Published online Sep 7, 2017. doi: 10.3748/wjg.v23.i33.6077
Peer-review started: May 12, 2017
First decision: June 5, 2017
Revised: June 23, 2017
Accepted: July 24, 2017
Article in press: July 24, 2017
Published online: September 7, 2017
Processing time: 120 Days and 5.4 Hours
To efficiently replicate the biology and pathogenesis of human esophageal adenocarcinoma (EAC) using the modified Levrat model of end-to-side esophagojejunostomy.
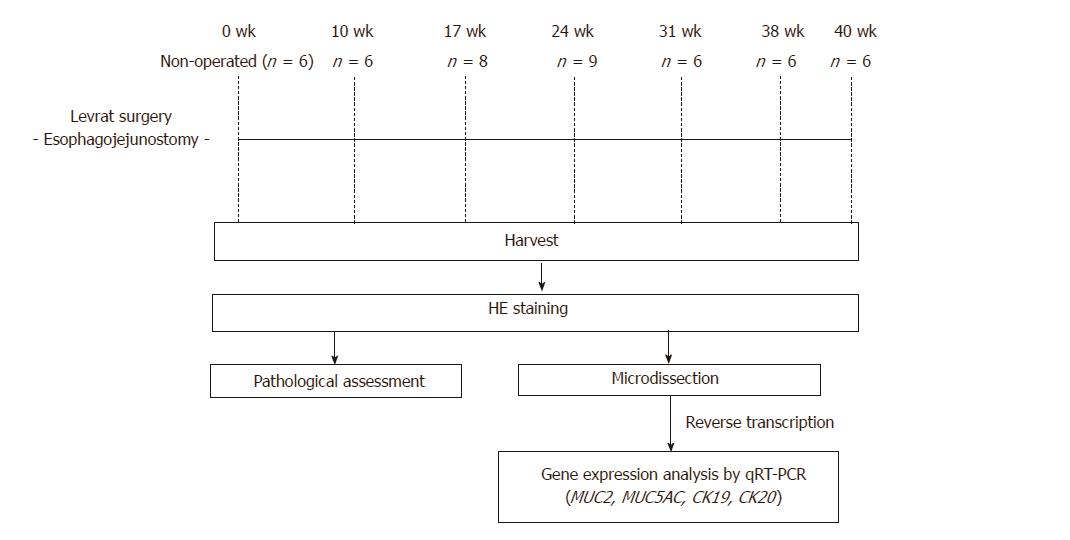
End-to-side esophagojejunostomy was performed on rats to induce gastroduodenoesophageal reflux to develop EAC. Animals were randomly selected and serially euthanized at 10 (n = 6), 17 (n = 8), 24 (n = 9), 31 (n = 6), 38 (n = 6), and 40 (n = 6) wk postoperatively. The esophagi were harvested for downstream histopathology and gene expression. Histological evaluation was completed to determine respective rates of carcinogenic development. Quantitative reverse transcription-polymerase chain reaction was performed to determine gene expression levels of MUC2, CK19, and CK20, and results were compared to determine significant differences throughout disease progression stages.
The overall study mortality was 15%. Causes of mortality included anastomotic leak, gastrointestinal hemorrhage, stomach ulcer perforation, respiratory infection secondary to aspiration, and obstruction due to tumor or late anastomotic stricture. 10 wk following surgery, 100% of animals presented with esophagitis. Barrett’s esophagus (BE) was first observed at 10 wk, and was present in 100% of animals by 17 wk. Dysplasia was confirmed in 87.5% of animals at 17 wk, and increased to 100% by 31 wk. EAC was first observed in 44.4% of animals at 24 wk and increased to 100% by 40 wk. In addition, two animals at 38-40 wk post-surgery had confirmed macro-metastases in the lung/liver and small intestine, respectively. MUC2 gene expression was progressively down-regulated from BE to dysplasia to EAC. Both CK19 and CK20 gene expression significantly increased in a stepwise manner from esophagitis to EAC.
Esophagojejunostomy was successfully replicated in rats with low mortality and a high tumor burden, which may facilitate broader adoption to study EAC development, progression, and therapeutics.
Core tip: The current study reportsrefined surgical techniques with improved tumor burdens for the modified Levrat model of end-to-side esophagojejunostomy in a rat for future in vivo studies of esophageal adenocarcinoma (EAC). For the first time, the model was established with significantly reduced mortality and morbidity and further validated through evaluation of conserved EAC disease progression markers, such as mucin and cytokeratins. The reported approach will allow for broader adoption of the model to allow for greater understanding of the complete disease progression spectrum from Barrett’s esophagus to metastatic EAC and aid in the development of novel therapeutics.
- Citation: Matsui D, Omstead AN, Kosovec JE, Komatsu Y, Lloyd EJ, Raphael H, Kelly RJ, Zaidi AH, Jobe BA. High yield reproducible rat model recapitulating human Barrett’s carcinogenesis. World J Gastroenterol 2017; 23(33): 6077-6087
- URL: https://www.wjgnet.com/1007-9327/full/v23/i33/6077.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i33.6077
Esophageal adenocarcinoma (EAC) incidence has increased dramatically in the western world in recent decades, with a 600% increase seen in the United States since the mid-1970s[1]. Despite recent advances in the development of multimodality patient-centered care plans, EAC still carries a poor prognosis with an overall 5-year survival rate of less than 15%[2]. Due to the increasing incidence and lethality of EAC, a better understanding of the underlying cancer biology and improved methods for prevention and treatment are urgently needed.
Barrett’s esophagus (BE) is induced by chronic gastroesophageal reflux disease (GERD) and is a premalignant precursor to the progression of dysplasia and EAC[3,4]. GERD stimulates the replacement of squamous epithelium of the distal esophagus by intestinal-type metaplastic columnar epithelium, pathologically characterized by goblet cells and the expression of intestinal and differentiation markers, such as mucin genes (MUC2, MUC5AC), cytokeratins (CK7, CK20) and villin[5-9]. Furthermore, several studies have shown that transcription factors involved in the establishment of tissue differentiation, such as CDX2 and SOX2, play a key role in the development of BE and precede morphological changes[10,11]. Although there is great interest the underlying biology of BE carcinogenesis, the exact molecular mechanisms involved in the longitudinal progression to EAC have not been fully elucidated. Therefore, molecular biomarkers that are dysregulated across the progression spectrum would have significant clinical utility for early detection, risk identification, prognosis and therapeutic intervention.
Well-established small animal models efficiently recapitulate human de novo disease progression at the histological and molecular levels, providing clinical utility for development of effective treatment strategies. In 1962, Levrat et al[12] reported a surgical model of esophagoduodenostomy (ED) in rats to induce chronic gastroduodenal reflux. This model was further refined to demonstrate that ED with or without gastrectomy, esophagojejunostomy (EJ) with or without gastrectomy, and pancreatico-esophageal anastomosis could recreate disease progression to EAC without the administration of exogenous carcinogens[13-18]. Additionally, the study of gene expression profiles from BE to EAC confirmed significant homology between human and rat disease[19]. The modified Levrat model of end-to-side EJ with vagal nerve preservation to induce EAC through chronic gastroduodenoesophageal reflux (GDER) currently represents the gold standard of the model and has been extensively utilized to evaluate EAC disease progression and novel therapeutics. Therefore, this surgical approach provides a representative and translatable model for the study of reflux-induced carcinogenesis and downstream genetic alterations associated with EAC.
Through our extensive use of the modified Levrat model over recent years to study EAC, we have successfully optimized our protocols to maximize tumor burden and minimize mortality. The aim of the present study was to describe efficient use of the modified Levrat model through improved surgical techniques, perioperative care, management of complications, and analysis of molecular profiles. We feel the successful broader implementation of a reliable and replicable model that mimics human disease will enable other laboratories to reproduce it effortlessly and advance further research into the pathophysiology and biology of EAC.
This study was conducted with approval from the Institutional Animal Care and Use Committee of Allegheny General Hospital in Pittsburgh, Pennsylvania under Protocol #992. All animals received humane care in compliance with the standards set forth in “The Guide for the Care and Use of Laboratory Animals.” All animals were weighed weekly and euthanized if acute decompensation occurred prior to study endpoint.
The cohort for this study was randomly preassigned from a larger protocol of 225 animals. Mortality and morbidity were calculated from the larger sample set to proportionally reflect the range and incidence rates of causes across sub-studies. The modified Levrat surgery of end-to-side EJ was performed on 200 to 250 g 6-wk to 8-wk-old male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) to induce chronic GDER and the subsequent spectrum of de novo premalignant lesions leading to EAC formation. Animals were randomly selected for serial euthanasia at 10 (n = 6), 17 (n = 8), 24 (n = 9), 31 (n = 6), 38 (n = 6), and 40 (n = 6) wk postoperatively. Additionally, six animals served as controls and were harvested with no surgical intervention. The higher effective n at 17 and 24 wk is reflective of animals that were prematurely sacrificed from the original designated time point due to health considerations. Pathological assessment was performed on all harvested esophageal specimens stained with hematoxylin and eosin (HE). Tissue samples were further evaluated for MUC2, MUC5AC, CK19, and CK20 gene expression using quantitative reverse transcription-polymerase chain reaction (qRT-PCR) to compare each level of disease progression. These markers were selected based on known roles in human esophageal carcinogenesis. Study schema of experimental design is represented in Figure 1.
Preoperative management: Prior to intervention, animals were acclimatized for at least one week and housed on a 12-h alternating light-dark cycle. Standard feed included a solid pellet diet and free access to tap water. One day prior to surgery, rats were provided with a gel-based diet (BioServ, Flemington, NJ; # S5769) to encourage stomach clearing to reduce the risk of anastomotic leak. Animals were nil per os (NPO) for 2-4 h before surgery. For sedation, animals were placed in an acrylic anesthetizing chamber for induction with 5% isoflurane and transferred to a nose cone mask for maintenance with 2% isoflurane in 1 mL/L of oxygen. The anesthetized rats were then placed on a circulating water heating bed to maintain adequate body temperature during surgery and were given prophylactic ketoprofen (3 mg/kg) and enfloxacin (5 mg/kg) immediately prior to surgery.
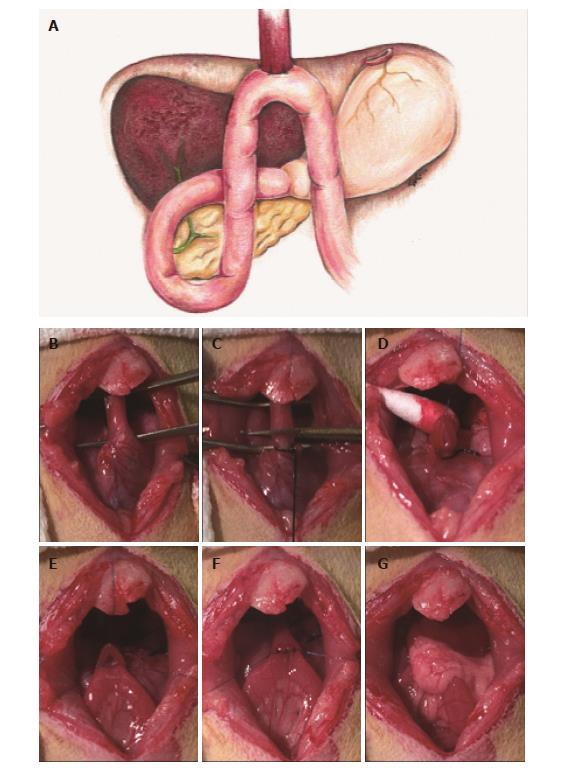
Surgical techniques: All animals underwent an end-to-side EJ with gastric preservation through upper midline abdominal incision. The esophagus was mobilized, preserving the left and right vagus nerves (Figure 2B), and the gastroesophageal junction was ligated using 3-0 silk. A temporary 7-0 prolene suture (Ethicon, Somerville, NJ, United States) was delicately threaded through muscle layer only of the distal esophagus 1 cm above the silk knot to prevent retraction of the esophagus through the diaphragm. Care was taken to minimize tension on the esophagus to prevent recoiling of the mucosal layer into the lumen, and the esophagus was cut just above the gastroesophageal junction (Figure 2C). A loop of jejunum was identified 4 cm distal to the ligaments of Treitz and 3 mm jejunostomy was made using a #11 surgical blade. Residual intestinal fluid in the immediate area was drained using a sterile swab to minimize risk of postoperative abscess formation and infection. An end-to-side anastomosis was constructed between the distal esophagus and jejunum in an antecolic manner with eight interrupted full-thickness 7-0 prolene sutures. Special care was taken to include the esophageal mucosa to ensure adequate mucosal-to-mucosal apposition (Figure 2D-F). The first 5 sutures were placed on the anterior wall, and the entire anastomosis was carefully flipped 180° to expose the posterior wall. Before placing the posterior sutures, patency of the proximal and distal lumen was confirmed. The final 3 sutures were placed, and the anastomosis was returned to anatomical position. This delicate flip allowed all suture knots to remain external to the lumen and minimized anastomotic failure due to postoperative obstruction. The omentum was wrapped around the completed anastomosis to prevent anastomotic leakage (Figure 2G), and the celiotomy was closed using 4-0 vicryl (Ethicon, Somerville, NJ, United States). All rats received a 25-30 mg/kg bolus of Lactated Ringer’s Solution (LRS), were placed on 100% oxygen until awake, and placed in a cage with a raised wire mesh grate to eliminate direct exposure to obstructive bedding. Rats remained NPO for at least 1 h postoperatively to prevent aspiration.
Postoperative management: For the duration of the study, postoperative animals were housed in cages with raised grates to eliminate access to bedding. Any ingestion of foreign material post-surgery results in anastomotic failure and leak or obstruction. Durable plastic huts were provided as enrichment, and rubber stoppers on water bottles were continuously monitored for integrity. Animals continued to receive daily injections of LRS, ketoprofen (3 mg/kg), and enfloxacin (5 mg/kg) until postoperative day 3. Additionally, all rats were placed on a 10-d diet modification plan designed to gradually shift from liquid diet to full solid diet, in an effort to facilitate proper healing of the anastomosis, minimize aspiration-related complication, and encourage feeding after surgery. On postoperative days 0 to 3, rats received a nutritional diluted liquid supplement diet (Ensure; Abbot, Columbus, OH, United States) with acetaminophen (Tylenol®) for pain control. Animals were then transitioned to a gel diet (BioServ, Flemington, NJ; # S5769) (days 4-6), followed by a mushed pellet diet (days 7-9), and then regular solid pellet diet on postoperative day 10. The days on a specific modified diet were extended by 1-2 d if the animal seemed slow to recover from surgery. During the postoperative window, all rats were individually housed to closely monitor health, consumption, and stool production and to reduce chances of wound damage. On day 14, all rats were ear-tagged and pair-housed. At 12 wk, all rats were treated with iron dextran (50 mg/kg, i.m.) every two weeks prophylactically for anemia. All animals received a weight check at least once a week, and during periods of weight loss, rats were weighed more frequently, and modified diets were provided in a supplemental fashion. Animals having more than 45% weight loss or acute decompensation in the postoperative period were sacrificed, and all remaining rats were euthanized at their respective time points for histological evaluation.
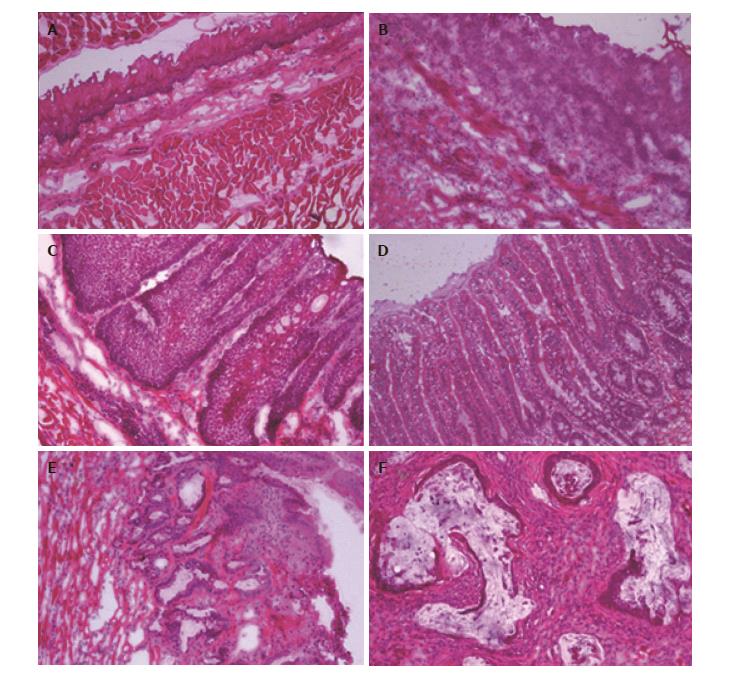
Tissue preparation and pathological assessment: Upon necropsy, the entire esophagus and jejunum, to a length approximately 1 cm distal to the anastomosis was harvested. After the specimen was cut open longitudinally, samples were rinsed in ice-cold phosphate buffered saline to remove debris, oriented to maximize exposure of suspicious areas, and flash frozen in Tissue-Tek Optimal Cutting Temperature (OCT) compound (Sakura Finetek, Torrance, CA; #4583). Next, frozen esophagi in OCT blocks were cut into 5 micron sections using a cryostat (Fisher Scientific, Waltham MA; Microm HM 550) and stained with HE for pathological assessment and gene expression analysis. Two experienced pathology experts independently performed the histological analysis to identify areas of disease. Histological changes were defined on the basis of the following established classification criteria: (1) esophagitis: intraepithelial inflammation, thick basal cell layer, elongated lamina propria papillae, and spongiosis; (2) proliferative hyperplasia: Increased thickness of the squamous epithelium (sometimes hyperkeratotic) with no cellular atypia; (3) BE: Replacement of normal esophageal squamous epithelium with columnar-lined epithelium containing goblet cells; (4) dysplasia: dysplastic squamous cell epithelium with enlarged, atypical nuclei and an increased number of mitotic figures, which may invade lamina propria of the epithelium but does not invade the submucosal layer; and (5) EAC: Mucinous, dysplastic glandular cell growth with both atypia and invasion through the basement membrane.
In order to evaluate the expression of a subset of four biomarkers at each esophageal disease level, qRT-PCR was performed on macrodissected esophageal tissues, including 3 normal esophageal epithelium (controls), 5 esophagitis, 5 BE, 11 dysplasia, 19 EAC and 2 EAC (primary profiled) with metastasis. Briefly, two experienced pathology experts independently confirmed areas of the highest disease for each sample and marked HE slides for macrodissection. Based on the marked areas, 200 μmol/L of tissue was macrodissected using a cryostat, and special care was taken to ensure all collections were highly representative of the disease states. RNA was isolated from the tissue using a miRNeasy kit (Qiagen, Valencia, CA; #217004), and a reverse transcription reaction was performed using a RT2 first strand kit (Qiagen, Valencia, CA, United States; #330401), according to the manufacturer’s protocol. qRT-PCR was performed with the RT2 SYBR Green ROX qPCR MasterMix (Qiagen, Valencia, CA, United States; #330523) in a total volume of 25 μL using the following RT2 Primer Assays: MUC2 (Qiagen, Valencia, CA, United States; #PPR69984A), MUC5AC (Qiagen, Valencia, CA, United States; #PPR59660B), CK19 (Qiagen, Valencia, CA, United States; #PPR44322A) and CK20 (Qiagen, Valencia, CA, United States; #PPR44539A). Real-time PCR reactions were conducted at 95 °C for 15 min, followed by 40 cycles of 94 °C for 15 s, 55 °C for 30 s, and 70 °C for 30 s, using a StepOnePlus real-time quantitative system (Applied Biosystems; Carlsbad, CA, United States). Raw data was exported from the real-time instrument software and relative gene expression was calculated using the ∆∆-Ct method. B-Actin (Qiagen, Valencia, CA, United States; #PPR0650C-200) and RPLP1 (Qiagen, Valencia, CA, United States; #PPR42363C-200) were selected as endogenous controls. All samples were normalized against pathologically confirmed normal squamous esophagus and run in technical triplicates.
Statistical analysis was conducted by a biomedical statistician using SPSS software (IBM, Armonk, NY, United States; Version 23). The gene expression levels in normal squamous esophagus, esophagitis, BE, dysplasia, and EAC tissues were compared by an independent 2-tailed t test to identify significant differences in expressions. A P < 0.05 was considered to be statistically significant.
Fifteen percent of the operated animals died before the intended endpoint of the experiment, and necropsies were performed on all euthanized and found dead animals to identify the cause of death. Of these, 23.5% animals died within 2 wk post-surgery due to surgical and procedure-related complications, such as anastomotic leaks and continuous gastrointestinal hemorrhage. An additional 26.5% of the animal deaths were between 2-10 wk following surgery, with the major cause of death being stomach ulcer perforation. The remaining 50% of animal deaths were 10 wk after surgery, and the major causes of mortality were attributed to respiratory infection secondary to aspiration, stomach ulcer perforation, and obstruction due to tumor or late anastomotic stricture. Overall, the anastomotic leak rate for the study was 2.2%. A randomized cohort of 47 animals was preselected and utilized for histopathology and gene expression. The effective numbers of rats examined for study endpoints were as follows: non-operated (n = 6), 10 wk (n = 6), 17 wk (n = 8), 24 wk (n = 9), 31 wk (n = 6), 38 wk (n = 6), and 40 wk (n = 6) after surgery.
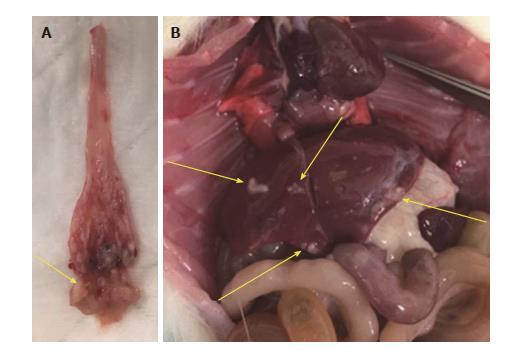
The results of histological findings for each disease level at each time point are shown in Table 1. All animals that underwent EJ showed histological features of esophagitis and proliferative hyperplasia from 10 wk following surgery (Figure 3B). BE was observed as early as 10 wk (16.7%; 1/6 animals) following surgery, and 100% of animals at 17, 31, and 38 wk showed the presence of BE (Figure 3C). Dysplasia was first recognized at 17 wk post-surgery (87.5%; 7/8 animals), and the incidence increased over time to 100% at 31-40 wk (Figure 3D). EAC was first observed at 24 wk (44.4%; 4/9 animals) post-surgery and sequentially increased to 100% at 40 wk. All of the neoplastic cases were localized to the esophagus just above the esophagojejunal anastomosis. Five of 6 (83.3%) EAC cases at 40 wk were well-differentiated mucinous carcinomas (Figure 3E). In addition, two animals at 38-40 wk post-surgery had confirmed macro-metastases in the lung/liver and small intestine, respectively (Figure 4).
| Histology | Postoperative wk | ||||||
| 0 wk | 10 wk | 17 wk | 24 wk | 31 wk | 38 wk | 40 wk | |
| n = 6 | n = 6 | n = 8 | n = 9 | n = 6 | n = 6 | n = 6 | |
| Esophagitis | 0 (0) | 6 (100) | 8 (100) | 9 (100) | 5 (83.3) | 5 (83.3) | 6 (100) |
| Proliferative hyperplasia | 0 (0) | 6 (100) | 8 (100) | 9 (100) | 6 (100) | 6 (100) | 6 (100) |
| Barrett's esophagus | 0 (0) | 1 (16.7) | 8 (100) | 8 (88.9) | 6 (100) | 6 (100) | 5 (83.3) |
| Dysplasia | 0 (0) | 0 (0) | 7 (87.5) | 8 (88.9) | 6 (100) | 6 (100) | 6 (100) |
| Adenocarcinoma | 0 (0) | 0 (0) | 0 (0) | 4 (44.4) | 4 (66.7) | 5 (83.3) | 6 (100) |
| Metastasis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 1 (16.7) |
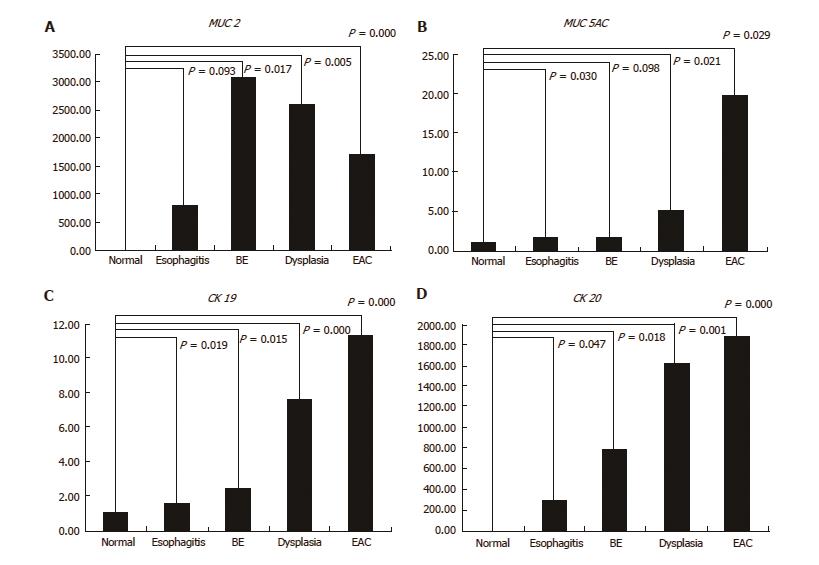
The relative MUC2 gene expression was highest in BE, and it was progressively down-regulation across the BE-dysplasia-adenocarcinoma spectrum. When compared to normal squamous esophagus, BE, dysplasia, and EAC had significantly higher expression levels of MUC2 (P = 0.017, 0.005 and < 0.001, respectively). Additionally, there was significant difference in MUC2 gene expression between esophagitis and BE (P = 0.028) (Figure 5A). The relative MUC5AC gene expression was highest in EAC and lowest in BE among esophageal disease types. Esophagitis, dysplasia, and EAC had significantly higher expression levels of MUC5AC, compared to normal squamous esophagus (P = 0.03, 0.021, and 0.029, respectively) (Figure 5B). Both CK19 and CK20 gene expression increased in a stepwise manner from esophagitis to EAC. All four histological stages (esophagitis, BE, dysplasia, and EAC) had significantly higher CK19 and CK20 expression levels than normal squamous esophagus (CK19: P = 0.019, 0.015, < 0.001 and < 0.001, respectively; CK20: P = 0.047, 0.018, 0.001 and < 0.001, respectively) (Figure 5C and D).
The present study describes a detailed surgical technique and efficient perioperative management for the modified Levrat surgery of end-to-side esophagojejunostomy with gastric preservation. Implementation of the outlined standards provided maximal utilization of the model to produce a high tumor burden balanced with minimal mortality. Additionally, successful management of perioperative complications allowed us to extend the postoperative window to 40 wk to evaluate well-differentiated EAC and metastasis.
The overall mortality rate in this study was 15%, with 23.5% of the total mortality within the first 2 wk post-surgery, resulting in an overall procedure-related mortality of only 3.5%. This rate was lower than that reported by other investigators for the modified Levrat model[20,21]. Improved and aseptic surgical techniques and perioperative managements may have also contributed to the reduction of operative mortality. Of note, taking special care to include the esophageal mucosa when constructing the anastomosis was essential to obtain adequate mucosal-to-mucosal apposition. Additionally, covering the anastomotic site with omentum minimized the risk of anastomotic leakage post-surgery. A postoperative progressive modified diet plan greatly contributed to the protection of the surgical site for the first 10 d after surgery to minimize leak rate. Additionally, supplemental diets provided during periods of weight loss or illness minimized acute morbidity, as anastomotic stricture or tumor growth presented a significant risk factor for obstruction.
As shown in the present study, the rat reflux model induced by EJ displayed sequential changes in the esophageal epithelium at the site of anastomosis and distal esophagus, similar to those seen in human esophagus. Dysplastic changes were first recognized near the anastomosis in 87.5% rats at 17 wk post-surgery. Additionally, 83.3% (5/6 animals) and 100% (6/6 animals) of the animals evaluated at 38 wk and 40 wk post-surgery showed histological evidence of EAC at the site of anastomosis with an adjacent area of dysplastic BE, respectively. The overall incidence of EAC reported in this study was higher compared to other previously reported rates of only 17.4% at 30 wk and 74% at 40 wk for the rat surgical reflux model[22,23]. This difference is likely a direct result of improved surgical techniques and successful management of health complications that allowed for the extension of the perioperative window to 40 wk. Additionally, frequent iron injections may enhance esophageal carcinogenesis by increasing oxidative stress[24]. In other words, oxidase damage could be a contributing factor in the formation of EAC in the rat reflux model, and may similarly occur in human patients with GERD and iron overload. Moreover, the high incidence of EAC and the presence of distant metastasis at 38-40 wk post-surgery may indicate that the 38-40 wk time point is ideal for the study of EAC progression and metastasis in the modified Levrat model.
Since Levrat and colleagues first described the surgically induced reflux model of esophagitis by performing ED in rats, it has been extensively used to study esophageal carcinogenesis and to evaluate novel preventive and treatment strategies[25-27]. In our previous studies, we have utilized the modified Levrat’s surgical model for studying both chemoprevention and targeted therapy and have demonstrated potent efficacy for multiple cancer mechanistic inhibitors against EAC progression[20,28,29]. For the first time, we demonstrated macro-metastatic lesions originating from the primary tumors in the model[30]. Additionally, we further enhanced the utility of the model through combined magnetic resonance imaging (MRI) and small animal endoscopic biopsy to simultaneously track in vivo tumor volumes and molecular correlates longitudinally[31]. With these advancements, the animal model has been highly efficient, not only to develop preventative and treatment strategies, but also to provide a platform to better understand the molecular mechanisms that affect disease progression from inflammation to EAC.
The identification and validation of molecular biomarkers that modulate expression across the progression spectrum may serve as powerful tools for early detection, risk stratification, prognosis, and the development of treatment strategies for EAC. The present study assessed the expression levels of MUC2, MUC5AC, CK19, and CK20 at each esophageal disease level harvested from surgically induced rat reflux model. Interestingly, compared to normal squamous esophagus and esophagitis, statistically significant MUC2 expression was highest in BE and then progressively down-regulated through the neoplastic sequence.
Mucin genes are expressed in a site specific manner in the human gastrointestinal tract, and all MUC subtypes are aberrantly expressed in Barrett’s metaplasia[32,33]. Although BE had a low MUC5AC expression level in this study, the expression pattern of MUC2 was consistent with reported human esophageal literature[6,7,34]. Furthermore, this study demonstrated a relative stepwise increase in expression of CK19 and CK20 across the EAC progression. This result may support the possible role of these markers for early detection and risk stratification.
Limitations of the study included small sample size and precision of histological macrodissection. Although the sample set utilized for molecular analysis was small, a quantitative RT-PCR approach was utilized to determine gene expression. Additionally, an inherent limitation of macrodissection included possible inadvertent inclusion of heterogenous tissue that could conceal a specific cell-type signature. Therefore, further investigation with a larger sample size, or applying a microdissection technique to isolate a highly-enriched pure cell population may be beneficial.
In summary, the present study displays improved surgical technique and successful perioperative management of the modified Levrat model to enhance the value in context of a low mortality rate and consistently higher disease burden. This will enable broader adoption of the model to facilitate further research into the pathophysiology and biology of EAC. We further demonstrated a unique expression pattern of MUC2, CK19, and CK20 that was relevant to human EAC progression, reinforcing the possible utility as conserved diagnostic molecular markers of BE and EAC.
Samantha Martin for providing statistical support.
Esophageal adenocarcinoma (EAC) is an extremely lethal disease with an overall survival rate of less than 20%. Small animal models are required to efficiently replicate and study the biology and pathogenesis of human EAC.
Currently, the treatment options for EAC are outdated, reflected by lack of improvement in patient outcomes over the last three decades, thereby establishing the need for the development of new and improved therapeutic paradigms. The modified Levrat surgery in a rat model has been previously utilized successfully to accelerate development of such agents for chemoprevention, but significant improvements are required to expand the applicability for treatment of established-disease.
These findings report a validated highly replicable protocol to study EAC carcinogenesis and subsequent utilization for treatment efficacy studies. For the first time, the authors demonstrate a significantly reduced mortality rate and increased tumor burden. Additionally, they further validate the translatable nature of the model through gene expression analysis of conserved markers of human Barrett’s carcinogenesis, such as mucin and cytokeratin.
The reported methodology will allow for more efficient utilization of the model to study EAC disease progression and treatment options, which in turn will be translated to clinical settings to improve patient prognosis.
This is a well-designed basic study. Authors in this study generated modified End-to-side esophagojejunostomy (EJ) rat model. Using this model, authors determine respective rates of carcinogenic development and gene expression levels of MUC2, CK19, and CK20. In order to better understand the underlying biology and prevent and treat EAC, the modified EJ model generated in the present study and the data obtained are important for understand disease progression spectrum from Barrett’s esophagus to metastatic EAC.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dinc T, Wittmann T, Zhao J S- Editor: Gong ZM L- Editor: A E- Editor: Xu XR
| 1. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 397] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 2. | Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63:232-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Cameron AJ, Lomboy CT, Pera M, Carpenter HA. Adenocarcinoma of the esophagogastric junction and Barrett’s esophagus. Gastroenterology. 1995;109:1541-1546. [PubMed] |
| 4. | Appelman HD, Matejcic M, Parker MI, Riddell RH, Salemme M, Swanson PE, Villanacci V. Progression of esophageal dysplasia to cancer. Ann N Y Acad Sci. 2014;1325:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett’s metaplasia. Lancet. 2000;356:2079-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 227] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Chinyama CN, Marshall RE, Owen WJ, Mason RC, Kothari D, Wilkinson ML, Sanderson JD. Expression of MUC1 and MUC2 mucin gene products in Barrett’s metaplasia, dysplasia and adenocarcinoma: an immunopathological study with clinical correlation. Histopathology. 1999;35:517-524. [PubMed] |
| 7. | Arul GS, Moorghen M, Myerscough N, Alderson DA, Spicer RD, Corfield AP. Mucin gene expression in Barrett’s oesophagus: an in situ hybridisation and immunohistochemical study. Gut. 2000;47:753-761. [PubMed] |
| 8. | Regalado SP, Nambu Y, Iannettoni MD, Orringer MB, Beer DG. Abundant expression of the intestinal protein villin in Barrett’s metaplasia and esophageal adenocarcinomas. Mol Carcinog. 1998;22:182-189. [PubMed] |
| 9. | Su Y, Chen X, Klein M, Fang M, Wang S, Yang CS, Goyal RK. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: similarity to human Barrett’s esophagus. Lab Invest. 2004;84:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Moons LM, Bax DA, Kuipers EJ, Van Dekken H, Haringsma J, Van Vliet AH, Siersema PD, Kusters JG. The homeodomain protein CDX2 is an early marker of Barrett’s oesophagus. J Clin Pathol. 2004;57:1063-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Barros R, Pereira D, Callé C, Camilo V, Cunha AI, David L, Almeida R, Dias-Pereira A, Chaves P. Dynamics of SOX2 and CDX2 Expression in Barrett’s Mucosa. Dis Markers. 2016;2016:1532791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Levrat M, Lambert R, Kirshbaum G. Esophagitis produced by reflux of duodenal contents in rats. Am J Dig Dis. 1962;7:564-573. [PubMed] |
| 13. | Fein M, Peters JH, Chandrasoma P, Ireland AP, Oberg S, Ritter MP, Bremner CG, Hagen JA, DeMeester TR. Duodenoesophageal reflux induces esophageal adenocarcinoma without exogenous carcinogen. J Gastrointest Surg. 1998;2:260-268. [PubMed] |
| 14. | Chen X, Yang Gy, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20:1801-1808. [PubMed] |
| 15. | Pera M, Trastek VF, Carpenter HA, Fernandez PL, Cardesa A, Mohr U, Pairolero PC. Influence of pancreatic and biliary reflux on the development of esophageal carcinoma. Ann Thorac Surg. 1993;55:1386-1392; discussion 1392-1393. [PubMed] |
| 16. | Clark GW, Smyrk TC, Mirvish SS, Anselmino M, Yamashita Y, Hinder RA, DeMeester TR, Birt DF. Effect of gastroduodenal juice and dietary fat on the development of Barrett’s esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol. 1994;1:252-261. [PubMed] |
| 17. | Li H, Walsh TN, O’Dowd G, Gillen P, Byrne PJ, Hennessy TP. Mechanisms of columnar metaplasia and squamous regeneration in experimental Barrett’s esophagus. Surgery. 1994;115:176-181. [PubMed] |
| 18. | Miyashita T, Tajima H, Munemoto M, Shah FA, Harmon JW, Watanabe T, Shoji M, Okamoto K, Nakanuma S, Sakai S. Impact of histone deacetylase 1 and metastasis-associated gene 1 expression in esophageal carcinogenesis. Oncol Lett. 2014;8:758-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Cheng P, Gong J, Wang T, Chen J, Liu GS, Zhang R. Gene expression in rats with Barrett’s esophagus and esophageal adenocarcinoma induced by gastroduodenoesophageal reflux. World J Gastroenterol. 2005;11:5117-5122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Gibson MK, Zaidi AH, Davison JM, Sanz AF, Hough B, Komatsu Y, Kosovec JE, Bhatt A, Malhotra U, Foxwell T. Prevention of Barrett esophagus and esophageal adenocarcinoma by smoothened inhibitor in a rat model of gastroesophageal reflux disease. Ann Surg. 2013;258:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Yamashita Y, Homma K, Kako N, Clark GW, Smyrk TC, Hinder RA, Adrian TE, DeMeester TR, Mirvish SS. Effect of duodenal components of the refluxate on development of esophageal neoplasia in rats. J Gastrointest Surg. 1998;2:350-355. [PubMed] |
| 22. | Realdon S, Dassie E, Fassan M, Dall’Olmo L, Hatem G, Buda A, Arcidiacono D, Diamantis G, Zhang H, Greene MI. In vivo molecular imaging of HER2 expression in a rat model of Barrett’s esophagus adenocarcinoma. Dis Esophagus. 2015;28:394-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Miyashita T, Shah FA, Marti GP, Wang J, Bonde P, Gibson MK, Ohta T, Montgomery EA, Duncan M, Harmon JW. Rabeprazole impedes the development of reflux-induced esophageal cancer in a surgical rat model. Dig Dis Sci. 2011;56:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Chen X, Ding YW, Yang Gy, Bondoc F, Lee MJ, Yang CS. Oxidative damage in an esophageal adenocarcinoma model with rats. Carcinogenesis. 2000;21:257-263. [PubMed] |
| 25. | Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265-2270. [PubMed] |
| 26. | Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101-1112. [PubMed] |
| 27. | Buskens CJ, Hulscher JB, van Gulik TM, Ten Kate FJ, van Lanschot JJ. Histopathologic evaluation of an animal model for Barrett’s esophagus and adenocarcinoma of the distal esophagus. J Surg Res. 2006;135:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kosovec JE, Zaidi AH, Kelly LA, Rotoloni CL, Vytlacil C, DiCarlo C, Matsui D, Komatsu Y, Boyd NH, Omstead A. Preclinical Study of AUY922, a Novel Hsp90 Inhibitor, in the Treatment of Esophageal Adenocarcinoma. Ann Surg. 2016;264:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Zaidi AH, Kosovec JE, Matsui D, Omstead AN, Raj M, Rao RR, Biederman RWW, Finley GG, Landreneau RJ, Kelly RJ. PI3K/mTOR Dual Inhibitor, LY3023414, Demonstrates Potent Antitumor Efficacy Against Esophageal Adenocarcinoma in a Rat Model. Ann Surg. 2017;266:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Zaidi AH, Saldin LT, Kelly LA, Bergal L, Londono R, Kosovec JE, Komatsu Y, Kasi PM, Shetty AA, Keane TJ. MicroRNA signature characterizes primary tumors that metastasize in an esophageal adenocarcinoma rat model. PLoS One. 2015;10:e0122375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Kosovec JE, Zaidi AH, Komatsu Y, Kasi PM, Cothron K, Thompson DV, Lynch E, Jobe BA. Establishing magnetic resonance imaging as an accurate and reliable tool to diagnose and monitor esophageal cancer in a rat model. PLoS One. 2014;9:e93694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Audie JP, Janin A, Porchet N, Copin MC, Gosselin B, Aubert JP. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479-1485. [PubMed] |
| 33. | Jass JR. Mucin core proteins as differentiation markers in the gastrointestinal tract. Histopathology. 2000;37:561-564. [PubMed] |
| 34. | Wang KK, Sampliner RE; Practice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 786] [Article Influence: 46.2] [Reference Citation Analysis (1)] |