Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.6983
Peer-review started: July 3, 2017
First decision: July 27, 2017
Revised: August 17, 2017
Accepted: September 6, 2017
Article in press: September 5, 2017
Published online: October 14, 2017
Processing time: 108 Days and 2.4 Hours
To investigate whether fecal microbiota transplantation (FMT) prevents hepatic encephalopathy (HE) in rats with carbon tetrachloride (CCl4)-induced acute hepatic dysfunction.
A rat model of HE was established with CCl4. Rat behaviors and spatial learning capability were observed, and hepatic necrosis, intestinal mucosal barrier, serum ammonia levels and intestinal permeability were determined in HE rats receiving FMT treatment. Furthermore, the expression of tight junction proteins (Claudin-1, Claudin-6 and Occludin), Toll-like receptor (TLR) 4/TLR9, interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α was examined.
FMT improved rat behaviors, HE grade and spatial learning capability. Moreover, FMT prevented hepatic necrosis and intestinal mucosal barrier damage, leading to hepatic clearance of serum ammonia levels and reduced intestinal permeability. The expression of TLR4 and TLR9, two potent mediators of inflammatory response, was significantly downregulated in the liver of rats treated with FMT. Consistently, circulating pro-inflammatory factors such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α were remarkably decreased, indicating that FMT is able to limit systemic inflammation by decreasing the expression of TLR4 and TLR9. Importantly, HE-induced loss of tight junction proteins (Claudin-1, Claudin-6 and Occludin) was restored in intestinal tissues of rats receiving FMT treatment.
FMT enables protective effects in HE rats, and it improves the cognitive function and reduces the liver function indexes. FMT may cure HE by altering the intestinal permeability and improving the TLR response of the liver.
Core tip: In this article, we first established a rat model of hepatic encephalopathy, and then carried on the fecal microbiota transplantation (FMT). Our results suggest that FMT can serve as a kind of new method for the treatment of hepatic encephalopathy, probably better than VSL#3.
- Citation: Wang WW, Zhang Y, Huang XB, You N, Zheng L, Li J. Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J Gastroenterol 2017; 23(38): 6983-6994
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/6983.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.6983
Hepatic encephalopathy (HE) is a common and serious disorder with a wide spectrum of neuropsychiatric abnormalities due to chronic and acute liver dysfunction[1]. HE is associated with poor quality of life and increased mortality, thus representing a major healthcare burden in patients with liver cirrhosis[2,3]. The estimated one-year survival rate is 42% after the first episode of overt HE, and the three-year survival rate is 23%[1,4,5]. Therefore, HE with cirrhosis is considered to have a poor prognosis for subjects who do not undergo liver transplantation. The death rate is substantially lower for patients who receive a transplant. The survival rate is more than 70% in the first 5 years after transplantation[1,6].
Although the pathogenesis of HE remains incompletely understood, gut-derived neurotoxin ammonia that accumulates in the central nervous system (CNS) is the most frequent precipitating factor[7-11]. In cases of normal hepatic function, ammonia enters the portal circulation of the liver and is subsequently converted to urea through the urea cycle[1].
Gut microbiota has emerged as a critical factor in the development of HE. Dysbiosis or an altered gut microbiota population promotes a systemic pro-inflammatory milieu, resulting in neuro-inflammation and ultimately neuronal dysfunction including HE[12-17]. Lactulose and lactitol, which target gut microbiota, have been demonstrated to be effective as first-line therapies for both acute and chronic HE. However, lactulose and lactitol have significant gastrointestinal side effects[18,19]. Furthermore, approximately 20% of patients with chronic liver failure and HE have been found to be non-responsive to lactulose treatment. A systemic review demonstrated that lactulose and lactitol failed to impart any survival benefit to cirrhotic patients with HE[20].
Probiotics (e.g., VSL#3, a clinically tested probiotic formula consisting of Lactobacilli, Bifidobacteria, and Streptococci) contain living beneficial bacteria and have been shown to improve mental status and cognitive functioning. Therefore, probiotics may prevent HE recurrence by decreasing urease-producing bacteria populations and ammonia absorption while also increasing hepatic ammonia clearance[21]. Although probiotics are thought to have no adverse effects when used as long-term therapeutics, they are not widely utilized clinically due to the potential risk of introducing live bacteria into immunosuppressed patients. Therefore, the use of probiotics for cirrhotic patients with HE cannot be currently recommended, and rigorous clinical evaluation in randomized controlled trials is required[22-25].
Given the limitations of current HE therapy, research efforts are now aimed at improving existing treatments or developing novel therapies. Fecal microbiota transplantation (FMT) is rapidly being accepted as a viable, safe, and effective treatment for chronic gastrointestinal infections, recurrent inflammatory bowel diseases (IBD) and Clostridium difficile infection (CDI)[26-29]. It has also gained attention for its therapeutic potential for cardiometabolic, autoimmune, and other extra-intestinal conditions that were not previously considered to be associated with the intestinal microbiota[30-32]. However, whether FMT has a healing effect on HE has not been investigated.
In this study, we present in vivo evidence that FMT is effective for improving HE symptoms as evidenced by increased rat motor activity, spatial learning and memory following FMT. Moreover, FMT reduces systematic inflammation, leading to restored hepatic and intestinal dysfunction. Mechanically, FMT reduced liver expression of Toll-like receptor (TLR) 4 and TLR9, which are two critical factors implicated in the accumulation of ammonia and regulating the levels of circulating pro-inflammatory mediators; therefore, FMT decreased liver inflammation and damage. Additionally, FMT recovered the expression of tight junction proteins such as Claudin-1, Claudin-6 and Occludin, resulting in attenuated intestinal permeability. Taken together, our study demonstrates for the first time the efficacy of FMT in the treatment of rats with HE and reveals the mechanisms underlying this process. Notably, we also provide an experimental basis for the potential use of FMT in HE patients.
Male Sprague-Dawley rats weighing 240-270 g were used for the experiments. Rats were fed regular chow and water ad libitum in cages placed in a room with a 12-h light/dark cycle at constant humidity and temperature (25 °C). All of the animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by The National Academy of Sciences and published by The National Institutes of Health.
The fecal material was collected and isolated as previously reported[33,34]. Briefly, approximately 30 g of fecal material was collected from a healthy female donor in a non-menstrual period with no history of hypertension, diabetes or cytomegalovirus, hepatitis or HIV infection. The volunteer also must not have presented with fever, abdominal pain, diarrhea, constipation, etc. In addition, the volunteer must not have used antibiotics, antiviral drugs and any drugs that may affect the function of the gut and bacteria within 2 wk of fecal matter donation. The volunteer was told to eat a liquid diet the day before the bacteria were extracted, and the fecal matter was then passed through 2.0-, 1.0-, 0.5- and 0.25-mm stainless steel laboratory sieves (WS Tyler, Mentor, OH) to remove undigested food and smaller particulate material. The resulting material passing through the 0.25-mm sieve was centrifuged at 6000 g for 15 min and homogenized in 150 mL of sterile normal saline. Glycerol (85%) was added to obtain a final concentration of 10%. The resulting fecal bacteria were resuspended and quantified by calculating the optical density (OD) relative to that of Enterococcus faecalis (E. faecalis) stored at -80 °C.
The rat model of HE was established as previously described. Briefly, rats were given subcutaneous injections of 5 mL/kg of CCl4 solution (a mixture of CCl4 and peanut oil at a ratio of 2:3) twice a week and were fed 5% alcohol in drinking water with a week normal complete diet for 9 consecutive weeks. Control animals received normal saline. Nine weeks after injection, the rats were randomized into five groups (four rats per group) to receive saline, low-dose FMT (containing 2.7 billion fecal bacteria), moderate-dose FMT (containing 5.4 billion fecal bacteria), high-dose FMT (containing 7.1 billion fecal bacteria) or probiotics (VSL#3200 μL/d) for 3 wk. Each capsule of VSL#3 contained 112.5 billion freeze-dried bacteria (Streptococcus thermophilus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus bulgaricus) suspended in corn starch dissolved in 8.33 mL phosphate buffered saline (PBS). The rats received 200 μL/d of this solution (containing 2.7 billion CFU) via intestinal intubation. Prior to introducing an intestinal tube, rats were anesthesized and incised to allow duodenal exposure. Then a small cut was made in the duodenum, allowing a PE50 tube for implantation, followed by stitching the cut. The tube was made to pass into the air and stuffed with sterilized cotton for further administration.
Rat behaviors were observed one week before or after administrations and recorded in an open field. Behaviors include mortality, body weight, motor activity, stools, hematochezia and infection. During the activity measurements, the animals had no access to food or chow. All studies were performed under strictly standardized conditions in a dark room for 30 min. The numbers of total movements, ambulatory movements, and vertical movements were separately recorded to reflect the motor activities of rats with hepatic failure. The motor activities were defined as zero in dead mice.
HE rats were evaluated for spatial learning and memory capabilities using a Morris water maze as described previously. Two training trials a day were conducted on 3 consecutive days during the 8th week of the study. The experimental apparatus consisted of a cylindrical water tank (145 cm in diameter, 60 cm in height) filled with water maintained at 21 ± 1 °C. The water was made opaque with black ink. A platform (10 cm in diameter) was submerged 2 cm below the water surface and placed at the midpoint of one quadrant. Room lights illuminated the pool, and the visual cues around the room (window, cabinets, furniture) were kept consistent. A video camera was placed above the center of the pool and connected to a video tracking system. During each training session, the rats were placed in the pool at a specified starting position and allowed to swim freely until they found the Morris water maze. The time required to escape (escape latency) was recorded. Rats that found the platform within 120 s were allowed to remain on it for 20 s and were then returned to their home cages. If a rat did not reach the platform within 120 s, it was gently guided there by the experimenter, and allowed to stay on it for 20 s. The test was performed again at 12 wk to assess spatial cognitive function.
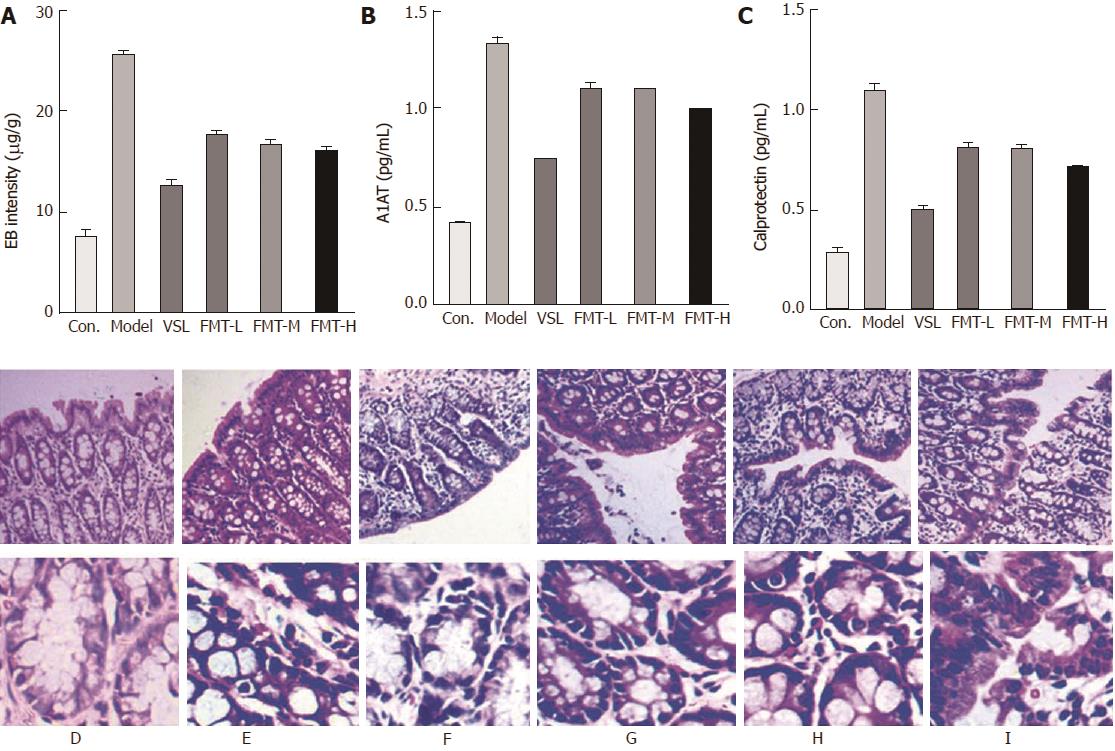
For histological examination, liver and distal ileum samples were fixed in 4% paraformaldehyde, dehydrated using graded ethanol, and then embedded in paraffin. The paraffin blocks were sectioned and stained with hematoxylin and eosin (HE) using standard histological techniques.
Intestinal permeability was determined by Evans blue staining as previously described. Rats were anesthetized and transcardiacally perfused with Evans Blue dye. Colonic tissue was removed and maintained in formamide solution at 60 °C for 24 h, followed by centrifugation at 5000 r/min for 20 min. Optical density at 632 nm was measured and calculated.
Biochemical parameters were measured using standard clinical methods, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (ALB), total bilirubin (TBIL) and direct bilirubin (DBIL). Blood samples (0.5 mL) were collected from the portal and tail veins and analyzed with an automatic biochemical analyzer.
For detection of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α, serum samples from each individual group were collected and separated from portal and tail veins, followed by analysis with ELISA kits according to the manufacturer’s instructions (Sangon, Shanghai). For Calprotectin and alpha-1-antitrypsin (A1AT) detection, equal weight stools from each group were collected and dissolved in PBS and then separated by centrifugation for ELISA testing according to the manufacturer’s instructions (Sangon, Shanghai).
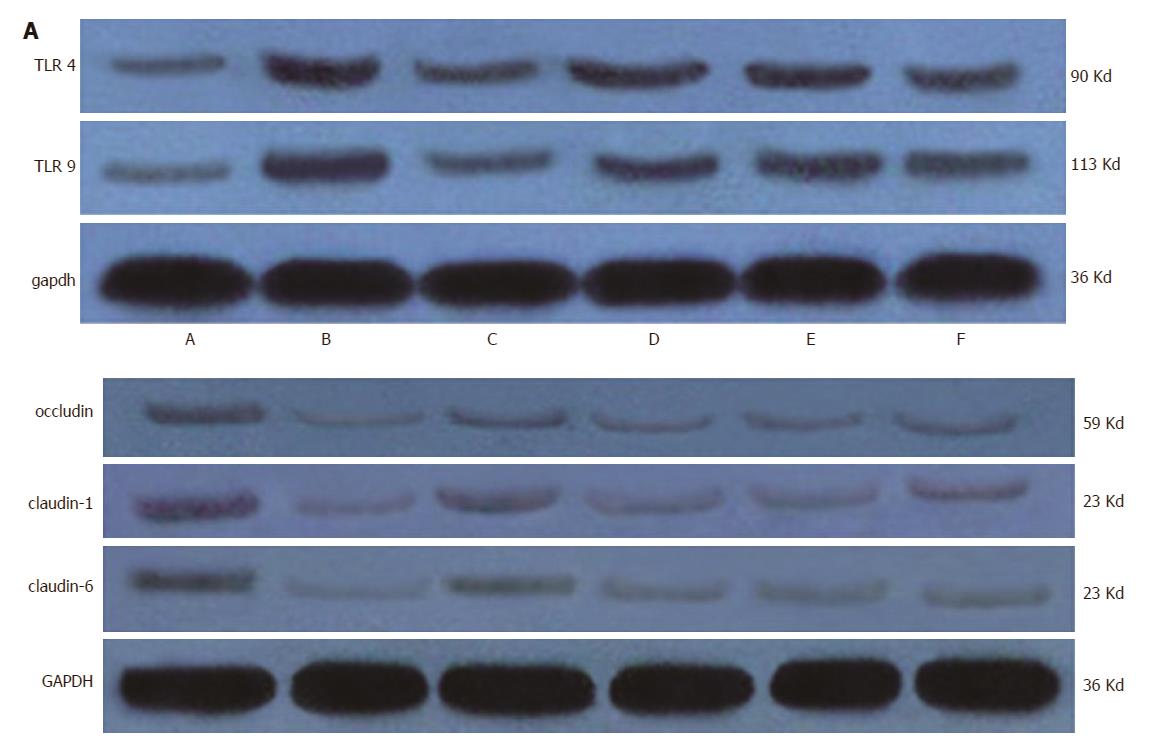
Protein expression was determined in lysates of liver and intestinal tissues using rabbit anti-TLR4, anti-TLR9, anti-Claudin-1, anti-Claudin-6 and anti-Occludin antibodies (Abcam) diluted in blocking buffer (5% milk in 0.2% Tween 20/TBS, 4 °C, overnight). The results were normalized to GAPDH expression (mouse anti-GAPDH, Abcam).
SPSS statistical software, version 19.0, was used for statistical analyses. The Pearson χ2 test or Fisher’s exact test was used to compare qualitative variables and Student’s t-test was used for comparisons of quantitative variables. All statistical tests were two-sided. P < 0.05 was considered statistically significant
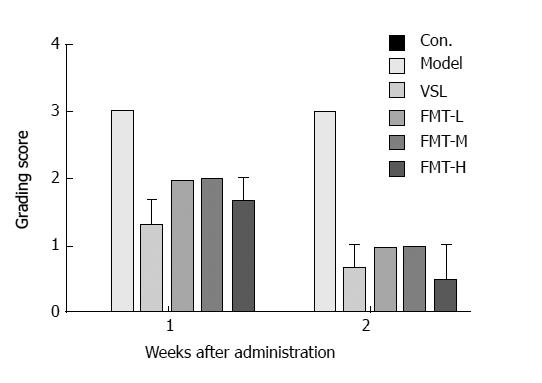
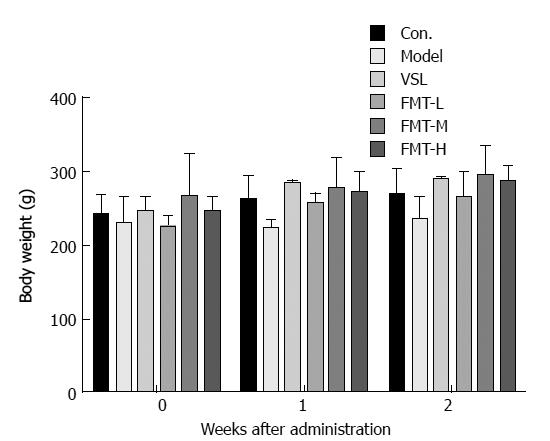
Chronic liver failure-induced HE was induced in rats by treatment with carbon tetrachloride-alcohol as previously described. All rats treated for 9 wk showed similar symptoms of HE, such as impaired spontaneous movement, cachexia and somnolence, when compared with saline-treated control mice. Histological examination verified the successful establishment of the rat HE model. The rats were then randomly divided into five groups including the model group, the probiotic-treated group (termed VSL) and the FMT treated groups across three doses (termed FMT-L, FMT-M and FMT-H for the low, mid and high FMT doses, respectively). Infections were observed during the experimental period; one rat each in the VSL, FMT-L, FMT-M and FMT-H groups died from infection. Data from these rats were excluded from the final statistical analysis. The behaviors of each group were observed clinically until the end of the experiment and were compared for mortality rate and the clinical grade by the scoring method represented in Table 1 and as previously described[35,36]. Significant weight loss and decreased appetite were observed in the control group relative to the other treated groups, whereas rats treated with either probiotics or any of the three FMT doses, but not the model group, started to increase food intake and gain weight during the first week. The feeding behavior of rats in the high-dose FMT treatment group normalized during the second week (Figures 1 and 2). Together, the data presented here demonstrate that FMT enables the healing of chronic liver failure-induced HE.
| Clinical score | Definition |
| 0 | Normal behavior |
| 1 | Mild lethargy |
| 2 | Decreased motor activity, poor gesture control, diminished pain perception |
| 3 | Severe ataxia, no spontaneous righting reflex |
| 4 | No righting reflex, no reaction to pain stimuli |
| 5 | Death |
The Morris water maze was used to evaluate the spatial learning of rats from different treatment groups. The Morris water maze test lasted six days. The first five days of acquisition training with an invisible platform were followed by 4 d of reversal training with an invisible platform. A probe trial was then carried out with no escape platform. Finally, four trials were conducted using the visible platform.
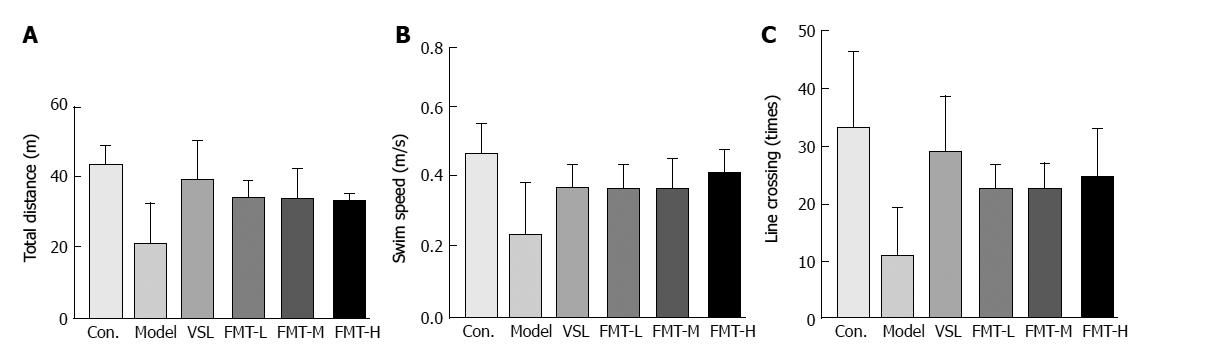
In contrast to the control group, the total travel distance was significantly decreased in the model group (P = 0.014), whereas the VSL or FMT treated rats showed longer travel distances compared with the model group (P < 0.003, Figure 3A). The swim speed was remarkably reduced in the model group, whereas it was markedly increased in rats that received probiotics or FMT (Figure 3B). The VSL and FMT groups exhibited twice as many line crossings than did the model group (P < 0.05, Figure 3C). Although crossing times increased along with FMT dose, this relationship did not reach statistical significance among FMT-L, FMT-M and FMT-H. Collectively, these observations indicate that FMT is capable of improving learning and memory deficits in the rat HE model.
Since FMT effectively improved the behavior and spatial cognitive capability of rats with HE, we next determined whether FMT affected rat hepatic function in the HE model. It was noted that FMT improved hepatic function in a dose-dependent manner as revealed by hematoxylin and eosin staining.
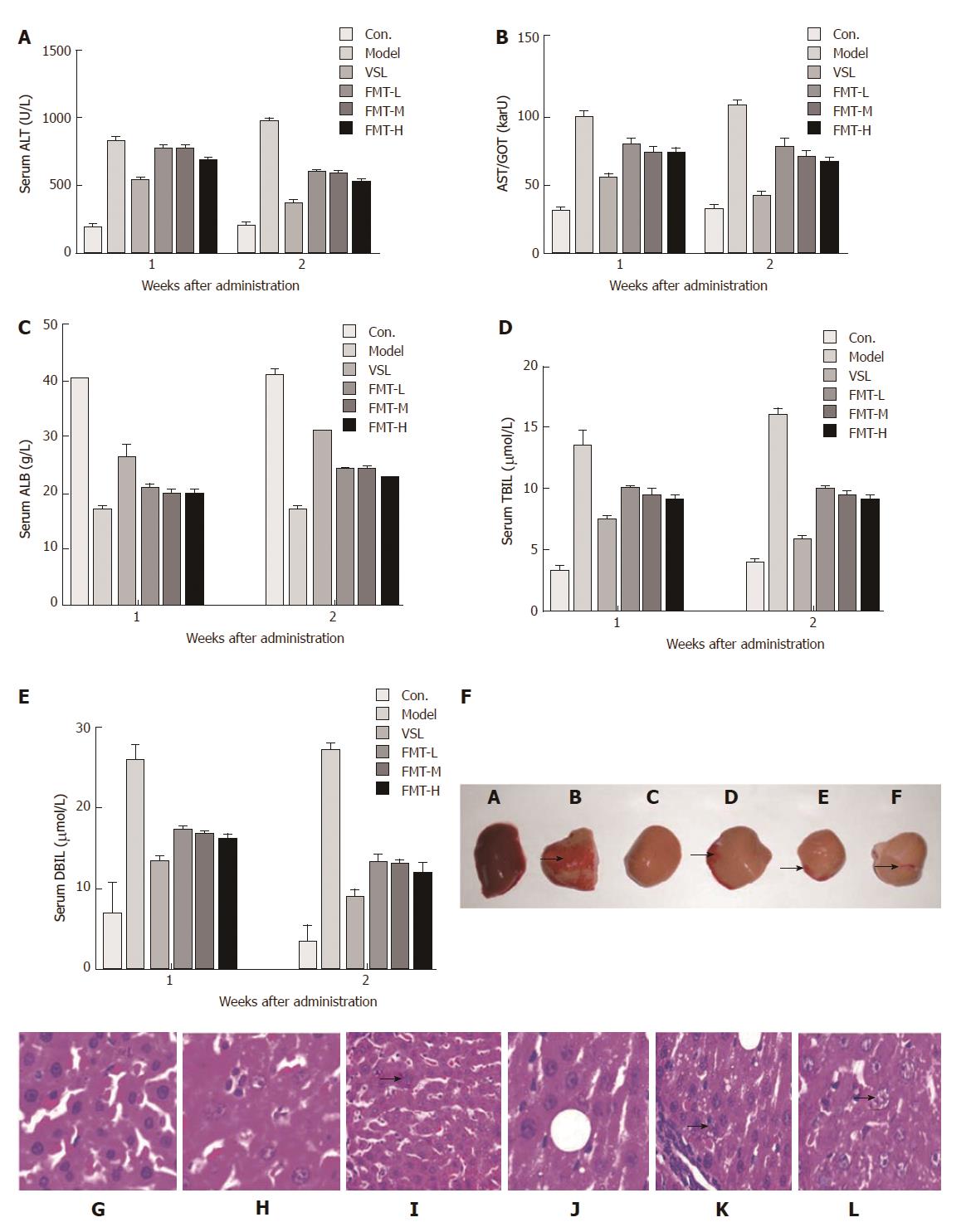
Hepatic function was examined by liver histology and serological parameters including ALT, AST, ALB, TBIL and DBIL. All of these parameters were significantly elevated in the model group (P < 0.0001), which was indicative of severe liver damage. However, rats that received probiotics or FMT showed reduced levels of ALT, AST, ALB, TBIL and DBIL (P < 0.0001) compared with the model rats (Figure 4A-E). Notably, the FMT-H group revealed a marked decrease in these parameters when compared with FMT-L and FMT-M groups (P < 0.0002), suggesting a dose-dependent improvement in hepatic function. Moreover, liver hepatomegaly and severe liver disease were clearly observed on the liver surfaces of the model group whose livers also turned brown, indicating liver damage. On the contrary, liver necrosis of probiotic-treated rats was dramatically relieved. When treated with FMT, the livers showed decreased necrosis as the concentration of FMT increased (Figure 4F). However, the liver color was still brown. In agreement with altered liver morphology, liver histology analysis suggested that inflammation and spotty or patchy necrosis were observed in the model group, whereas none of these manifestations were observed in the control group. Livers from rats that received probiotics or FMT showed less necrotic areas than did the model rats (Figure 4G-L).
The intestinal barrier controls physical and biochemical activities to maintain a balance with the external environment. Patients with liver cirrhosis develop a series of alterations in the intestinal barrier associated with the severity of liver disease that ultimately increase intestinal permeability. We therefore examined whether FMT could protect intestinal integrity in the HE model. As showed in Figure 5A, the intestinal permeability tripled in the model group compared with the control group (P < 0.0001), as detected by Evans blue extravasation method. FMT treatment remarkably decreased the intestinal permeability (P < 0.0001), whereas the VSL-treated group appeared to have even less intestinal permeability. Histological observations suggested mild edema and mucosal separation in the model group. FMT-treated HE rats displayed decreased edema, mucosal damage and inflammatory infiltration. Similar effects were observed when the HE rats were treated with probiotics (Figure 5F). Intestinal permeability was not different between the VSL and FMT treatment groups, irrespective of dose. Taken together, these findings support a protective role for FMT in maintaining intestinal integrity and attenuating the mucosal barrier dysfunction induced by HE. In accordance with altered intestinal permeability, expression ofA1AT in the stool was elevated in the model rats, which was indicative of excessive gastrointestinal protein loss. A1AT levels decreased in the rats that received probiotics or FMT, suggesting improved intestinal permeability and reduced gastrointestinal protein loss (Figure 5B). Elevated Calprotectin levels in the stool, which are commonly used in the clinic to measure the intestinal inflammation index, were found to be reversed by probiotics and FMT, implying attenuated intestinal inflammation (Figure 5C). Overall, the data presented here indicate that FMT preserves intestinal mucosal barrier function.
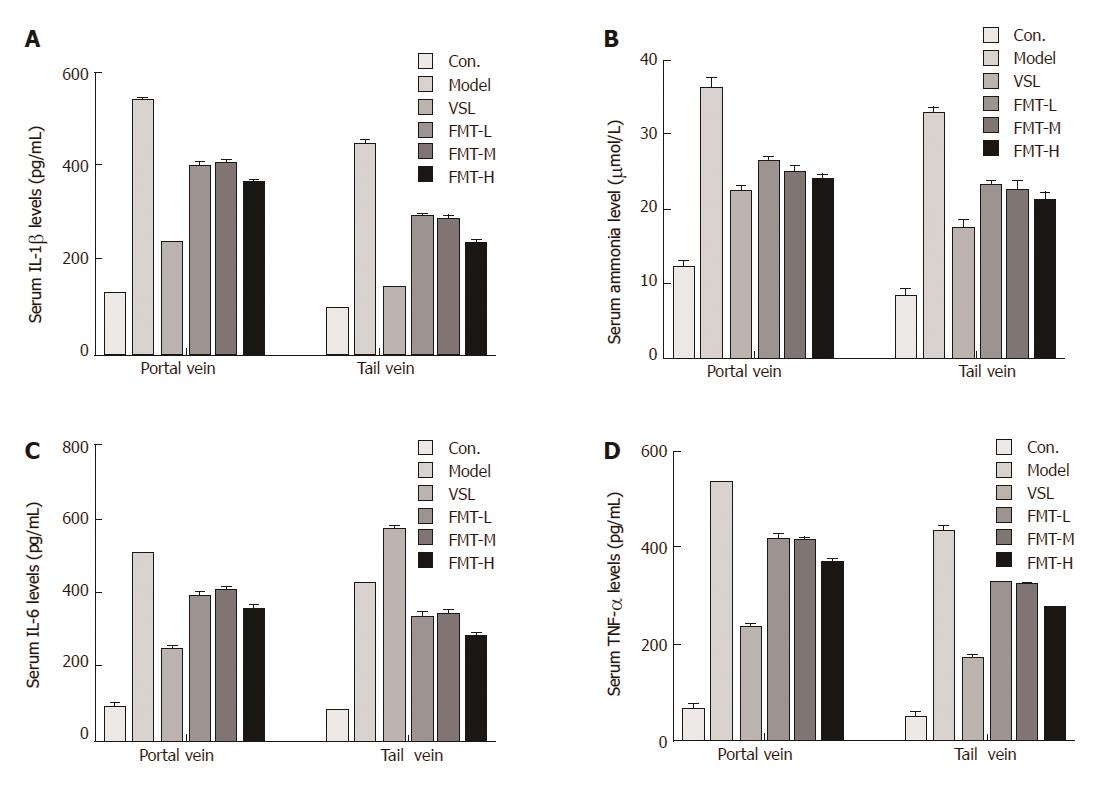
It is well known that ammonia serves as the most important precipitating factor leading to HE. In addition, accumulating evidence has demonstrated that inflammation, including systemic inflammation, neuroinflammation and endotoxemia, acts concomitantly with ammonia to drive HE pathogenesis in cirrhotic patients. We next determined whether FMT affects serum ammonia levels and proinflammatory responses. Serum ammonia levels and proinflammatory cytokines including IL-1β, IL-6 and TNF-α were detected using Berthelot reaction and ELISA assay. The mean ammonia level from the portal vein was 12.37 μmol/L in the control group, which was increased to 36.28 μmol/L in HE rats. When the HE rats were given probiotics, the mean serum ammonia level decreased to 22 μmol/L. FMT therapy resulted in a notable decline in ammonia levels (P < 0.0001). In particular, high-dose FMT administration was almost as effective as probiotic treatment in decreasing ammonia levels. Moreover, similar effects were observed when the ammonia level was evaluated from the tail vein, indicating that FMT enables effective clearance of serum ammonia and ultimately improves clinical symptoms (Figure 6A). Consistent with the decreased serum ammonia concentration, circulating levels of proinflammatory mediators such as IL-1β, IL-6 and TNF-α were elevated in the model group and reduced in groups that received probiotics or FMT treatment (P < 0.0001, Figure 6B-D). Significant differences in proinflammatory mediator levels were observed among the groups that received different FMT doses (P < 0.002). The data described here demonstrate a systematic relief of HE severity in rats, and it was clear that systemic inflammation, but not ammonia, was strongly correlated with increasing grades of HE.
Although FMT has been shown to be effective in a rat model of HE, leading to improved hepatic and intestinal function and impaired systemic inflammation, the underlying mechanisms of these processes have not been investigated. Liver TLRs are reported to be important determinants of HE severity and are intimately related to arterial ammonia concentration and levels of circulating pro-inflammatory mediators[37-39]. Additionally, tight junction proteins (Occludin and Claudins) are implicated in the regulation of intestinal permeability[40,41].
Therefore, we examined the protein expression levels of TLR4 and TLR9 in the liver tissues, and the expression levels of Claudin-1, Claudin-6 and Occludin were analyzed in the intestinal tissues (Figure 7). As expected, liver dysfunction stimulated the expression of TLR4 and TLR9 in the liver, which was downregulated in liver tissue from rats treated with probiotics and FMT, indicating that FMT can limit systemic inflammation by decreasing the expression of TLR4 and TLR9. TLR4 and TLR9 are potent mediators that promote the expression of pro-inflammatory factors such as IL-6 and TNF-α. Accordingly, the expression of Claudin-1, Claudin-6 and Occludin was lost in the intestinal tissues from the model rats, suggesting impaired tight junction function. Tight junction protein loss was reversed by probiotic as well as FMT treatment. High dose FMT appeared to induce more expression of Claudin-1, Claudin-6 and Occludin than did other doses of FMT (Figure 6A and B). In summary, these data demonstrate that FMT delays HE progression in rats by reducing the liver expression of TLR4 and TLR9 and triggering tight junction protein expression, resulting in attenuated systemic inflammation and decreased intestinal permeability.
HE is a potentially reversible spectral neuropsychiatric complication caused by acute and chronic liver diseases, significantly affecting the prognosis. Although decades of clinical practice have demonstrated that nonabsorbable disaccharides, such as lactulose or lactitol, are effective for approximately 80% of HE patients, little survival benefit in cirrhotic HE patients has been observed following treatment with these agents. Probiotics, thought to have a therapeutic effect with no adverse effects, are not widely used clinically due to the potential risk of introducing live bacteria[42]. Acute liver organ shortage and increased morbidity have expanded research efforts aimed at improved treatment[28,43].
FMT is the introduction of a fecal suspension derived from a healthy donor into the gastrointestinal tract of a diseased individual. The clinical application of FMT has grown significantly within the last decade and is now gaining mainstream acceptance as a valuable, low cost procedure with apparently safe, and readily available materials, especially for the treatment of recurrent or refractory CDI[26,28,29]. Whether FMT is effective for HE patients has not yet been investigated. Here we provide experimental evidence that FMT has potent protective effects in improving motor activity in a rat model of HE and was comparable to probiotic administration. A significant negative correlation was found between the FMT dose and behavioral score. In addition, FMT has been shown to enhance spatial learning and memory as revealed by the Morris water maze assay. Since FMT effectively improves behavior and spatial cognitive capabilities, we next determined whether it affected hepatic function in the rat HE model. Hepatic function was examined by liver histology and serological parameters. Rats that received FMT showed reduced levels of ALT, AST, ALB, TBIL, and DBIL (P < 0.0001) compared with the model rats. Notably, the FMT-H group revealed a marked decrease in these parameters when compared with the FMT-L and FMT-M groups (P < 0.0002). Moreover, liver necrosis in the FMT-treated rats was dramatically relieved compared with the model rats, suggesting a dose-dependent improvement in hepatic function. However, in general, the levels of cognitive function of rats that received FMT were altered more than other indicators.
Alterations in the intestinal barrier were associated with the severity of liver disease, leading to increased intestinal permeability[44-46]. We therefore examined whether FMT could protect against the intestinal permeability induced by HE. FMT treatment remarkably decreased the intestinal permeability (P < 0.0001), whereas histological observations suggested that FMT treatment in HE rats displayed decreased edema, mucosal damage and inflammatory infiltration. In accordance with decreased intestinal permeability, A1AT levels in the stool were reduced following FMT, suggesting improved intestinal permeability and reduced gastrointestinal protein loss. Elevated Calprotectin levels in the stool were also found to be reversed by FMT, implying that FMT prevents intestinal mucosal barrier dysfunction. Furthermore, FMT was found to attenuate serum ammonia levels and impair systematic inflammation as demonstrated by reduced proinflammatory cytokines including IL-1β, IL-6 and TNF-α, indicating a systematic relief of HE severity in rats. It was clear that systemic inflammation, but not ammonia, was strongly correlated with increasing grades of HE. Taken together, the data in present study clearly show a potent healing effect for FMT in the rat HE model. Next we tried to determine the possible mechanisms underlying the protective functions of FMT. It has been widely accepted that an impaired gut-liver-brain axis in patients with liver disease is the leading cause of complications including HE. In HE patients, bacterial translocation and increased intestinal permeability are frequently found, the latter of which promotes bacterial translocation, such as migration of microbes or their products including pathogen-associated molecular patterns (PAMPs), the natural ligands for TLRs[40]. Liver expression of TLR4 and TLR9 is a critical determinant of HE severity, triggers the inflammation cascade and is intimately related to arterial ammonia concentration and circulating proinflammatory mediators such as IL-1β, IL-6 and TNF-α. We therefore evaluated the levels of liver TLR4 and TLR9 and circulating IL-1β, IL-6 and TNF-α in the HE model and FMT-treated rats.
Our data here suggest that VSL treatment efficiently reduced circulating IL-1β and TNF-α but not IL-6, a well-established independent risk factor for HE that was negatively associated with cognitive functions in HE patients[47-49]. Serum IL-6 was more evidently decreased in the FMT group than the VSL group in our study, which was consistent with more improved spatial learning and motor activity, suggesting that FMT is more effective than VSL, at least partially. Lata et al[50] also reported the limited effectiveness of VSL for HE.
Note that our study was the first to provide experimental data for potential use of FMT for HE, yet more accurate investigations are supposed to be done for optimized usage of FMT. Instead, VSL is commercially available with more detailed study.
FMT significantly reduced the liver expression levels of TLR4 and TLR9 and proinflammatory mediators, suggesting that FMT improves HE symptoms by impairing liver inflammation and reducing systemic inflammation. Importantly, the expression of tight junction proteins Claudin-1, Claudin-6 and Occludin was increased in the intestinal tissues of rats that were given FMT, indicating improved intestinal mucosal barrier function. The limitation of this study was the similar infection rate observed between the probiotics group and the FMT group. Our experiment still cannot fully explain whether FMT is superior to VSL#3 or not, which requires more experimental or clinical evidence.
Collectively, our study provided experimental evidence for the first time that supports an FMT-enabled protective role in treating HE rats and revealed the mechanisms behind FMT function, thus providing a basis for potential clinical application of FMT in HE patients.
Hepatic encephalopathy (HE) is a disease of liver dysfunction, and there is currently no effective therapy for treatment of this condition. To all knowledge, gut microbiota has emerged as a critical factor in the development of HE. Fecal microbiota transplantation (FMT) has been confirmed to treat some diseases. However, the effect of FMT on HE has not been researched deeply.
Previous clinical evidence has shown that many diseases of the digestive tract, including liver diseases, have intestinal flora abnormalities, while FMT can improve the composition and quantity of intestinal bacteria to achieve therapeutic purposes.
This is the first experimental study to confirm that FMT can be used to treat hepatic encephalopathy, and it also reflects the superiority of FMT relative to VSL#3.
According to the results of the article, FMT has the potential to become a new method for treating hepatic encephalopathy. At the same time, our results suggest that FMT has special advantages in some areas, providing more possibilities for further searching for new targets for treatment of hepatic encephalopathy.
FMT is the introduction of a fecal suspension derived from a healthy donor into the gastrointestinal tract of a diseased individual. Initially as a treatment for Clostridium difficile infection, it has been shown to be effective in many diseases of the human body.
This is a very interesting paper about FMT for HE. The authors demonstrated that FMT has good effects in improving intestinal barrier function, by regulating the expression of tight junction proteins (Claudin-1, Claudin-6 and Occludin). Therefore, FMT has a protective role in treating HE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Accepted: September 6, 2017
P- Reviewer: Hashimoto N, Kharbanda KK S- Editor: Ma YJ L- Editor: A E- Editor: Ma YJ
| 1. | Wijdicks EFM. Hepatic Encephalopathy. N Engl J Med. 2017;376:186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 3. | Lauridsen MM, Bajaj JS. Hepatic encephalopathy treatment and its effect on driving abilities: A continental divide. J Hepatol. 2015;63:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Bustamante J, Rimola A, Ventura PJ, Navasa M, Cirera I, Reggiardo V, Rodés J. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30:890-895. [PubMed] |
| 5. | Dhiman RK, Kurmi R, Thumburu KK, Venkataramarao SH, Agarwal R, Duseja A, Chawla Y. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, Durkalski V, Larson AM, Liou I, Fix O. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 7. | Gubbins GP, Moritz TE, Marsano LS, Talwalkar R, McClain CJ, Mendenhall CL. Helicobacter pylori is a risk factor for hepatic encephalopathy in acute alcoholic hepatitis: the ammonia hypothesis revisited. The Veterans Administration Cooperative Study Group No. 275. Am J Gastroenterol. 1993;88:1906-1910. [PubMed] |
| 8. | Kramer L, Tribl B, Gendo A, Zauner C, Schneider B, Ferenci P, Madl C. Partial pressure of ammonia versus ammonia in hepatic encephalopathy. Hepatology. 2000;31:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Wright G, Jalan R. Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: Pandora’s box? Hepatology. 2007;46:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Ge PS, Runyon BA. Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312:643-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 14. | Victor DW 3rd, Quigley EM. Hepatic encephalopathy involves interactions among the microbiota, gut, brain. Clin Gastroenterol Hepatol. 2014;12:1009-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Dhiman RK. Gut microbiota and hepatic encephalopathy. Metab Brain Dis. 2013;28:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Garcovich M, Zocco MA, Roccarina D, Ponziani FR, Gasbarrini A. Prevention and treatment of hepatic encephalopathy: focusing on gut microbiota. World J Gastroenterol. 2012;18:6693-6700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Dhiman RK. Gut microbiota, inflammation and hepatic encephalopathy: a puzzle with a solution in sight. J Clin Exp Hepatol. 2012;2:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Maharshi S, Sharma BC, Srivastava S, Jindal A. Randomised controlled trial of lactulose versus rifaximin for prophylaxis of hepatic encephalopathy in patients with acute variceal bleed. Gut. 2015;64:1341-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Kalaitzakis E, Björnsson E. Lactulose treatment for hepatic encephalopathy, gastrointestinal symptoms, and health-related quality of life. Hepatology. 2007;46:949-950; author reply 951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328:1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Cesaro C, Tiso A, Del Prete A, Cariello R, Tuccillo C, Cotticelli G, Del Vecchio Blanco C, Loguercio C. Gut microbiota and probiotics in chronic liver diseases. Dig Liver Dis. 2011;43:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003-1008.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 23. | McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev. 2011;11:CD008716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Xu J, Ma R, Chen LF, Zhao LJ, Chen K, Zhang RB. Effects of probiotic therapy on hepatic encephalopathy in patients with liver cirrhosis: an updated meta-analysis of six randomized controlled trials. Hepatobiliary Pancreat Dis Int. 2014;13:354-360. [PubMed] |
| 25. | Zhao LN, Yu T, Lan SY, Hou JT, Zhang ZZ, Wang SS, Liu FB. Probiotics can improve the clinical outcomes of hepatic encephalopathy: An update meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, Weese JS, Collins S, Moayyedi P, Crowther M. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2016;315:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 27. | Ho N, Prasad V. Clostridium difficile diarrhea and fecal transplantation. J Clin Gastroenterol. 2011;45:742-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing Clostridium difficile infection. Therap Adv Gastroenterol. 2012;5:403-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 29. | Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 30. | Abdollahi-Roodsaz S, Abramson SB, Scher JU. The metabolic role of the gut microbiota in health and rheumatic disease: mechanisms and interventions. Nat Rev Rheumatol. 2016;12:446-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Groen AK, Nieuwdorp M. An evaluation of the therapeutic potential of fecal microbiota transplantation to treat infectious and metabolic diseases. EMBO Mol Med. 2017;9:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Smits LP, Bouter KE, de Vos WM, Borody TJ, Nieuwdorp M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology. 2013;145:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 473] [Article Influence: 39.4] [Reference Citation Analysis (1)] |
| 33. | Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 544] [Article Influence: 41.8] [Reference Citation Analysis (2)] |
| 34. | Satokari R, Mattila E, Kainulainen V, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection--an observational cohort study. Aliment Pharmacol Ther. 2015;41:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 35. | Farjam M, Dehdab P, Abbassnia F, Mehrabani D, Tanideh N, Pakbaz S, Imanieh MH. Thioacetamide-induced acute hepatic encephalopathy in rat: behavioral, biochemical and histological changes. Iran Red Crescent Med J. 2012;14:164-170. [PubMed] |
| 36. | Zimmermann C, Ferenci P, Pifl C, Yurdaydin C, Ebner J, Lassmann H, Roth E, Hörtnagl H. Hepatic encephalopathy in thioacetamide-induced acute liver failure in rats: characterization of an improved model and study of amino acid-ergic neurotransmission. Hepatology. 1989;9:594-601. [PubMed] |
| 37. | Tranah TH, Vijay GK, Ryan JM, Shawcross DL. Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis. 2013;28:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 38. | , Chouhan M, Hughes RD. The role of infection and inflammation in the pathogenesis of hepatic encephalopathy and cerebral edema in acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2006;3:118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Blei AT. Infection, inflammation and hepatic encephalopathy, synergism redefined. J Hepatol. 2004;40:327-330. [PubMed] |
| 40. | Aguirre Valadez JM, Rivera-Espinosa L, Méndez-Guerrero O, Chávez-Pacheco JL, García Juárez I, Torre A. Intestinal permeability in a patient with liver cirrhosis. Ther Clin Risk Manag. 2016;12:1729-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Benjamin J, Singla V, Arora I, Sood S, Joshi YK. Intestinal permeability and complications in liver cirrhosis: A prospective cohort study. Hepatol Res. 2013;43:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Luo M, Guo JY, Cao WK. Inflammation: A novel target of current therapies for hepatic encephalopathy in liver cirrhosis. World J Gastroenterol. 2015;21:11815-11824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Pathak R, Enuh HA, Patel A, Wickremesinghe P. Treatment of relapsing Clostridium difficile infection using fecal microbiota transplantation. Clin Exp Gastroenterol. 2013;7:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, Miranda A, Portincasa P, Carbonara V, Palasciano G. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011;35:14S-20S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 47. | Luo M, Li L, Yang EN, Dai CY, Liang SR, Cao WK. Correlation between interleukin-6 and ammonia in patients with overt hepatic encephalopathy due to cirrhosis. Clin Res Hepatol Gastroenterol. 2013;37:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Li W, Li N, Wang R, Li Q, Wu H. Interferon gamma, interleukin-6, and -17a levels were correlated with minimal hepatic encephalopathy in HBV patients. Hepatol Int. 2015;9:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Tsai CF, Chu CJ, Huang YH, Wang YP, Liu PY, Lin HC, Lee FY, Lu CL. Detecting minimal hepatic encephalopathy in an endemic country for hepatitis B: the role of psychometrics and serum IL-6. PLoS One. 2015;10:e0128437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | Lata J, Jurankova J, Kopacova M, Vitek P. Probiotics in hepatology. World J Gastroenterol. 2011;17:2890-2896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |