Published online Sep 28, 2017. doi: 10.3748/wjg.v23.i36.6628
Peer-review started: February 28, 2017
First decision: April 28, 2017
Revised: May 25, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: September 28, 2017
Processing time: 212 Days and 1 Hours
Inflammatory bowel diseases (IBDs), such as ulcerative colitis and Crohn’s disease, are chronic pathologies associated with a deregulated immune response in the intestinal mucosa, and they are triggered by environmental factors in genetically susceptible individuals. Exogenous glucocorticoids (GCs) are widely used as anti-inflammatory therapy in IBDs. In the past, patients with moderate or severe states of inflammation received GCs as a first line therapy with an important effectiveness in terms of reduction of the disease activity and the induction of remission. However, this treatment often results in detrimental side effects. This downside drove the development of second generation GCs and more precise (non-systemic) drug-delivery methods. Recent clinical trials show that most of these new treatments have similar effectiveness to first generation GCs with fewer adverse effects. The remaining challenge in successful treatment of IBDs concerns the refractoriness and dependency that some patients encounter during GCs treatment. A deeper understanding of the molecular mechanisms underlying GC response is key to personalizing drug choice for IBDs patients to optimize their response to treatment. In this review, we examine the clinical characteristics of treatment with GCs, followed by an in depth analysis of the proposed molecular mechanisms involved in its resistance and dependence associated with IBDs. This thorough analysis of current clinical and biomedical literature may help guide physicians in determining a course of treatment for IBDs patients and identifies important areas needing further study.
Core tip: Glucocorticoids (GCs) are widely used in patients with Inflammatory Bowel Diseases who have moderate or severe disease activity; however, some of them do not respond to treatment or become dependent. Knowledge of both the clinical approach of GCs treatment as well as the molecular basis underlying their effects will help physicians prescribe drugs that will lead to better outcomes for patients.
- Citation: Dubois-Camacho K, Ottum PA, Franco-Muñoz D, De la Fuente M, Torres-Riquelme A, Díaz-Jiménez D, Olivares-Morales M, Astudillo G, Quera R, Hermoso MA. Glucocorticosteroid therapy in inflammatory bowel diseases: From clinical practice to molecular biology. World J Gastroenterol 2017; 23(36): 6628-6638
- URL: https://www.wjgnet.com/1007-9327/full/v23/i36/6628.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i36.6628
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC) and Crohn’s disease (CD), are multifactorial disorders comprised of both environmental and genetic factors. Even though they are different disease entities, UC and CD are grouped under the generic term of IBDs given their similar activity/remission stages, chronicity, immunological pathophysiology and uncertain etiology[1-3]. The prevalence of UC is 505 per 100000 people in Europe and 249 per 100000 people in North America, and CD is 322 per 100000 people in Europe and 319 per 100000 people in North America[4]. An increased incidence has been reported in some South American and mid-eastern Asian countries such as Chile[5], Brazil[6], Israel[7], and Malaysia[8]. This tendency might be related to the industrialization phenomenon and the influence of environmental risk factors[4].
Various genetic and environmental factors have been associated with development of IBDs. Polymorphisms in a vast number of genes which impair functions such as lymphocyte activation, autophagy, pathogen sensing, stress response, antigen presentation, and chemotaxis, among others have been described[9]. The presence of such genetic variants may contribute to an imbalance in the immune response and increased predisposition to IBDs[10,11]. Moreover, environmental factors, such as diet, smoking, appendectomy, breastfeeding behavior in childhood, vitamin D deficiency and infections, play key roles in inflammatory manifestations, and have been described as risk factors for IBDs[12,13]. Furthermore, intestinal microbiome composition differs in IBDs patients compared to healthy subjects: IBD patients have a more predominant Proteobacteria population and decreased Firmicutes and Bacteroidetes populations[14,15]. Environmental factors might be involved in creating such imbalance in microbiome composition, and this may give rise to a constant inflammatory antigenic stimulus in the gut that causes chronic inflammation in genetically susceptible individuals[14].
These multifactorial components involved in the origin and progression of IBDs, has prevented the development of a specific treatment applicable to all patients. Current treatment options seek to diminish inflammation in order to control symptoms and keep the patient in a state of remission or symptom improvement. For example, aminosalicylates (5-asa derivatives) are used to treat mild disease, while immunosuppressants such as azathioprine/6-mercaptopurine and methotrexate, and biological therapy such as anti-TNF antibodies or anti-integrin are prescribed for moderate to severe disease[16]. Finally, glucocorticoids (GCs) are used to achieve, but not to maintain, remission in patients with moderate to severe activity[17].
The first study that demonstrated the usefulness of GCs (cortisone) in controlling severe UC attacks was published in 1955[18]. Since the 70s, the first generation of GCs (prednisone, methylprednisolone, hydrocortisone) has been used to induce clinical remission in IBDs patients. However, significant adverse effects have led to the development of second generation GCs (budesonide, budesonide MMX, Beclomethasone dipropionate)[19-21]. A characteristic of GC treatments and their use is summarized in Table 1.
| Glucocorticoids | Component | Indications | Ref. |
| 1st generation | Prednisone | Moderate to Severe cases | [3,25,27] |
| Methyl-Prednisolone | of IBDs | [26] | |
| Hydrocortisone | Short duration of treatment | [29] | |
| 2nd generation | Budesonide | Moderate CD cases | [32-34] |
| Budesonide MMX | Mild to moderate UC cases | [36,37] | |
| Beclomethasone dipropionate | Topical administration | [40,41] | |
| Erythrocyte - Mediated Delivery of Dexamethasone | In research for long term treatments | [42] |
Several studies have compared the efficacy and safety of traditional GCs to other treatments by measuring the rates of remission and adverse side effects. Two independent placebo-controlled double-blind randomized trials showed that 47% of active CD patients treated with prednisone (1 mg/kg, ranging from 40-60 mg/d) achieved clinical remission, defined as CDAI score < 150 (for a review on clinical and endoscopy scoring see references[22-24]), compared to 26% of patients receiving placebo after 4 mo of treatment. Neither sulfasalazine (0.5-1 gr/15 kg) nor azathioprine (2-2.5 mg/kg per day) achieved significant effectiveness[25]. Later, a placebo-controlled study showed that high doses of methylprednisolone (12-48 mg/d), sulfasalazine (3 gr/d), or both in active CD patients, were significantly effective compared to the placebo, and patients treated with methylprednisolone (alone or in combination with sulfasalazine) showed faster improvement compared to sulfasalazine[26].
Despite the effectiveness of GCs demonstrated in patients with severe CD, a prospective multicenter study comprised of patients with active CD on prednisone treatment (1 mg/kg per day) concluded that while 92% achieved clinical remission, only 29% of them reached endoscopic remission[27]. The presence of stenosis or fistula secondary to CD which occurs in patients who do not reach endoscopic remission, indicates a poor response to GC therapy and requires surgical intervention[27]. This suggests that GCs are efficient in improving the inflammatory response but other strategies are needed to increase mucosal healing.
Regarding UC, systemic GCs are the first choice therapy in cases of severe colitis[3]. In acute and critical UC, 76% of the patients treated with prednisolone (20 mg/d), plus a nightly rectal drip of hydrocortisone succinate sodium (100 mg in solution), presented clinical remission vs 52% of patients who were treated with sulfasalazine (8 gr/d the first week, 4 gr/d the second week)[28] demonstrating the clinical efficacy of this treatment. Likewise, two consecutive trials in UC patients with left side colitis determined that oral prednisone (1 mg/kg per day) was effective in severe stages of the disease compared with calcium lactate (1-3 gr; placebo), and prednisone treatment led to clinical remission more quickly and more often than those treated with salazopyrin (4 gr/d; a sulfasalazine derivate) and topic hydrocortisone treatments (100 mg/150 mL of sterile water administered as an enema)[29].
Despite the effectiveness of first generation GCs, they are characterized by several serious side effects that limit their long-term use. Among these, some of the most important are: metabolic (altered distribution of fat, Cushing’s face or steroid-induced diabetes), eye-related (cataracts and glaucoma), dermatological (bruising and urticarial), gastrointestinal (gastric ulcer and gastrointestinal bleeding), and musculoskeletal side effects (osteopenia to osteoporosis). Additionally, adverse effects on the central nervous system have been described (insomnia, anxiety, hyperactivity, psychotic processes), along with hypertension, hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections due to immunosuppression[19]. However, its use in IBDs patients has not be associated with atherosclerosis progression[30]. Finally, there is evidence that GC use increases the likelihood of sepsis of gastrointestinal origin; therefore it should be prescribed with caution in these patients[31].
The previously described serious side-effects related to first-generation GCs drove the development of second-generation steroids, which maximize the amount of corticosteroid locally available for distal ileum and proximal colon and minimize systemic bioavailability[3,26]. These new formulations of GCs with limited absorption include budesonide, budesonide MMX (multimatrix delivery system MMX) and beclomethasone dipropionate[3,19].
Budesonide, similar effectiveness with fewer adverse effects: Budesonide undergoes hepatic inactivation before reaching the systemic circulation (first-pass metabolism) which reduces its bioavailability[3]. Various placebo-controlled clinical studies have provided evidence about the efficacy of budesonide in IBDs patients. A multicenter randomized, placebo-controlled, double-blind clinical trial was conducted in CD patients who received three different budesonide doses (3, 9 and 15 mg/d)[32]. After 8 wk of treatment, 51% of those receiving 9 mg budesonide progressed to remission in CD patients with ileum and proximal colon disease activity. Thus, this dose was effective and safe but depended on disease location. A randomized, double-blind, double-dummy controlled trial conducted in patients with mild to moderately active CD demonstrated that budesonide (9 mg/d for 2 mo) was as effective as prednisone (40 mg/d for the first 2 wk, 30 mg/d the third week, and gradually tapered to 5 mg/wk at the end of the study lasting 2 mo) in patients with terminal ileum, cecum, and/or ascending colon inflammation[33].
Subsequently, a multicenter double-blind, randomized trial was conducted in CD patients with moderate activity in the distal ileum and ascending colon. More patients that received budesonide (4.5 mg twice daily, or 9 mg once daily) achieved clinical remission compared to placebo 2 wk after beginning treatment, and this difference was statistically significant[34]. In addition, a pooled analysis of trials in CD patients found significantly fewer GC-related adverse events compared to patients treated with conventional corticosteroids (RR = 0.64, 95%CI: 0.54-0.76)[35].
An improved oral formulation of budesonide uses the colonic delivery technology Multi-Matrix System (MMX) to extend drug release in the colon (Budesonide MMX). Due to this characteristic, it is indicated for the treatment of UC patients that do not respond to standard doses of salicylates. A placebo-controlled study demonstrated that a higher proportion of UC patients with mild to moderate activity treated with budesonide MMX (9 mg), reached clinical remission and symptom resolution compared to placebo. In contrast, a lower dose of budesonide MMX (6 mg) and mesalamine, another salicylate treatment (2.4 gr/d), did not significantly improve either parameter[36]. Furthermore, the higher dose of Budesonide MMX was efficient in inducing remission in patients with mild to moderate UC disease who did not achieve remission with 5-asa treatments such as mesalamine or sulfasalazine[36,37]. Currently, physicians prescribe Budesonide MMX in UC patients that do not respond to traditional maintenance therapy before treating with azathioprine/6-mercaptopurin.
In 2015, data from 5 clinical studies, including double-blind, randomized, and open label studies, showed that rates of adverse effects were similar between budesonide MMX 9 mg and 6 mg (54.5% and 60.6% respectively) and placebo (50.5%) in patients with mild to moderate UC[38]. Furthermore, the open label studies showed less frequency of adverse effects with budesonide MMX (3 mg or 9 mg) compared to placebo. The most common adverse effects in these studies were headache, nausea and urinary tract infection demonstrating that second generation GCs not only have fewer side effects, but those effects are less severe compared to first generation GCs side effects.
Beclomethasone dipropionate, a second generation option with low systemic activity: Beclomethasone dipropionate (BDP) is a second-generation corticosteroid with topical effects and minimal systemic activity. It is administered as a pro-drug and is partially metabolized in the lower gastrointestinal tract[39]. The effectiveness and tolerability of BDP vs budesonide have been evaluated in CD patients whose clinical characteristics did not include complications such as stenosis or fistulas. A study showed that BDP (10 mg/d) was less effective than budesonide (9 mg/d) in CD patients treated for 2 mo. They concluded that this was due to the pharmacokinetic properties of budesonide[40].
A study was conducted to evaluate the efficacy and safety of BDP enemas vs prednisone enemas in active distal UC after 1 mo of treatment. They determined that clinical and endoscopic remission occurred in 29% of patients who received BDP and in 25% of patients who had standard topical GC treatment, and both groups experienced few adverse effects[41]. This evidence leads to the conclusion that BDP has similar efficacy to traditional GCs.
Novel drug delivery systems capable of slowly releasing drugs into the bloodstream at a low concentration such as the erythrocyte-mediated drug delivery have been proposed. The membrane characteristics of autologous erythrocytes render them ideal drug carriers because their permeability allows for the diffusion of small molecules. This has been demonstrated using dexamethasone 21-phosphate (Dex 21-P), a biologically inactive compound[42]. Once encapsulated, an enzyme resident in the red blood cell dephosphorylates Dex 21-P to form the corresponding active metabolite, dexamethasone (Dex), which is then released into the bloodstream by passive diffusion through the cytoplasmic membrane of erythrocytes[42].
A study recruited 40 patients with mild to moderate UC, who had not responded to mesalamine and randomly assigned them to one of the following three treatment groups: Dex 21-P encapsulated into erythrocytes (DEE) delivered via two infusions 14 d apart, oral prednisolone infusions (0.5 mg/kg for 2 wk by 6 mg/wk tapering), and placebo. The group of DEE and oral prednisolone achieved a higher rate of clinical and endoscopic remission (75%) after 2 mo compared to placebo[42]. DEE-treated patients showed no adverse effects associated with GCs, in contrast to 80% of prednisone group, in whom acne, hirsutism and weight gain were reported. This delivery tool is an attractive choice for patients with IBDs to avoid most of GCs’ adverse effects.
More than half of IBDs patients on GC therapy respond to treatment, approximately 28% have a partial response, and 19% are non-responders (GC-resistant). However, about 20% of IBDs patients on long-term GC therapy become dependent[43,44]. Therefore, clarifying this GC resistance/dependency is crucial to making appropriate decisions regarding patient treatment. GC resistance or refractoriness is the inability of GCs to exert their effects on target tissues, thus limiting the efficacy of the therapy. In IBDs, GC resistance is defined as the persistence of an active manifestation of the pathology, despite having received standard treatment of 0.75 mg/kg per day for 4 or more weeks[44].
On the other hand, GC dependency is the need for GCs to maintain remission. Patients are considered GC-dependent if they fail to taper to steroid doses below 10 mg within 4 mo (starting dose 0.75-1 mg/kg oral prednisone-equivalent) or if they relapse within 3 mo after the discontinuation of GC treatment[44]. Usually, long-term treatment with GCs is associated with its resistance or dependency and a more aggressive clinical phenotype in IBD patients[45].
Exogenous GCs are highly lipophilic compounds, making them widely bioavailable. Similar to endogenous cortisol, GCs are primarily transported in the bloodstream bound to corticosteroid-binding globulin and, to a lesser extent, to albumin[46]. GCs have the ability to passively diffuse through cell membranes and interact with the glucocorticoid receptor (GR), a member of the nuclear receptor superfamily of ligand-dependent transcription factors[47].
GR is a cytosolic protein with a molecular weight of 94 kDa encoded by the NR3C1 gene (5q31.3). It contains nine exons that mainly code for two transcripts formed by alternative splicing: the α and β isoforms[48]. In addition to GRβ, three less-well-characterized isoforms have been reported: GRγ, GR-A and GR-P[49]. Moreover, GRα and GRβ can also undergo alternative translation initiation in exon 2, which generates eight additional GR isoforms with truncated N-terminals giving them distinct properties[49].
The amino terminal region of GRα contains a ligand-independent transactivation domain (AF-1), a highly conserved central DNA binding domain (DBD), and a hinge segment. The carboxyl-terminal region contains the ligand-binding domain, which includes an AF-2 region that interacts with co-regulators in a ligand-dependent manner[50]. The GRβ is a shorter protein which differs from GRα in its C-terminal domain and antagonizes the activity of GRα[51]. After GC binding, the receptors undergo conformational changes and expose the DBD, interact with chromatin, and regulate gene expression[50].
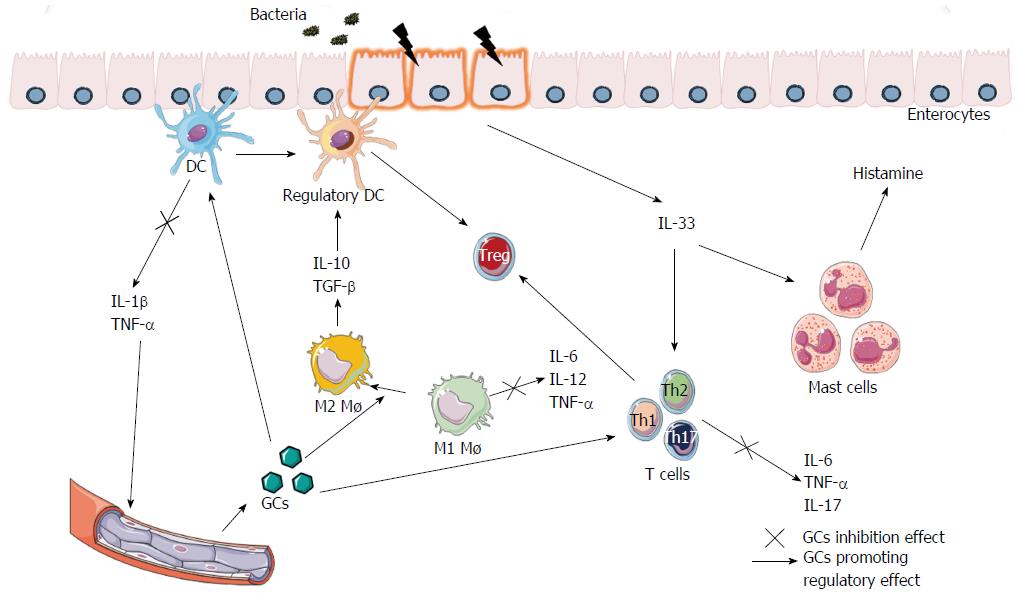
The GR-GC complex can inhibit proinflammatory proteins such as nuclear factor κB and AP-1 through protein-protein interactions. These molecular mechanisms down-regulate the expression of proinflammatory cytokines and chemokines, such as IL-1α, IL-1β and IL-8. In addition, GCs up-regulate the expression of other cytokines that suppress the production of inflammatory mediators: such as transforming growth factor-β3 (TGF-β) and IL-10, increasing its anti-inflammatory function. They also inhibit T and B lymphocyte proliferation, and promote a tolerant macrophage profile (M2) (Figure 1)[46,52,53].
The molecular mechanisms of GC resistance in IBDs have been associated with changes in the GR isoform levels, polymorphisms in NR3C1 or genes involved in GC bioavailability, and impaired cytokine production[46,48,54,55]. The most important genes associated to GC resistance are summarized in Table 2. Changes in levels of GRα and β isoforms have been associated with GC resistance. The α-isoform is the most well-studied protein, but only increased levels of the β-isoform have been associated to GC resistance in clinical trials in UC, asthma, nasal polyposis, cancer and chronic lymphocytic leukemia[53]. The GRβ protein is located primarily in the nucleus and is found mostly in T lymphocytes, macrophages, neutrophils, eosinophils, and peripheral blood mononuclear cells; however, it has also been reported in brain, lung, and heart tissue[56,57]. It has been proposed that GRβ may act as a dominant negative to GRα, since GRβ does not bind to GCs, but rather it interacts with glucocorticoid element response (GRE) causing GC resistance. This mechanism alters GRα signaling independent of GRα-GRβ heterodimer formation[58]. An in vitro study showed that overexpression of GRβ in a colonocyte cell model induces a vast deregulation of gene expression without Dex treatment, and a proportion of these genes have been shown to be altered in IBDs patients[59]. Thus, GRβ may directly change transcriptional gene activity of proinflammatory molecules. This effect is independent of GRα function and may promote the GC-resistance.
| Gene/protein | Function | Molecular alteration or genetic variant | Effect on GC response | Ref. |
| NR3C1 | GC receptor | Increased expression of the isoform | Block signaling of the isoform/GC resistance | [58] |
| rs6189 | Altered transactivation of GC receptor/GC resistance | [54,63] | ||
| rs6190 | ||||
| rs6195 | Enhanced sensitivity to GC | [65] | ||
| rs41423247 | Alternative splicing of receptor/GC resistance | [54,63] | ||
| MDR1 | Export of drugs from the cell | rs1045642 | Decreased MDR1 levels/GC resistance in Brazilian CD patients | [68] |
| rs1128503 | Possible misfolding/GC dependence in Chinese CD patients | [75-77] | ||
| rs1045642 | ||||
| IL-10 | Anti-inflammatory cytokine | rs1800896 | Lowered production of IL-10/GC dependence in UC and CD patients | [45] |
| EBF3 | Transcription factor | Decreased levels of EBF3 | More severely inflamed phenotype/GC dependence | [72] |
| PAR2 | G protein coupled receptor | Methylation of gene promoter | GC resistance/dependence in UC patients. | [82] |
Furthermore, GRβ can bind to RU-486, a GRα antagonist, in such a way as to change the cellular location of this GRα, and alter its ability to regulate gene expression[60]. In addition, it has previously been shown that the expression of GRβ can be transcriptionally activated in regulatory T lymphocytes and neutrophils by proinflammatory cytokines[61]. Hence, GRβ expression may affect the immune response of these cells promoting inflammation and non-response to GC[55,57,62].
In 2009, a study was conducted to measure the expression of GRβ in intestinal mucosa of IBD patients that were refractory to treatment with GCs[55]. High levels of the GRα isoform were found in both GC-resistant and responsive patients; however, GC-resistant patients expressed higher levels of GRβ, in comparison with the GC-responder group. In the intestinal mucosa, the cells that primarily expressed the GRβ receptor were CD4+ and CD8+ T lymphocytes, macrophages and B cells. Fibroblasts and vascular endothelial cells expressed GRβ in a minor proportion. These results suggest that a higher ratio of GRβ/GRα in inflammatory mononuclear cells disturbs the effects of GCs resulting in refractory outcomes.
Single nucleotide polymorphisms (SNPs) in NR3C1 gene, have been associated with GC resistance[54], and these SNPs may result in different effects on the receptor structure and its signal transduction[54]. The most-studied SNPs that could induce a refractory phenotype in IBDs patients are: ER22/23EK (rs6189 and rs6190), N363S (rs6195), and BcII (rs41423247)[54]. The ER22/23EK is produced by a change in the amino acid residues 22 and 23 in the GR, where two glutamic acids (E) are replaced by an arginine (R) in position 22, and a lysine (K) in position 23[63]. These SNPs affect the transactivation process in in vitro assays, where the receptor is less likely to change its conformation and induce gene transcription in the presence of GCs[64]. The SNP N363S is in codon 363 of exon 2 and results in an amino acid change from asparagine (N) to serine (S). This SNP was reported to be associated with enhanced sensitivity to GCs, as was demonstrated by a Dex suppression test[65]. The BclI SNP was identified as a cytosine-guanine substitution 646 nucleotides downstream from exon 2, yielding 2.2 and 3.9 kb long fragments[63], which could suggest alternative splicing. A meta-analysis that included a mostly Caucasian population, analyzed the association of each of these 3 SNPs with GC resistance in IBDs patients[54]. Although no association was found, the analysis of the presence of all three SNPs, (or combinations of two SNPs) as a polygenic additive effect, could show risk associations of these variants with GC resistance.
Like the GR, the P-glycoprotein (P-gp) has also been associated with GC resistance in IBDs. P-gp is a trans-membrane glycoprotein with a molecular weight of 170 kDa encoded by the MDR1 gene, which is located on the long arm of chromosome 7. P-gp is responsible for the absorption, distribution, metabolism and excretion of various drugs including GC[66]. A relationship between increased P-gp levels in circulating lymphocytes and intestinal epithelial cells with GC-resistant IBDs patients has been reported[67]. Polymorphisms in the MDR1 gene have been shown to alter P-gp function, which in turn modifies the response to GCs[67]. A case-control study in a Brazilian population demonstrated that SNP C3435T (rs1045642), located in exon 26 of MDR1 was associated with GC resistance in CD but not in UC patients[68].
Additionally, higher expression of proinflammatory cytokines has been associated with GC resistance. Overexpression of IL-6 and IL-8 has been associated with non-responsiveness to GC treatment[69]. In pediatric patients with UC, a similar correlation was found between the expression levels of IL-6 and unresponsiveness to GC[70]. In this study, the patients who did not respond to GC had higher levels of IL-6, compared to responders. Given the pleiotropic nature of IL-6, this correlation could reflect a cause-effect relationship between the signaling of the GC receptor and the expression of proinflammatory cytokines, but the real reason for the failure to respond remains unclear[70].
Similarly, in CD a correlation between cytokine production and resistance to GC was found. A clinical study showed that in intestinal mucosa from GC-resistant patients, the rate of apoptosis of T and B cells, presence of caspase-3 and IL-10 production were diminished compared to healthy and GC-responsive individuals[71]. These findings suggest a strong relationship between cytokine signaling and the inhibition of the GR effect in inflammatory diseases.
Analyses of gene expression using microarrays have been conducted to associate gene deregulation with GC resistance from colonoscopic biopsies of IBDs patients. ATG16L1, a gene related to the autophagy process was down-regulated in GC-resistant patients[72]. A low expression of the ATG16L1 protein has been associated with altered secretion of mucus by Paneth cells[73]. Furthermore, inhibition of autophagy led to Dex-resistance in a lymphoma cell line and in a mouse model[74]. This evidence indicates the importance of autophagy in the GC-resistant phenotype.
Contrary to GC refractoriness, the molecular mechanisms behind GC dependence are poorly studied. There is evidence that associates MDR1 SNPs (rs1128503 and rs1045642) with GC-dependent CD patients in a Chinese population[75]. Both SNPs correspond to a change from cytosine (C) to thymine (T) and are in coding regions of the gene (exon 12 and 26, respectively). Based on computational protein analysis, these SNPs, as haplotypes, seem to affect protein folding which could alter P-gp levels[76], but this mechanism has not been experimentally demonstrated. Nevertheless, the rs1045642 - CC was frequently found in individuals with significantly higher P-gp levels in plasma along with increased protein activity (measured by rhodamine efflux in CD56+ natural killer cells), compared to carriers of the TT genotype[77]. This study suggests that increased GC transportation out of cells might drive a decreased GC response leading to the dependent phenotype[75].
SNPs in the IL-10 gene have been associated with GC dependency as well. The SNP -1082 A/G (rs1800896) is located in the promoter sequence of the IL-10 gene, and a case control study of this variant showed an association with GC dependence in UC and CD patients[45]. This SNP was also related to a lower production of IL-10 in IBDs patients. Together, this evidence suggests that intrinsically inferior levels of IL-10 may generate GC dependency because exogenous GCs would be necessary to reach the anti-inflammatory IL-10 content necessary to achieve sustained remission. Other mechanisms, similar to those of refractoriness, such as altered expression of GR that impair intracellular signaling, have been proposed[78].
Downregulation of EBF3 was related to GC dependence in IBD patients[72]. The transcription factor EBF3 is a downstream target of SMAD2/3, which are components of the TGF-β signaling pathway[79]. This anti-inflammatory mechanism might be impaired in the context of low EBF3 expression[80]. Moreover, EFB3 down-regulation has been associated with a low rate of apoptosis and increased cell proliferation[81]. This evidence suggests that decreased EBF3 levels might allow for a more severely inflamed phenotype making necessary a continual GC administration to maintain remission in IBD patients and driving their dependence.
Epigenetic changes may also cause GC dependence/resistance. In the context of IBDs, higher methylation of the Protease-Activated Receptor 2 (PAR2) gene, has been associated with steroid-dependent and resistance phenotypes in UC patients[82]. PAR2 is a G protein coupled receptor that is involved in pro-inflammatory responses and play a role in IBDs pathogenesis[82]. However, its specific role in GCs dependence/resistance has not been described.
Various studies relate the epigenetics changes such as hypermethylation of promoter genes with severe disease and risk of neoplasia[83-85]. There is evidence that epigenetic shifts can occur in the early stages of embryonic development in genes known as metastable epialleles, and these events can appear, as shown in rat and murine models, by environmental factors such as stress[86-88]. However, more analyses are needed to clarify the role of epigenetics in controlling the molecular mechanisms that underlie dependence or resistant to GCs in IBDs patients.
It has been over half a century since the first use of GC in IBD, yet amazingly, it currently remains a widely used therapeutic tool, specifically in disease exacerbations. The latest pharmacological applications, known as second-generation steroids, have contributed to the reduction of adverse reactions, however, increased effectiveness would need to be demonstrated in order for them to replace traditional GC therapy.
Despite findings showing positive GC responses, some patients remain resistant or become dependent to this drug. Deepening our understanding of the molecular basis of these undesired effects will help us generate novel, personalized GC therapies, depending on a patient’s genetic background and cytokine profiles.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Chile
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciccone MM, Quetglas EG S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Ananthakrishnan AN. Environmental risk factors for inflammatory bowel diseases: a review. Dig Dis Sci. 2015;60:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Rietdijk ST, D’Haens GR. Recent developments in the treatment of inflammatory bowel disease. J Dig Dis. 2013;14:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | De Cassan C, Fiorino G, Danese S. Second-generation corticosteroids for the treatment of Crohn’s disease and ulcerative colitis: more effective and less side effects? Dig Dis. 2012;30:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 5. | Simian D, Fluxá D, Flores L, Lubascher J, Ibáñez P, Figueroa C, Kronberg U, Acuña R, Moreno M, Quera R. Inflammatory bowel disease: A descriptive study of 716 local Chilean patients. World J Gastroenterol. 2016;22:5267-5275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 6. | Victoria CR, Sassak LY, Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Abu Freha N, Schwartz D, Elkrinawi J, Ben Yakov G, Abu Tailakh M, Munteanu D, Abu Ganim A, Fich A. Inflammatory bowel disease among Bedouin Arabs in southern Israel: urbanization and increasing prevalence rates. Eur J Gastroenterol Hepatol. 2015;27:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Hilmi I, Jaya F, Chua A, Heng WC, Singh H, Goh KL. A first study on the incidence and prevalence of IBD in Malaysia--results from the Kinta Valley IBD Epidemiology Study. J Crohns Colitis. 2015;9:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1882] [Article Influence: 134.4] [Reference Citation Analysis (2)] |
| 10. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1092] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 11. | Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, Tosi I, Capon F, Trembath RC, Peris K. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS One. 2011;6:e17160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 12. | Pappa HM, Langereis EJ, Grand RJ, Gordon CM. Prevalence and risk factors for hypovitaminosis D in young patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53:361-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2154] [Article Influence: 102.6] [Reference Citation Analysis (1)] |
| 14. | Sartor RB, Mazmanian SK. Intestinal Microbes in Inflammatory Bowel Diseases. Am J Gastroenterol Suppl. 2012;1:15-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3430] [Article Influence: 190.6] [Reference Citation Analysis (1)] |
| 16. | Leitner GC, Vogelsang H. Pharmacological- and non-pharmacological therapeutic approaches in inflammatory bowel disease in adults. World J Gastrointest Pharmacol Ther. 2016;7:5-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 17. | Kozuch PL, Hanauer SB. Treatment of inflammatory bowel disease: a review of medical therapy. World J Gastroenterol. 2008;14:354-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 165] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, Moayyedi P. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590-599; quiz 600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 20. | Danese S, Hart A, Dignass A, Louis E, D’Haens G, Dotan I, Rogler G, D’Agay L, Iannacone C, Peyrin-Biroulet L. Effectiveness of budesonide MMX (Cortiment) for the treatment of mild-to-moderate active ulcerative colitis: study protocol for a prospective multicentre observational cohort study. BMJ Open Gastroenterol. 2016;3:e000092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Sherlock ME, MacDonald JK, Griffiths AM, Steinhart AH, Seow CH. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015;CD007698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Paine ER. Colonoscopic evaluation in ulcerative colitis. Gastroenterol Rep (Oxf). 2014;2:161-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 24. | Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 2188] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 25. | Summers RW, Switz DM, Sessions JT, Becktel JM, Best WR, Kern F, Singleton JW. National Cooperative Crohn’s Disease Study: results of drug treatment. Gastroenterology. 1979;77:847-869. [PubMed] |
| 26. | Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, Jesdinsky H. European Cooperative Crohn’s Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 27. | Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, Rene E. Picture of attacks of Crohn’s evolution on prednisolone. Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 477] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 28. | Truelove SC, Watkinson G, Draper G. Comparison of corticosteroid and sulphasalazine therapy in ulcerative colitis. Br Med J. 1962;2:1708-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Lennard-jones JE, Longmore AJ, Newell AC, Wilson CW, Jones FA. An assessment of prednisone, salazopyrin, and topical hydrocortisone hemisuccinate used as out-patient treatment for ulcerative colitis. Gut. 1960;1:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 149] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Principi M, Mastrolonardo M, Scicchitano P, Gesualdo M, Sassara M, Guida P, Bucci A, Zito A, Caputo P, Albano F. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis. 2013;7:e427-e433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The efficacy of corticosteroid therapy in inflammatory bowel disease: analysis of a 5-year UK inception cohort. Aliment Pharmacol Ther. 2006;24:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Greenberg GR, Feagan BG, Martin F, Sutherland LR, Thomson AB, Williams CN, Nilsson LG, Persson T. Oral budesonide for active Crohn’s disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994;331:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 348] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Bar-Meir S, Chowers Y, Lavy A, Abramovitch D, Sternberg A, Leichtmann G, Reshef R, Odes S, Moshkovitz M, Bruck R. Budesonide versus prednisone in the treatment of active Crohn’s disease. The Israeli Budesonide Study Group. Gastroenterology. 1998;115:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Tremaine WJ, Hanauer SB, Katz S, Winston BD, Levine JG, Persson T, Persson A. Budesonide CIR capsules (once or twice daily divided-dose) in active Crohn’s disease: a randomized placebo-controlled study in the United States. Am J Gastroenterol. 2002;97:1748-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Rezaie A, Kuenzig ME, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH, Kaplan GG, Seow CH. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;CD000296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Sandborn WJ, Travis S, Moro L, Jones R, Gautille T, Bagin R, Huang M, Yeung P, Ballard ED. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology. 2012;143:1218-1226.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 37. | Danese S, Siegel CA, Peyrin-Biroulet L. Review article: integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2014;39:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Lichtenstein GR, Travis S, Danese S, D’Haens G, Moro L, Jones R, Huang M, Ballard ED, Bagin R, Hardiman Y. Budesonide MMX for the Induction of Remission of Mild to Moderate Ulcerative Colitis: A Pooled Safety Analysis. J Crohns Colitis. 2015;9:738-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Prantera C, Marconi S. Glucocorticosteroids in the treatment of inflammatory bowel disease and approaches to minimizing systemic activity. Therap Adv Gastroenterol. 2013;6:137-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Tursi A, Giorgetti GM, Brandimarte G, Elisei W, Aiello F. Beclomethasone dipropionate for the treatment of mild-to-moderate Crohn’s disease: an open-label, budesonide-controlled, randomized study. Med Sci Monit. 2006;12:PI29-PI32. [PubMed] |
| 41. | Campieri M, Cottone M, Miglio F, Manenti F, Astegiano M, D’Arienzo A, Manguso F, D’Albasio G, Bonanomi A, Galeazzi R. Beclomethasone dipropionate enemas versus prednisolone sodium phosphate enemas in the treatment of distal ulcerative colitis. Aliment Pharmacol Ther. 1998;12:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Bossa F, Latiano A, Rossi L, Magnani M, Palmieri O, Dallapiccola B, Serafini S, Damonte G, De Santo E, Andriulli A. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol. 2008;103:2509-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 792] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 44. | Manz M, Vavricka SR, Wanner R, Lakatos PL, Rogler G, Frei P, Safroneeva E, Schoepfer AM. Therapy of steroid-resistant inflammatory bowel disease. Digestion. 2012;86 Suppl 1:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Castro-Santos P, Suarez A, López-Rivas L, Mozo L, Gutierrez C. TNFalpha and IL-10 gene polymorphisms in inflammatory bowel disease. Association of -1082 AA low producer IL-10 genotype with steroid dependency. Am J Gastroenterol. 2006;101:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17:1095-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Lu NZ, Wardell SE, Burnstein KL, Defranco D, Fuller PJ, Giguere V, Hochberg RB, McKay L, Renoir JM, Weigel NL. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 48. | Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1237] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 49. | Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 50. | Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005:pe48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 51. | Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66:3435-3448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 52. | Almawi WY, Melemedjian OK. Molecular mechanisms of glucocorticoid antiproliferative effects: antagonism of transcription factor activity by glucocorticoid receptor. J Leukoc Biol. 2002;71:9-15. [PubMed] |
| 53. | Gross KL, Cidlowski JA. Tissue-specific glucocorticoid action: a family affair. Trends Endocrinol Metab. 2008;19:331-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Chen HL, Li LR. Glucocorticoid receptor gene polymorphisms and glucocorticoid resistance in inflammatory bowel disease: a meta-analysis. Dig Dis Sci. 2012;57:3065-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Fujishima S, Takeda H, Kawata S, Yamakawa M. The relationship between the expression of the glucocorticoid receptor in biopsied colonic mucosa and the glucocorticoid responsiveness of ulcerative colitis patients. Clin Immunol. 2009;133:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Oakley RH, Webster JC, Sar M, Parker CR, Cidlowski JA. Expression and subcellular distribution of the beta-isoform of the human glucocorticoid receptor. Endocrinology. 1997;138:5028-5038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Pujols L, Mullol J, Roca-Ferrer J, Torrego A, Xaubet A, Cidlowski JA, Picado C. Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am J Physiol Cell Physiol. 2002;283:C1324-C1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 58. | Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996;271:9550-9559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 398] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 59. | Nagy Z, Acs B, Butz H, Feldman K, Marta A, Szabo PM, Baghy K, Pazmany T, Racz K, Liko I. Overexpression of GRß in colonic mucosal cell line partly reflects altered gene expression in colonic mucosa of patients with inflammatory bowel disease. J Steroid Biochem Mol Biol. 2016;155:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Lewis-Tuffin LJ, Jewell CM, Bienstock RJ, Collins JB, Cidlowski JA. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci U S A. 2001;98:6865-6870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 347] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 62. | Boivin MA, Ye D, Kennedy JC, Al-Sadi R, Shepela C, Ma TY. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2007;292:G590-G598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 63. | van Rossum EFC, Lamberts SWJ. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 64. | Russcher H, Smit P, van den Akker EL, van Rossum EF, Brinkmann AO, de Jong FH, Lamberts SW, Koper JW. Two polymorphisms in the glucocorticoid receptor gene directly affect glucocorticoid-regulated gene expression. J Clin Endocrinol Metab. 2005;90:5804-5810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Marti A, Ochoa MC, Sánchez-Villegas A, Martínez JA, Martínez-González MA, Hebebrand J, Hinney A, Vedder H. Meta-analysis on the effect of the N363S polymorphism of the glucocorticoid receptor gene (GRL) on human obesity. BMC Med Genet. 2006;7:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Farrell RJ, Menconi MJ, Keates AC, Kelly CP. P-glycoprotein-170 inhibition significantly reduces cortisol and ciclosporin efflux from human intestinal epithelial cells and T lymphocytes. Aliment Pharmacol Ther. 2002;16:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 894] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 68. | Carvalho AT, Fróes RS, Esberard BC, Santos JC, Rapozo DC, Grinman AB, Simão TA, Nicolau Neto P, Luiz RR, Carneiro AJ. Multidrug resistance 1 gene polymorphisms may determine Crohn’s disease behavior in patients from Rio de Janeiro. Clinics (Sao Paulo). 2014;69:327-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Wine E, Mack DR, Hyams J, Otley AR, Markowitz J, Crandall WV, Leleiko N, Muise AM, Griffiths AM, Turner D. Interleukin-6 is associated with steroid resistance and reflects disease activity in severe pediatric ulcerative colitis. J Crohns Colitis. 2013;7:916-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Santaolalla R, Mañé J, Pedrosa E, Lorén V, Fernández-Bañares F, Mallolas J, Carrasco A, Salas A, Rosinach M, Forné M. Apoptosis resistance of mucosal lymphocytes and IL-10 deficiency in patients with steroid-refractory Crohn’s disease. Inflamm Bowel Dis. 2011;17:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Montero-Meléndez T, Llor X, García-Planella E, Perretti M, Suárez A. Identification of novel predictor classifiers for inflammatory bowel disease by gene expression profiling. PLoS One. 2013;8:e76235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1256] [Cited by in RCA: 1244] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 74. | Jiang L, Xu L, Xie J, Li S, Guan Y, Zhang Y, Hou Z, Guo T, Shu X, Wang C. Inhibition of autophagy overcomes glucocorticoid resistance in lymphoid malignant cells. Cancer Biol Ther. 2015;16:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Yang QF, Chen BL, Zhang QS, Zhu ZH, Hu B, He Y, Gao X, Wang YM, Hu PJ, Chen MH. Contribution of MDR1 gene polymorphisms on IBD predisposition and response to glucocorticoids in IBD in a Chinese population. J Dig Dis. 2015;16:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta. 2009;1794:860-871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 77. | Hitzl M, Drescher S, van der Kuip H, Schäffeler E, Fischer J, Schwab M, Eichelbaum M, Fromm MF. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics. 2001;11:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 78. | Chikanza IC, Kozaci D, Chernajovsky Y. The molecular and cellular basis of corticosteroid resistance. J Endocrinol. 2003;179:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Bond HM, Mesuraca M, Amodio N, Mega T, Agosti V, Fanello D, Pelaggi D, Bullinger L, Grieco M, Moore MA. Early hematopoietic zinc finger protein-zinc finger protein 521: a candidate regulator of diverse immature cells. Int J Biochem Cell Biol. 2008;40:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 80. | Park SH, Kim SK, Choe JY, Moon Y, An S, Park MJ, Kim DS. Hypermethylation of EBF3 and IRX1 genes in synovial fibroblasts of patients with rheumatoid arthritis. Mol Cells. 2013;35:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Zhao LY, Niu Y, Santiago A, Liu J, Albert SH, Robertson KD, Liao D. An EBF3-mediated transcriptional program that induces cell cycle arrest and apoptosis. Cancer Res. 2006;66:9445-9452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Tahara T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, Okubo M, Maruyama N, Kamano T, Kamiya Y, Fujita H. Promoter methylation of protease-activated receptor (PAR2) is associated with severe clinical phenotypes of ulcerative colitis (UC). Clin Exp Med. 2009;9:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Kuester D, Guenther T, Biesold S, Hartmann A, Bataille F, Ruemmele P, Peters B, Meyer F, Schubert D, Bohr UR. Aberrant methylation of DAPK in long-standing ulcerative colitis and ulcerative colitis-associated carcinoma. Pathol Res Pract. 2010;206:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Balasa A, Gathungu G, Kisfali P, Smith EO, Cho JH, Melegh B, Kellermayer R. Assessment of DNA methylation at the interferon regulatory factor 5 (IRF5) promoter region in inflammatory bowel diseases. Int J Colorectal Dis. 2010;25:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Sato F, Harpaz N, Shibata D, Xu Y, Yin J, Mori Y, Zou TT, Wang S, Desai K, Leytin A. Hypermethylation of the p14(ARF) gene in ulcerative colitis-associated colorectal carcinogenesis. Cancer Res. 2002;62:1148-1151. [PubMed] |
| 86. | Harris RA, Nagy-Szakal D, Kellermayer R. Human metastable epiallele candidates link to common disorders. Epigenetics. 2013;8:157-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Weinhouse C, Anderson OS, Jones TR, Kim J, Liberman SA, Nahar MS, Rozek LS, Jirtle RL, Dolinoy DC. An expression microarray approach for the identification of metastable epialleles in the mouse genome. Epigenetics. 2011;6:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4264] [Cited by in RCA: 3784] [Article Influence: 180.2] [Reference Citation Analysis (0)] |