Published online Aug 28, 2017. doi: 10.3748/wjg.v23.i32.5829
Peer-review started: April 1, 2017
First decision: May 5, 2017
Revised: June 25, 2017
Accepted: August 1, 2017
Article in press: August 2, 2017
Published online: August 28, 2017
Processing time: 153 Days and 0.8 Hours
Colorectal cancer (CRC) is a multifactorial disease characterized by several genetic and epigenetic alterations occurring in epithelial cells. It is increasingly recognized that tumour progression is also regulated by tumour microenvironment (TME). The bidirectional cross-talk between tumour resident adipocytes and cancer cells within TME has been proposed as active contributor to carcinogenesis. Tumour resident adipocytes exhibit an activated phenotype characterized by increased secretion of pro-tumorigenic factors (angiogenic/inflammatory/immune) which contribute to cancer cell proliferation, invasion, neoangiogenesis, evasion of immune surveillance and therapy resistance. Furthermore, adipocytes represent a fuel rich source for increasing energy demand of rapidly proliferating tumour cells. Interestingly, a relationship between obesity and molecular variants in CRC has recently been identified. Whether adipose tissue promotes cancer progression in subsets of molecular phenotypes or whether local tissue adipocytes are involved in inactivation of tumour suppressor genes and/or activation of oncogenes still needs to be explored. This editorial highlights the major findings related to cross-talk between adipocytes and colon cancer cells and how local paracrine interactions may promote cancer progression. Furthermore, we provide future strategies in studying colonic TME which could provide insights in bidirectional cross-talk mechanisms between adipocytes and colonic epithelial cells. This could enable to decipher critical signalling pathways of both early colonic carcinogenesis and cancer progression.
Core tip: The tumor microenvironment (TME) has been implicated in cancer progression and chemoresistance. Adipocytes are active components of the TME. Bidirectional cross-talk between adipocytes and cancer cells has recently been postulated to actively contribute to tumor initiation and progression. This Editorial highlights the role of local paracrine interactions between adipocytes and colon cancer cells. Discovery of signalling pathways activated by tumor resident adipocytes in colon cancer will allow better understanding of carcinogenesis and provide potential therapeutic targets.
- Citation: Tabuso M, Homer-Vanniasinkam S, Adya R, Arasaradnam RP. Role of tissue microenvironment resident adipocytes in colon cancer. World J Gastroenterol 2017; 23(32): 5829-5835
- URL: https://www.wjgnet.com/1007-9327/full/v23/i32/5829.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i32.5829
Colorectal carcinogenesis is multifactorial involving interactions between genetic mutations (APC, TP53, PI3K, KRAS, BRAF, PTEN), microsatellite instability, chromosomal instability, epigenetic alterations (locus-specific CpG island methylation, global DNA hypomethylation)[1] and environmental factors (obesity, diabetes, metabolic syndrome, intestinal microbiome)[2]. Moreover the importance of “field cancerisation” has been highlighted in terms of cancer development in the macroscopically normal colon[3]. Epidemiologic studies support an association between high BMI and colorectal cancer (CRC) incidence and mortality[4]. Adipose tissue has been recognised as a major endocrine organ, secreting adipokines (leptin, adiponectin, visfatin), growth factors and immune/inflammatory/angiogenic factors [tumour necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-8 and vascular-endothelial-growth-factor (VEGF)] involved in the regulation of systemic energy and metabolic homeostasis. Altered adipose tissue secretion of such factors has been implicated in dysregulation of inflammatory and immune responses leading not only to systemic low-grade inflammation and metabolic dysfunction, but also to local tissue inflammation contributing to cancer progression[5].
The tumour microenvironment (TME) comprising stromal cells (adipocytes, macrophages, fibroblasts, monocytes, neutrophils, infiltrating immune T-cells and B-cells) and extracellular matrix is a fertile soil for cancer cells. Increasing evidence suggests that paracrine bi-directional cancer cell-stromal cell cross-talk is a key driver not only of local inflammation, but also activation and recruitment of immune cells, neo-angiogenesis and extracellular matrix remodelling. This in turn contributes to cancer cell proliferation, invasion and migration[6].
This Editorial highlights the contribution of adipose tissue secreted factors in colon cancer progression with a focus on bidirectional cross-talk between adipocytes and colon cancer cells.
The mesentery, constituted by loose connective tissue and adipocytes, is the primitive envelope of the colon. Therefore, the colon lies in close proximity to adipose tissue depots. Submucosal distribution of adipocytes has also been reported[7], although the presence and proportion of submucosal fat in normal colonic wall is not well known. Increased submucosal fat deposition is a known characteristic of inflammatory bowel disease[8]. However, this has also been described in subjects without intestinal pathology[9]. Understanding normal colonic wall histology is essential in order to appreciate the close spatial relationship between adipocytes and colonic epithelial cells, progenitor cells of CRC.
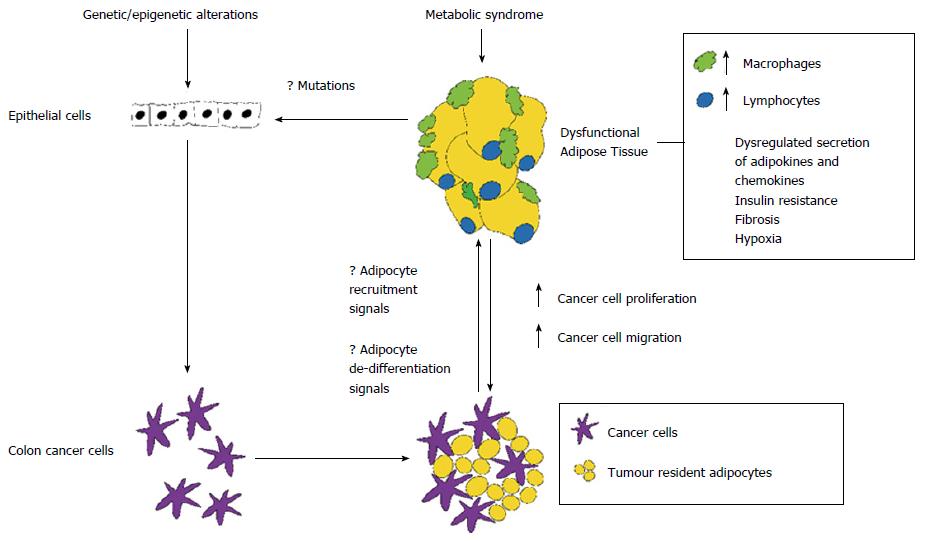
Recently, an interesting area of research termed “molecular pathologic epidemiology” (MPE) has detected epigenetic modifications associated with obesity, providing insights into the relationship between obesity and molecular variants in CRC[10]. It has been proposed that microenvironment-derived signals trigger heritable genetic changes within cancer cells, contributing to tumour evolution[11]. Studies in breast cancer, suggest that bidirectional interactions induce sequential epigenetic modifications in both cancer and stromal cells with progression from in situ ductal carcinoma to invasive carcinoma[12]. Epigenetic modifications induced by tumour resident adipocytes in colon cancer cells have not been reported, although MPE studies have identified a relationship between obesity and molecular variants in CRC[10].
The main component of adipose tissue is white adipose tissue (WAT). Expansion of WAT is consequence of an increase in size (hypertrophy) and/or increase in number (hyperplasia) of adipocytes. Healthy adipose tissue expansion consists in hypertrophic and hyperplastic white adipocytes, with appropriate angiogenic response, extracellular matrix remodelling and minimal inflammation. In contrast, pathological expansion of adipose tissue consists of adipocytes hypertrophy resulting in hypoxia, reduced angiogenesis, infiltration of macrophages and immune cells, low-grade inflammation, excessive production of reactive oxygen radicals, endoplasmic reticulum stress, mithocondrial dysfunction and remodelling of extracellular matrix[13].
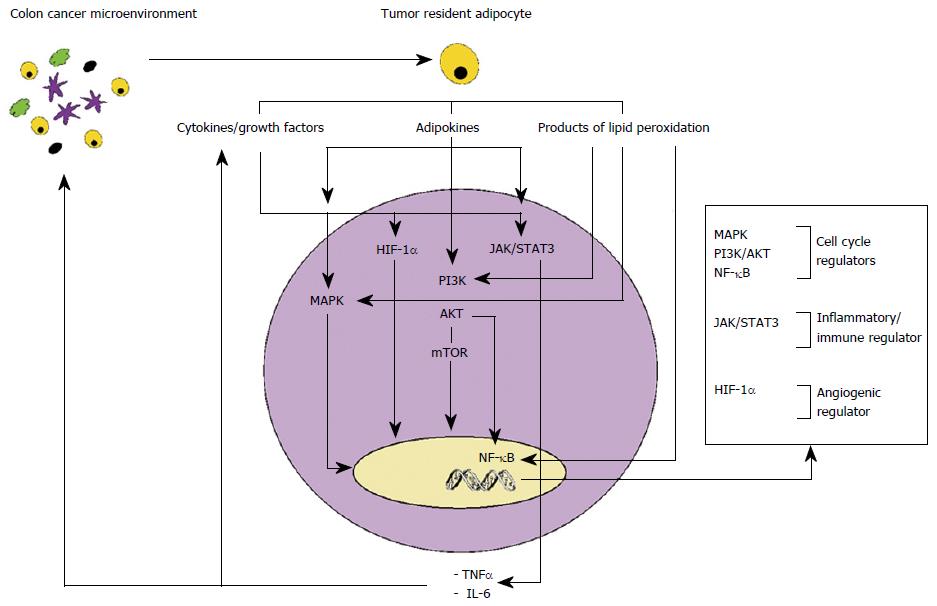
Inflammation is a recognised hallmark of cancer and pre-existing pro-inflammatory microenvironments are associated with increased cancer risk[14]. Increasing evidence, in breast, prostatic, ovarian and colon cancer, suggests that dysfunctional adipocytes are involved in cancer cell proliferation and migration through dysregulation of local and systemic inflammatory-immune-angiogenic response system[15]. Inflammation is initiated by adipose tissue hypertrophy leading to localized hypoxia which activates hypoxia-inducible factor 1-alpha (HIF-1α). HIF-1α up-regulates secretion of chemokines and proangiogenic factors including TNF-α, IL-6, IL-1, monocyte chemoattractant protein (MCP-1), plasminogen activator inhibitor-1 and VEGF, which are involved in the recruitment of macrophages and initiation of angiogenesis. Recruited macrophages contribute further to up regulation of inflammatory/immune cytokines favouring the acquisition of a systemic and local inflammatory phenotype[16].
The adipose tissue secreted factors, lipid metabolites and signalling pathways have been summarized in Table 1.
| AT secreted factors | Function | Signalling pathway | Ref. |
| TNF-α | Pro-inflammatory, | PI3K, NF-κB | Pikarsky et al[17], 2004 |
| cell proliferation, anti-apoptotic, angiogenetic | Huang et al[18], 2009 | ||
| Viatour et al[19], 2005 | |||
| IL-6 | Pro-inflammatory, cell proliferation and anti- apoptotic | JAK/STAT3 | Hodge DR et al[20], 2005 |
| Leptin | Promotion of cell survival, proliferation, differentiation, pro-inflammatory | JAK/STAT, PI3K, MAPK | Hefetz-Sela et al[22], 2013 |
| Hoda et al[23], 2007 | |||
| Adiponectin | Anti-inflammatory, anti-proliferative and pro-apoptotic effect | Inhibition of PI3K, AMPK/mTOR, JAK/STAT3, NF-κB | Hefetz-Sela et al[22], 2013 |
| Visfatin | Pro-inflammatory, angiogenic, promotion of cell survival and migration | ERK/MAPK, PI3K/AKT, NF-κB, β1-integrin | Adya et al[25], 2008 |
| Huang et al[26], 2013 | |||
| Lipid peroxidation products | Promotion of cell proliferation, differentiation, survival, migration, angiogenesis | PI3K/AKT/mTOR | Ayala et al[27], 2014 |
| NF-κB, PPAR, MAPK |
TNF-α, secreted by dysfunctional adipose tissue, has been shown to support cancer cell proliferation, angiogenesis and metastasis through activation of key transcription factors, including PI3K/AKT/mTOR and nuclear transcription factor NF-κB[17-19]. TNF-α and hypoxic conditions also induce secretion of the proinflammatory cytokine IL-6, activator of Janus Kinase and signal transducers and activators of transcription 3 (Jak/STAT3) pathways, key regulators of cell proliferation and apoptosis[20].
Adipocyte secreted hormones, including leptin, adiponectin and visfatin, have also been implicated in colon cancer progression (Figure 1).
Leptin is a potent inflammatory agent involved in up regulation of pro-inflammatory cytokines such as TNF-α, MCP-1, and reactive oxygen species from endothelial cells and peripheral blood mononuclear cells[21]. In vitro studies, in colon cancer cell lines, have demonstrated that leptin exerts pro-inflammatory, mitogenic, anti-apoptotic and angiogenic properties[22,23].
Adiponectin has a potent anti-inflammatory, anti-proliferative and pro-apoptotic activity. However, proliferative and pro-inflammatory properties of adiponectin on colonic epithelial cancer cells have also been reported. Several studies suggest local-paracrine pro tumorigenic effects of adiponectin according to tissue-specific expression of its receptor subtypes (ADIPOR1 and ADIPOR2). Increased AdipoR1 and AdipoR2 expression has been associated with cancer progression linked with the pro-angiogenic activity of adiponectin[22,24].
Visfatin has been shown to exhibit pro-inflammatory and pro-angiogenic effects in endothelial cells[25]. Studies have demonstrated a role of visfatin in CRC. CRC cells express chemokine receptors (CXCR4 and CXCR7), activated by visfatin, which bind stromal cell-derived factor-1, promoter of survival and migration of cancerous cells[26].
The chronic low-grade inflammatory state of dysfunctional adipocytes leads to activation of lipid peroxidation with the production and secretion of 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde. The secreted 4-HNE is responsible of deregulation of multiple pathways involved in tumour cell proliferation, differentiation, cell survival, migration, apoptosis and angiogenesis including MAPK, PI3K-AKT-mTOR, NF-κB. This also results in upregulation of prostaglandin E2 (PGE2) and cyclooxygense-2, implicated in CRC[27].
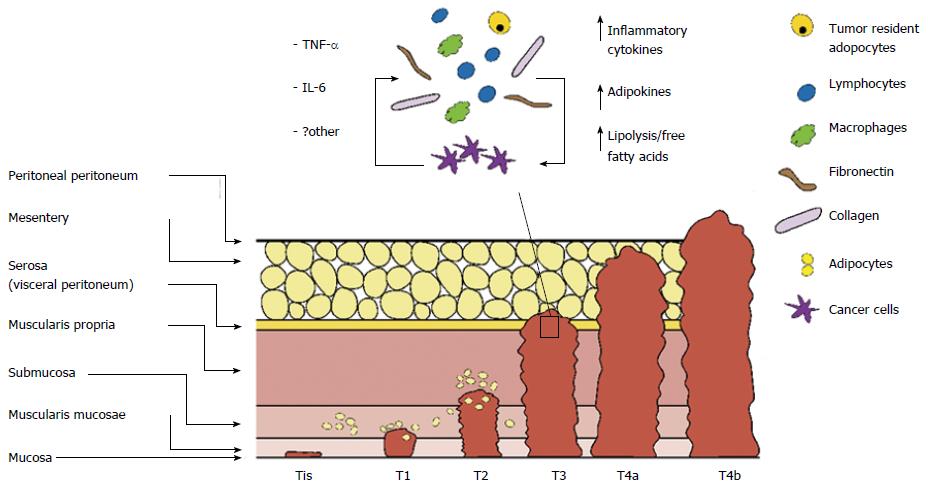
Recently great interest has emerged in reciprocal signalling between tumour resident adipocytes and cancer cells. CRC progresses through sequential stages involving multiple layers of the colonic wall. TNM staging system is currently used for classifying CRC in 4 stages according to local invasion depth (T stage), lymph node involvement (N stage) and presence or absence of distant metastasis (M stage), providing indication for prognosis and therapeutic strategies. With cancer progression activation of complex signalling networks modify both cancer cells and stromal cells[28]. Cancer cells and activated stromal cells communicate by autocrine/paracrine pathways contributing to dynamic modulation of TME through persistent recruitment of inflammatory and stromal cells in the TME. As a result, TME becomes increasingly populated with infiltrating innate immune cells (macrophages, neutrophils), adaptive immune cells (T and B lymphocytes) pericytes and stem cells contributing to cancer cell proliferation and invasion (Figure 2).
Adipocytes located in close proximity to tumour invasive front are reprogrammed by cancer cells into activated fibroblast-like cells. Tumour resident adipocytes exhibit morphological and functional modifications, known as adipocyte dedifferentiation, consisting in size reduction, due to enhanced lipolytic activity, decreased adipocyte-differentiation markers (adiponectin, resistin, fatty acid binding protein-4, adipocyte protein 2), and increased secretion of inflammatory factors (IL-6, IL-8, IL-1 β, TNF-α), growth factors (insulin-like growth factor 1, IGF1 binding proteins), angiogenic factors (VEGF) and MCP-1 (CCL2)[15]. In vitro studies, in breast and prostate cancer, have demonstrated that tumour resident adipocyte secreted factors activate signalling pathways involved in cancer cell survival, proliferation, invasion, epithelial to mesenchimal transition, angiogenesis and extracellular matrix remodelling, promoting cancer initiation and metastasis[15,22]. Active recruitment of adipocytes to TME has not been reported, although it has been reported that bone marrow derived mesenchymal stem cells (progenitors of adipocytes) may be recruited to specific sites of neoplasia inducing metastatic properties[29]. An open question is whether adipose tissue promotes cancer progression in subsets of molecular phenotypes or whether local tissue adipocytes are involved in inactivation of tumour suppressor genes and/or activation of oncogenes (Figure 3).
Adipocytes serve as a fuel rich source for increasing energy demand of rapidly proliferating tumour cells. Advanced stages of gastrointestinal malignancies often present with cancer-associated-cachexia as a result of lipolysis induced by cancer cells. Studies have described increased lipid droplets in colon adenocarcinoma and it has been implicated in PGE2 synthesis. Inhibition of lipid droplet formation by fatty acid synthase inhibitors reduces cancer cell proliferation in vitro, suggesting a role of lipid droplets in colon adenocarcinoma[30].
Metabolic and transcriptomic expression profile and direct paracrine effects of tumour resident adipocytes in colon cancer have not been evaluated. We have preliminary data (unpublished) indicating increased expression of pro-inflammatory/immune/angiogenic factors in colon cancer resident adipocytes, isolated from paraffin embedded sections using laser micro dissection system, compared to adipocytes isolated from the distal non neoplastic mucosa.
TME signalling pathways have recently been implicated in inducing chemoresistance in breast and prostate cancer[31]. There is also evidence, in colon cancer cell lines, that leptin inhibits cytotoxic effects of 5-fluorouracil[32].
Two dimensional (2D) cell culture models, widely used in basic science research, do not reproduce the complex interactions between host cells of TME. Recently, three-dimensional (3D) organoid models, derived from mouse and human intestinal tissue ex vivo, have been described. These in vitro organ-like cultures reproduce intestinal tissue microenvironment. Furthermore, they can be co-cultured with stromal components[33]. Reproduction of colonic microenvironment in vitro will allow to decipher ex vivo bidirectional cross-talk mechanisms between adipocytes and colonic epithelial cells.
The bidirectional cross talk between tumour resident adipocytes and colon cancer cells contributes to the progressive evolution of tumour microenvironment and cancer progression. It is therefore important to decipher the metabolic and transcriptomic expression profiles of colon cancer resident adipocytes in different stages of tumour progression. Colon organoid cultures combined with adipocytes and/or tumour resident adipocyte secreted factors will allow to identify critical signalling pathways of both early colonic carcinogenesis and cancer progression providing diagnostic biomarkers and novel therapeutic targets for colon cancer.
We would like to thank Jason McAllister for the creation of the figures.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abdel-Rahman WM, Harmanci O, Zhu YL S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 522] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 2. | Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 456] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 3. | Patel A, Tripathi G, Gopalakrishnan K, Williams N, Arasaradnam RP. Field cancerisation in colorectal cancer: a new frontier or pastures past? World J Gastroenterol. 2015;21:3763-3772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3505] [Cited by in RCA: 3679] [Article Influence: 216.4] [Reference Citation Analysis (1)] |
| 5. | van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 518] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 6. | Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 7. | Eroschenko VP. diFiore’s Digestive System: small and large intestines. In: diFiore’s Atlas of Histology. Philadelphia: Lippioncott Williams Wilkins, 2005: 270-271. . |
| 8. | Jones B, Fishman EK, Hamilton SR, Rubesin SE, Bayless TM, Cameron JC, Siegelman SS. Submucosal accumulation of fat in inflammatory bowel disease: CT/pathologic correlation. J Comput Assist Tomogr. 1986;10:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Harisinghani MG, Wittenberg J, Lee W, Chen S, Gutierrez AL, Mueller PR. Bowel wall fat halo sign in patients without intestinal disease. AJR Am J Roentgenol. 2003;181:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Comaills V, Kabeche L, Morris R, Buisson R, Yu M, Madden MW, LiCausi JA, Boukhali M, Tajima K, Pan S. Genomic Instability Is Induced by Persistent Proliferation of Cells Undergoing Epithelial-to-Mesenchymal Transition. Cell Rep. 2016;17:2632-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 571] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 13. | Trayhurn P. Hypoxia and adipocyte physiology: implications for adipose tissue dysfunction in obesity. Annu Rev Nutr. 2014;34:207-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47109] [Article Influence: 3364.9] [Reference Citation Analysis (5)] |
| 15. | Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 568] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 16. | Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 17. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 2016] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 18. | Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1215] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 20. | Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 717] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 21. | Adya R, Tan BK, Randeva HS. Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res. 2015;2015:648239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Hefetz-Sela S, Scherer PE. Adipocytes: impact on tumor growth and potential sites for therapeutic intervention. Pharmacol Ther. 2013;138:197-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 23. | Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg. 2007;94:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Byeon JS, Jeong JY, Kim MJ, Lee SM, Nam WH, Myung SJ, Kim JG, Yang SK, Kim JH, Suh DJ. Adiponectin and adiponectin receptor in relation to colorectal cancer progression. Int J Cancer. 2010;127:2758-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 333] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Huang WS, Chen CN, Sze CI, Teng CC. Visfatin induces stromal cell-derived factor-1 expression by β1 integrin signaling in colorectal cancer cells. J Cell Physiol. 2013;228:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2367] [Cited by in RCA: 3550] [Article Influence: 322.7] [Reference Citation Analysis (0)] |
| 28. | Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 460] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 29. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2442] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 30. | Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008;68:1732-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 31. | Villanueva MT. Cell signalling: Stuck in the middle of chemoresistance and metastasis. Nat Rev Clin Oncol. 2012;9:490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Bartucci M, Svensson S, Ricci-Vitiani L, Dattilo R, Biffoni M, Signore M, Ferla R, De Maria R, Surmacz E. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G. Three-Dimensional Gastrointestinal Organoid Culture in Combination with Nerves or Fibroblasts: A Method to Characterize the Gastrointestinal Stem Cell Niche. Stem Cells Int. 2016;2016:3710836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |