
Peer-review started: August 19, 2017
First decision: September 6, 2017
Revised: September 22, 2017
Accepted: September 29, 2017
Article in press: September 26, 2017
Published online: October 28, 2017
Processing time: 71 Days and 4.2 Hours
To explore the role of macrophages in chronic pancreatitis (CP) and the effect of Dachaihu decoction (DCHD) on pancreatic fibrosis in mice.
KunMing mice were randomly divided into a control group, CP group, and DCHD group. In the CP and DCHD groups, mice were intraperitoneally injected with 20% L-arginine (3 g/kg twice 1 d/wk for 6 wk). Mice in the DCHD group were administered DCHD intragastrically at a dose of 14 g/kg/d 1 wk after CP induction. At 2 wk, 4 wk and 6 wk post-modeling, the morphology of the pancreas was observed using hematoxylin and eosin, and Masson staining. Interleukin-6 (IL-6) serum levels were assayed using an enzyme-linked immunosorbent assay. Double immunofluorescence staining was performed to observe the co-expression of F4/80 and IL-6 in the pancreas. Inflammatory factors including monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and IL-6 were determined using real time-polymerase chain reaction. Western blot analysis was used to detect fibronectin levels in the pancreas.
Compared with the control group, mice with 20% L-arginine-induced CP had obvious macrophage infiltration and a higher level of fibrosis. IL-6 serum concentrations were significantly increased. Double immunofluorescence staining showed that IL-6 and F4/80 were co-expressed in the pancreas. With the administration of DCHD, the infiltration of macrophages and degree of fibrosis in the pancreas were significantly attenuated; IL-6, MCP-1 and MIP-1α mRNA, and fibronectin levels were reduced.
The dominant role of macrophages in the development of CP was mainly related to IL-6 production. DCHD was effective in ameliorating pancreatic fibrosis by inhibiting macrophage infiltration and inflammatory factor secretion in the pancreas.
Core tip: Macrophages, important inflammatory cells, can also promote fibrogenesis by interfering with the synthesis and degradation of the extracellular matrix, as confirmed in both liver fibrosis and renal fibrosis models. Our study suggested that macrophages also play an important role in the development of pancreatic fibrosis. We found that macrophages are an important source of interleukin-6, which is involved in the progression of chronic pancreatitis (CP). Dachaihu decoction (DCHD), a traditional Chinese medicinal formula, effectively improves the clinical symptoms of CP patients, but the underlying mechanism remains unclear. We found that DCHD ameliorates pancreatic fibrosis by inhibiting macrophage infiltration and inflammatory factor secretion.
- Citation: Duan LF, Xu XF, Zhu LJ, Liu F, Zhang XQ, Wu N, Fan JW, Xin JQ, Zhang H. Dachaihu decoction ameliorates pancreatic fibrosis by inhibiting macrophage infiltration in chronic pancreatitis. World J Gastroenterol 2017; 23(40): 7242-7252
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7242
Chronic pancreatitis (CP) is a progressive inflammatory disease that is characterized by irreversible injury of the pancreas leading to endocrine and exocrine dysfunction[1]. It has been reported that pancreatic fibrosis is a common pathological feature and characterizes the end-stage of CP of different etiologies[2]. The progressive fibrotic cascade can eventually lead to loss of pancreatic function as well as systemic complications, including malabsorption, diabetes mellitus, and others. However, the underlying molecular mechanisms of CP remain unclear, and the available therapeutic strategies are very few in number[3].
Based on the necrosis-fibrosis sequence playing a key role in the underlying pathogenesis of CP, pancreatic fibrosis commonly arises from inflammation or tissue injury[4]. Recent studies have highlighted the role of macrophages in promoting wound healing and fibrosis[5,6]. Large numbers of macrophages have been observed around areas of pancreatic injury in rat models of CP[7,8]. Detlefsen and colleagues[9] demonstrated that many infiltration macrophages are also found in the pancreas of alcoholic CP patients, which facilitate fibrosis formation by producing transforming growth factor (TGF)-β and platelet derived growth factor (PDGF)-β. Thus, we propose that macrophages may play a critical role in the progression of pancreatic fibrosis, but its definite mechanism remains unclear.
Interleukin-6 (IL-6) is a key pro-inflammatory cytokine that is involved in inflammation and the immune response. It is mainly released by macrophages, fibroblasts, endothelial cells, and myeloid cells[10,11]. In a previous study, Zhang et al[12] found that acinar injury results in the infiltration of myeloid cells (i.e., macrophages and neutrophils) into the pancreas during severe acute pancreatitis, and infiltrating macrophages further release IL-6, which can lead to pancreatitis-associated acute lung injury. A study by Lesina et al[13] demonstrated that tumor-associated macrophages appear to be the major source of IL-6 in murine and human pancreatic ductal adenocarcinoma. Several reports have confirmed that serum level of IL-6 in patients with CP was significantly increased compared with that in control subjects[12,14]. However, the contribution of IL-6 and the relationship between IL-6 and macrophages in pancreatic fibrosis have not been analyzed in detail.
Dachaihu decoction (DCHD; major Radix bupleuri decoction) is a traditional Chinese medicine formula that was first described by Zhongjing Zhang in “Treatise on Febrile Disease Caused by Cold (Shanghan Lun)”. DCHD has been widely used in the clinical treatment of acute pancreatitis (AP)[15]. Recently, it was used to treat patients with CP, and it was found that the effects included improving the clinical symptoms of CP patients. But whether it can inhibit pancreatic fibrosis and its underlying mechanism remain uncertain. The aim of the present study was to ascertain the role of macrophages in pancreatic fibrosis induced by 20% L-arginine, as well as to determine the effects and mechanism of DCHD on inhibiting pancreatic fibrosis.
KunMing mice (20-28 g) were obtained from the Experimental Animal Center of Xi’an Jiaotong University (Certificate No. 0011744) and housed under specific-pathogen-free conditions. The mice received humane care in accordance with the Shaanxi University of Chinese Medicine Animal Care Committee guidelines. Antibodies against fibronectin (FN), F4/80, and IL-6 were provided by Santa Cruz Biotechnology Inc. (Santa Cruz, CA, United States). All secondary antibodies and immunofluorescence and immunohistochemistry reagents were purchased from Boster Biotechnology Inc. (Wuhan, China). L-arginine was obtained from Sigma-Aldrich Co. (St. Louis, MO, United States). Crude DCHD, including Radix bupleuri, Radix scutellariae, Fructus aurantii immaturus, Paeonia lactiflora, Rhizomapinelliae, Rheum palmatum, Rhizoma zingiberis recens and Fructus jujubae (Table 1), was purchased from the Department of Pharmaceutical Preparation of Chinese Medicine of Shaanxi University. The components of DCHD were extracted twice by boiling in distilled water for 1 h, and the drug was subsequently filtered and reduced by boiling to a volume of 70 mL (1 g crude drug/mL) and stored at 4 °C for later use. Masson staining and amylase enzyme-linked immunosorbent assay (ELISA) kits were purchased from the Nanjing Jiancheng Biotechnology Company (Nanjing, China). Reverse transcriptase polymerase chain reaction (PCR) kits and primers were provided by Transgen Biotech Co. (Beijing, China).
| Chinese name | Botanical name | Common name | Genus | Family | Weight, g | Part used |
| Chai hu | Bupleurumabchasicum Manden | Radix bupleuri | Bupleurum | umbelliferae | 15 | Root |
| Huang qin | Scutellaria baicalensis Georgi baicalensis | Radix scutellariae | Scutellaria | Labiatae | 9 | Root |
| Zhishi | Citrus × aurantium L. | Fructus aurantii immaturus | Citrus | Rutaceae | 9 | Fruit |
| Shaoyao | Paeonia × suffruticosa Andrews | Paeonia lactiflora | Paeonia | Paeoniaceae | 9 | Root |
| Ban xia | Pinelliaternate (Thunb.) Makino | Rhizomapinelliae | pinellia | Araceae | 9 | Root and rhizoma |
| Da huang | Rheum palmatum L. | Rheum palmatum | Rheum | polygonaceae | 6 | Root and rhizome |
| Sheng jiang | Zingiber officinale Roscoe | Rhizoma zingiberis recens | Zingiber Adans . | zingiberaceae | 15 | Root and rhizome |
| Da zao | Ziziphus jujube MIll | Fructus jujuba | Ziziphus | Rhamnales | 20 | fruit |
The KunMing mice were randomly divided into three groups (n = 36): the control group, the CP group, and the DCHD group. Mice in the CP and DCHC groups were injected intraperitoneally with 20% L-arginine (two 3 g/kg injections separated by 1 h, weekly for 6 wk), while the mice in the control group were injected with saline. Mice in the DCHD group were treated with DCHD (14 g/kg per day) intragastrically 1 wk after the 20% L-arginine injections.
Mice were anesthetized and sacrificed at 2 wk, 4 wk and 6 wk after 20% L-arginine or saline injection (n = 9 at each time point). Blood samples were obtained from the inferior vena cava, centrifuged at 1500 rpm for 10 min, and serum was collected and stored at -80 °C for IL-6 analysis. The pancreas was removed, weighed, and mixed well. One part of the pancreas was fixed in 4% formaldehyde solution for hematoxylin and eosin (HE) and immunofluorescence staining, while the other part was frozen in liquid nitrogen and stored at -80 °C for RNA and protein extraction.
Plasma was collected at 2 wk, 4 wk and 6 wk after modeling and serum was obtained by centrifuging samples at 1500 rpm for 10 min. IL-6 levels were evaluated using an ELISA kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
To observe morphological and fibrosis changes in the pancreas, pancreatic sections were subjected to HE or Masson staining. Samples were dehydrated and embedded in paraffin, sectioned (3 μm) after fixation in 4% formaldehyde solution for 12 h. Images were collected using an optical microscope.
Immunohistochemical staining and immunofluorescence staining were performed. For immunohistochemistry, antibodies were applied at the following concentrations: F4/80, 1:150 dilution, overnight at 4 °C; and biotinylated secondary antibody, 1:400 dilution, for 1 h. Bound peroxidase was visualized after a 3,3-diaminobenzidine reaction for 2-5 min and after light counterstaining with hematoxylin, dehydration, and mounting. The slides were examined using a Zeiss Axio1 imager (Carl-Zeiss-Promenade, Jena, Germany). For immunofluorescence, antibodies were applied at the following concentrations: F4/80 and IL-6 antibodies, 1:150 dilution, overnight at 4 °C in the dark; FITC-labeled and PE-labeled secondary antibody, 1:400 dilution, for 1 h. DAPI (1:1000) was used to counterstain the nuclei for 5-15 s, and the slides were mounted on coverslips with anti-fade mounting medium. Images were collected using a fluorescent microscope (Olympus IX51, Shinjuku-ku, Tokyo, Japan).
Total RNA of pancreatic tissue was extracted using the Trizol reagent (RNeasy Minicolumns, Transgen, Beijing, China) and reverse- transcribed into cDNA using reverse transcriptase kits. RT-PCR was performed using SYBR Green reaction mix and the ABI-7500 Sequence Detection System (Thermo Fisher Scientific, Shanghai, China). The primers, number of cycles, and annealing temperatures are shown in Table 2.
| Gene | Forward primer | Reverse primer | Cycles | Temperature, °C |
| IL-6 | 5’-GAAGTAGGGAAGGCCGTGG-3’ | 5’-CTCTGCAAGAGACTTCCATCCAGT-3’ | 40 | 60 |
| MCP-1 | 5’-GTTGGCCTCAGCCAGATGCA-3’ | 5’-AGCGTACTCATTGGGATCATCTTG-3’ | 40 | 60 |
| MIP-1α | 5’-TGCCCTTGCTHTTCTTCTCTGCACCATGGC-3’ | 5’-GGCAAATTCCACGAAAATTCATTGCTGACT-3’ | 40 | 60 |
| β-actin | 5’-CATCCTGCGTCTGGACCT-3’ | 5’-TCAGGAGGAGCAATGATCTTG-3’ | 40 | 60 |
Protein was extracted from pancreas tissue using standard methods. The protein concentrations were measured using a BCA protein assay kit (Boster Biotechnology Inc.) and adjusted to 4 μg/μL. Before loading, the samples were boiled at 95 °C for 5 min, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA, United States), and transferred onto PVDF membranes (EMD Millipore, Darmstadt, Germany). The membranes were blocked with 5% non-fat milk followed by incubation with anti-actin (Boster Biotechnology Inc.) and anti-FN antibodies (sc-101759; Santa Cruz Biotechnology Inc.). The sizes of the detected bands were determined by reference to a 10-180 kDa protein marker ladder.
Values are presented as the mean ± SD and were analyzed using the unpaired Student’s t-test and one-way analysis of variance (commonly known as ANOVA). A value of P < 0.05 was considered to indicate statistical significance.
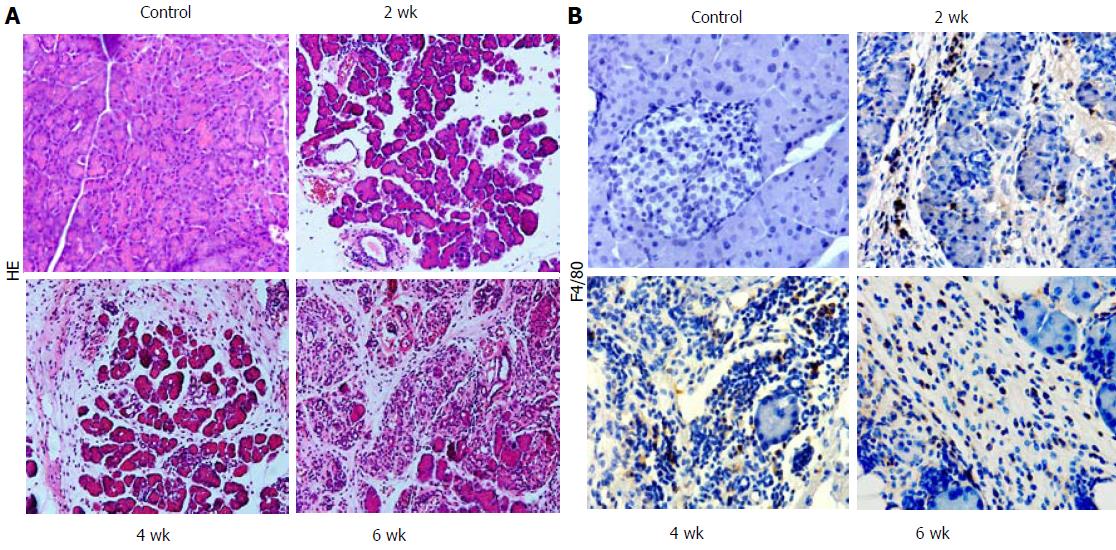
We induced CP in mice using repeated 20% L-arginine injections over 6 wk. Two weeks after modeling, morphological signs of CP were found, including leukocyte infiltration, acinar cell necrosis, and a small amount of connective tissue deposition. At 4 wk and 6 wk, loss of acinar cells, collagen deposition, and aggravated fibrosis in the pancreas were observed (Figure 1A).
Immunohistochemistry using the macrophage marker F4/80 was performed. Two weeks after L-arginine injection, F4/80-positive cells were predominant in mice with CP compared with the normal group (Figure 1B). The positive macrophage ratio was highest at week 4, and levels were sustained until week 6. Thus, macrophages were abundant during CP and were involved in the development of fibrosis (Figure 1B).
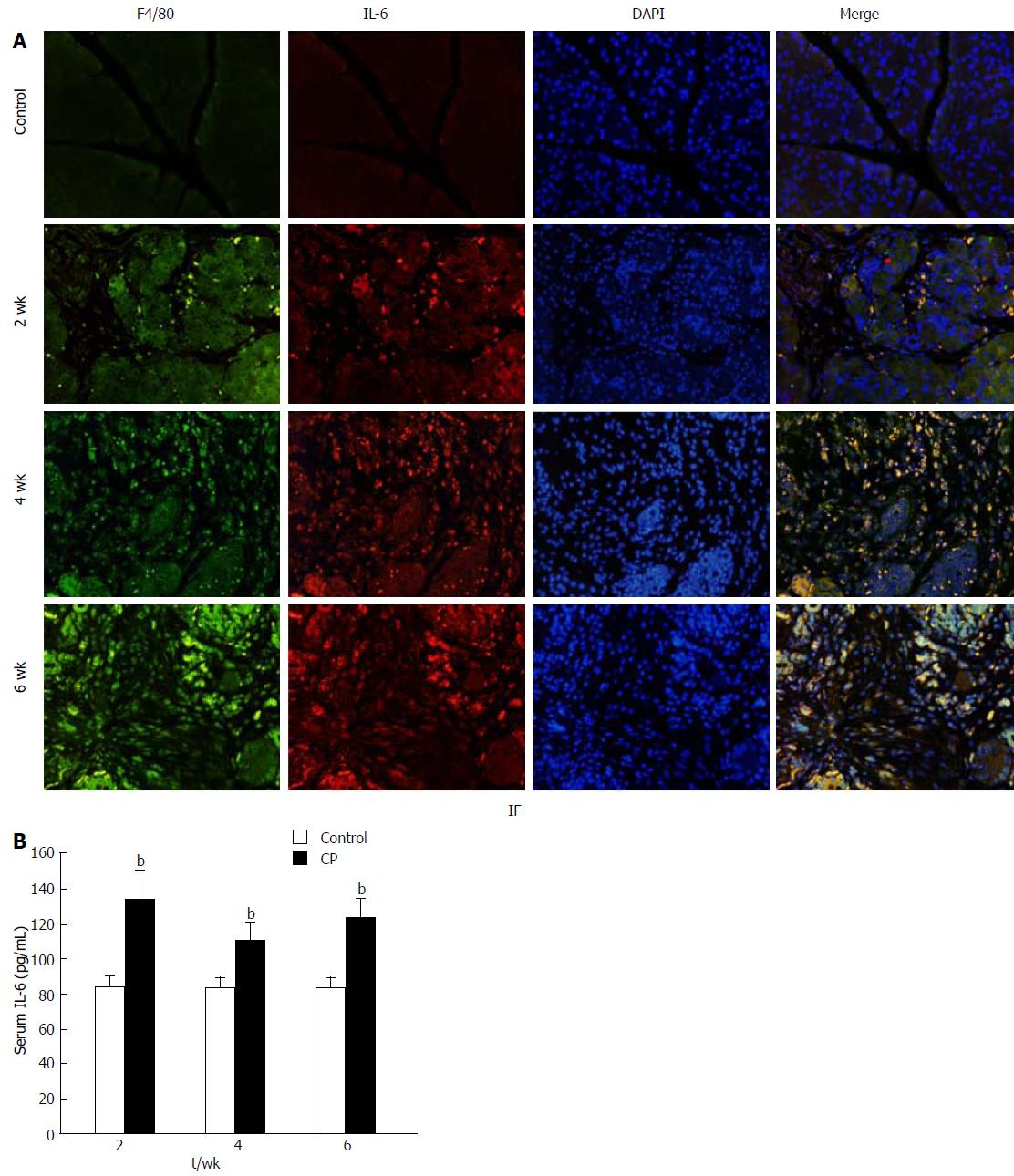
The serum concentration of IL-6 was significantly increased 2 wk after 20% L-arginine injection. At 4 wk, the IL-6 level was somewhat decreased but remained elevated to week 6 (Figure 2A). These findings demonstrate that high IL-6 levels contribute to the development of CP.
While macrophage infiltration and IL-6 levels were increased in CP, their relationship remains unclear. To determine whether IL-6 is mainly secreted by macrophages, double staining of F4/80 and IL-6 labeled with different fluorescence markers was performed on pancreas paraffin sections. As shown in Figure 2B, no positive F4/80 and IL-6 cells were found in the pancreas of the control group. However, L-arginine administration resulted in a significant increase in the expression of F4/80 and IL-6 in the pancreas. While there was only weak expression in the samples at 2 wk, co-expression of F4/80 and IL-6 increased markedly at 4 wk and 6 wk. These results demonstrate that IL-6 is mainly secreted by macrophages in CP.
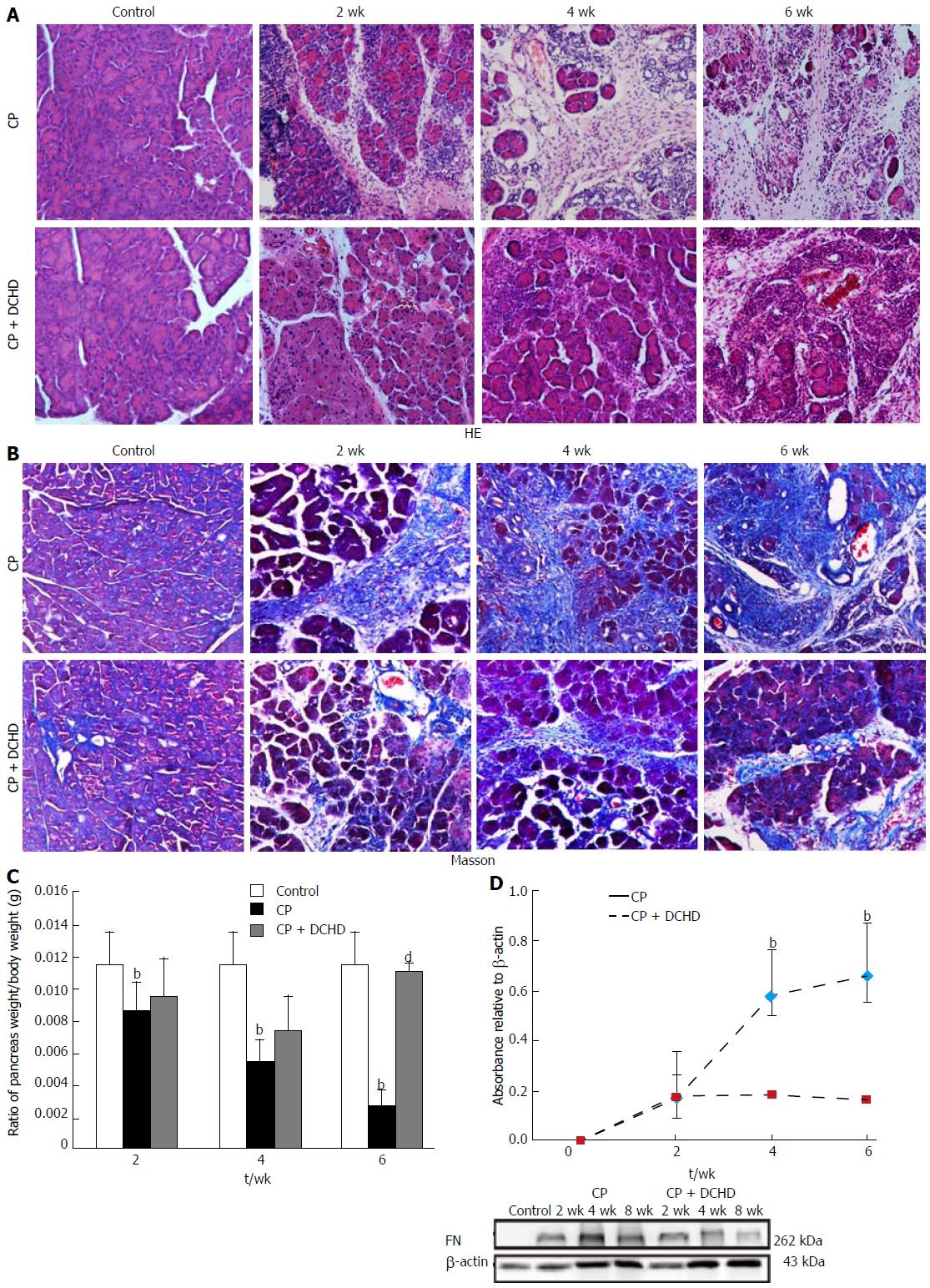
We next sought to investigate whether pancreatic injury and subsequent fibrosis were alleviated by DCHD treatment. DCHD administration resulted in significantly improved tissue damage, pancreatic fibrosis, and ratio of pancreas weight/body weight (Figure 3A-C). Moreover, Masson staining revealed that the area of pancreas fibrosis markedly decreased in the DCHD group (Figure 3B).
FN is a major indicator of extracellular matrix deposition and the degree of fibrosis. Western blotting revealed no FN expression in the pancreas of control mice; however, 20% L-arginine treatment resulted in increased FN expression in the pancreas at 2 wk, 4 wk, and (maximally) 6 wk. Interestingly, DCHD treatment attenuated FN levels compared with those in the CP group at the same time point, illustrating that DCHD can suppress the deposition of FN in pancreatic fibrosis (Figure 3D).
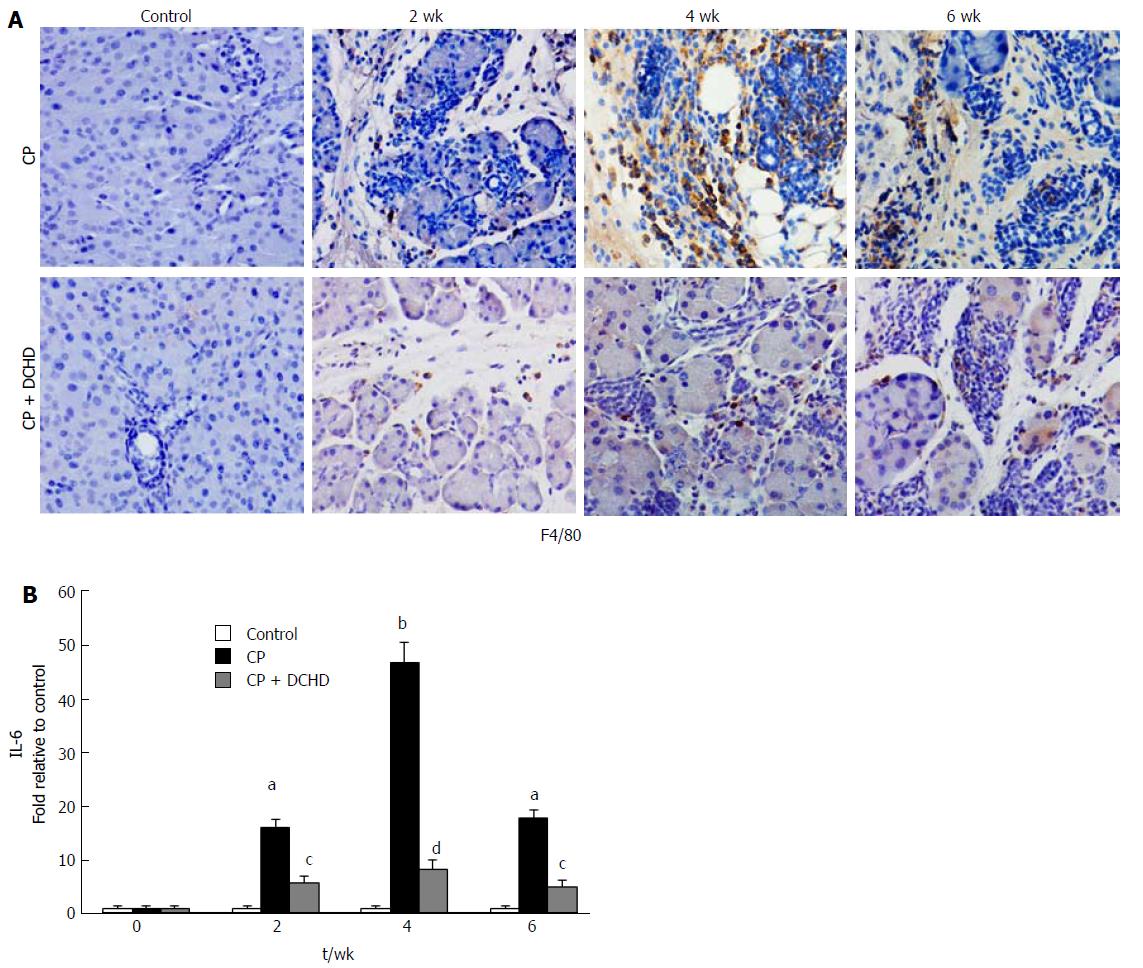
To understand the mechanism underlying the effects of DCHD treatment in CP, we used immunohistochemistry to detect macrophage infiltration. Compared with the CP group, DCHD treatment significantly decreased pancreatic macrophage infiltration at 2 wk. Accompanied with evident and an improvement in pancreatic fibrosis at 4 wk and 6 wk, macrophage infiltration was also significantly reduced (Figure 4A). Thus, limiting macrophage accumulation in the pancreas may be an underlying mechanism of DCHD in inhibiting the development of pancreatic fibrosis.
Considering the association between IL-6 and macrophages, the expression of IL-6 mRNA in the pancreas was assessed using RT-PCR. IL-6 expression levels were increased in CP and were highest at week 4. Compared with those in the CP group, IL-6 levels in the DCHD group were significantly decreased (Figure 4B). This finding demonstrates that IL-6 is produced by macrophages and thus may be a potential treatment target for DCHD.
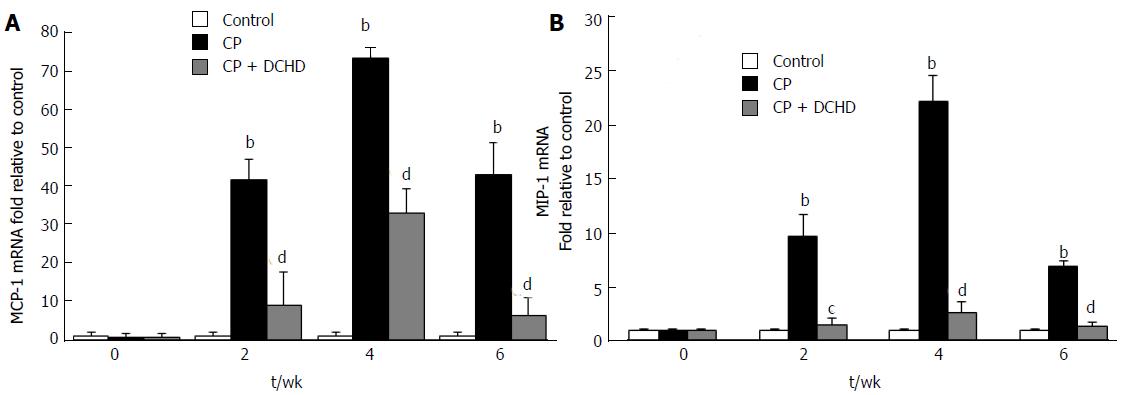
To further explore the mechanism underlying macrophage accumulation, pancreatic mRNA levels of the macrophage-associated chemokines MCP-1 and MIP-1α were detected using RT-PCR. MCP-1 and MIP-1α were up-regulated in the CP group compared with the control group at 2 wk, 4 wk and 6 wk (Figure 5A and B), while they were significantly decreased in the DCHD group at the same time points.
Generally, pancreatic fibrosis is a common pathological feature of CP. Recurrent acute pancreatic insults trigger necro-inflammation, and inflammatory cells, once in their active states, release cytokines that in turn promote fibrogenesis, which is involved in the progression of pancreatic fibrosis[16]. Recent studies have confirmed the dominant role played by activated macrophages in liver fibrosis by interfering with the synthesis and degradation of the extracellular matrix[17]. In a rat model of liver cirrhosis, activated macrophages were shown to accelerate fibrogenesis by activating hepatic myofibroblasts, while the suppression of macrophage infiltration inhibited liver fibrogenesis[17]. Treiber et al[18] found large numbers of macrophages among inflammatory cells infiltrating the pancreas of a mouse model of CP. In our study, the development of pancreatic fibrosis was accompanied by a significant increase in F4/80-positive cells in pancreatic tissue, suggesting that activated macrophages may play a role in the progression of pancreatic fibrosis.
A study by Saeki et al[19] revealed that infiltration of macrophages in AP originates mainly from peripheral blood mononuclear cells (PBMCs) due to chemokines. Our CP model was based on repetitively inducing AP, and we speculated that infiltration of macrophages in CP was attributable to PBMCs’ recruitment. MCP-1 and MIP-1α are potent C-C type chemokines that are mainly secreted by activated macrophages and fibroblasts. They recognize the C-C chemokine receptor type (CCR)-2 or CCR-3 on PBMCs and recruit macrophages to injured tissues[20,21]. Therefore, we measured MCP-1 and MIP-1α mRNA levels and found that they were up-regulated in mice with CP, which may contribute to macrophage accumulation in CP.
To obtain a deeper understanding of the role of macrophages in pancreatic fibrosis, other researchers previously stimulated PBMCs with lipopolysaccharide, and found that TGF-β1 and IL-6 were significantly elevated in culture supernatant. Moreover, further co-culture of macrophages with pancreatic stellate cells resulted in increased FN and collagen type I levels[22]. TGF-β1 is considered one of the strongest fibrosis factors[9], but the role of IL-6 in CP has not been recognized. Numerous studies have shown that IL-6 was related to fibrosis progress in the liver and lung[23,24]. Recent experiments also demonstrated that the serum level of IL-6 in CP patients was significantly increased[14].
In our present study, IL-6 levels in serum and IL-6 mRNA levels in the pancreas were also significantly increased in the progression of CP mice. These results indicated that IL-6 participates in the development of pancreatic fibrosis. To determine the source of IL-6 production, we preformed immunofluorescent double-staining of F4/80 and IL-6 to evaluate F4/80 and IL-6 expression in the pancreas and found large numbers of doubly positive cells in the pancreas of the CP group. We can thus infer that macrophage infiltration is the main source of IL-6 secretion, and an excess of IL-6 might aggravate inflammation and further accelerate pancreatic fibrogenesis.
The traditional Chinese herbal compound DCHD is composed mainly of Radix bupleuri, Radix scutellariae, Fructus aurantii immaturus, Paeonia lactiflora, Rhizomapinelliae, Rheum palmatum, Rhizoma zingiberis recens, and Fructus jujubae[25]. In the clinic, DCHD has been effective in treating the characteristics of the “shao-yang signs”, such as fever, chills and rib-side fullness, and “yang-ming” signs, such as abdominal pain and fullness[15]. Therefore, DCHD is used mainly to treat acute and chronic digestive diseases, such as AP, inflammatory bowel disease, hepatitis and disorders of the gallbladder[15].
Clearly, CP, a common disease of the digestive system, is associated with the shao-yang and yang-ming signs of abdominal pain and rib-side distention. According to the traditional Chinese medical theory, many doctors consider that the mechanism underlying CP involves “liver-spleen disharmony, qi and blood stasis, and dampness-heat obstruction”[26], while the main actions of DCHD are removal of heat and toxic materials and elimination of blood stasis[27]. DCHD may be effective in treating CP. Modern medical studies have shown that petunidin and quercetin from Radix bupleuri exert anti-inflammatory effects and act as antioxidants[15,26,28]. Therefore, DCHD has also been used to treat patients of CP, and some related clinical symptoms such as abdominal pain, abdominal distension and loss of appetite, with significant improvement[15], but the underlying molecular mechanisms remain unclear.
In this study, the effects of DCHD on the development of pancreatic fibrosis in CP were evaluated systematically using an L-arginine-induced CP mouse model. Following DCHD administration, morphological changes indicative of CP, including pancreatic acinar cell injury, inflammatory cells infiltration, and pancreatic fibrosis were improved. This suggests that DCHD can attenuate inflammatory lesions and intervene in the progression of pancreatic fibrosis, and led us to perform further study on its underlying mechanism of action.
We next found that DCHD treatment significantly attenuated macrophage infiltration and IL-6 expression in the pancreas. Combined with our previous results, DCHD may suppress pancreatic fibrosis via the inhibition of macrophage infiltration, which in turn reduces the release of IL-6. In addition, MCP-1 and MIP-1α expression was also significantly decreased, suggesting that the DCHD-induced decrease in macrophage infiltration was due to a reduction in the release of macrophage-associated chemokines.
The present study suggests that macrophages play a critical role in CP and pancreatic fibrosis, and their dominant role in CP may be related to the release of cytokines, specifically IL-6. We demonstrated that macrophages are the main source of IL-6 production in CP. In addition, DCHD can ameliorate pancreatic fibrosis by inhibiting the macrophage infiltration of CP, which provides a new experimental basis for the clinical promotion of DCHD.
However, our work had certain limitations. We confirmed that the effect of macrophages on CP is related to IL-6, but the detailed mechanism of IL-6 on CP progression was not further explored. In addition, DCHD is effective in CP treatment, but its in-depth mechanism is not further studied, and these are our next steps.
Pancreatic fibrosis is a common pathological feature characteristic of chronic pancreatitis (CP). As the necrosis-fibrosis sequence plays an important role in the underlying pathogenesis of CP, pancreatic fibrosis commonly develops after repeated inflammation. Macrophages are important inflammatory cells that can also promote fibrogenesis by interfering with the synthesis and degradation of the extracellular matrix. This has been confirmed in models of liver fibrosis and renal fibrosis, but any role of macrophages in pancreatic fibrosis has remained unclear. A traditional Chinese medicinal formula, Dachaihu decoction (DCHD), has been widely used in the clinical treatment of AP, and recently, DCHD was used to treat CP, which significantly improved the patient’s clinical symptoms, but, again, the underlying mechanism has been unclear.
CP is a progressive inflammatory disease characterized by irreversible injury to the pancreas leading to endocrine and exocrine dysfunctions that pose serious threats to human health, because the pathogenesis is unclear, and few treatments are available. Recent studies suggested that DCHD can significantly improve the clinical symptoms of CP patients, such as abdominal pain, abdominal distension, loss of appetite and others. However, DCHD cannot be widely used clinically because the therapeutic mechanism of action remains unclear.
The aim was to explore the role played by macrophages in the pathogenesis of CP to further elucidate the mechanisms of CP. In addition, they assessed the efficacy of DCHD in treating CP in animal experiments to determine the mechanism involved, providing an experimental basis for promoting the use of DCHD in clinical practice.
KunMing mice were randomly divided into a control group, CP group, and DCHD group. In the CP and DCHD groups, mice were intraperitoneally injected with 20% L-arginine (3 g/kg twice a day, 1 d a week). Mice in the DCHD group were given DCHD (14 g/kg per day) intragastrically for 1 wk after CP induction. The animals were anesthetized and sacrificed at 2 wk, 4 wk and 6 wk after study commencement. Pancreatic morphology and the extent of fibrosis were assessed using hematoxylin and eosin and Masson staining. Serum interleukin-6 (IL-6) levels were assessed by enzyme-linked immunosorbent assay. Double immunofluorescence staining was performed to assess the co-expression of F4/80 and IL-6 in the pancreas. The mRNA levels of inflammatory factors including monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α) and IL-6 were determined by real time-polymerase chain reaction. Western blotting was used to measure fibronectin (FN) levels in the pancreas.
We found that macrophage infiltration was significantly increased in the CP group, and the IL-6 levels in serum and IL-6 mRNA levels in the pancreas were also increased in the CP group. Further, immunofluorescent double-staining of F4/80 and IL-6 revealed that macrophages were the main source of the IL-6. In addition, DCHD ameliorated pancreatic fibrosis, inhibited the pancreatic infiltration of macrophages in the CP group, and reduced the release of cytokines IL-6, MCP-1 and MIP-1α mRNA, and FN levels. However, our work had certain limitations. We confirmed that the effect of macrophages on CP is related to IL-6, but the detailed mechanism of IL-6 on CP progression was not further explored. In addition, DCHD is effective in CP treatment, but its in-depth mechanism was not studied further.
The authors confirmed that macrophages are involved in the development of pancreatic fibrosis and may constitute a new therapeutic target. In addition, DCHD can ameliorate pancreatic fibrosis by inhibiting macrophage infiltration and inflammatory factor secretion in the pancreas.
We would like to thank our colleagues and postgraduates in the Department of Pathophysiology and the Medical Experimental Center of Shaanxi University of Chinese Medicine for their help in supporting this research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A,
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akamatsu N, Bramhall S, Tsoulfas G S- Editor: Wei LJ L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Muniraj T, Aslanian HR, Farrell J, Jamidar PA. Chronic pancreatitis, a comprehensive review and update. Part I: epidemiology, etiology, risk factors, genetics, pathophysiology, and clinical features. Dis Mon. 2014;60:530-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Manohar M, Verma AK, Venkateshaiah SU, Sanders NL, Mishra A. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8:10-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 134] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (2)] |
| 3. | Lew D, Afghani E, Pandol S. Chronic Pancreatitis: Current Status and Challenges for Prevention and Treatment. Dig Dis Sci. 2017;62:1702-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Madro A, Slomka M, Celinski K. Can we expect progress in the treatment of fibrosis in the course of chronic pancreatitis? Adv Med Sci. 2011;56:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1116] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 6. | Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1127] [Cited by in RCA: 1078] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 7. | Reding T, Bimmler D, Perren A, Sun LK, Fortunato F, Storni F, Graf R. A selective COX-2 inhibitor suppresses chronic pancreatitis in an animal model (WBN/Kob rats): significant reduction of macrophage infiltration and fibrosis. Gut. 2006;55:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Nakamura Y, Kanai T, Saeki K, Takabe M, Irie J, Miyoshi J, Mikami Y, Teratani T, Suzuki T, Miyata N. CCR2 knockout exacerbates cerulein-induced chronic pancreatitis with hyperglycemia via decreased GLP-1 receptor expression and insulin secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G700-G707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Detlefsen S, Sipos B, Feyerabend B, Klöppel G. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod Pathol. 2006;19:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Lutz HH, Sackett SD, Kroy DC, Gassler N, Trautwein C. Deletion of gp130 in myeloid cells modulates IL-6-release and is associated with more severe liver injury of Con A hepatitis. Eur J Cell Biol. 2012;91:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Lesina M, Wörmann SM, Neuhöfer P, Song L, Algül H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin Immunol. 2014;26:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, Treiber M, Wartmann T, Regnér S, Thorlacius H. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123:1019-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 13. | Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 689] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 14. | Mroczko B, Groblewska M, Gryko M, Kedra B, Szmitkowski M. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal. 2010;24:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Li B, Tao W, Zheng C, Shar PA, Huang C, Fu Y, Wang Y. Systems pharmacology-based approach for dissecting the addition and subtraction theory of traditional Chinese medicine: An example using Xiao-Chaihu-Decoction and Da-Chaihu-Decoction. Comput Biol Med. 2014;53:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Ramadori G, Saile B. Inflammation, damage repair, immune cells, and liver fibrosis: specific or nonspecific, this is the question. Gastroenterology. 2004;127:997-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Alzaid F, Lagadec F, Albuquerque M, Ballaire R, Orliaguet L, Hainault I, Blugeon C, Lemoine S, Lehuen A, Saliba DG. IRF5 governs liver macrophage activation that promotes hepatic fibrosis in mice and humans. JCI Insight. 2016;1:e88689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Treiber M, Neuhöfer P, Anetsberger E, Einwächter H, Lesina M, Rickmann M, Liang S, Kehl T, Nakhai H, Schmid RM. Myeloid, but not pancreatic, RelA/p65 is required for fibrosis in a mouse model of chronic pancreatitis. Gastroenterology. 2011;141:1473-1485, 1485.e1-1485.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Saeki K, Kanai T, Nakano M, Nakamura Y, Miyata N, Sujino T, Yamagishi Y, Ebinuma H, Takaishi H, Ono Y. CCL2-induced migration and SOCS3-mediated activation of macrophages are involved in cerulein-induced pancreatitis in mice. Gastroenterology. 2012;142:1010-1020.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Steinhauser ML, Kunkel SL, Hogaboam CM, Evanoff H, Strieter RM, Lukacs NW. Macrophage/fibroblast coculture induces macrophage inflammatory protein-1alpha production mediated by intercellular adhesion molecule-1 and oxygen radicals. J Leukoc Biol. 1998;64:636-641. [PubMed] |
| 21. | Hoh BL, Hosaka K, Downes DP, Nowicki KW, Fernandez CE, Batich CD, Scott EW. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1α and macrophage inflammatory protein-2-dependent pathway. Circulation. 2011;124:2243-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Michalski CW, Gorbachevski A, Erkan M, Reiser C, Deucker S, Bergmann F, Giese T, Weigand M, Giese NA, Friess H. Mononuclear cells modulate the activity of pancreatic stellate cells which in turn promote fibrosis and inflammation in chronic pancreatitis. J Transl Med. 2007;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Mair M, Blaas L, Österreicher CH, Casanova E, Eferl R. JAK-STAT signaling in hepatic fibrosis. Front Biosci (Landmark Ed). 2011;16:2794-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Knight D, Mutsaers SE, Prêle CM. STAT3 in tissue fibrosis: is there a role in the lung? Pulm Pharmacol Ther. 2011;24:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Mao S, Zhang MZ. [Clinical experience and thinking of treating abdominal compartment syndrome by dachaihu decoction]. Chin J Integr Trad West Med. 2013;33:845-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Cheng YX, Wang M, Cheng X. [Effect of dachaihu decoction in treating acute mild pancreatitis of Gan-qi stagnant type]. Chin J Integr Trad West Med. 2008;28:793-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Yu W, Ma M, Chen X, Min J, Li L, Zheng Y, Li Y, Wang J, Wang Q. Traditional Chinese Medicine and Constitutional Medicine in China, Japan and Korea: A Comparative Study. Am J Chin Med. 2017;45:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Lin M, Zhang W, Su J. Toxic polyacetylenes in the genus Bupleurum (Apiaceae) - Distribution, toxicity, molecular mechanism and analysis. J Ethnopharmacol. 2016;193:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |