Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5925
Peer-review started: February 1, 2017
First decision: April 5, 2017
Revised: April 26, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: August 28, 2017
Processing time: 214 Days and 23.6 Hours
To evaluate endoscopic ultrasound (EUS)-guided biopsies for the pretreatment characterization of gastrointestinal stromal tumors (GIST) to personalize the management of patients.
All patients with lesions suspected to be GIST who were referred for EUS-sampling at a tertiary Swedish center were eligible for inclusion 2006-2015. During the observational study phase (2006-2011), routine fine-needle-aspiration (EUS-FNA) was performed. In 2012-2015, we converted to an interventional, randomized protocol with dual sampling EUS-FNA and fine-needle-biopsy-sampling (EUS-FNB) for all lesions. c-KIT- and DOG-1-immunostaining was attempted in all samples and a manual count of the Ki-67-index was performed. FNB-sampled tissue and the resected specimens were subjected to Sanger sequencing of the KIT and platelet-derived growth factor alpha (PDGFRA) genes.
In all, 64 unique patients with GIST were included, and of these, 38 were subjected to pretreatment dual sampling. EUS-FNB had a higher diagnostic sensitivity when compared head-to-head with EUS-FNA (98% vs 58%, P < 0.001) and was more adequate for Ki-67-indexing (Ki-67EUS) (92% vs 40%, P < 0.001). Sequencing of EUS-biopsies was successful in 43/44 (98%) patients, and the mutation profiles (KIT-mutation 73%, PDGFRA-mutation 18%, wild-type 7%) were fully congruent with those detected in the corresponding resected specimens. In imatinib-naïve patients, the Ki-67EUS was comparable with the Ki-67-index in the corresponding surgical specimens (Ki-67SURG) (2.7% vs 2.9%, P = 0.68). In patients treated with neoadjuvant imatinib who also carried mutations indicating sensitivity, the Ki-67EUS was higher than the Ki-67SURG (2.5% vs 0.2%, P = 0.005), with a significant reduction in the Ki-67-index of -91.5% (95%CI: -82.4 to -96.0, P = 0.005).
EUS-guided biopsy sampling is accurate for the pretreatment diagnosis and characterization of GISTs and allows the prediction and evaluation of tumor response to neoadjuvant imatinib therapy.
Core tip: Personalization of the management and treatment of gastrointestinal stromal tumors (GIST) requires an extensive characterization of individual tumors. Information on the tumor proliferation rate and the KIT- and platelet-derived growth factor alpha (PDGFRA)-mutation profile is essential. While endoscopic ultrasound (EUS)-FNA is reported to be suboptimal for the diagnosis of GIST, EUS-guided biopsy sampling (EUS-FNB) has not been evaluated for the characterization of GISTs. This prospective, long-term study showed that EUS-FNB was safe and highly accurate for the pretreatment diagnosis of GISTs, for the sequencing of KIT and PDGFRA, and for the assessment of the tumor proliferation rate (Ki-67-index). By obtaining this information, we managed to guide and evaluate neoadjuvant imatinib therapy in patients with GIST.
- Citation: Hedenström P, Nilsson B, Demir A, Andersson C, Enlund F, Nilsson O, Sadik R. Characterizing gastrointestinal stromal tumors and evaluating neoadjuvant imatinib by sequencing of endoscopic ultrasound-biopsies. World J Gastroenterol 2017; 23(32): 5925-5935
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5925
In personalized medicine, a detailed characterization of tumors is essential for accurate patient management. A gastrointestinal stromal tumor (GIST) is an example of a tumor entity that illustrates the potential for genotype-driven targeted therapy[1]. However, to turn this potential into clinical reality, an extensive characterization of the tumor is needed.
First, GISTs are difficult to diagnose preoperatively. A sufficient quantity of tumor material is required for a conclusive diagnosis, which is reached by immunostaining for c-KIT (CD117), anoctamin 1 (DOG-1), or CD34[2].
Second, the tumor response to the tyrosine kinase inhibitor imatinib depends upon the mutation profile of the individual tumor. The genes commonly mutated in GIST are KIT proto-oncogene receptor tyrosine kinase (KIT) (exon 9, 11, 13 or 17), and less frequently, platelet-derived growth factor alpha (PDGFRA) (exon 12, 14 or 18). Primary resistance, or reduced sensitivity to imatinib, is related to mutations in exon 9, 13, or 17 of KIT, exon 18 of PDGFRA, or to the wild type profile (WT)[3]. Secondary resistance may evolve during imatinib treatment due to additional mutations[4]. Imatinib treatment of GISTs has led to a significant improvement in survival[5,6], and neoadjuvant imatinib is valuable, especially in advanced tumors[7,8]. The mutation profile is also a predictor of overall survival[9].
Third, the prognostic risk of GIST varies from excellent to poor[10]. In resected GIST specimens, the National Institutes of Health prognostic risk classification is used to assess the prognosis based on the tumor size and the tumor proliferation rate (the mitotic index [11]. The Ki-67-index is an alternative indicator of the tumor proliferation rate in GIST as well as in many other tumor entities. The level of the Ki-67-index in GIST strongly correlates with the prognosis[12-16].
Endoscopic ultrasound (EUS) enables the visualization of tumors such as GIST and the sampling with fine-needle aspiration (FNA) for cytology. The analysis of EUS-FNA-samples by mass spectrometry has been shown to facilitate the challenging assessment of cystic pancreatic lesions, a potential precursor of pancreatic adenocarcinoma[17]. In GISTs however, EUS-FNA-samples are often non-diagnostic[18-20], which also leads to an evident lack of prognostic information based on the tumor mutation profile and the tumor proliferation rate. This drawback of EUS-FNA is a major obstacle for the early personalized management of patients with GIST. Confronted with the difficulties in the characterization of GISTs, clinicians have to decide on surgical resection based on the mere suspicion of malignancy and without knowledge of the tumor proliferation rate. Finally, the decision on expensive neoadjuvant imatinib treatment can only be based on probability and not on the actual mutation profile of KIT and PDGFRA.
The primary aim of the study was to evaluate EUS-guided sampling for the diagnosis and the pretreatment characterization of GIST with respect to the tumor proliferation rate and the mutation profile of KIT and PDGFRA. The secondary aim was to evaluate the Ki-67-index in EUS-biopsies and in resected specimens as a marker for individual tumor response to neoadjuvant imatinib therapy.
Sahlgrenska University Hospital is a tertiary center for advanced endoscopy and for the management of GIST in the region of west Sweden (population: 1.6 million). All patients who were referred to the unit for a diagnostic EUS-guided sampling of a suspicious GIST were eligible for inclusion in this prospective study as consecutive subjects. Findings suspected to be GIST were defined as lesions previously detected at gastroscopy or cross-sectional imaging with a probable origin from within the gastric or duodenal wall and with a hypoechoic appearance on ultrasound. Ongoing treatment with imatinib was a criterion for non-eligibility. Subjects were later excluded if the follow-up was consistent with an alternative diagnosis of the suspected lesion or if the GIST diagnosis could never be firmly established by conclusive histopathology including positive immunostaining for c-KIT or DOG-1.
The time frame for this study was February 2006 to December 2015. The medical records and the data from a parallel, prospective study on the long-term outcome of all patients with GIST in the region[15] were used to assess the results of EUS-guided sampling with respect to the clinical follow-up and the surgical outcome.
This study was reviewed and approved by the Regional Ethical Review Board of Gothenburg. Written informed consent was obtained from all patients.
This study is registered at ClinicalTrials. gov. The registration identification number is NCT02360839.
All study subjects were examined by EUS under conscious sedation. Linear echoendoscopes [2006-2012: Pentax EG3830UT (Tokyo, Japan), 2012-: Pentax EG3870UTK] and an ultrasound processor (HI VISON Ascendus, Hitachi, Tokyo, Japan) were used for this purpose. The examinations were performed by the study endosonographer (RS). The tumor location, size, echogenicity, and vascularization were assessed before the optimal sampling route was chosen.
The Baseline Period (2006-2011): During the baseline period (BP), the study design was observational. All patients were prospectively included except for the first nine patients of the study start-up phase.
The suspicious GISTs were sampled at the discretion of the endosonographer with no specific interventional procedure. EUS-FNA was performed with either a 22 G or a 25 G needle (Olympus, Aomori, Japan/Boston Scientific, Spencer, United States/Wilson-Cook Medical, Limerick, Ireland), while a 19 G trucut-needle (TCB) was used (Wilson-Cook Medical) for biopsies.
The Study Period (2012-2015): We designed an interventional study protocol in 2011 and modified the sampling procedure in 2012. From 2012 to 2015 (Study Period, SP), dual sampling was performed on each individual subject using both EUS-FNA for cytology (needles as described above) and EUS-guided core biopsy sampling (EUS-FNB) for histology (22 G Procore or 19 G Procore, Wilson-Cook Medical)[21]. In blocks of four and by using sealed envelopes, the patients were randomized to a first pass with FNA or FNB. This was performed to eliminate the introduction of a bias related to the sampling sequence. Further passes were performed by alternating the needles. A non-necrotic area of the tumors was targeted and sampling was performed by fanning. If the yield was poor, the sampling time and the suction were increased.
The first six subjects of the Study Period underwent EUS-FNB only to accustom the endosonographer to the new sampling technique. With some limitations, a cytotechnician was present for rapid on-site cytology evaluation.
FNA-samples and FNB-biopsies were processed and analyzed as described in the Supplementary Methods.
Representative samples were subjected to immunostaining for Ki-67 as described in the Supplementary Methods. The quality and the adequacy of the FNA-samples and the FNB-biopsies for the assessment of the Ki-67-index were categorized as adequate or non-adequate by the study cytopathologist (AD) and pathologist (ON).
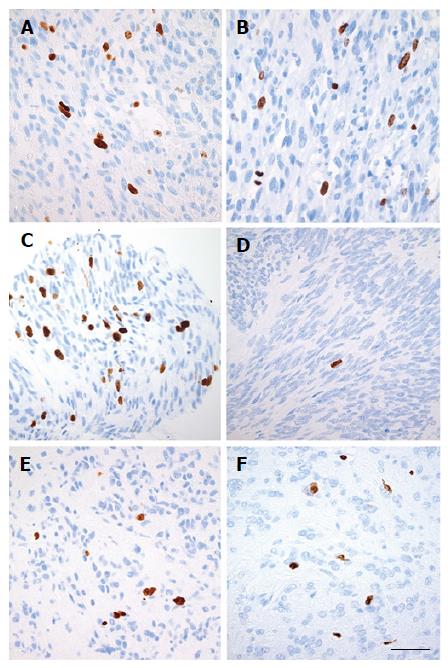
Given the superior quality of the FNB-biopsies compared with FNA-samples, only the Ki-67-index of FNB-biopsies (Ki-67EUS) was calculated in detail on printouts of digital images captured via an x40-magnification objective (Eclipse E1000, Nikon, Japan) with a ProgResC7-camera (Jenoptik, Germany). Manual counting of positive nuclei including 2000 tumor cells was performed. Counting by eyeballing and digital counting are considered less accurate and were not used[22]. The result was recorded as the fraction of positive tumor cells (%). Similarly, the Ki-67-index of the corresponding surgical specimens (Ki-67SURG) was analyzed in subjects who underwent resection.
In each case sampled by EUS-FNB and subjected to surgical resection, we calculated the following parameters: (1) The pairwise difference in the Ki-67-index (%-units): Ki-67DIFF = Ki-67EUS - Ki-67SURG; and (2) The pairwise reduction in the Ki-67-index (%): Ki-67RED = -100 × [1-(Ki-67SURG)/(Ki-67EUS)]
No sequencing of FNA-samples was performed since the sample quantity and quality were poor compared with that of FNB-biopsies. All FNB-biopsies were subjected to mutational analysis by Sanger sequencing as were the corresponding resected specimens (in subjects who underwent resection). In the early part of the SP, the sequencing of FNB-biopsies was performed for research purposes after EUS. In the latter part of the SP, the procedure was implemented into clinical practice and was performed directly after EUS to supply the genetic information to the clinician (BN).
The preparation of FNB-biopsies for DNA-extraction followed by sequencing is described in the Supplementary Methods.
Subjects were followed-up by the clinician (BN) for 5 year or until death. Neoadjuvant imatinib therapy was considered and initiated by the clinician (BN). Patients having small tumors (size < 20 mm) were not evaluated for neoadjuvant imatinib. The cases subjected to surgical resection, either treated or not treated with neoadjuvant imatinib, were designated as: (1) Neo- (no neoadjuvant imatinib therapy); (2) Neo + s (neoadjuvant imatinib and imatinib-sensitive mutation profile); or (3) Neo + r (neoadjuvant imatinib therapy and imatinib-resistant mutation profile) according to the table in the Supplementary Methods. The tumor response was evaluated on a clinical basis in some cases via the comparison of the fluorodeoxyglucose positron emission tomography (18FDG-PET) signal at baseline and at 3-8 wk after the start of imatinib treatment.
Resected specimens were used to validate the diagnosis of GIST. In patients not subjected to surgery, the GIST-diagnosis was considered established if cytopathology or histopathology of tumor sampling was conclusive for GIST including positive immunostaining for KIT or DOG-1.
The FNA-samples and FNB-biopsies were classified as diagnostic only if they contained adequate GIST material for accurate diagnostic KIT or DOG-1 immunostaining. Samples with adequate tumor yield but with failed or inconclusive immunostaining were classified as suggestive of GIST. Samples without adequate tumor yield were considered non-diagnostic.
The primary outcome of this study was the diagnostic sensitivity of EUS-guided sampling for GIST. The secondary outcome was the EUS-sample adequacy (1) for the assessment of the Ki-67-index; (2) for the sequencing of KIT and PDGFRA (FNB-biopsies only); and (3) for the evaluation of response to neoadjuvant imatinib therapy (FNB-biopsies only), which was measured as the difference in the Ki-67-index of FNB-biopsies compared with that of resected specimens.
Demographics, tumor characteristics, and procedures were compared using Fisher’s exact test and the Mann-Whitney U-test. Prior to the interventional phase (the SP), a sample size calculation was performed for paired, dichotomous variables (statistical power = 80%, alpha error = 0.05), which aimed to detect a difference in sensitivity of 35% in order to compare EUS-FNA and EUS-FNB at dual sampling. A sample size of 33 cases was returned.
The diagnostic sensitivity for GIST as a binary outcome was compared between sampling groups using Fisher’s exact test (unpaired data) and McNemar’s test (paired data) in an intention-to-treat analysis. The Ki-67-index of FNB-biopsies and resected specimens was compared using the Wilcoxon signed-rank test. The mutation profile of FNB-biopsies and resected specimens was compared on a case-by-case basis. The (95%CI) was calculated when possible. The statistical significance level was set at P < 0.05. All authors had access to the study data and approved the manuscript. The STARD protocol was applied throughout the study.
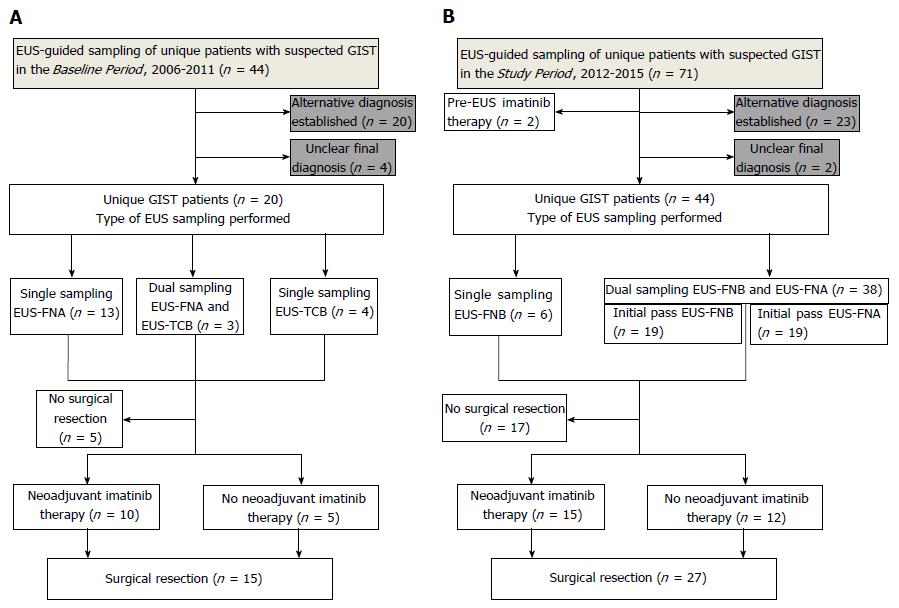
In total, 64 patients [34 women/30 men, median age 70 (range: 23-89)] were included (Figure 1). Validation specimens were available in 43/64 (67%) cases (resected specimen: 42 cases, endoscopy forceps: one case). The baseline characteristics are shown in Table 1.
| Parameter | Baseline period | Study period | P value |
| Age, median (range) | 75 (23-89) | 68 (49-89) | 0.07 |
| Gender (M/F) | 11/9 | 19/25 | 0.43 |
| Study patients (n) | 20 | 44 | |
| Tumor location (n) | |||
| Stomach | 18 | 40 | |
| Duodenum | 2 | 4 | |
| Tumor size (mm), median (range) | 60 (12-200) | 38 (13-220) | 0.29 |
| Tumor endosonographic appearance | |||
| Homogenous (solid) | 8 | 17 | |
| Heterogeneous (necrotic) | 12 | 27 | |
| EUS-FNA (n) | 16 | 38 | |
| Needle (22 G/25 G) | 12/4 | 26/12 | 0.75 |
| Passes (n), median (range) | 2 (1-3) | 3 (1-4) | 0.10 |
| EUS-FNB (n) | 7 | 44 | |
| Needle (TCB 19 G/FNB 19 G/FNB 22 G) | 7/0/0 | 0/5/39 | < 0.001 |
| Passes (n), median (range) | 1 (1-4) | 2 (1-4) | 0.15 |
| ROSE1 | 9 (56) | 26 (68) | 0.53 |
| Study cytologist | 5 (31) | 32 (84) | < 0.001 |
| Study pathologist | 2 (29) | 38 (86) | 0.003 |
| Resected cases | 15 (75) | 27 (61) | 0.40 |
| Resection margin (R0/R1/R2) | 13/1/1 | 24/3/0 | |
| Follow-up time2, mo (range) | 72 (16-105) | 19 (1-45) | |
| Overall survival (OS)3, 12 mo | 20/20 (100) | 31/31 (100) | 1.00 |
| OS, 24 mo | 19/20 (95) | 17/18 (94) | 1.00 |
| OS, 36 mo | 18/20 (90) | 10/11 (91) | 1.00 |
| Patients deceased | 9/20 (45) | 2/44 (5) |
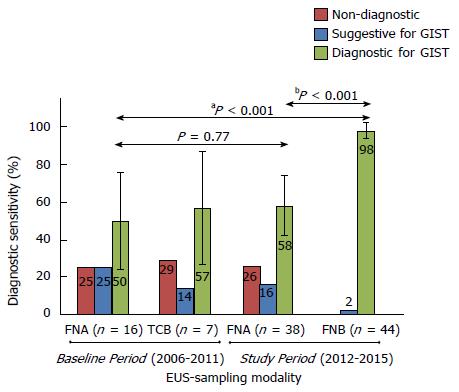
The diagnostic sensitivity of EUS-FNB (dual procedures FNB+FNA: n = 38, single FNB-procedures: n = 6) was superior both compared with routine EUS-FNA performed during the Baseline Period [43/44 (98%) vs 8/16 (50%), aP < 0.001] and compared with EUS-FNA in a head-to-head comparison of dual sampling procedures during the SP [37/38 (97%) vs 22/38 (58%), bP < 0.001], as shown in Figure 2.
Supposing that the two cases in Figure 1, which had an unclear final diagnosis during the Study Period, were actually true GISTs, the worst scenario of the sensitivity of EUS-FNB would still be superior to that of EUS-FNA in a head-to-head comparison (37/40, 93% vs 22/40, 55%, P < 0.001). The sensitivity of FNA-samples was not affected by the recorded variables, as shown in Table 2.
| Parameter | P value | ||
| Tumor echogenicity | Homogenous (solid) | Heterogeneous (necrotic) | |
| EUS-FNA-sensitivity | 11/20 (55) | 18/32 (56) | 1.0 |
| Tumor size | < 30 mm | ≥ 30 mm | |
| EUS-FNA-sensitivity | 10/18 (56) | 19/34 (56) | 1.0 |
| ROSE | ROSE | non-ROSE | |
| EUS-FNA-sensitivity | 21/34 (62) | 8/18 (44) | 0.26 |
| FNA-needle | 22 gauge | 25 gauge | |
| EUS-FNA-sensitivity | 20/37 (54) | 9/15 (60) | 0.77 |
| FNA-passes | < 3 passes | ≥ 3 passes | |
| EUS-FNA-sensitivity | 9/17 (53) | 20/35 (57) | 1.0 |
| Sampling order1 | EUS-FNA first | EUS-FNB first | |
| EUS-FNA-sensitivity | 12/19 (61) | 10/19 (53) | 0.63 |
One minor adverse event was recorded (1/64, complication rate 1.6%). Patient #33, who had a 3-cm GIST in the stomach, experienced local bleeding post-EUS, which was stopped by adrenalin injection. No technical failure was observed for any needle. No tumor seeding was observed in any of the patients during follow-up.
Ki-67-indexing: During the BP, the FNA-samples were of adequate quality for the assessment of the Ki-67-index in 3/16 (19%) cases. In the dual sampling procedures during the SP, FNB-biopsies were more often of adequate quality (37/38, 92%) compared with FNA-samples (15/38, 40%, P < 0.001), and the FNB-biopsies were adequate for the assessment of the Ki-67-index in all cases subjected to surgical resection 27/27 (100%).
In non-resected cases with adequate FNB-biopsies (n = 14), the mean Ki-67EUS was 6.1% (95%CI: 2.5 to 9.7).
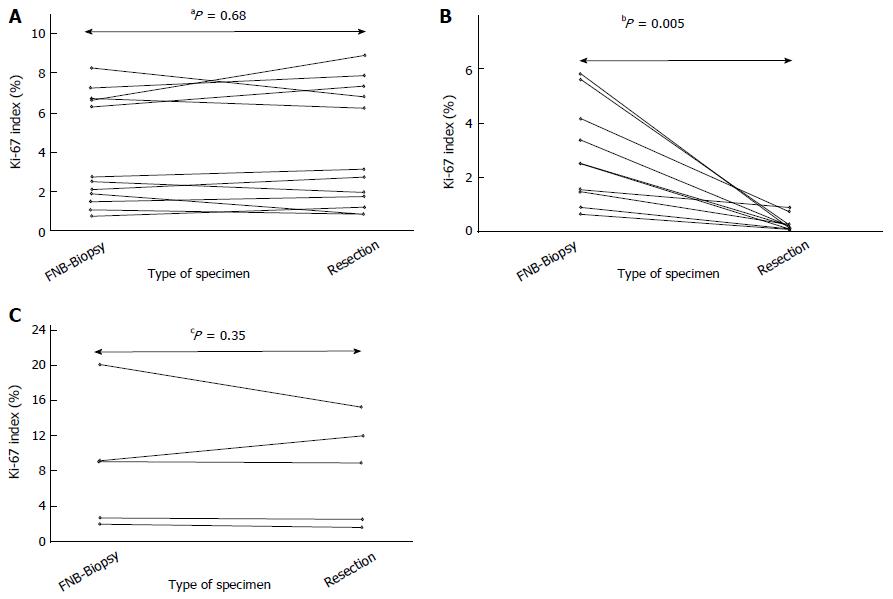
In resected cases not treated with neoadjuvant imatinib (n = 12, Neo- Group), the median Ki-67EUS was not significantly different from the median Ki-67SURG [2.7% vs 2.9%, aP = 0.68, median Ki-67DIFF = -0.30 (95CI: -0.62 to 0.57, P = 0.64)] (Figures 3A, 4A and B). No significant reduction was observed in the Ki-67-index [median Ki-67RED = 10.7% (95%CI: -22.3 to 26.5, P = 0.70)].
Sequencing of KIT and PDGFRA: The FNB-biopsies were adequate for successful Sanger sequencing of KIT and PDGFRA in 43/44 (98%) cases (Table 3). Among resected cases, full congruence (100%) was found in the comparison of the mutations detected in the FNB-biopsies and the mutations detected in the corresponding resected specimens (n = 27). Additional mutations in KIT or PDGFRA were not observed in any of the resected specimens. The sole FNB-biopsy (case #2) with inadequate material for diagnostic immunohistochemistry still contained sufficient material for successful sequencing.
| Case | Mutation gene and exon | Mutation | Treatment neoadj1 | Surgery | Group | EUS-surgery (mo) | Ki67EUS | Ki67SURG | Ki67RED |
| 1 | KIT exon 11 | p.V560del | No | Yes | Neo- | 2 | 2.2 | 2.8 | 26 |
| 2 | PDGFRA exon 18 | p.D842V2 | No | No | NA | - | NC | - | - |
| 3 | Wild Type | Wild Type | Yes | Yes | Neo + r | 2 | 2.1 | 1.7 | -19 |
| 4 | KIT exon 11 | p.V559D | Yes | Yes | Neo + s | 6 | 4.2 | 0.7 | -82 |
| 5 | KIT exon 11 | p.Y553-Q556del | Yes | Yes | Neo + s | 13 | 1.5 | 0.2 | -84 |
| 6 | Unknown | Unknown | No | No | NA | - | NC | - | - |
| 7 | KIT exon 11 | V559D | No | No | NA | - | 2.4 | - | - |
| 8 | KIT exon 11 | p.P577-R586dupl | No | Yes | Neo- | 2 | 6.3 | 7.4 | 17 |
| 9 | KIT exon 11 | V559del | Yes | Yes | Neo + s | 9 | 2.5 | 0.1 | -96 |
| 10 | KIT exon 11 | p.V560D | No | Yes | Neo- | 1 | 1.5 | 1.8 | 17 |
| 11 | KIT exon 11 | p.V560D | No | Yes | Neo- | 2 | 0.8 | 1.2 | 47 |
| 12 | KIT exon 11 | p.V560del | Yes | No | NA | - | 19.3 | - | - |
| 13 | KIT exon 11 | p.V559D | No | Yes | Neo- | 3 | 1.9 | 0.9 | -52 |
| 14 | KIT exon 11 | V559G | Yes | Yes | Neo + s | 16 | 1.6 | 0.9 | -43 |
| 15 | PDGFRA exon 18 | p.D842V | Yes | Yes | Neo + r | 2 | 9.1 | 9.0 | -1 |
| 16 | KIT exon 11 | p.W557G | Yes | Yes | Neo + s | 12 | 0.6 | 0.1 | -93 |
| 17 | KIT exon 11 | p551-W557delinsR | Yes | Yes | Neo + s | 12 | 3.4 | 0.2 | -94 |
| 18 | KIT exon 11 | D579del | No | Yes | Neo- | 2 | 6.7 | 6.3 | -6 |
| 19 | PDGFRA exon 12 | E556-I565dupl | Yes | Yes | Neo + s | 2 | 0.9 | 0.1 | -89 |
| 20 | KIT exon 13 | p K642E | Yes | Yes | Neo + r | 4 | 20.1 | 15.3 | -24 |
| 21 | KIT exon 11 | p.W557R | No | Yes | Neo- | 2 | 7.2 | 7.8 | 9 |
| 22 | KIT exon 11 | V559D | Yes | Yes | Neo + s | 2 | 5.6 | 0.3 | -95 |
| 23 | KIT exon 11 | P551-E554delinsQ | Yes | Yes | Neo + s | 12 | 5.8 | 0.1 | -98 |
| 24 | KIT exon 11 | K558-G565delinsR | Yes | No | NA | - | 21.5 | - | - |
| 25 | PDGFRA exon 18 | p.D842V | No | No | NA | - | 1.5 | - | - |
| 26 | PDGFRA exon 18 | p.D842V | Yes | Yes | Neo + r | 1 | 2.7 | 2.5 | -10 |
| 27 | KIT exon 11 | V559D | No | Yes | Neo- | 3 | 2.8 | 3.1 | 13 |
| 28 | KIT exon 11 | p.V559D | No | Yes | Neo- | 2 | 1.1 | 0.8 | -25 |
| 29 | KIT exon 11 | p.V559D | Yes | Yes | Neo + s | 17 | 2.5 | 0.2 | -90 |
| 30 | KIT exon 11 | pQ575-L576dupl | No | No | NA | - | 2.7 | - | - |
| KIT exon 13 | V654A | ||||||||
| 31 | KIT exon 11 | V559D | No | No | NA | - | 1.4 | - | - |
| 32 | KIT exon 11 | p.L576P | No | No | NA | - | 1.8 | - | - |
| 33 | PDGFRA exon 18 | p.D842V | No | Yes | Neo- | 2 | 6.6 | 8.9 | 35 |
| 34 | PDGFRA exon 18 | p.D846Y | No | Yes | Neo- | 2 | 2.6 | 2.0 | -22 |
| 35 | KIT exon 11 | p P551-W560del | No | No | NA | - | 3.0 | - | - |
| 36 | KIT exon 11 | V560E | Yes | No | NA | - | NC | - | - |
| 37 | Wild type | Wild type | Yes | Yes | Neo + r | 3 | 9.2 | 12.0 | 31 |
| 38 | KIT exon 11 | pL567del | Yes | No | NA | - | 10.1 | - | - |
| 39 | PDGFRA exon 12 | M578-S584del | Yes | No | NA | - | 11.0 | - | - |
| 40 | KIT exon 11 | p.P551-Q556del | Yes | No | NA | - | 4.6 | - | - |
| 41 | KIT exon 11 | p.N567-T574del | No | Yes | Neo- | 1 | 8.3 | 6.8 | -18 |
| 42 | KIT exon 11 | 57-E561del | Yes | No | NA | - | 4.6 | - | - |
| 43 | Wild type | Wild type | No | No | NA | - | 0.1 | - | - |
| 44 | KIT exon 9 | A502-Y503dupl | Yes | No | NA | - | 0.7 | - | - |
Evaluation of neoadjuvant imatinib therapy: (1) Neoadjuvant imatinib + imatinib-sensitive mutation detected (Neo + s Group): In resected patients who were treated with neoadjuvant imatinib and who carried a mutation suggestive of primary sensitivity to imatinib [n = 10: KIT exon 11 (n = 9); PDGFRA exon 12 (n = 1)], the median Ki-67EUS was significantly higher than the median Ki-67SURG [2.5% vs 0.2%, P = 0.005, median Ki-67DIFF = 2.3 (95%CI: 0.67 to 5.37, P = 0.005)] (Figures 3B, 4C and D). Consequently, a significant reduction was observed in the Ki-67-index [median Ki-67RED = -91.5% (95%CI: -82.4 to -96.0, P = 0.005)].
In the five patients with a positive baseline 18FDG-PET, a signal reduction was recorded in the post-treatment 18FDG-PET signal.
(2) Neoadjuvant imatinib + imatinib-resistant mutation detected (Neo + r Group): Five resected patients who were treated with neoadjuvant imatinib carried a mutation suggestive of primary resistance to imatinib [n = 5: PDGFRA exon 18 D842V (n = 2); WT (n = 2); KIT exon 13 p. K642E (n = 1)]. The median Ki-67EUS was not significantly different from the median Ki-67SURG (9.1% vs 9.0%, P = 0.35) (Figures 3C, 4E and F). In addition, no significant reduction was observed in the Ki-67-index (median Ki-67RED = -10.2%, P = 0.50). The baseline 18FDG-PET signal was measured and was positive in two patients. In one patient, no reduction was observed in the post-treatment 18FDG PET-signal, while a weak reduction was recorded in the other (case #20).
This study provides new knowledge on the ability to perform extensive preoperative and pretreatment characterization of gastrointestinal stromal tumors. This knowledge enables the introduction of an early personalized management and treatment of patients with GIST.
According to the results of this work, endoscopic ultrasound-guided biopsy sampling is a safe and accurate method for the purpose of diagnosis and for further analyses of the tumor material in GIST.
A correct and reliable diagnosis of GIST is important to avoid unnecessary resections of benign lesions that are merely suspected GISTs. A non-diagnostic sample will result in uncertain management and a resection based on suspicion alone. Prospective studies that evaluate the accuracy of EUS-guided sampling of GIST are scarce. Studies have reported a diagnostic sensitivity of approximately 50%[18], which is in agreement with the sensitivity of EUS-FNA in our work. A sensitivity of 80% was reported in a recent study that excluded small tumors (< 20 mm)[23].
In the present study, a new method of dual sampling with both EUS-FNA and EUS-FNB was used on all tumors during a 4-year period, and these modalities were compared head-to-head. As a result, we have now shown that EUS-FNB can be used for the reliable and safe diagnosis of GIST in up to 98% of cases including small tumors.
The treatment decision for GIST requires a balance between the benefits and drawbacks of both surgical and pharmacological therapies. The initiation of adjuvant and neoadjuvant treatment can vary in between institutions and the prognostic risk needs to be addressed since there are potential side-effects of imatinib. Nevertheless, the treatment with tyrosine kinase inhibitors should be prescribed only to patients who carry sensitive mutations. Without the mutational status of KIT and PDGRFA, the clinician randomly selects the therapy. The mutation profile is also valuable for the prediction of survival[9].
This study shows that a mutational analysis of pretreatment FNB-biopsies by Sanger sequencing provides the genetic information needed. In the early part of the SP the clinician had to decide on neoadjuvant imatinib therapy without information on the mutational status. Consequently, in five patients who were treated with neoadjuvant imatinib, the sequencing of FNB-biopsies later showed a genetic profile consistent with primary resistance to imatinib, which led to a modification in the treatment regimen. To assist clinicians during the preoperative management of patients with GIST, we implemented the immediate sequencing of FNB-biopsies during the latter part of the SP. One recent retrospective study revealed that it is possible to obtain the mutation profile of GISTs in a selected pool of EUS-FNA-samples using next-generation sequencing[24]. No comparison was made between the sequencing of EUS-FNA-samples and the sequencing of any corresponding resected specimens.
The prognosis of an individual patient with GIST is dependent on the tumor proliferation rate and the size of the tumor[15,25]. In our study, the FNB-biopsies were highly accurate for the precise assessment of the Ki-67-index by manual counting. The Ki-67-index measured in the FNB-biopsies seems reliable since it was in agreement with the Ki-67-index of the resected specimens of the study patients who were not treated with neoadjuvant imatinib. More importantly, in patients who are sensitive to imatinib and who are treated with neoadjuvant imatinib, the Ki-67-index of FNB-biopsies probably better reflects the accurate proliferation rate of tumors compared with the Ki-67-index of resected specimens, which may erroneously be found to be low. A substantial danger of the overestimation of survival and the under-prescription of adjuvant therapy can emerge in these groups of patients. An assessment of the mitotic rate of specimens obtained by FNB-biopsy is probably challenging, and it was not an aim of this study. The maximum quantity of FNB-material obtained in this study reached 40 high-power fields.
The pretreatment assessment of the Ki-67-index has a range of clinical applications. This assessment provides clinicians with prognostic information for a discussion of therapeutic options with their patients. The tumor response to neoadjuvant treatment by measurement of the reduction in the Ki-67-index, as described in the current study, may guide adjuvant treatment in patients who undergo resection. 18FDG-PET is expensive and some tumors may have a negative baseline signal; the demonstration of the Ki-67-indexing of repeated EUS-biopsies is an attractive method by which the therapeutic response may be evaluated.
This prospective study was conducted in a large Swedish region over several years and involved dedicated experts and the use of advanced techniques. The centralized management of GIST facilitated good control of patients and reliable follow-up data. We used pretreatment tumor tissue not only to diagnose GIST but also to clarify the sensitivity to imatinib, to assess the tumor proliferation rate, and finally, to evaluate the treatment response to imatinib. To the best of our knowledge, the presented results are more detailed and accurate than those of any comparable publications in the literature.
A limitation of EUS is that GISTs in the jejunum or ileum can be punctured only if they are visible from the stomach or the duodenum. However, the majority of GISTs are located in the stomach. Some study patients were treated with neoadjuvant imatinib even if they carried mutations with primary resistance to imatinib, which highlights the importance of sequencing prior to therapy. Sampling errors may result in an erroneously low Ki-67-index. However, such a phenomenon was probable only in two patients in this study (case #33 and #37).
The described pretreatment characterization of tumors should be incorporated in future management guidelines of GIST to facilitate personalized treatment. Moreover, the work-up of complex tumors such as GISTs should be centralized to high-volume centers in order to enable a rational and effective treatment.
We conclude that this study provides clear support for endoscopic ultrasound as the front-line diagnostic procedure in GIST, as it enables an early diagnosis and a personalized, genotype-driven targeted therapy of patients. The presented approach with the extensive characterization of GISTs based on the analysis of EUS-guided biopsies may also serve as a model for other tumor entities.
Thanks to all staff at the GEA Endoscopy unit, Sahlgrenska University Hospital; Professor Henrik Sjövall and Professor Hasse Abrahamsson for the critical revision of the manuscript; John Hedenström, MSc, for proof-reading.
The early personalized management and treatment of gastrointestinal stromal tumors (GISTs) require an extensive characterization of individual tumors. Information on the tumor proliferation rate and the KIT- and platelet-derived growth factor alpha-mutation profile is essential.
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been reported to be suboptimal for the diagnosis of GIST, but endoscopic ultrasound (EUS)-guided biopsy sampling (EUS-FNB) has not been evaluated for the characterization of GISTs. Neither the Ki-67 index nor KIT/PDGFRA-sequencing has been evaluated in EUS-FNB-tissue.
This prospective, long-term study showed that EUS-FNB was safe and highly accurate for the pretreatment diagnosis of GISTs, for the sequencing of KIT and PDGFRA, and for the assessment of the tumor proliferation rate (Ki-67-index). To the best of our knowledge, other relevant publications in this field demonstrate a diagnostic accuracy of EUS-FNA of approximately 50%. The sequencing of EUS-FNA-smears of GIST has not been evaluated in a prospective cohort but only in a single, retrospective study that included 20 patients.
By obtaining the extensive, preoperative diagnostic and prognostic information described in the present study, it will be possible to personalize the clinical and surgical management of patients with GIST especially with respect to the guidance and evaluation of neoadjuvant imatinib therapy.
This manuscript is about endoscopic ultrasound-guided biopsy in the diagnosis of gastrointestinal stromal tumors and evaluating neoadjuvant imatinib by sequencing of EUS-biopsies. It’s an interesting and valuable manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Deng MM, Lee SW S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360-5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 459] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Balachandran VP, DeMatteo RP. Gastrointestinal stromal tumors: who should get imatinib and for how long? Adv Surg. 2014;48:165-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, McKinley A, Ou WB, Fletcher JA. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352-5359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 577] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 5. | DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58. [PubMed] |
| 6. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1323] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 7. | Rutkowski P, Gronchi A, Hohenberger P, Bonvalot S, Schöffski P, Bauer S, Fumagalli E, Nyckowski P, Nguyen BP, Kerst JM. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Sjölund K, Andersson A, Nilsson E, Nilsson O, Ahlman H, Nilsson B. Downsizing treatment with tyrosine kinase inhibitors in patients with advanced gastrointestinal stromal tumors improved resectability. World J Surg. 2010;34:2090-2097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Rossi S, Gasparotto D, Miceli R, Toffolatti L, Gallina G, Scaramel E, Marzotto A, Boscato E, Messerini L, Bearzi I. KIT, PDGFRA, and BRAF mutational spectrum impacts on the natural history of imatinib-naive localized GIST: a population-based study. Am J Surg Pathol. 2015;39:922-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 671] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 11. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [PubMed] |
| 12. | Cerski MR, Pereira F, Matte US, Oliveira FH, Crusius FL, Waengertner LE, Osvaldt A, Fornari F, Meurer L. Exon 11 mutations, Ki67, and p16(INK4A) as predictors of prognosis in patients with GIST. Pathol Res Pract. 2011;207:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-S41; quiz S42-S44. [PubMed] |
| 14. | Kemmerling R, Weyland D, Kiesslich T, Illig R, Klieser E, Jäger T, Dietze O, Neureiter D. Robust linear regression model of Ki-67 for mitotic rate in gastrointestinal stromal tumors. Oncol Lett. 2014;7:745-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 874] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 16. | Zhao WY, Xu J, Wang M, Zhang ZZ, Tu L, Wang CJ, Lin TL, Shen YY, Liu Q, Cao H. Prognostic value of Ki67 index in gastrointestinal stromal tumors. Int J Clin Exp Pathol. 2014;7:2298-2304. [PubMed] |
| 17. | Jabbar KS, Verbeke C, Hyltander AG, Sjövall H, Hansson GC, Sadik R. Proteomic mucin profiling for the identification of cystic precursors of pancreatic cancer. J Natl Cancer Inst. 2014;106:djt439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Fernández-Esparrach G, Sendino O, Solé M, Pellisé M, Colomo L, Pardo A, Martínez-Pallí G, Argüello L, Bordas JM, Llach J. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: a randomized crossover study. Endoscopy. 2010;42:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR. EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc. 2009;70:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Watson RR, Binmoeller KF, Hamerski CM, Shergill AK, Shaw RE, Jaffee IM, Stewart L, Shah JN. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, Balci S, Gucer H, Jang KT, Tajiri T. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol. 2015;28:686-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Lee JH, Cho CJ, Park YS, Ahn JY, Kim DH, Na HK, Choi KD, Song HJ, Lee GH, Jung HY. EUS-guided 22-gauge fine needle biopsy for the diagnosis of gastric subepithelial tumors larger than 2 cm. Scand J Gastroenterol. 2016;51:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Gleeson FC, Kipp BR, Kerr SE, Voss JS, Graham RP, Campion MB, Minot DM, Tu ZJ, Klee EW, Lazaridis KN. Kinase genotype analysis of gastric gastrointestinal stromal tumor cytology samples using targeted next-generation sequencing. Clin Gastroenterol Hepatol. 2015;13:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Dematteo RP, Gold JS, Saran L, Gönen M, Liau KH, Maki RG, Singer S, Besmer P, Brennan MF, Antonescu CR. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 345] [Article Influence: 20.3] [Reference Citation Analysis (0)] |