Published online Sep 28, 2017. doi: 10.3748/wjg.v23.i36.6571
Peer-review started: July 15, 2017
First decision: August 10, 2017
Revised: August 21, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: September 28, 2017
Processing time: 73 Days and 2 Hours
In the last years new evidence has accumulated on nonalcoholic fatty liver disease (NAFLD) challenging the paradigms that had been holding the scene over the previous 30 years. NAFLD has such an epidemic prevalence as to make it impossible to screen general population looking for NAFLD cases. Conversely, focusing on those cohorts of individuals exposed to the highest risk of NAFLD could be a more rational approach. NAFLD, which can be diagnosed with either non-invasive strategies or through liver biopsy, is a pathogenically complex and clinically heterogeneous disease. The existence of metabolic as opposed to genetic-associated disease, notably including ”lean NAFLD” has recently been recognized. Moreover, NAFLD is a systemic condition, featuring metabolic, cardiovascular and (hepatic/extra-hepatic) cancer risk. Among the clinico-laboratory features of NAFLD we discuss hyperuricemia, insulin resistance, atherosclerosis, gallstones, psoriasis and selected endocrine derangements. NAFLD is a precursor of type 2 diabetes (T2D) and metabolic syndrome and progressive liver disease develops in T2D patients in whom the course of disease is worsened by NAFLD. Finally, lifestyle changes and drug treatment options to be implemented in the individual patient are also critically discussed. In conclusion, this review emphasizes the new concepts on clinical and pathogenic heterogeneity of NAFLD, a systemic disorder with a multifactorial pathogenesis and protean clinical manifestations. It is highly prevalent in certain cohorts of individuals who are thus potentially amenable to selective screening strategies, intensive follow-up schedules for early identification of liver-related and extrahepatic complications and in whom earlier and more aggressive treatment schedules should be carried out whenever possible.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is a pandemic disease. Recent evidence highlights new concepts in clinical and pathogenic heterogeneity of NAFLD, a systemic disorder with a multifactorial pathogenesis and protean clinical manifestations. Other than the classical obese phenotype of NAFLD, a lean though metabolically abnormal variant has been recognized. Simple steatosis is no more considered a benign condition; insulin resistance is necessary but not sufficient for the disease progression, and NAFLD is not only a mere hepatic manifestation of metabolic syndrome, but may forerun the development of metabolic syndrome and cardio-renal complications. Several non-invasive diagnostic tests are now available and new drug treatment options are coming.
- Citation: Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE. Nonalcoholic fatty liver disease: Evolving paradigms. World J Gastroenterol 2017; 23(36): 6571-6592
- URL: https://www.wjgnet.com/1007-9327/full/v23/i36/6571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i36.6571
Nonalcoholic fatty liver disease (NAFLD) features excess intrahepatic ectopic triglyceride deposition[1] in patients who are free of competing etiologies of liver disease[2]. It is the most frequent liver disease and is associated with a wide spectrum of hepatic disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma (HCC)[3]. Moreover, frequent co-morbidities of NAFLD include specific cardio-renal-metabolic conditions and increased hepatic/extrahepatic cancer risk[4].
NAFLD is a pandemic disease worldwide, which has been paralleling the ongoing epidemics of obesity, type 2 diabetes (T2D), and metabolic syndrome (MetS)[4]. The prevalence of NAFLD approaches 25%-30% in the Europe and United States general populations; but this figure surges to 80%-90% in selected cohorts of dysmetabolic individuals[5-7]. Patients with NAFLD have an increased risk of premature cardiovascular as well as of liver-related mortality[8]. Of concern, up to 50% of cases NAFLD-HCC may occur in the absence of cirrhosis, a circumstance which will often worsen the outcome[9,10].
Knowledge accumulated since 1980, when Ludwig named and described NASH[11], has challenged most of the paradigms that had been holding the scene over the previous 30 years. For example, NAFLD is now considered a multifaceted complex systemic condition[12,13] which exhibits a sexual dimorphisms[14] and follows a variable hepatic and extra-hepatic course[15,16]. In addition, recent evidence suggests that simple steatosis may progress to NASH and advanced liver fibrosis[17] challenging the previous dogma that “steatosis is a benign condition”.
Insulin resistance (IR) is often associated with NAFLD but it is “necessary but not sufficient” for the disease to progress[18]. Moreover, NAFLD may occur in lean individuals[19,20] and it is not only a mere “hepatic manifestation of MetS”, but may forerun the development of MetS[21,22]. NAFLD is associated with secretion of diabetogenic hepatokines and inflammatory biomarkers that increase the risk of incident T2D by adversely affecting glucose homeostasis[23]. Of concern, NAFLD and T2D are linked by a “vicious circle” which carries to accelerated worsening of liver disease[24], more difficult metabolic control and earlier appearance of T2D complications[25,26].
The historical “two-hit” pathogenic theory[27] has been replaced by a multi-factorial model, which more incisively recapitulates the complexity of the pathogenesis of the disease[28] by emphasizing the myriads of pathways leading to the same hepatic phenotype[29]. A better definition of the role played by genetics in the development of NAFLD[30] has led to the notion that, probably, two different NAFLD types exist: NAFLD associated with and NAFLD dissociated from MetS, and that each may differentially affect cardio-metabolic risk[31,32]. In addition, the declining gradient of risk of NAFLD in Hispanic patient populations compared to non-Hispanic whites and African Americans may potentially be accounted for by polymorphisms in genes that control metabolism[33]. The impressive amount of data implicating gut microbiota in the development of NAFLD may well illustrate the role of diet in increasing NAFLD risk[34-36] and significantly impact our understanding of NAFLD pathogenesis and treatment[4].
Over time, the diagnostic approach has also undergone substantial changes. Non-invasive physical and chemical biomarkers are now available[37]. Liver biopsy is reserved to selected NAFLD cases in clinical practice and is more extensively performed and repeated to evaluate novel drug agents[3,4,38,39]. Finally, new histological classifications have recently been proposed[40] and the pros and cons of each system must be carefully evaluated[41].
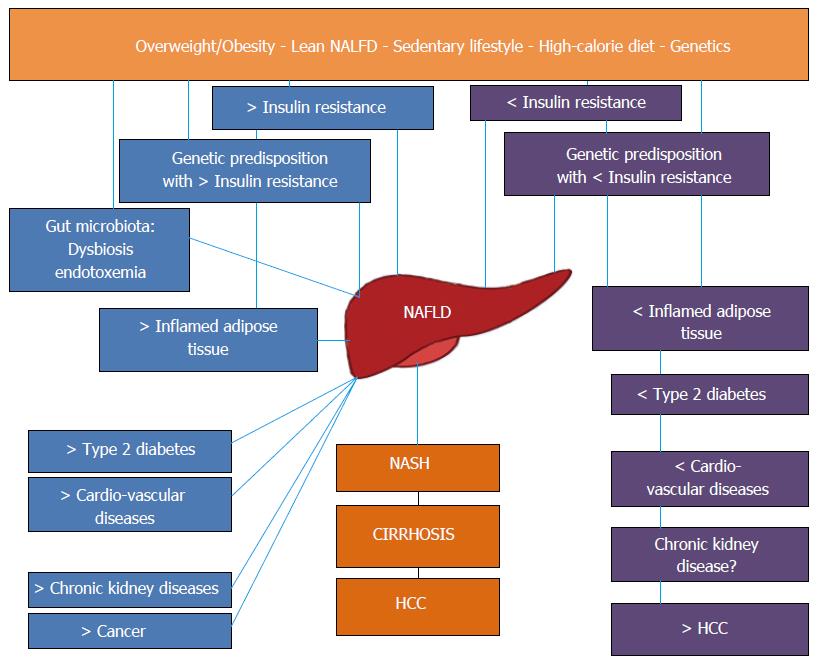
Within the complex frame of pathogenic and clinical heterogeneity of different NAFLD populations synthesized in Figure 1, this review aims at disseminating the most updated paradigms on NAFLD and the possible implications for both clinical practice and research purposes.
An ongoing debate regards whether any screening campaigns should be ever conducted to identify NAFLD individuals[38,42]. Those supporting screening would argue that NAFLD patients are prone to excess liver-related morbidity/mortality and cardiovascular risk and thus in need of intensive follow-up schedules; those against would reply that there is no point in identifying NAFLD, a condition for which no licensed drug treatment schedule is available. At any rate, given the overwhelming prevalence of NAFLD in the general population worldwide, any sensitive approach should best be conducted based on our understanding of which specific cohorts of individuals are exposed to the highest risk of disease.
In the general population, the prevalence of NAFLD has been reported to widely range from 6.3% to 51% related to the different population/ethnicity evaluated as well as to the diagnostic methods utilized to assess the amount of intrahepatic fat content[43]. In particular, the diagnostic technique used to capture cases of NAFLD has undoubtedly affected the results. For example, liver enzymes are far less sensitive than ultrasonographic scanning; in its turn, MR spectroscopy is the most sensitive although this technique is expensive and not universally available[3]. Based on meta-analytic data, Younossi et al[6] have reported that, in Europe and in United States, the overall prevalence of NAFLD in the general population approximates 25%. However, there are areas of the world, such as south America and middle East, wherein the average prevalence peaks > 30%; conversely, Africa has the minimum prevalence of NAFLD worldwide[6]. These impressive data clearly imply that no society worldwide can invest so many resources as to screen the general population for NAFLD. It is of interest, therefore, that modifiers of risk might assist health authorities as well as practicing physician in identifying individuals who are particularly prone to developing NAFLD. For example: male gender, middle age and Hispanic ethnicity, all reinforce the risk of NAFLD and, conversely, young women of Afro-American descent have the lowest risk of this disease[7]. Further to the above physiological modifiers, a set of metabolic modifiers have also been identified. They include arterial hypertension and the MetS (whose presence doubles the prevalence of NAFLD up to 50% of individuals); obesity, dyslipidemia and T2D[7]. In the general population, women are protected from developing NAFLD owing to their hormonal profile and/or set of chromosomes. Conversely, women with T2D are exposed to the same risk of NAFLD as men, indicating that T2D abrogates such a gender-related protection[7,14].
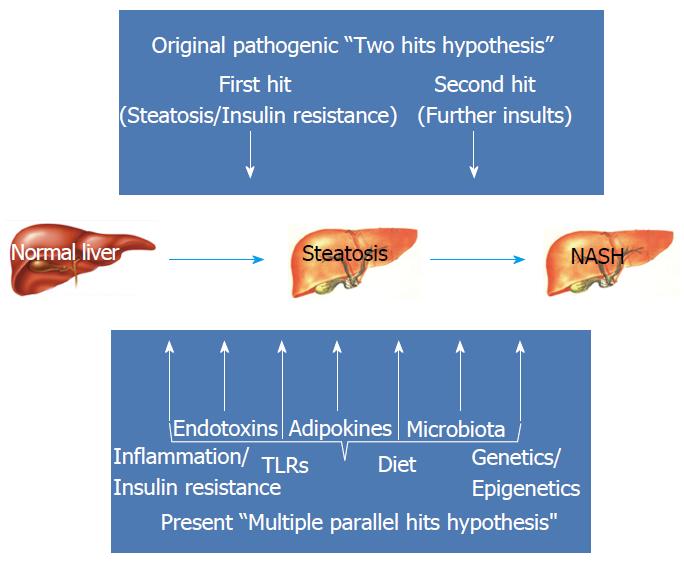
In recent years, it has increasingly become evident that what had initially been called the NAFLD “two hit hypothesis”, in which IR acted as the “first hit” by inducing lipid accumulation in the hepatocytes and increasing the vulnerability of the liver to further insults, referred to as the “second hit”, that, in their turn, promoted hepatic injury, inflammation and fibrosis, is probably over-simplistic[27,44]. Human NAFLD is rather a multi-factorial, non-communicable disease resulting from a complex interaction between multiple environmental and metabolic “hits” and a predisposing genetic background[45] (Figure 2). A “multiple parallel hits hypothesis” seems more appropriate to recapitulate the complexity of NAFLD pathogenesis[28], which results from numerous events originating within liver, adipose tissue, gastrointestinal tract and the muscle[45].
An unhealthy lifestyle, characterized by sedentariness and high-calorie diet, is crucial for NAFLD development and progression. The unbalance between caloric intake and expenditure determines the expansion of fat depots, which become inflamed and insulin resistant, and release increased amounts of free fatty acids into the bloodstream, leading to ectopic fat accumulation in the liver, skeletal muscles and pancreas[46]. The hepatic lipid content mainly derives from the increased lipolysis of triglycerides in the adipose tissue and, to a lesser extent, from dietary fats and sugars, and from de novo lipogenesis[47]. Moreover, the dysfunctional adipose tissue overproduces pro-inflammatory cytokines, such as leptin and resistin, whereas the release of anti-inflammatory adipokines, such as adiponectin, is reduced, so further enhancing NAFLD progression[48]. The role of intra-hepatocytic accumulation of specific toxic lipids, via lipotoxicity and inflammation, has emerged as a key player in the pathogenesis of NASH and its systemic complications[49]. Saturated fatty acids have been found elevated in NASH patients and induce inflammation and hepatocyte apoptosis through activation of JNK/endoplasmic reticulum stress and oxidative stress/mitochondrial dysfunction[50]. The accumulation of diacylglycerol and ceramide in the liver impairs hepatic insulin signaling, fuels gluconeogenesis and promotes the development of persisting hyperglycemia and, eventually, T2D[51].
Unhealthy lifestyle also results in dysbiosis, i.e. quantitative and qualitative changes of gut microbiota composition[52]. In NAFLD an increased Firmicutes/Bacteroidetes ratio and changes in metagenomic-based functional aspects of gut microbiota have all been described[53]. Gut microbiota may contribute to the development and progression of NAFLD by triggering (both directly or via end-products of bacterial metabolism) different signaling pathways, by increasing the efficiency of caloric extraction from the food, and by inducing translocation of bacterial products via increased gut permeability[54-57]. Circulating pathogen-associated molecular pattern (PAMPs) and damage-associated molecular patterns (DAMPs) interact with the family of pattern recognition receptors (toll-like receptors, TLRs) within the liver and induce several pro-inflammatory pathways, including over-expression of cytokines/chemokines, production of reactive oxygen species and activation of the inflammasome[58,59]. Of note, activation of Nod-like receptor protein 3 inflammasome, upregulated by TLRs in response to the presence of PAMPs and DAMPs, has been associated with a novel cell death mechanism, referred to as hepatocyte pyroptosis[60].
Several nuclear receptors with their molecular cascades are promising pharmacological targets for the treatment of NAFLD, given their pivotal role in the regulation of energy homeostasis and metabolic pathways. Owing to the development of specific targeted drugs, peroxisome proliferator-activated receptors (PPARs), farnesoid X receptor (FXR) and liver X receptors (LXRs), now deserve full attention. The PPARs superfamily includes PPAR-α, PPAR-β/δ and PPAR-γ. PPAR-α, mainly expressed in liver and muscle, modulates the rates of fatty acids catabolism, lipogenesis and ketone body synthesis by acting as a nutrient sensor. PPAR-γ, abundantly expressed in the adipose tissue, promotes adipocyte differentiation and storage of triglycerides, and regulates glucose homeostasis. PPAR-β/δ, universally expressed in all organ tissues, regulates glucose and lipoprotein metabolism and exerts an anti-inflammatory role. FXR, mainly expressed in the liver and gut, acts as a sensor of bile acids and mediates the signaling effects exerted by bile acid on glucose and lipid metabolism. LXRs serve as lipid sensors in the liver and participate in regulating the metabolism of cholesterol and fatty acids. The role of nuclear receptors in NAFLD pathophysiology has been comprehensively reviewed elsewhere[15].
The role of skeletal muscle in the pathogenesis of NAFLD is a matter of increasing research[23]. Intra-myocellular lipid storage is an early step of ectopic fat accumulation and systemic IR, leads to increased delivery of glucose to the liver, where it becomes substrate for hepatic de novo lipogenesis, and has been associated with NAFLD[61,62]. Accordingly, sarcopenia, a pathological condition featured by generalized loss of skeletal mass and strength, has recently been proposed as an additional key factor in the pathogenesis of NAFLD[63-65]. The myokines, such as irisin, which is produced in response to physical activity and exerts several beneficial metabolic effects, may partly account for these associations[66,67].
NAFLD is a complex disorder with a variable natural history. Subtle inter-patient genetic variation and environmental factors combine to determine variation in disease progression[68]. Several clinical features are clues to genetics playing a major role in NAFLD. First, up to a quarter of the general population are at risk of progressive disease; however only a minority will experience associated liver-related morbidity[69]. Second, both the development and the progression of disease tend to cluster as distinct traits among “NAFLD families”[7,70]. Third, among non-diabetics, NAFLD will typically affect men more often than women[7] which, further to hormonal variations, may mirror genetic factors. Fourth, although NAFLD is strictly linked with obesity[7] not all obese subjects will develop NAFLD and, more importantly, NAFLD can also be found in non-obese individuals[20]. On these grounds, in the last few years scientific attention has focused on NAFLD genetic features.
Among all analyzed genes patatin-like phospholipase domain-containing protein 3 (PNPLA3, also called adiponutrin) and trans-membrane 6 super family 2 (TM6SF2) are deemed to play the most significant role. More recently, membrane bound O-acyltransferase domain-containing 7 gene (MBOAT7) has also gained attention. They are all involved in the process of intrahepatic accumulation of triglycerides.
PNPLA3 gene encodes for a 481-aminoacid protein expressed in the liver and adipose tissues, with function of triglyceride hydrolase and acetytil-CoA-indipendent transacylase, suggesting a double role of catabolic lipase activity and anabolic lipogenic activity[71-73]. The nonsynonymous variant rs738409 of PNPLA3 is a genetic polymorphism characterized by the substitution of isoleucine to methionine at position 148 (I148M). Studies consistently show a strong association between I148M variant and hepatocellular triglycerides accumulation[74-76]. I148M variant is a loss of function allele that determines an altered hepatic lipid metabolism, eventually leading to hepatic droplet lipid accumulation, lower hepatic VLDL secretion and lower adiponectin production[77]. Adiponectin is a protein with anti-inflammatory activity and onco-suppressive properties; lower circulating levels of adiponectin can account for the propensity of I148M variant to the progression from NAFLD to NASH and the increased risk of HCC[77,78].
TM6SF2, also known as KIAA 1926, is a gene at the 19p13.11 locus, with unknown biological function that encodes a protein high expressed in the small intestine, kidney and liver. It is involved in the pathophysiology of NAFLD by modifying hepatic lipid secretion; a decreased hepatic expression of this protein is associated with an increased size and number of hepatocytic lipid droplets[79]. In particular, studies have shown that a variant of TM6SF2, rs58542926 c.449 C>T (E167K), is associated with NAFLD evolution, also with dyslipidemia and cardiovascular risk. The minor variant allele (T) is linked with a reduction of the excretion of hepatic VLDL and a lower level of circulating triglyceride and LDL-cholesterol. Conversely, C-allele is associated with a higher hepatic excretion of VLDL, serum triglyceride and cholesterol. Thus, the minor allele exhibits a higher risk of advanced hepatic disease, though protecting from metabolic and cardiovascular disease[80]. Conversely, increased metabolic risk is associated with C-allele[81]. Morris et al[82]. based on their large meta-analysis have hypothesized a correlation with T-allele variant and risk developing T2D, but this theory remains to be confirmed[82]. Recently, it has been shown that TM6SF2 (E167K) gene variant is associated with an altered lipidomic profile featuring deficiency of polyunsaturated phosphatidylcholine and excess of polyunsaturated free fatty acids in the liver. This lipidomic profile leads to decreased concentrations of circulating VLDL and increased liver fat content[83]. Moreover, the same polymorphism has been found to modulate postprandial lipidmetabolism, nutrient oxidation and glucose homeostasis in response to dietary fat[84]. These findings are compatible with the above reported notion that carriers of TM6SF2 polymorphisms may be specifically exposed to liver-related as opposed to vascular manifestations of NAFLD.
Finally, it has been recently shown that the rs641738 C>T variant in MBOAT7 gene, which is involved in phosphatidylinositol acyl-chain remodeling, increases the risk of developing NAFLD; inflammation and fibrosis[85,86].
As previously reported in this review, genetic factors play a major role in development and progression of NAFLD. Such a role of genetics is further reinforced when lean NAFLD patients are taken into consideration. It is well-known that NAFLD is often found in obese and dysmetabolic patients. Accordingly, the “Lean NAFLD” phenotype has attracted interest among researchers in as much as it features selectively expanded visceral adiposity though preserving a normal body mass index and, sometimes, even a normal waist circumference. Worryingly, these individuals develop IR and atherogenic dyslipidemia similarly to obese patients and have, therefore, in the past been alluded to as “metabolically obese, normal weight”[87]. Lean NAFLD cases have been described among different ethnic population, mainly among Asians, who tend to develop the metabolic complications of obesity for BMI values inferior to those of Caucasians[88,89]. The mechanism(s) through which lean NAFLD develop(s) is/are poorly characterized and the disease goes often under-recognized. Though the role of genetic factors is likely to be more substantial in “lean NAFLD” than that found in “obese NAFLD”[90], a recent statistically sophisticated study has suggested that both lean and obese NAFLD patients share cardio-metabolic risk commonalities and that the former, despite normal body weight, nonetheless have excess of abdominal adiposity and other MetS features[19]. Accordingly, also lean NAFLD patients may benefit from increased physical activity which is effective in reducing visceral fat[91]. Of note, the findings of a very recent study confirm that NAFLD development and progression derive from a complex interplay between genetic and environmental factor, by clearly demonstrating the presence of a synergy between adiposity and the genetic risk of NAFLD conferred by multiple loci[92].
Diagnosis and staging of NAFLD fully rely on liver biopsy. Histological examination of liver tissue specimens is the gold-standard for quantitating steatosis, diagnosing NASH and staging fibrosis. The last of these three tasks bears major prognostic significance given that fibrosis is associated with long-term liver-related and, probably, cardiovascular outcomes[93-95]. Over the past two decades, different criteria have been suggested for scoring and staging histological lesions, and different definitions of NASH have been used[40,96-99]. Besides discrepancies and subjectivity in interpretation, liver biopsy has additional limitations such as cost, invasiveness and sampling variability which limit the feasibility of this procedure in all the patients with suspected NAFLD and have called for the development of alternative non-invasive diagnostic and staging procedures. Despite some intrinsic and specific drawbacks[100,101], these non-invasive tools, especially if used in combination or sequentially, may help in restricting the number of and assist in a better selection of potential candidates to liver biopsy[102].
Steatosis, namely a minimal threshold of 5% of hepatocytes containing fat droplets in biopsy specimen, is a prerequisite for the diagnosis of NAFLD. The histological definition of adult NASH is based on a combination of three elementary lesions, i.e., steatosis, hepatocellular ballooning and lobular inflammation[41,97].
Four main pathologic classifications of NAFLD have been proposed: Matteoni’s, Brunt’s, Kleiner’s classification with the NAFLD Activity Score (NAS) and the recent Fatty Liver Inhibition of Progression (FLIP) algorithm with the Steatosis, Activity, and Fibrosis (SAF) score[40,96-99].
In 2005 the NASH Clinical Research Network designed and validated a histological feature scoring system that addressed the full spectrum of lesions of NAFLD. Currently, NAS is the most widely used histological classification for NAFLD/NASH and its use is recommended for defining and quantifying disease activity in clinical trials[98]. NAS, ranging from 0 to 8, is derived from the sum of steatosis (0-3), ballooning (0-2) and lobular inflammation (0-3). However, at least two out of its three components show a high variability among pathologists; and data increasingly suggest that NAS is poorly correlated with metabolic risk factors[103], is unable to predict fibrosis progression[104] and to prognosticate liver-related and overall mortality in NAFLD patients[93,94,105].
More recently, Bedossa and the FLIP Consortium created a simple histological algorithm (FLIP algorithm) based on a scoring system, the SAF score (Steatosis, Activity, Fibrosis) intended for pathologists to reliably diagnose NASH, assess disease severity and limit interobservervariation[40,99]. The SAF score separately assesses steatosis, activity and fibrosis. Activity, which ranges from 0 to 4, is derived from the combination of the semi-quantitative values of hepatocellular ballooning (0-2) and lobular inflammation (0-2). NASH according to the FLIP algorithm is diagnosed when steatosis, ballooning and lobular inflammation are all scored ≥ 1. The main discrepancy between NAS and SAF scores is that SAF does not include steatosis in the activity score, owing to the questionable role of steatosis per se as a marker of disease progression and prognosis. Moreover, fibrosis stage, the strongest predictor of outcomes in chronic liver disease, was not included in the NAS. This novel histological classification has shown excellent applicability and reproducibility among pathologists for the assessment of liver lesions in morbidly obese patients and in NAFLD patients with MetS[40,99]. However, it will have to undergo further validation in clinical practice. Table 1 shows the main features of NAS and SAF scores and highlights the main differences between these scoring systems.
| NAS | SAF | |
| Scoring system | Steatosis + Lobular Inflammation + Ballooning: 0-8 | Steatosis, Activity (Lobular Inflammation + Ballooning), Fibrosis: S0-3, A0-4, F0-4 |
| Details of scoring | Steatosis | Steatosis |
| 0: < 5% | 0: < 5% | |
| 1: 5%-33% | 1: 5%-33% | |
| 2: 34%-66% | 2: 34%-66% | |
| 3: > 67% | 3: > 67% | |
| Lobular inflammation | Lobular inflammation | |
| 0:00 | 0:00 | |
| 1: < 2/20X | 1: < 2/20X | |
| 2: 2-4/20X | 2: ≥ 2/20X | |
| 3: > 4/20X | ||
| Ballooning (number of ballooned hepatocytes) | Ballooning (size and shape of hepatocytes) | |
| 0: None | 0: normal hepatocytes | |
| 1: Few | 1: clusters, reticulated cytoplasm | |
| 2: Many | 2: enlarged hepatocytes | |
| Fibrosis stage | Fibrosis stage | |
| F0: None | F0: 0 | |
| F1a: zone 3 perisinusoidal, delicate | F1: zone 3 perisinusoidal (all), or portal only | |
| F1b: perisinusoidal, dense | F2: zone 3 + portal | |
| F1c: portal only | F3: bridging | |
| F2: 1a or 1b + portal | F4: cirrhosis | |
| F3: bridging | ||
| F4: cirrhosis |
Imaging methods are the most commonly used non-invasive tools for the diagnosis of steatosis. The main advantages of ultrasonography are its safety, low cost, wide availability and the overall scanning of abdominal organs. Traditionally, ultrasonography has been considered a technique with a low sensitivity in identifying fatty liver infiltration when less than 30% of hepatocytes are steatotic. Of note, a recent study has demonstrated that ultrasonography, especially when implemented with standardized measurements and semi-quantitative scores, is sensitive for an amount of steatosis as low as 10%[106] and may predict metabolic derangements and liver histology changes[106-108]. Further studies are necessary to confirm these novel findings. The main limitations of ultrasonography are its inability in differentiating steatosis from fibrosis, the issues with morbid obese individuals, and its operator and machinery dependency.
The controlled attenuation parameter (CAP) is a new promising screening method that measures ultrasound attenuation in the liver using signals obtained by the Vibration Controlled Transient Elastometry (TE) (Fibroscan®). Interestingly, CAP appears to be more sensitive in detecting lesser degrees of steatosis compared to other widely available imaging methods. Moreover, being coupled with liver stiffness measurements, it has the advantage of simultaneously estimating liver fibrosis[108-110]. However, the underlying disease, BMI and T2D may affect the findings and thus must be taken into consideration in the interpretation of CAP results[111].
Over the last 10 years, several ultrasound elastographic techniques have been implemented, including TE, acoustic radiation force impulse (ARFI) elastography, 2D shear-wave elastography and real-time strain elastography. TE, is the most studied elastographic method for the estimation of liver fibrosis in chronic liver disease; in NAFLD, TE can be used to confidently rule out severe fibrosis and cirrhosis (with a nearly 90% negative predictive value) and for monitoring disease progression[11,112]. Of note, the amount of steatosis has emerged as a significant factor affecting the performance of liver stiffness measurements with TE[112,113]. For this reason, it has been suggested that taking into account CAP values may improve the prediction of liver fibrosis by TE[113].
Magnetic resonance imaging techniques, and particularly magnetic resonance spectroscopy, are able to accurately quantify liver fat content; the advantages include high sensitivity for assessing small amounts of intrahepatic triglycerides and sampling a large liver volume[114]. More recently, magnetic resonance elastography and multiparametric magnetic resonance offer the possibility of performing a thorough characterization of liver tissue by assessing steatosis, fibrosis and haemosiderosis at the same time[115,116], and have been shown to be able to predict clinical outcomes in patients with various chronic liver disease, notably including NAFLD[117]. Cost and limited availability, however, restrict their use to research purposes.
Several serum biomarker panels have been developed for predicting steatosis, diagnosing NASH and estimating fibrosis[118] which are, in theory, the preferred diagnostic tool for large-scale screening and epidemiological studies on NAFLD[4,38]. For example, Bedogni’s Fatty Liver Index (FLI) has been largely used to capture steatosis in epidemiological studies[119]. The strength of FLI as a surrogate marker comes from the demonstration that it can predict clinical outcomes related to the MetS, such as risk of T2D, accelerated atherosclerosis and cardiovascular risk, as well as hepatic, cardiovascular and cancer-related mortality[120,121]. While clinically relevant, these associations might be conveyed independently of steatosis per se since FLI incorporatesvariables that are part of the MetS phenotype[101]. In fact, recent studies have found only a limited correlation between FLI and the amount of liver fat per se, as defined by liver biopsy or magnetic resonance spectroscopy[101,122,123]. As for the FLI, other steatosis biomarkers, such as the NAFLD liver fat score, the Hepatic Steatosis Index and the Lipid Accumulation Product, generally show good performance in identifying steatosis, though they are unable to predict liver fat content and are confounded by hepatic histological changes other than steatosis, mainly necro-inflammation and fibrosis[101,123].
With regard to the possibility of non-invasively differentiating NASH from simple steatosis, several biomarkers (acute-phase proteins, cytokines, markers of oxidative stress and apoptosis, as is the case of cytokeratin-18 fragments) and multiple complex models have been investigated, but the majority of them lack external validation, have only been tested among selected populations and have been inconsistent in their performance of detecting NASH[102,118].
Several simple non-invasive fibrosis scoring systems, notably including NAFLD Fibrosis Score and Fib-4 can, however, reliably exclude advanced fibrosis in a high proportion of NAFLD patients, allowing a more targeted use of liver biopsy[124]. Their use in clinical practice is recommended by updated hepatological guidelines[4,38], and they reliably predict liver-related and cardiovascular complications and death in NAFLD patients[125-127]. However, they were principally developed and validated in patients aged < 65 years of age, hence their cut-offs need to be adapted in the elderly[128]. It has been shown that the combination or the sequential use of different scoring systems or different non-invasive techniques (TE plus biomarker) improve the diagnostic accuracy in detecting severe liver fibrosis, thus further reducing the need for liver biopsy[129,130].
Recently, several miRNAs[131], epigenetic biomarkers[132], lipidomic[133,134], metabolomics[135] and proteomic[136] profiles have been found differently expressed in NAFLD patients and could be used as surrogate markers of progressive liver injury. Probably, a multi-domain, multi-component approach which integrates variables from a diverse set of data sources deriving from phenotypic, genomic, lipidomic, metabolomic and proteomic domains will be the avenue for future investigations in the noninvasive assessment of NAFLD[136,137].
Usually asymptomatic, NAFLD is a phenotypically polymorphic disease which, owing to its systemic nature, has a variable clinical presentation, a multitude of potentially associated disease and a rich spectrum of laboratory features[4,12,138]. Understanding this remarkable phenotypic polymorphism is key in guiding the diagnostic process in the individual patient.
Most patients will seek medical advice on the grounds of otherwise inexplicably deranged liver tests/ultrasonographic findings compatible with fatty changes. Collectively, these laboratory/ultrasonographic abnormalities tend to be often discovered either during routine medical check-up performed by asymptomatic individuals or by patients who have nonspecific and causally unrelated complaints[139,140].
The association of NAFLD with IR and its close connection with the MetS was independently reported by three unrelated groups of investigators as early as 1999[141-143]. In particular, Marchesini et al[141] by comparing 46 patients with NAFLD to 92 age and sex-matched healthy controls with the homeostasis model assessment method found that NAFLD was associated with IR and hyperinsulinemia even in lean subjects with normal glucose tolerance. Consistently, Cortez-Cortez-Pinto et al[142] in 30 patients with NAFLD found that 80% were either obese or dyslipidemic; 50% had arterial hypertension and 33% T2D; moreover, glucose metabolism was altered in 69% and hyperinsulinemia and hyperleptinemia were common. Along the same line, Lonardo, based on his systematic search of the available literature, concluded that fatty liver most commonly affected middle-aged men with obesity, altered glucose metabolism, hyperlipidemia, and hypertension[143].
Over time, these pioneer studies suggesting an intimate connection of NAFLD with IR and the MetS have extensively been confirmed and carried further. For example Ballestri et al[144] have recently shown that HOMA-IR, a widely used index of IR, along with serum uric acid concentrations, is an independent predictor of both steatosis and ballooning; waist circumference predicts both ballooning and hepatic fibrosis[144]. Moreover, the same group of investigators, also found that baseline NAFLD predicts the development of incident T2D and MetS over a median 5-year follow-up[22]. Taken collectively, these findings strongly support the notion that NAFLD should no longer be considered a mere “hepatic manifestation of the MetS” but rather, and more appropriately, both a precursor and a consequence of the MetS[21,31,145-147].
Lonardo et al[139] were first in reporting that both fasting insulin (a sensitive marker of IR) and uric acid were independent predictors of NAFLD. These findings have been independently confirmed by several studies[148-152], including two meta-analytic reviews[153,154]. Of interest, although serum uric acid concentrations are associated with indices of IR, both parameters are independently associated with NASH, at least in children and adolescents[155], suggesting that each probably carries an independent contribution to the development of progressive liver disease.
NAFLD is typically characterized by atherogenic dyslipidemia featuring larger and triglyceride over-enriched circulating very-low-density lipoproteins (VLDLs), small dense low-density lipoproteins (LDLs) and low and dysfunctional high-density lipoproteins (HDLs). Of note, when NAFLD progresses to severe fibrosis and cirrhosis, dyslipidemia will apparently improve, probably owing to failing hepatic synthetic capacity[144,156-159].
Liver tests are typically normal or mildly elevated in NAFLD. Gamma-glutamyltransferase (GGT) may be slightly elevated and is increasingly recognized as a marker of metabolic disturbances and cardiovascular risk[160]. Aminotransferases do not identify progressive disease, given that patients with normal liver enzymes are spared neither from NASH nor significant fibrosis[161].
The diagnostic process followed to investigate a suspect liver disease may include the determination of parameters of iron metabolism and non-organ specific autoantibodies (NOSA). Elevated serum ferritin, closely associated with hepatic iron deposition, is common in NAFLD and is strongly correlated with IR, more advanced disease and increased mortality[162-164]. Whether therapeutic strategies aimed at correcting iron metabolism may be beneficial in NAFLD remains controversial.
It is important to highlight that the positivity of NOSA, in the appropriate clinical and epidemiological context, is compatible with NAFLD. Loria et al[165], in their pilot study, were first in reporting that NOSA positivity in NAFLD was more prevalent than in the general population and that high-titer ANA was associated with IR. Bringing this line of research further, the clinical research network, in a multicenter study including a total of 864 NAFLD patients found that NOSA are frequently positive in NAFLD in the absence of autoimmune hepatitis and their occurrence is not associated with more advanced histological features[166].
As early as 1995, was it first suggested that NAFLD was a systemic condition with a specific cardio-metabolic involvement[167], a notion which is now universally accepted[12,168].
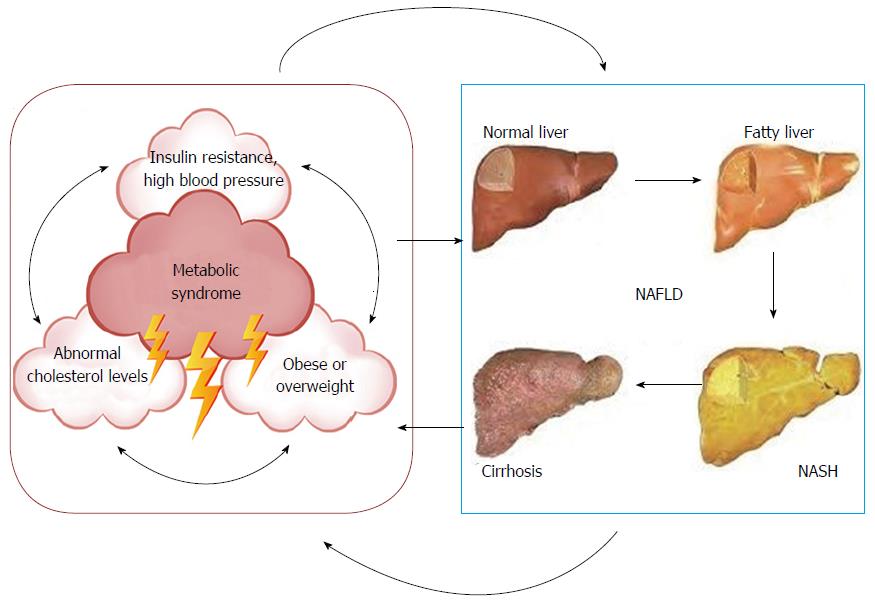
In particular, our understanding of the close and mutual relationship of NAFLD with the MetS has evolved and the bidirectional relationship among the two conditions is now largely acknowledged (Figure 3)[4,31,146,147]. IR, in its turn, will drive the course of liver disease to progressively fibrosing liver disease culminating with cirrhosis, but also promote the development of T2D[169]. NAFLD almost doubles the risk of incident T2D[22]. However, NAFLD patients usually die of extra-hepatic causes, frequently for cardiovascular diseases, which underpins the importance to early diagnose and aggressively treat CVD risk factors, as extensively discussed elsewhere[170]. A meta-analysis of 27 cross-sectional studies has shown a robust association between NAFLD and several markers of subclinical atherosclerosis burden, such as an increased risk, independent of classical CVD risk factors, of coronary artery calcification, carotid intima-media thickness, impaired flow-mediated vasodilatation and arterial stiffness[171]. Moreover, studies have shown that NAFLD is also associated with increased risk of atrial fibrillation and aortic valve sclerosis[172-175].
While cardiovascular mortality is the leading cause of death in NAFLD patients, malignancies, mainly affecting the gastrointestinal tract (liver, colon, esophagus, stomach, and pancreas) and extra-intestinal sites (kidney in men, and breast in women) rank second[176,177]. The association between NAFLD and colorectal cancer (CRC) has been investigated: some studies have shown a higher prevalence of CRC in NAFLD population than patients without, and this association is increased in presence of NASH[178].
T2D accounts for more than 90% of those 415 million people worldwide who live with diabetes; T2D may lead to micro-macrovascular complications that cause profound sufferings and put a heavy burden on health-care systems worldwide[179].
The prevalence of NAFLD is increased among those with T2D and T2D rescinds the typical gender dimorphism of NAFLD[7,180,181]. Conversely, NAFLD is associated with a 2-to-5-fold risk of developing T2D after adjustment for several metabolic confounders[22,169,182].
Common features in the epidemiology, risk factors and natural history of NAFLD and T2D can be identified: overweight and obesity[183-185]; a hypercaloric diet coupled with a sedentary behavior leading to weight gain[186,187]; IR and MetS components, including combined dyslipidemia[147,188].
As previously highlighted, NAFLD is not only a consequence, but also a cause of T2D and MetS generating a “vicious circle” and worsens the course of T2D[21,24,31,189]. It is mandatory to interrupt this circle and in this regard, it is encouraging that even transient remission/improvement of NAFLD is independently associated with reduced incidence of T2D[190,191].
T2D and NAFLD interact in enhancing the risk of promoting endothelial dysfunction, atherosclerosis, cardiovascular diseases, chronic kidney disease and advanced retinopathy[21,168,192,193]. The coexistence of T2D and NAFLD predicts the development of hepatic fibrosis[194-198]. Treatment of NAFLD, by affecting those pathogenic factors linking fatty liver with atherosclerosis (hence the so called “atherogenic liver”) was predicted to be able to reduce cardiovascular risk a few years ago[199]. Consistent with such a prediction, a recent study reported that reduced CIMT progression in the entire cohort of 92 patients was independently associated with decreased liver fat content assessed with magnetic resonance spectroscopy[200].
T2D and NAFLD are also associated with an increased incidence and mortality from several cancer types and, similarly, mortality for malignancies is the second cause of mortality in patients with NAFL[178,201-203]. Similar to NAFLD, T2D enhances the risk of developing HCC and may aggravates HCC outcomes[204-206].
NAFLD in those with T2D will follow a worse prognosis via hastened progression to NASH, liver cirrhosis and its complications and increased risk of developing NAFLD-HCC[43,207-210]. Specularly, concurrent NAFLD will often worsen the course of T2D[211].
An early diagnosis and treatment of NAFLD may potentially increase life expectancy of T2D patients[209]. Conversely, in subjects with NAFLD, periodical screening for T2D is encouraged, given that poor metabolic control will lead to progressive liver disease which,in its turn, is associated with enhanced cardiometabolic risk[38,193,212].
An impressively heterogeneous spectrum of clinical conditions have been associated with NAFLD spanning from carotid atherosclerosis through polycystic ovarian syndrome[23]. Some will be shortly reviewed here; others have recently been covered in detail elsewhere[4,213,214].
A pioneer study by Lonardo et al[215] evaluated the triangular association of NAFLD, gallstones and atherosclerosis. A decade later, we now know that the most severe NAFLD forms are strongly associated with excess cardiovascular mortality, probably as a direct/indirect result of hepatic fibrosis[193,212]. Concerning the NAFLD-gallstone duo, a systematic review and meta-analysis conducted in 12 observational studies (9 cross-sectional studies, 2 cohort studies and 1 case-control study) found that gallstones are significantly associated with NAFLD[216]. Further studies should be conducted regarding the underlying mechanism of this association. However, it is now clear that bile acids are no longer “a detergent which facilitates the absorption of fatty foodstuffs” such as it was deemed in the past; but rather, they must be regarded as signaling molecules, which coordinately regulate metabolism and inflammation acting not only in entero-hepatic tissues, but in peripheral organs as well[217].
This line of research was almost anecdotally inaugurated by a report of three cases from the POLI.ST.E.N.A study[218]. Several systematic studies have subsequently confirmed this novel association and a recent systematic review and meta-analysis supports an association between psoriasis and NAFLD suggesting that screening for NAFLD may be warranted among those with psoriasis[219].
Again, this topic was originally initiated by pioneer clinical observations. First, it was reported that endocrine derangements may eventually be conducive to NAFLD as a result of hormones being master regulators of body fat distribution and cell metabolism[220]. Next, endocrine pathways specifically leading to NASH were identified[221]. Among these, hypothyroidism has been associated to the whole NAFLD spectrum, steatosis to NASH-HCC[156,221-224].
A large Rotterdam population-based survey, aimed at investigating the association between variations in thyroid function and NAFLD, prospectively, in 9419 adults with a median follow-up time of 10 years; the presence of fibrosis in those with NAFLD was assessed with TE[225]. Data have shown that, compared to euthyroid individuals, hypothyroid ones were exposed to a higher risk of NAFLD and of clinically relevant fibrosis; consistently, on the opposite side of the spectrum, hyperthyroidism was associated with a protection from developing NAFLD[225]. These findings may potentially disclose novel treatment strategies.
It is widely accepted that lifestyle changes, namely diet and physical exercise are the mainstay of treatment of NAFLD, a condition no drugs are specifically licensed for[4,38].
A 3%-5% weight loss will improve steatosis; however, 5%-7% reductions are necessary to decrease hepatic inflammatory changes and 7%-10% to obtain remission of NAFLD/NASH and regression of fibrosis. Calorie restriction per se rather than specific diets are deemed to be beneficial[226]. In particular, despite emphasis is usually put in restricting dietary carbohydrates, moderate-carbohydrate diets do not induce a more profound reduction in liver enzymes (a non-validated surrogate index of NAFLD severity) than low/moderate-fat diets[227].
Exercise effectively reduces visceral and perhaps liver fat. A recent meta-analysis found that aerobic exercise but not resistance training exercise was effective in reducing visceral fat in overweight/obese adults with T2D[228]. The benefits of exercise in reducing intrahepatic triglyceride content will typically occur with minimal or no body weight loss[227,229], suggesting that diet and physical exercise will improve NAFLD and should be associated whenever possible given that they act via different pathways. Indeed, diet combined with exercise is associated with improvement in NAFLD activity score[227].
Drug treatment (better if within the frame of a clinical trial) should be reserved to those NASH individuals who do not respond to lifestyle changes. Drugs used to treat concurrent metabolic disorders (e.g., statins and antidiabetics) should be differentiated from drugs aimed at treating NASH per se.
Statins, glitazones and liraglutide belong to the first group. Our appreciation of the role of statins in hepatology has undergone a dramatic evolution from under-prescription based on fears of hepatotoxicity[230] to their identification as potentially protectors from the full spectrum of liver damage in NASH, notably including liver fibrosis and HCC[231-238].
As regards the oral antidiabetic agents glitazones, a meta-analysis of five eligible randomized controlled trials showed that these drugs improved neither fibrosis nor IR; however, lobular inflammation decreased in NASH patients who received TZD treatment[239]. However, a more recent meta-analytic review was specifically aimed at investigating the role of thiazolidinediones in advanced hepatic fibrosis in those with NASH analyzed 8 RCTs (5 evaluating pioglitazone and 3 evaluating rosiglitazone) enrolling 516 patients with biopsy-proven NASH for a duration of 6 to 24 mo. Among all studies combined, thiazolidinedione therapy was associated with improved advanced fibrosis, fibrosis of any stage and NASH resolution. Analyses restricted to RCTs enrolling patients without diabetes yielded similar results. All effects were accounted for by pioglitazone use[240]. Weight gain and lower limb edema occurred more frequently with thiazolidinedione therapy[240]. These novel data indicate that pioglitazone improves advanced fibrosis in NASH, even in patients without diabetes.
A phase 2 randomized study conducted on 52 patients (26 per arm) tested the efficacy of liraglutide (1.8 mg/daily via subcutaneous route) vs placebo. The use of liraglutide was associated with a 39% NASH resolution rate vs 9% in the placebo arm (RR = 4.3, 95%CI: 1.0-17.7, P = 0.019). This study was funded by the manufacturers of liraglutide[241].
A few drugs, such as vitamin E and obeticholic acid (OCA) have specifically been tested against NASH. Based on a meta-analysis of five RCTs vitamin E significantly reduced liver enzymes (AST, ALT, ALP); steatosis, inflammation and hepatocellular ballooning compared to the control group. Vitamin E treatment in adult NASH was also associated with reduction of fibrosis[242]. Concerns have been raised, however, on the long-term safety of Vitamin E and the lack of data in patients with T2D and cirrhosis. A network meta-analysis found that pentoxyphylline and OCA improve fibrosis, and vitamin E, TZDs, and OCA improve ballooning degeneration in patients with NASH[243]. A subsequent network meta-analysis substantially confirmed these findings by showing that OCA significantly improves fibrosis, while vitamin E and TZDs result in a significant increase in NASH resolution[244]. Table 2 compares the findings from the above two meta-analytic studies[243,244].
| Sawangjit et al[244] | Singh et al[243] | |
| Fibrosis | OCA1 improves fibrosis.PTX2, TZDs plus Metformin2, TZDs plus Losartan2 might potentially be effective. | PTX1 and OCA1 improve fibrosis |
| Resolution of NASH | TZDs1 and Vitamin E1 use is associated with resolution of NASH | Vitamin E1, TZDs1, and OCA1 improve ballooning degeneration |
| NAS | PTX1, OCA1, vitamin E1, and TZDs1 improve NAS | |
| Steatosis | PTX1, Vitamin E2, TZD1 and OCA2 significantly improve steatosis | |
| Lobular inflammation | PTX1, TZDs1 and OCA2 significantly improve lobular Inflammation |
Further to the above discussed notions on the relationship of the entire NAFLD spectrum with endocrine and metabolic derangements, a line of innovative treatment options can be envisaged. For example, administration of thyroid receptor agonists (GC-1 and KB2115) improves NAFLD in a ob/ob mouse model and a xantine oxidase inhibitor (febuxostat) antagonizes the development of disease in a rodent NASH model[245,246].
Also our understanding of the role of nuclear receptors in the pathogenesis and natural course of NAFLD may lead to novel treatments[15]. The above-mentioned OCA exerts its effects by agonizing FXR. Elafibranor, a dual PPAR-α/δ agonist, has been shown to resolve NASH without fibrosis worsening and to improve the cardiometabolic profile in a recent phase 2 randomized trial of patients with NASH[247]. Oltipraz is a synthetic inhibitor of LXR-α whose antisteatosic properties have previously been shown in a mouse model. A recent phase 2 randomized controlled trial found that, in patients with NAFLD, a 24-wk course of oltipraz significantly reduced intrahepatic fat content assessed with magnetic resonance spectroscopy in a dose-dependent manner; however IR, liver enzymes, lipids and cytokines were unaltered[248].
NAFLD is an emerging multifaceted systemic disease with a heavy epidemiological burden. Further to hepatic risks, NAFLD also affects cardiovascular, metabolic, renal and endocrine systems as well as cancer development with a significant hepatic and extra-hepatic impact on morbidity and mortality[4]. The bi-directional relationship between NAFLD and MetS-T2D is an important emerging topic underpinning the mutual influence and the need to interrupt the vicious circle associating liver and dysmetabolism. However, we now know that IR is a necessary though not sufficient requisite of the development of NAFLD and that gender, genetic background and microbiota also play a major role[4,14]. It is anticipated that pathogenic complexity and multi-factoriality will have to be translated into more finely tailored diagnostic and treatment strategies. This implies that “ad hoc” studies are necessary to more precisely identify the individual target populations to be addressed. Presently, lifestyle changes effected via dietary restrictions and physical exercising remain the only recommended treatment of NAFLD; in all overweight/obese patients, weight loss is mandatory[4]. However, given that only a small minority of such individuals will succeed in maintaining their ideal body weight, and that no currently marketed drugs are specifically licensed for NASH, data of ongoing studies are eagerly awaited to offer these numerous and heterogeneous patients effective and safe tailored drug treatment options.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cordeiro A, Jamali R, Mikolasevic I, Sherif Z, Vanni E S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Petäjä EM, Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int J Mol Sci. 2016;17:pii: E633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, Gasbarrini A, Loguercio C, Lonardo A, Marchesini G. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 4. | Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis. 2017;49:471-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 5. | Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 438] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 6. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7538] [Article Influence: 837.6] [Reference Citation Analysis (0)] |
| 7. | Non-alcoholic Fatty Liver Disease Study Group, Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, Cortez-Pinto H, Grieco A, Machado MV, Miele L, Targher G. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 8. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17:pii: E774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 9. | Giannini EG, Marabotto E, Savarino V, Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F, Caturelli E, Chiaramonte M; Italian Liver Cancer (ITALICA) Group. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 465] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 11. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 12. | Petta S, Valenti L, Bugianesi E, Targher G, Bellentani S, Bonino F; Special Interest Group on Personalised Hepatology of the Italian Association for the Study of the Liver (AISF); Special Interest Group on Personalised Hepatology of Italian Association for Study of Liver AISF. A “systems medicine” approach to the study of non-alcoholic fatty liver disease. Dig Liver Dis. 2016;48:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 14. | Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv Ther. 2017;34:1291-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 411] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 15. | Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Lonardo A. The Role of Nuclear Receptors in the Pathophysiology, Natural Course, and Drug Treatment of NAFLD in Humans. Adv Ther. 2016;33:291-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease - atherosclerosis as a major player in the natural course of NAFLD. Curr Pharm Des. 2013;19:5177-5192. [PubMed] |
| 17. | Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643-654.e1-e9; quiz e39-e40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 1237] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 18. | Lonardo A, Bellentani S, Ratziu V, Loria P. Insulin resistance in nonalcoholic steatohepatitis: necessary but not sufficient - death of a dogma from analysis of therapeutic studies? Expert Rev Gastroenterol Hepatol. 2011;5:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Sookoian S, Pirola CJ. Systematic review with meta-analysis: risk factors for non-alcoholic fatty liver disease suggest a shared altered metabolic and cardiovascular profile between lean and obese patients. Aliment Pharmacol Ther. 2017;46:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Kim D, Kim WR. Nonobese Fatty Liver Disease. Clin Gastroenterol Hepatol. 2017;15:474-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (1)] |
| 21. | Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 505] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 22. | Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 541] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 23. | Ballestri S, Nascimbeni F, Romagnoli D, Baldelli E, Targher G, Lonardo A. Type 2 Diabetes in Non-Alcoholic Fatty Liver Disease and Hepatitis C Virus Infection--Liver: The “Musketeer” in the Spotlight. Int J Mol Sci. 2016;17:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Loria P, Lonardo A, Anania F. Liver and diabetes. A vicious circle. Hepatol Res. 2013;43:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 25. | Valenti L, Bugianesi E, Pajvani U, Targher G. Nonalcoholic fatty liver disease: cause or consequence of type 2 diabetes? Liver Int. 2016;36:1563-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 26. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 804] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 27. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. [PubMed] |
| 28. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 29. | Larrain S, Rinella ME. A myriad of pathways to NASH. Clin Liver Dis. 2012;16:525-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Dongiovanni P, Valenti L. Genetics of nonalcoholic fatty liver disease. Metabolism. 2016;65:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 937] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 32. | Lonardo A, Nascimbeni F, Ponz de Leon M. Nonalcoholic fatty liver disease and COPD: is it time to cross the diaphragm? Eur Respir J. 2017;49:pii: 1700546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Kalia HS, Gaglio PJ. The Prevalence and Pathobiology of Nonalcoholic Fatty Liver Disease in Patients of Different Races or Ethnicities. Clin Liver Dis. 2016;20:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 34. | Betrapally NS, Gillevet PM, Bajaj JS. Changes in the Intestinal Microbiome and Alcoholic and Nonalcoholic Liver Diseases: Causes or Effects? Gastroenterology. 2016;150:1745-1755.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Zhu L, Baker RD, Zhu R, Baker SS. Gut microbiota produce alcohol and contribute to NAFLD. Gut. 2016;65:1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1564] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 37. | Boursier J, Vergniol J, Guillet A, Hiriart JB, Lannes A, Le Bail B, Michalak S, Chermak F, Bertrais S, Foucher J. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol. 2016;65:570-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 38. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3180] [Article Influence: 353.3] [Reference Citation Analysis (4)] |
| 39. | Bugianesi E, Rosso C, Cortez-Pinto H. How to diagnose NAFLD in 2016. J Hepatol. 2016;65:643-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Bedossa P; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 459] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 41. | Brunt EM. Nonalcoholic Fatty Liver Disease: Pros and Cons of Histologic Systems of Evaluation. Int J Mol Sci. 2016;17:pii: E97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 42. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1357] [Article Influence: 104.4] [Reference Citation Analysis (4)] |
| 43. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2293] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 44. | Day CP, Saksena S. Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol. 2002;17 Suppl 3:S377-S384. [PubMed] |
| 45. | Caligiuri A, Gentilini A, Marra F. Molecular Pathogenesis of NASH. Int J Mol Sci. 2016;17:pii: E1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 46. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 47. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2597] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 48. | Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;60:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 49. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 50. | Leamy AK, Egnatchik RA, Young JD. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog Lipid Res. 2013;52:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 51. | Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 644] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 52. | Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126-2132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 592] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 54. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8760] [Article Influence: 461.1] [Reference Citation Analysis (1)] |
| 55. | Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3224] [Cited by in RCA: 3543] [Article Influence: 208.4] [Reference Citation Analysis (0)] |
| 56. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3084] [Article Influence: 237.2] [Reference Citation Analysis (0)] |
| 57. | Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100-1101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 395] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 58. | Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28 Suppl 1:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 59. | Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:7381-7391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 252] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 60. | Wree A, Marra F. The inflammasome in liver disease. J Hepatol. 2016;65:1055-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 928] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 62. | Kitajima Y, Hyogo H, Sumida Y, Eguchi Y, Ono N, Kuwashiro T, Tanaka K, Takahashi H, Mizuta T, Ozaki I, Eguchi T, Kimura Y, Fujimoto K, Anzai K; Japan Nonalcoholic Fatty Liver Disease Study Group (JSG-NAFLD). Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol. 2013;28:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, Kang ES, Han KH, Lee HC, Cha BS. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008-2011). J Hepatol. 2015;63:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 64. | Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, Lee KL, Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 65. | Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, Marchesini G, Craxì A. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 66. | Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, Han CK, Zhuang XJ, Lu Y, Li XJ. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 67. | Arias-Loste MT, Ranchal I, Romero-Gómez M, Crespo J. Irisin, a link among fatty liver disease, physical inactivity and insulin resistance. Int J Mol Sci. 2014;15:23163-23178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, Alexander GJ, Piguet AC, Anty R. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 461] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 69. | Anstee QM, Day CP. The Genetics of Nonalcoholic Fatty Liver Disease: Spotlight on PNPLA3 and TM6SF2. Semin Liver Dis. 2015;35:270-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 70. | Bhadoria AS, Kedarisetty CK, Bihari C, Kumar G, Jindal A, Bhardwaj A, Shasthry V, Vyas T, Benjamin J, Sharma S. Impact of family history of metabolic traits on severity of non-alcoholic steatohepatitis related cirrhosis: A cross-sectional study. Liver Int. 2017;37:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Wilson PA, Gardner SD, Lambie NM, Commans SA, Crowther DJ. Characterization of the human patatin-like phospholipase family. J Lipid Res. 2006;47:1940-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 72. | Naik A, Košir R, Rozman D. Genomic aspects of NAFLD pathogenesis. Genomics. 2013;102:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Browning JD. Common genetic variants and nonalcoholic Fatty liver disease. Clin Gastroenterol Hepatol. 2013;11:1191-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 75. | Hernaez R, McLean J, Lazo M, Brancati FL, Hirschhorn JN, Borecki IB, Harris TB; Genetics of Obesity-Related Liver Disease (GOLD) Consortium, Nguyen T, Kamel IR, Bonekamp S, Eberhardt MS, Clark JM, Kao WH, Speliotes EK. Association between variants in or near PNPLA3, GCKR, and PPP1R3B with ultrasound-defined steatosis based on data from the third National Health and Nutrition Examination Survey. Clin Gastroenterol Hepatol. 2013;11:1183-1190.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 76. | Takaki A, Kawai D, Yamamoto K. Molecular mechanisms and new treatment strategies for non-alcoholic steatohepatitis (NASH). Int J Mol Sci. 2014;15:7352-7379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 77. | Severson TJ, Besur S, Bonkovsky HL. Genetic factors that affect nonalcoholic fatty liver disease: A systematic clinical review. World J Gastroenterol. 2016;22:6742-6756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 78. | Trépo E, Nahon P, Bontempi G, Valenti L, Falleti E, Nischalke HD, Hamza S, Corradini SG, Burza MA, Guyot E. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology. 2014;59:2170-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 79. | Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 930] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 80. | Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, Motta BM, Kaminska D, Rametta R, Grimaudo S. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 418] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 81. | Holmen OL, Zhang H, Fan Y, Hovelson DH, Schmidt EM, Zhou W, Guo Y, Zhang J, Langhammer A, Løchen ML. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat Genet. 2014;46:345-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 82. | Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen AC, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L; Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network-Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1703] [Cited by in RCA: 1477] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 83. | Luukkonen PK, Zhou Y, Nidhina Haridas PA, Dwivedi OP, Hyötyläinen T, Ali A, Juuti A, Leivonen M, Tukiainen T, Ahonen L. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J Hepatol. 2017;67:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 84. | Musso G, Cipolla U, Cassader M, Pinach S, Saba F, De Michieli F, Paschetta E, Bongiovanni D, Framarin L, Leone N. TM6SF2 rs58542926 variant affects postprandial lipoprotein metabolism and glucose homeostasis in NAFLD. J Lipid Res. 2017;58:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219-1230.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 86. | Luukkonen PK, Zhou Y, Hyötyläinen T, Leivonen M, Arola J, Orho-Melander M, Orešič M, Yki-Järvinen H. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2016;65:1263-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 87. | Conus F, Rabasa-Lhoret R, Péronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Deurenberg-Yap M, Deurenberg P. Is a re-evaluation of WHO body mass index cut-off values needed? The case of Asians in Singapore. Nutr Rev. 2003;61:S80-S87. [PubMed] |
| 89. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8272] [Article Influence: 393.9] [Reference Citation Analysis (0)] |
| 90. | Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, Chan HL, Chim AM, Woo J, Chu WC. Prevalence and Severity of Nonalcoholic Fatty Liver Disease in Non-Obese Patients: A Population Study Using Proton-Magnetic Resonance Spectroscopy. Am J Gastroenterol. 2015;110:1306-1314; quiz 1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 91. | Wattacheril J, Sanyal AJ. Lean NAFLD: An Underrecognized Outlier. Curr Hepatol Rep. 2016;15:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 92. | Stender S, Kozlitina J, Nordestgaard BG, Tybjærg-Hansen A, Hobbs HH, Cohen JC. Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. Nat Genet. 2017;49:842-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 93. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-397.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2231] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 94. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1703] [Article Influence: 170.3] [Reference Citation Analysis (1)] |
| 95. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 1431] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 96. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. [PubMed] |
| 97. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2887] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 98. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8239] [Article Influence: 412.0] [Reference Citation Analysis (5)] |
| 99. | Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 648] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 100. | Nascimbeni F, Lebray P, Fedchuk L, Oliveira CP, Alvares-da-Silva MR, Varault A, Ingiliz P, Ngo Y, de Torres M, Munteanu M. Significant variations in elastometry measurements made within short-term in patients with chronic liver diseases. Clin Gastroenterol Hepatol. 2015;13:763-771.e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 102. | Bedossa P, Patel K. Biopsy and Noninvasive Methods to Assess Progression of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150:1811-1822.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 103. | Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 997] [Cited by in RCA: 953] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 104. | Ekstedt M, Franzén LE, Mathiesen UL, Kechagias S. Low clinical relevance of the nonalcoholic fatty liver disease activity score (NAS) in predicting fibrosis progression. Scand J Gastroenterol. 2012;47:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 105. | Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, Goodman Z. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 477] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 106. | Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Targher G, Lonardo A. Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/histological parameters in various liver diseases. Metabolism. 2017;72:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 107. | Ballestri S, Lonardo A, Romagnoli D, Carulli L, Losi L, Day CP, Loria P. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32:1242-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 108. | Ballestri S, Romagnoli D, Nascimbeni F, Francica G, Lonardo A. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol. 2015;9:603-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 109. | Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, Duarte-Rojo A, Wong D, Crotty P, Elkashab M. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 110. | de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 111. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 838] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 112. | Petta S, Maida M, Macaluso FS, Di Marco V, Cammà C, Cabibi D, Craxì A. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015;62:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 113. | Petta S, Wong VW, Cammà C, Hiriart JB, Wong GL, Marra F, Vergniol J, Chan AW, Di Marco V, Merrouche W. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017;65:1145-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 114. | Nasr P, Forsgren MF, Ignatova S, Dahlström N, Cedersund G, Leinhard OD, Norén B, Ekstedt M, Lundberg P, Kechagias S. Using a 3% Proton Density Fat Fraction as a Cut-Off Value Increases Sensitivity of Detection of Hepatic Steatosis, Based on Results From Histopathology Analysis. Gastroenterology. 2017;153:53-55.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 115. | Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 359] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 116. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 543] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 117. | Pavlides M, Banerjee R, Sellwood J, Kelly CJ, Robson MD, Booth JC, Collier J, Neubauer S, Barnes E. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol. 2016;64:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 118. | Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol. 2013;58:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 119. | Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1238] [Cited by in RCA: 2035] [Article Influence: 107.1] [Reference Citation Analysis (0)] |
| 120. | Gastaldelli A, Kozakova M, Højlund K, Flyvbjerg A, Favuzzi A, Mitrakou A, Balkau B; RISC Investigators. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 121. | Calori G, Lattuada G, Ragogna F, Garancini MP, Crosignani P, Villa M, Bosi E, Ruotolo G, Piemonti L, Perseghin G. Fatty liver index and mortality: the Cremona study in the 15th year of follow-up. Hepatology. 2011;54:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 122. | Guiu B, Crevisy-Girod E, Binquet C, Duvillard L, Masson D, Lepage C, Hamza S, Krausé D, Verges B, Minello A. Prediction for steatosis in type-2 diabetes: clinico-biological markers versus 1H-MR spectroscopy. Eur Radiol. 2012;22:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Cuthbertson DJ, Weickert MO, Lythgoe D, Sprung VS, Dobson R, Shoajee-Moradie F, Umpleby M, Pfeiffer AF, Thomas EL, Bell JD. External validation of the fatty liver index and lipid accumulation product indices, using 1H-magnetic resonance spectroscopy, to identify hepatic steatosis in healthy controls and obese, insulin-resistant individuals. Eur J Endocrinol. 2014;171:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 124. | McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 681] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 125. | Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782-789.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 400] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 126. | Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 627] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 127. | Perazzo H, Munteanu M, Ngo Y, Lebray P, Seurat N, Rutka F, Couteau M, Jacqueminet S, Giral P, Monneret D. Prognostic value of liver fibrosis and steatosis biomarkers in type-2 diabetes and dyslipidaemia. Aliment Pharmacol Ther. 2014;40:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, Oliveira CP, Francque S, Van Gaal L, Schattenberg JM. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am J Gastroenterol. 2017;112:740-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 650] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 129. | Demir M, Lang S, Nierhoff D, Drebber U, Hardt A, Wedemeyer I, Schulte S, Quasdorff M, Goeser T, Töx U. Stepwise combination of simple noninvasive fibrosis scoring systems increases diagnostic accuracy in nonalcoholic fatty liver disease. J Clin Gastroenterol. 2013;47:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 130. | Petta S, Vanni E, Bugianesi E, Di Marco V, Cammà C, Cabibi D, Mezzabotta L, Craxì A. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2015;35:1566-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 131. | Panera N, Gnani D, Crudele A, Ceccarelli S, Nobili V, Alisi A. MicroRNAs as controlled systems and controllers in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15079-15086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, Garrett ME, Ashley-Koch A, Suzuki A, Tillmann HL. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 312] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 133. | Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 134. | Anjani K, Lhomme M, Sokolovska N, Poitou C, Aron-Wisnewsky J, Bouillot JL, Lesnik P, Bedossa P, Kontush A, Clement K. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J Hepatol. 2015;62:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 135. | Alonso C, Fernández-Ramos D, Varela-Rey M, Martínez-Arranz I, Navasa N, Van Liempd SM, Lavín Trueba JL, Mayo R, Ilisso CP, de Juan VG. Metabolomic Identification of Subtypes of Nonalcoholic Steatohepatitis. Gastroenterology. 2017;152:1449-1461.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 136. | Wood GC, Chu X, Argyropoulos G, Benotti P, Rolston D, Mirshahi T, Petrick A, Gabrielson J, Carey DJ, DiStefano JK. A multi-component classifier for nonalcoholic fatty liver disease (NAFLD) based on genomic, proteomic, and phenomic data domains. Sci Rep. 2017;7:43238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 137. | Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, Lundbom N, Rissanen A, Ridderstråle M, Groop L. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 601] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 138. | Lonardo A, Bagni A, Tarugi P, Loria P. The wide spectrum of steatohepatitis: a report of four cases and a review of the literature. Eur J Gastroenterol Hepatol. 2004;16:1043-1050. [PubMed] |
| 139. | Lonardo A, Loria P, Leonardi F, Borsatti A, Neri P, Pulvirenti M, Verrone AM, Bagni A, Bertolotti M, Ganazzi D. ST.E.N.A. Study Group. Policentrica Steatosi Epatica Non Alcolica. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig Liver Dis. 2002;34:204-211. [PubMed] |
| 140. | Nascimbeni F, Loria P, Ratziu V. Non-alcoholic fatty liver disease: diagnosis and investigation. Dig Dis. 2014;32:586-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 141. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] |
| 142. | Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 143. | Lonardo A. Fatty liver and nonalcoholic steatohepatitis. Where do we stand and where are we going? Dig Dis. 1999;17:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 198] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 144. | Ballestri S, Nascimbeni F, Romagnoli D, Lonardo A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res. 2016;46:1074-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 145. | Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 146. | Ma J, Hwang SJ, Pedley A, Massaro JM, Hoffmann U, Chung RT, Benjamin EJ, Levy D, Fox CS, Long MT. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 147. | Zhang Y, Zhang T, Zhang C, Tang F, Zhong N, Li H, Song X, Lin H, Liu Y, Xue F. Identification of reciprocal causality between non-alcoholic fatty liver disease and metabolic syndrome by a simplified Bayesian network in a Chinese population. BMJ Open. 2015;5:e008204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 148. | Liu CQ, He CM, Chen N, Wang D, Shi X, Liu Y, Zeng X, Yan B, Liu S, Yang S. Serum uric acid is independently and linearly associated with risk of nonalcoholic fatty liver disease in obese Chinese adults. Sci Rep. 2016;6:38605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 149. | Liu J, Xu C, Ying L, Zang S, Zhuang Z, Lv H, Yang W, Luo Y, Ma X, Wang L. Relationship of serum uric acid level with non-alcoholic fatty liver disease and its inflammation progression in non-obese adults. Hepatol Res. 2017;47:E104-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 150. | Zhao CC, Wang AP, Li LX, Li TT, Chen MY, Zhu Y, Yu TP, Bao YQ, Jia WP. Urine uric acid excretion is associated with nonalcoholic fatty liver disease in patients with type 2 diabetes. J Diabetes Complications. 2016;30:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 151. | Ozcelik F, Yiginer O. The relationship between serum uric acid levels and the major risk factors for the development of nonalcoholic fatty liver disease. Liver Int. 2016;36:768-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 152. | Lombardi R, Pisano G, Fargion S. Role of Serum Uric Acid and Ferritin in the Development and Progression of NAFLD. Int J Mol Sci. 2016;17:548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 153. | Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Biochem. 2016;49:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 154. | Jaruvongvanich V, Ahuja W, Wirunsawanya K, Wijarnpreecha K, Ungprasert P. Hyperuricemia is associated with nonalcoholic fatty liver disease activity score in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:1031-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 155. | Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, Alisi A, Byrne CD. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol. 2017;66:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 156. | Carulli L, Ballestri S, Lonardo A, Lami F, Violi E, Losi L, Bonilauri L, Verrone AM, Odoardi MR, Scaglioni F. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern Emerg Med. 2013;8:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 157. | Siddiqui MS, Fuchs M, Idowu MO, Luketic VA, Boyett S, Sargeant C, Stravitz RT, Puri P, Matherly S, Sterling RK. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol. 2015;13:1000-1008.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 158. | Jiang ZG, Tapper EB, Connelly MA, Pimentel CF, Feldbrügge L, Kim M, Krawczyk S, Afdhal N, Robson SC, Herman MA. Steatohepatitis and liver fibrosis are predicted by the characteristics of very low density lipoprotein in nonalcoholic fatty liver disease. Liver Int. 2016;36:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 159. | Lucero D, Miksztowicz V, Gualano G, Longo C, Landeira G, Álvarez E, Zago V, Brites F, Berg G, Fassio E. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin Chim Acta. 2017;473:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Lonardo A, Romagnoli D. Gamma glutamyl transferase: A novel cardiovascular outfit for an old liver test. Indian J Med Res. 2016;143:4-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 161. | Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, Bertelli C, Fatta E, Bignamini D, Marchesini G. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 162. | Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE; NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 163. | Ryan JD, Armitage AE, Cobbold JF, Banerjee R, Borsani O, Dongiovanni P, Neubauer S, Morovat R, Wang LM, Pasricha SR. Hepatic iron is the major determinant of serum ferritin in NAFLD patients. Liver Int. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 164. | Hagström H, Nasr P, Bottai M, Ekstedt M, Kechagias S, Hultcrantz R, Stål P. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver Int. 2016;36:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 165. | Loria P, Lonardo A, Leonardi F, Fontana C, Carulli L, Verrone AM, Borsatti A, Bertolotti M, Cassani F, Bagni A. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. 2003;48:2173-2181. [PubMed] |
| 166. | Vuppalanchi R, Gould RJ, Wilson LA, Unalp-Arida A, Cummings OW, Chalasani N, Kowdley KV; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol Int. 2012;6:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 167. | Lonardo A, Bellini M, Tondelli E, Frazzoni M, Grisendi A, Pulvirenti M, Della Casa G. Nonalcoholic steatohepatitis and the “bright liver syndrome”: should a recently expanded clinical entity be further expanded? Am J Gastroenterol. 1995;90:2072-2074. [PubMed] |
| 168. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 169. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 170. | Lonardo A, Ballestri S, Targher G, Loria P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:629-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 171. | Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, Erbel R, Blankstein R, Feldman T, Al-Mallah MH. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 172. | Targher G, Mantovani A, Pichiri I, Rigolon R, Dauriz M, Zoppini G, Morani G, Vassanelli C, Bonora E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond). 2013;125:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 173. | Targher G, Valbusa F, Bonapace S, Bertolini L, Zenari L, Rodella S, Zoppini G, Mantovani W, Barbieri E, Byrne CD. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS One. 2013;8:e57183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 174. | Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 175. | Rossi A, Targher G, Zoppini G, Cicoira M, Bonapace S, Negri C, Stoico V, Faggiano P, Vassanelli C, Bonora E. Aortic and mitral annular calcifications are predictive of all-cause and cardiovascular mortality in patients with type 2 diabetes. Diabetes Care. 2012;35:1781-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 176. | Angulo P. Long-term mortality in nonalcoholic fatty liver disease: is liver histology of any prognostic significance? Hepatology. 2010;51:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 177. | Tilg H, Moschen AR. Mechanisms behind the link between obesity and gastrointestinal cancers. Best Pract Res Clin Gastroenterol. 2014;28:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 178. | Sanna C, Rosso C, Marietti M, Bugianesi E. Non-Alcoholic Fatty Liver Disease and Extra-Hepatic Cancers. Int J Mol Sci. 2016;17:pii: E717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 179. | Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1703] [Article Influence: 212.9] [Reference Citation Analysis (1)] |
| 180. | Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM; Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 368] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 181. | Targher G, Marchesini G, Byrne CD. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: Causal association or epiphenomenon? Diabetes Metab. 2016;42:142-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 182. | Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 183. | Logue J, Walker JJ, Leese G, Lindsay R, McKnight J, Morris A, Philip S, Wild S, Sattar N; Scottish Diabetes Research Network Epidemiology Group. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. 2013;36:887-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 184. | Seo DC, Choe S, Torabi MR. Is waist circumference ≥102/88cm better than body mass index ≥30 to predict hypertension and diabetes development regardless of gender, age group, and race/ethnicity? Meta-analysis. Prev Med. 2017;97:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 185. | Camilleri M, Malhi H, Acosta A. Gastrointestinal Complications of Obesity. Gastroenterology. 2017;152:1656-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 186. | Lionetti L, Mollica MP, Lombardi A, Cavaliere G, Gifuni G, Barletta A. From chronic overnutrition to insulin resistance: the role of fat-storing capacity and inflammation. Nutr Metab Cardiovasc Dis. 2009;19:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 187. | Boden G, Homko C, Barrero CA, Stein TP, Chen X, Cheung P, Fecchio C, Koller S, Merali S. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Sci Transl Med. 2015;7:304re7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 188. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 615] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 189. | Loria P, Marchesini G, Nascimbeni F, Ballestri S, Maurantonio M, Carubbi F, Ratziu V, Lonardo A. Cardiovascular risk, lipidemic phenotype and steatosis. A comparative analysis of cirrhotic and non-cirrhotic liver disease due to varying etiology. Atherosclerosis. 2014;232:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 190. | Yamazaki H, Tsuboya T, Tsuji K, Dohke M, Maguchi H. Independent Association Between Improvement of Nonalcoholic Fatty Liver Disease and Reduced Incidence of Type 2 Diabetes. Diabetes Care. 2015;38:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 191. | Fukuda T, Hamaguchi M, Kojima T, Mitsuhashi K, Hashimoto Y, Ohbora A, Kato T, Nakamura N, Fukui M. Transient remission of nonalcoholic fatty liver disease decreases the risk of incident type 2 diabetes mellitus in Japanese men. Eur J Gastroenterol Hepatol. 2016;28:1443-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 192. | Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418-e426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 359] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 193. | Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1005] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 194. | Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, Leebeek FW, Hofman A, Stricker BH, Castera L. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 195. | Shima T, Uto H, Ueki K, Takamura T, Kohgo Y, Kawata S, Yasui K, Park H, Nakamura N, Nakatou T. Clinicopathological features of liver injury in patients with type 2 diabetes mellitus and comparative study of histologically proven nonalcoholic fatty liver diseases with or without type 2 diabetes mellitus. J Gastroenterol. 2013;48:515-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 196. | Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V; LIDO Study Group. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 384] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 197. | Nakahara T, Hyogo H, Yoneda M, Sumida Y, Eguchi Y, Fujii H, Ono M, Kawaguchi T, Imajo K, Aikata H. Type 2 diabetes mellitus is associated with the fibrosis severity in patients with nonalcoholic fatty liver disease in a large retrospective cohort of Japanese patients. J Gastroenterol. 2014;49:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 198. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 199. | Maurantonio M, Ballestri S, Odoardi MR, Lonardo A, Loria P. Treatment of atherogenic liver based on the pathogenesis of nonalcoholic fatty liver disease: a novel approach to reduce cardiovascular risk? Arch Med Res. 2011;42:337-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 200. | Bhatia L, Scorletti E, Curzen N, Clough GF, Calder PC, Byrne CD. Improvement in non-alcoholic fatty liver disease severity is associated with a reduction in carotid intima-media thickness progression. Atherosclerosis. 2016;246:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 201. | Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2022] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 202. | Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 505] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 203. | Adams LA, Harmsen S, St Sauver JL, Charatcharoenwitthaya P, Enders FB, Therneau T, Angulo P. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol. 2010;105:1567-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 204. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [PubMed] |
| 205. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 618] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 206. | Rinaldi L, Nascimbeni F, Giordano M, Masetti C, Guerrera B, Amelia A, Fascione MC, Ballestri S, Romagnoli D, Zampino R. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J Gastroenterol. 2017;23:1458-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 207. | Ortiz-Lopez C, Lomonaco R, Orsak B, Finch J, Chang Z, Kochunov VG, Hardies J, Cusi K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care. 2012;35:873-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 208. | Hamaguchi E, Takamura T, Sakurai M, Mizukoshi E, Zen Y, Takeshita Y, Kurita S, Arai K, Yamashita T, Sasaki M. Histological course of nonalcoholic fatty liver disease in Japanese patients: tight glycemic control, rather than weight reduction, ameliorates liver fibrosis. Diabetes Care. 2010;33:284-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 209. | Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol. 2014;109:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 210. | Davila JA. Diabetes and hepatocellular carcinoma: what role does diabetes have in the presence of other known risk factors? Am J Gastroenterol. 2010;105:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 211. | Giorda C, Forlani G, Manti R, Mazzella N, De Cosmo S, Rossi MC, Nicolucci A, Russo G, Di Bartolo P, Ceriello A. Occurrence over time and regression of nonalcoholic fatty liver disease in type 2 diabetes. Diabetes Metab Res Rev. 2017;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 212. | Lonardo A, Sookoian S, Pirola CJ, Targher G. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism. 2016;65:1136-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 213. | Targher G, Rossini M, Lonardo A. Evidence that non-alcoholic fatty liver disease and polycystic ovary syndrome are associated by necessity rather than chance: a novel hepato-ovarian axis? Endocrine. 2016;51:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 214. | Targher G, Lonardo A, Rossini M. Nonalcoholic fatty liver disease and decreased bone mineral density: is there a link? J Endocrinol Invest. 2015;38:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 215. | Lonardo A, Lombardini S, Scaglioni F, Ballestri S, Verrone AM, Bertolotti M, Carulli L, Ganazzi D, Carulli N, Loria P. Fatty liver, carotid disease and gallstones: a study of age-related associations. World J Gastroenterol. 2006;12:5826-5833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 216. | Jaruvongvanich V, Sanguankeo A, Upala S. Significant Association Between Gallstone Disease and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:2389-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 217. | Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:1679-1694.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 677] [Article Influence: 84.6] [Reference Citation Analysis (1)] |
| 218. | Lonardo A, Loria P, Carulli N. Concurrent non-alcoholic steatohepatitis and psoriasis. Report of three cases from the POLI.ST.E.N.A. study. Dig Liver Dis. 2001;33:86-87. [PubMed] |
| 219. | Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 220. | Lonardo A, Carani C, Carulli N, Loria P. ‘Endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J Hepatol. 2006;44:1196-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 221. | Loria P, Carulli L, Bertolotti M, Lonardo A. Endocrine and liver interaction: the role of endocrine pathways in NASH. Nat Rev Gastroenterol Hepatol. 2009;6:236-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 222. | Chi HC, Chen SL, Tsai CY, Chuang WY, Huang YH, Tsai MM, Wu SM, Sun CP, Yeh CT, Lin KH. Thyroid hormone suppresses hepatocarcinogenesis via DAPK2 and SQSTM1-dependent selective autophagy. Autophagy. 2016;12:2271-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 223. | Perra A, Plateroti M, Columbano A. T3/TRs axis in hepatocellular carcinoma: new concepts for an old pair. Endocr Relat Cancer. 2016;23:R353-R369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 224. | Frau C, Loi R, Petrelli A, Perra A, Menegon S, Kowalik MA, Pinna S, Leoni VP, Fornari F, Gramantieri L. Local hypothyroidism favors the progression of preneoplastic lesions to hepatocellular carcinoma in rats. Hepatology. 2015;61:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 225. | Bano A, Chaker L, Plompen EP, Hofman A, Dehghan A, Franco OH, Janssen HL, Darwish Murad S, Peeters RP. Thyroid Function and the Risk of Nonalcoholic Fatty Liver Disease: The Rotterdam Study. J Clin Endocrinol Metab. 2016;101:3204-3211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 226. | Hannah WN Jr, Harrison SA. Effect of Weight Loss, Diet, Exercise, and Bariatric Surgery on Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2016;20:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 227. | Katsagoni CN, Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism. 2017;68:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 228. | Sabag A, Way KL, Keating SE, Sultana RN, O’Connor HT, Baker MK, Chuter VH, George J, Johnson NA. Exercise and ectopic fat in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2017;43:195-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 229. | Keating SE, George J, Johnson NA. The benefits of exercise for patients with non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2015;9:1247-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 230. | Demyen M, Alkhalloufi K, Pyrsopoulos NT. Lipid-lowering agents and hepatotoxicity. Clin Liver Dis. 2013;17:699-714, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 231. | Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, Maggioni M, Kakela P, Wiklund O, Mozzi E. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 232. | Nascimbeni F, Aron-Wisnewsky J, Pais R, Tordjman J, Poitou C, Charlotte F, Bedossa P, Poynard T, Clément K, Ratziu V; LIDO study Group. Statins, antidiabetic medications and liver histology in patients with diabetes with non-alcoholic fatty liver disease. BMJ Open Gastroenterol. 2016;3:e000075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 233. | Zhou YY, Zhu GQ, Wang Y, Zheng JN, Ruan LY, Cheng Z, Hu B, Fu SW, Zheng MH. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016;7:21753-21762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 234. | Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 235. | Lonardo A, Loria P. Potential for statins in the chemoprevention and management of hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1654-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 236. | Björkhem-Bergman L, Backheden M, Söderberg Löfdal K. Statin treatment reduces the risk of hepatocellular carcinoma but not colon cancer-results from a nationwide case-control study in Sweden. Pharmacoepidemiol Drug Saf. 2014;23:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 237. | McGlynn KA, Divine GW, Sahasrabuddhe VV, Engel LS, VanSlooten A, Wells K, Yood MU, Alford SH. Statin use and risk of hepatocellular carcinoma in a U.S. population. Cancer Epidemiol. 2014;38:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 238. | Singh S, Singh PP, Roberts LR, Sanchez W. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 239. | He L, Liu X, Wang L, Yang Z. Thiazolidinediones for nonalcoholic steatohepatitis: A meta-analysis of randomized clinical trials. Medicine (Baltimore). 2016;95:e4947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 240. | Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern Med. 2017;177:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 342] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 241. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1470] [Article Influence: 163.3] [Reference Citation Analysis (1)] |
| 242. | Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 243. | Singh S, Khera R, Allen AM, Murad MH, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology. 2015;62:1417-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 244. | Sawangjit R, Chongmelaxme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, Chaiyakunapruk N. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): A PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore). 2016;95:e4529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 245. | Martagón AJ, Lin JZ, Cimini SL, Webb P, Phillips KJ. The amelioration of hepatic steatosis by thyroid hormone receptor agonists is insufficient to restore insulin sensitivity in ob/ob mice. PLoS One. 2015;10:e0122987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 246. | Nakatsu Y, Seno Y, Kushiyama A, Sakoda H, Fujishiro M, Katasako A, Mori K, Matsunaga Y, Fukushima T, Kanaoka R. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol. 2015;309:G42-G51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 247. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 248. | Kim W, Kim BG, Lee JS, Lee CK, Yeon JE, Chang MS, Kim JH, Kim H, Yi S, Lee J. Randomised clinical trial: the efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |