Published online Oct 7, 2017. doi: 10.3748/wjg.v23.i37.6877
Peer-review started: June 12, 2017
First decision: July 13, 2017
Revised: August 15, 2017
Accepted: September 6, 2017
Article in press: September 5, 2017
Published online: October 7, 2017
To investigate the clinical utility of biological age (BA) measurement in screening colonoscopy for the detection of colorectal adenomas in the average-risk population.
A consecutive series of asymptomatic subjects aged ≥ 30 years who underwent colonoscopy in routine check-ups were enrolled. Colorectal adenoma was classified according to size, number, and location. BAs were calculated using the MEDIAGETM Biological Age Measurement System.
A total of 2696 subjects were investigated (1876 men and 820 women). The mean chronological age (CA) was 46.0 years and the mean BA was 44.7 years. Metabolic syndrome (MS) was diagnosed in 218 subjects (8.1%). The prevalence of overall colorectal adenoma was 23.1% (622/2,696). When the subjects were divided into four groups based on BA (≤ 39 years; 40-49 years; 50-59 years; ≥ 60 years), the prevalence of colorectal adenoma was increased as BA increased (P < 0.001). Colorectal adenoma located in the proximal colon was more prevalent in the BA-dominant group (BA-CA ≥ 5 years) than the CA-dominant group (CA-BA ≥ 5 years) (P = 0.034). When the subjects were categorized into four groups according to MS and age gap between BA and CA, the incidence of colorectal adenoma increased with MS and BA-dominance (P < 0.05).
Measurement of BA may help to assess the risk of colorectal adenoma in screening colonoscopy.
Core tip: The present study was conducted on the basis of the hypothesis that colorectal adenoma recognized as a premalignant lesion of colorectal cancer is associated with the biological aging process, suggesting that biological age (BA) represents a biological activity in human. Thus, we intended to assess the risk of colorectal adenomas in the average-risk population from the biological perspective. As a result, the present study demonstrated that the prevalence of colorectal adenoma correlated well with BA, and was more common in the distal colon in BA -dominance.
- Citation: Kim SJ, Kim BJ, Kang H. Measurement of biological age may help to assess the risk of colorectal adenoma in screening colonoscopy. World J Gastroenterol 2017; 23(37): 6877-6883
- URL: https://www.wjgnet.com/1007-9327/full/v23/i37/6877.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i37.6877
Colorectal cancer (CRC) is one of the most common malignancies and a leading cause of cancer mortality in the world[1,2]. Colonoscopy is effective for detecting adenomatous polyps, and colonoscopy with polypectomy has been shown to significantly reduce the expected incidence of CRC by 76%–90% in multiple cohort studies[3,4].
The guidelines of the American Society of Gastrointestinal Endoscopy for colorectal cancer screening and surveillance recommend that screening colonoscopy should begin at the age of 50 for average-risk individuals[5,6]. The current cutoff age is based mainly on the fact that the incidence of CRC begins to rise during the 6th decade of life[7]. However, given the long lead time associated with adenoma-to-carcinoma progression, the increased number of CRCs diagnosed in this age group may reflect the end result of adenomas present in individuals under 50 years old[6]. In fact, nearly 1 in 15 of CRCs develops in adults under 50 years of age[8]. Therefore, reasonable guidelines for performing screening colonoscopy for colorectal cancer should be proposed for the average-risk population.
Aging is a highly individual process, and has a high degree of inter-individual and between-individual difference[9]. Chronological age (CA) is the age as determined by the passage of time since birth. However, biological age (BA) is determined by physiology rather than chronology[10]. As CA does not accurately reflect the functional state of the body, it does not provide an accurate indication of the aging process[11,12]. CA and BA may differ significantly, since both genetics and the environment contribute to BA[10]. Individuals might be healthier, more vital, and biologically younger than might be expected from their CA, and vice versa[13].
If subjects with an average risk of colon cancer undergo colonoscopy according to BA, colon cancers may be prevented by colonoscopy with polypectomy for colorectal adenomas and premalignant lesions at the optimal age of life. Thus, it is necessary to stratify the risks and identify high-risk groups in a specialized manner. In addition, there might be a rationale by which to recommend screening colonoscopy for early cancer detection and prevention besides CA determination.
To date, no worldwide studies have been conducted to estimate the clinical application of BA as a new indicator in CRC screening. Hence, we intended to assess the risk for colorectal adenomas in the average-risk population from the biological perspective. Therefore, the aim of the present study was to investigate the clinical utility of BA measurement in screening colonoscopy for the detection of colorectal adenomas in the average-risk population.
We conducted this study on a consecutive series of asymptomatic subjects ≥ 30 years of age who underwent colonoscopy during a routine check-up at the Health Promotion Center of Bundang Jesaeng Hospital in Gyungki-do, South Korea between August 2011 and August 2012. The exclusion criteria were a history of colonic disease, such as CRC, polyps, and inflammatory bowel disease, and a history of colorectal surgery or colonoscopy within the previous 10 years. Information regarding age, sex, medical history of diabetes mellitus and hypertension, and family history of colon cancer was collected from a standardized questionnaire. The study was approved by the Institutional Review Board of the Bundang Jesaeng Hospital in Korea (IMG 15-04).
BAs were calculated as described previously using models for predicting BA (MEDIAGETM Biological Age Measurement System, MEDIAGE Research Center, Gyeonggi-do, South Korea)[14,15]. This measurement system has been used as an adjunctive measure for easily predicting individual differences in health and aging status with the use of clinical parameters that are commonly used at many health check-up centers in South Korea.
We collected clinical profiles such as physical, biochemical, and hormonal parameters for the calculation of BA. A profile of physical parameters included height, weight, waist circumference, lean body mass, body fat measurement, body mass index, blood pressure, forced expiratory volume 1, and forced vital capacity. Waist circumference was measured at the high point of the iliac crest during minimal respiration. Blood samples were drawn from the antecubital space in the morning. Biochemical parameters included complete blood count with differential count, erythrocyte sedimentation rate, fasting blood glucose, liver function test, lipid profile, blood urea nitrogen, creatinine, bone turnover markers such as osteocalcin and deoxypyridinoline, and tumor markers such as prostate specific antigen, and total antioxidant status were measured using Randox TAS assay kits (Randox, United States) based on the reaction between metmyoglobin and hydrogen peroxide. Lastly, the hormonal parameters included thyroid-stimulating hormone, temporary threshold shift, sex hormone-binding globulin, follicle-stimulating hormone, insulin-like growth factor-1, and dehydroepiandrosterone sulphate.
Based on the ATP III-WPRO (Regional Office for the Western Pacific Region of WHO) definition according to the WPRO criteria, metabolic syndrome (MS) was defined as the presence of three or more of the following[16]: (1) abdominal obesity, waist circumference > 90 cm in men and > 80 cm in women; (2) hypertriglyceridemia, ≥ 150 mg/dL; (3) low high-density lipoprotein cholesterol level, < 40 mg/dL in men and < 50 mg/dL in women; (4) high blood pressure, ≥ 130 mm Hg systolic or ≥ 85 mm Hg diastolic; and (5) high serum fasting glucose level, ≥ 110 mg/dL.
We performed colonoscopies that reached the cecum after bowel preparation with 4 L of Colyte powder (TAEJOON PHARM Co., Ltd., South Korea). The Colyte powder was composed of polyethylene glycol (3350.29 g), anhydrous sodium sulfate (2.85 g), sodium hydrogen carbonate (0.84 g), sodium chloride (0.73 g), and potassium chloride (0.37 g). We examined colonoscopic features, including the size, location, number, and histologic findings of polyps. Polyp size was assessed using open colonoscope biopsy forceps (MTW-Endoskopie Manufaktur, Wesel, Germany). Adenoma size was classified into < 10 and ≥ 10 mm (the largest size was used for multiple adenomas). The location of colorectal adenomas was divided into three categories: the proximal colon, including the cecum, ascending colon, and transverse colon; the distal colon, including the splenic flexure, descending colon, sigmoid colon, and rectum; and both sides of the colon. The number of adenomas was classified as either single or multiple (≥ 2). Histological findings were classified according to their premalignant potential: colorectal adenomas included tubular, villous, or serrated adenomas; and controls had normal colonoscopic findings and non-polypoid benign lesions such as non-specific colitis or histologically confirmed hyperplastic polyps. In addition, advanced adenomas were defined as ≥ 1 cm in estimated diameter, containing ≥ 25% villous features, and/or high-grade dysplasia.
For continuous variables, data distribution was first evaluated for normality using the Shapiro-Wilk test. As all the variables did not pass not pass normality test, data were analyzed using Mann-Whitney’s U tests and presented as medians (P25–P75). Descriptive variables were analyzed using χ2 analyses or Fisher’s exact tests, as appropriate, and P < 0.05 were considered statistically significant. Statistical analyses were conducted using SPSS 21.0 (IBM Corp., Armonk, NY, United States).
We identified a total of 2699 subjects in the study period. Among them, three with colorectal carcinoma were excluded. Thus, the study population consisted of 2696 subjects. The demographic and clinical characteristics of the enrolled subjects are summarized in Table 1.
| All subjects (n = 2696) | ||
| Chronological age | Biological age | |
| Age [median (P25-P75), yr] | 46.0 (39.0-54.0) | 44.7 (38.2-53.1) |
| Gender | ||
| Male | 1876 | (69.6) |
| Female | 820 | (30.4) |
| High blood pressure | ||
| Yes (%) | 544 | (20.2) |
| No (%) | 2152 | (79.8) |
| Total cholesterol (mg/dL) | 193.00 | (172.00-217.75) |
| LDL cholesterol (mg/dL) | 118.00 | (99.00-141.00) |
| HDL cholesterol (mg/dL) | 53.00 | (45.0-64.00) |
| Triglyceride (mg/dL) | 98.00 | (66.00-152.00) |
| Fasting glucose (mg/dL) | 85.00 | (79.00-92.00) |
| HbA1c | 5.40 | (5.20-5.70) |
| CEA | 1.55 | (1.00-2.38) |
| Metabolic syndrome | ||
| Yes | 218 | (8.1) |
| No | 2478 | (91.9) |
The subjects comprised 1876 men (69.6%) and 820 women (30.4%). The median age of the subjects in CA was 46.0 years, whereas the median age og the subjects in BA was 44.7 years old.
MS was diagnosed in 218 subjects (8.1%). When the subjects were divided into four groups based on BA (≤ 39 years; 40-49 years; 50-59 years; ≥ 60 years), the prevalence of MS was increased with increasing BA (P < 0.001). Interestingly, the gradient of prevalence of MS in BA was higher than that of CA.
The prevalence of overall colorectal adenoma was 23.1% (622/2696). The colonoscopic findings of the subjects according to location, size, and number are summarized in Table 2.
| All subjects (n = 2696) | |
| Colorectal adenoma | |
| Yes | 622 (23.1) |
| No | 2074 (76.9) |
| Location | |
| Proximal | 489 (18.1) |
| Distal | 244 (9.1) |
| Both | 53 (2.0) |
| Size (mm) | |
| < 10 | 714 (26.5) |
| ≥ 10 | 73 (2.7) |
| Number | |
| 1 | 484 (18.0) |
| ≥ 2 | 138 (5.1) |
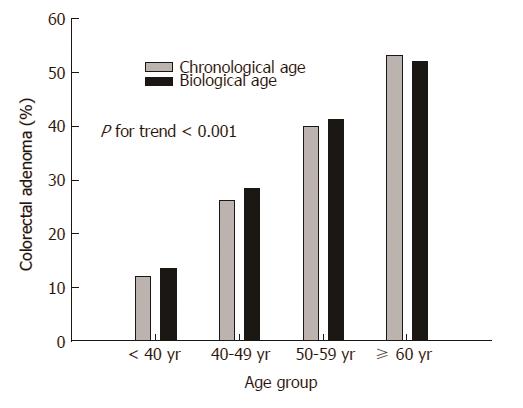
When the subjects were divided into four groups based on BA or CA (≤ 39 years; 40-49 years; 50-59 years; ≥ 60 years), the prevalence of colorectal adenoma was increased as BA and CA increased (P < 0.001) (Figure 1).
Clinical and colonoscopic characteristics were evaluated in accordance with the age gap between CA and BA. The prevalence of colorectal adenoma in dominant age groups was assessed. The subjects were divided into two groups based on an age gap between CA and BA of more than 5 years: BA-dominant group (BA-CA ≥ 5 years) and CA-dominant group (CA-BA ≥ 5 years). The incidences of MS as well as high blood pressure, fasting glucose level, and HbA1c in the BA-dominant group were significantly higher than those in the CA-dominant group (Table 3). As shown in Table 4, colorectal adenoma located in the distal colon was more prevalent in the BA-dominant group than the CA-dominant group (P = 0.034).
| BA-dominant group (n = 28) | CA-dominant group (n = 76) | P value | |
| Age (yr, mean ± SD) | 42.00 (35.25-49.75) | 52.00 (43.25-59.00) | 0.001 |
| Gender | 0.013 | ||
| Male | 25 (89.3) | 49 (64.5) | |
| Female | 3 (10.3) | 27 (35.5) | |
| High blood pressure | < 0.001 | ||
| Yes (%) | 11 (39.3) | 6 (7.9) | |
| No (%) | 17 (60.7) | 70 (92.1) | |
| Fasting glucose (mg/dL) | 88.00 (82.00-97.50) | 81.00 (75.25-87.00) | < 0.001 |
| HbA1c | 5.55 (5.30-5.80) | 5.30 (5.20-5.50) | 0.001 |
| CEA | 1.91 (0.97-3.20) | 1.67 (1.03-2.57) | 0.858 |
| Metabolic syndrome | < 0.001 | ||
| Yes | 7 (25.0) | 1 (1.3) | |
| No | 21 (75.0) | 75 (98.7) |
| BA-dominant group | CA-dominant group | P value | |
| (n = 28) | (n = 76) | ||
| Colorectal adenoma | 0.386 | ||
| Yes | 3 (10.7) | 15 (19.7) | |
| No | 25 (89.3) | 61 (80.3) | |
| Location | 0.034 | ||
| Proximal | 0 (0.0) | 18 (23.7) | |
| Distal | 4 (14.3) | 6 (7.9) | |
| Both | 1 (3.6) | 1 (1.3) | |
| Size (mm) | 0.088 | ||
| < 10 | 3 (10.7) | 23 (30.3) | |
| ≥ 10 | 2 (7.1) | 2 (2.6) | |
| Number | 0.433 | ||
| 1 | 2 (7.1) | 13 (17.1) | |
| ≥ 2 | 1 (3.6) | 2 (2.6) |
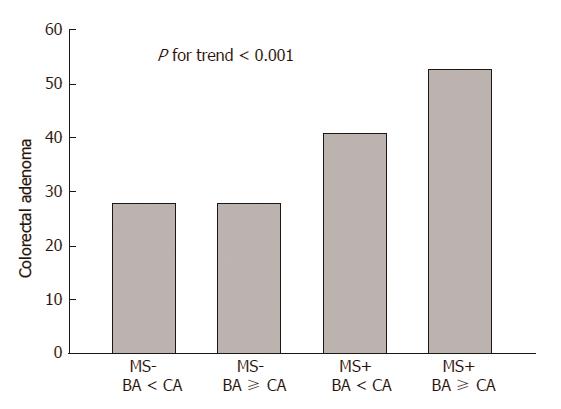
The prevalence of colorectal adenoma according to MS and age gap was evaluated. We categorized subjects into four groups according to MS and age gap between BA and CA. As a result, the incidence of colorectal adenoma increased with MS and BA-dominance: The incidence of colorectal adenoma was 21.5%, 22.8%, 34.5%, and 39.2% for the first group (MS- and BA < CA, n = 368/1711), the second group (MS- and BA ≥ CA, n = 175/767), third group (MS+ and BA ≥ CA, n = 31/79), and the fourth group (MS+ and BA < CA, n = 48/139) (P < 0.05) (Figure 2).
The present study was conducted on the basis of the hypothesis that colorectal adenoma recognized as a premalignant lesion of colorectal cancer is associated with the biological aging process, suggesting that BA represents a biological activity in human. Thus, we intended to assess the risk of colorectal adenomas in the average-risk population from the biological perspective. As a result, the present study demonstrated that the prevalence of colorectal adenoma correlated well with BA, and was more common in the distal colon in BA-dominance. Furthermore, the incidence of MS correlated more precisely with BA compared with CA.
Aging in humans refers to a multidimensional process of physical, psychological, and social changes that occur from birth[14]. Aging is usually assessed in terms of the CA, which is defined as the time elapsed since birth. However, CA fails as an accurate indicator of the aging process[17]. BA estimates the functional status of an individual with respect to their chronological peers on the basis of how well they function in comparison with others of the same CA[18]. Different individual rates of aging lead to differences between CA and BA, and individual values of BA can therefore vary widely at any given CA and are ultimately expected to correspond to the inter-individual variations in longevity and timing and/or magnitude of sequelae of the aging process[19,20]. It has been proposed that BA may serve as an indicator of an individual’s general health status, remaining healthy life span, and active life expectancy[21]. Considering that BA corresponds well with the aging process, BA may be a good indicator for starting screening colonoscopy at the most biologically optimal time in an individual’s life. If so, CRC may eventually be preventable using colonoscopy with polypectomy for premalignant lesions.
Many previous studies have revealed that colorectal adenoma is associated with MS. The clinical characteristics of MS, such as obesity, dyslipidemia, and insulin resistance, have been linked to an increased risk of CRC in several studies[16,22]. In this study, the subjects with MS had more colorectal adenomas compared to those without MS (44.9% vs 27.8%, P < 0.001). In particular, the prevalence of colorectal adenoma was increased along with MS and BA-dominance (P < 0.001) (Figure 2).
In this study, the prevalence of MS increased proportionally with BA compared with CA. This suggests that MS is related to BA more than to CA because the metabolic change is associated with aging. Soprano et al[23]. reported that MS can be considered an age-related disease by identifying a correlation between MS and aging. In addition, they highlighted aging-related dysfunctions that contribute to the onset of several metabolic diseases[23]. Therefore, physiological disorders such as MS should be measured based on BA rather than CA.
In this study, a CA-dominant subject was defined as an individual younger than their CA, showing that they are healthier than expected. On the other hand, a BA-dominant subject was defined as an individual older than their CA, showing that they are less healthy than expected. The prevalence of MS was higher in the BA-dominant group than in the CA-dominant group in this study. Therefore, this result also suggests the clinical application of BA to various metabolic diseases related with age.
In this study, the prevalence of colorectal adenoma increased with BA. As shown in Figure 1, the prevalence of colorectal adenoma rose sharply along with BA distribution rather than CA distribution with increasing age. The prevalence of colorectal adenoma was higher in the BA-dominant group than in the CA-dominant group, although the difference between the two groups was not statistically significant. Interestingly, colorectal adenomas in the distal colon were more common in the BA-dominant group compared with the CA-dominant group (P = 0.034).
Based on these results, BA deserves attention as an alternative indicator for screening for various cancers including CRC. Interestingly, the prevalence of colorectal adenoma in subjects aged < 50 years was as high as 19.9% (329/2696) in this study. Hence, the commencement of screening colonoscopy should be determined based on BA and physiological criteria rather than indiscriminately at 50 years of CA. This strategy will help in identifying those at risks of CRC at an early stage (before the age of 50 years).
This study was limited because the study population, who voluntarily attended health screening, may have been individuals more concerned about their health than average and trying harder to maintain their health than members of the general population. As a result, the age gap between BA and CA was not as large as expected. Therefore, further studies targeting members of the general population are needed in the future.
The present study highlights the important implications for the future planning of CRC screening in that the higher prevalence of BA-dominance was observed in subjects with colorectal adenoma. It should be emphasized that long-term prevention of both colorectal adenoma along with the biological aging process is of clinical importance.
In conclusion, measurement of BA may help to assess the risk of colorectal adenoma in screening colonoscopy. In particular, careful colonoscopic examination in the distal colon should be performed in BA-dominant individuals.
Current guidelines for colorectal cancer screening are based on chronological age (CA) of 50 years. However, a significant number of colorectal cancers have been found even under 50 years of age. Therefore, screening colonoscopy at a more reasonable age may be necessary from the biological perspective.
The authors investigated the clinical utility of biological age (BA) measurement in screening colonoscopy for the detection of colorectal adenomas in the average-risk population.
The present study demonstrated that the prevalence of colorectal adenoma correlated well with BA, and was more common in the distal colon in BA-dominance. Furthermore, the incidence of metabolic syndrome correlated more precisely with BA compared with CA.
Measurement of BA may help to assess the risk of colorectal adenoma in screening colonoscopy. In particular, careful colonoscopic examination in the distal colon should be performed in BA-dominant individuals.
BA is determined by physiology rather than chronology. BA estimates the functional status of an individual with respect to their chronological peers on the basis of how well they function in comparison with others of the same CA.
The author aimed to investigate the clinical utility of BA measurement in screening colonoscopy for the detection of colorectal adenomas in the average-risk population. The present paper is indeed interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Riss S, Shi HY, Lakatos PL S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Shonka NA, Anderson JR, Panwalkar AW, Reed EC, Steen PD, Ganti AK. Effect of diabetes mellitus on the epidemiology and outcomes of colon cancer. Med Oncol. 2006;23:515-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46:595-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Boroff ES, Gurudu SR, Hentz JG, Leighton JA, Ramirez FC. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol. 2013;108:993-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546-553. [PubMed] [Cited in This Article: ] |
| 6. | Blake GJ, Pradhan AD, Manson JE, Williams GR, Buring J, Ridker PM, Glynn RJ. Hemoglobin A1c level and future cardiovascular events among women. Arch Intern Med. 2004;164:757-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Kim BJ, Kim YH, Sinn DH, Kang KJ, Kim JY, Chang DK, Son HJ, Rhee PL, Kim JJ, Rhee JC. Clinical usefulness of glycosylated hemoglobin as a predictor of adenomatous polyps in the colorectum of middle-aged males. Cancer Causes Control. 2010;21:939-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Imperiale TF, Kahi CJ, Stuart JS, Qi R, Born LJ, Glowinski EA, Rex DK. Risk factors for advanced sporadic colorectal neoplasia in persons younger than age 50. Cancer Detect Prev. 2008;32:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Aihie Sayer A, Osmond C, Briggs R, Cooper C. Do all systems age together? Gerontology. 1999;45:83-86. [PubMed] [Cited in This Article: ] |
| 10. | Wiweko B, Prawesti DM, Hestiantoro A, Sumapraja K, Natadisastra M, Baziad A. Chronological age vs biological age: an age-related normogram for antral follicle count, FSH and anti-Mullerian hormone. J Assist Reprod Genet. 2013;30:1563-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Zhang WG, Bai XJ, Sun XF, Cai GY, Bai XY, Zhu SY, Zhang M, Chen XM. Construction of an integral formula of biological age for a healthy Chinese population using principle component analysis. J Nutr Health Aging. 2014;18:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Zhang WG, Zhu SY, Bai XJ, Zhao DL, Jian SM, Li J, Li ZX, Fu B, Cai GY, Sun XF. Select aging biomarkers based on telomere length and chronological age to build a biological age equation. Age (Dordr). 2014;36:9639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Freude G, Jakob O, Martus P, Rose U, Seibt R. Predictors of the discrepancy between calendar and biological age. Occup Med (Lond). 2010;60:21-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Bae CY, Kang YG, Piao MH, Cho B, Cho KH, Park YK, Yu BY, Lee SW, Kim MJ, Lee SH. Models for estimating the biological age of five organs using clinical biomarkers that are commonly measured in clinical practice settings. Maturitas. 2013;75:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Bae CY, Kang YG, Suh YS, Han JH, Kim SS, Shim KW. A model for estimating body shape biological age based on clinical parameters associated with body composition. Clin Interv Aging. 2013;8:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kim JH, Lim YJ, Kim YH, Sung IK, Shim SG, Oh SO, Park SS, Yang S, Son HJ, Rhee PL. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;16:1543-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Finkel D, Whitfield K, McGue M. Genetic and environmental influences on functional age: a twin study. J Gerontol B Psychol Sci Soc Sci. 1995;50:P104-P113. [PubMed] [Cited in This Article: ] |
| 18. | Borkan GA, Norris AH. Assessment of biological age using a profile of physical parameters. J Gerontol. 1980;35:177-184. [PubMed] [Cited in This Article: ] |
| 19. | Anstey KJ, Lord SR, Smith GA. Measuring human functional age: a review of empirical findings. Exp Aging Res. 1996;22:245-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. [PubMed] [Cited in This Article: ] |
| 21. | Borkan GA, Norris AH. Biological age in adulthood: comparison of active and inactive U.S. males. Hum Biol. 1980;52:787-802. [PubMed] [Cited in This Article: ] |
| 22. | Kim BC, Shin A, Hong CW, Sohn DK, Han KS, Ryu KH, Park BJ, Nam JH, Park JW, Chang HJ. Association of colorectal adenoma with components of metabolic syndrome. Cancer Causes Control. 2012;23:727-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Soprano M, Rusciano MR, Ciccarelli M, Maione AS, Formisano P, Illario M. Metabolic Syndrome and Aging: Calcium Signaling as Common Regulator. Curr Diabetes Rev. 2015;12:84-89. [PubMed] [Cited in This Article: ] |