Published online Aug 14, 2017. doi: 10.3748/wjg.v23.i30.5519
Peer-review started: April 10, 2017
First decision: June 8, 2017
Revised: June 26, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: August 14, 2017
Processing time: 129 Days and 10.8 Hours
To investigate interleukin (IL)-26 expression in the inflamed mucosa of patients with inflammatory bowel disease (IBD) and the function of IL-26.
Human colonic subepithelial myofibroblasts (SEMFs) were isolated from colon tissue surgically resected. The expression of IL-26 protein and its receptor complex was analyzed by immunohistochemistry. The gene expression induced by IL-26 was evaluated by real-time polymerase chain reaction. Intracellular signaling pathways were evaluated by immunoblotting and specific small interfering (si) RNA transfection.
The mRNA and protein expression of IL-26 were significantly enhanced in the inflamed mucosa of patients with IBD. IL-26 receptor complex was expressed in colonic SEMFs in vivo and in vitro. IL-26 stimulated the mRNA expression of IL-6 and IL-8 in colonic SEMFs. The inhibitors of mitogen-activated protein kinases and phosphoinositide 3-kinase, and siRNAs for signal transducers and activator of transcription 1/3, nuclear factor-kappa B and activator protein-1 significantly reduced the mRNA expression of IL-6 and IL-8 induced by IL-26.
These results suggest that IL-26 plays a role in the pathophysiology of IBD through induction of inflammatory mediators.
Core tip: We investigated interleukin (IL)-26 expression in the inflamed mucosa of patients with inflammatory bowel disease (IBD) and characterized the biological function of IL-26 using human colonic subepithelial myofibroblasts. To our knowledge, this is the first report to state that IL-26 activates STAT1/3 and leads to the induction of IL-6 and IL-8 expression in non-transformed cells derived from human colon. We suggest that IL-26 plays a role in the pathophysiology of IBD through the induction of inflammatory mediators.
- Citation: Fujii M, Nishida A, Imaeda H, Ohno M, Nishino K, Sakai S, Inatomi O, Bamba S, Kawahara M, Shimizu T, Andoh A. Expression of Interleukin-26 is upregulated in inflammatory bowel disease. World J Gastroenterol 2017; 23(30): 5519-5529
- URL: https://www.wjgnet.com/1007-9327/full/v23/i30/5519.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i30.5519
Inflammatory bowel diseases (IBD) comprise two major phenotypes, Crohn’s disease (CD) and ulcerative colitis (UC). Recent studies suggest that the chronic inflammation in IBD is mediated by uncontrolled immune responses against a subset of luminal bacteria and dietary factors[1-3]. This hypothesis is supported by the recent finding that the genes encoding innate immune responses are responsible for the susceptibility to IBD[4-6]. However, the precise etiology of IBD still remains unclear.
Interleukin (IL)-26 is a member of the IL-10 cytokine family. IL-26 was first discovered in herpesvirus saimiri-transformed T-cell lines by subtractive hybridization[7]. IL-26 has been reported to be co-expressed with IL-17 and IL-22 by Th17 cells[8-10], and recent studies have reported that natural killer cells[11,12], macrophages[13], and fibroblast-like cells[14,15] are sources of IL-26. A murine IL-26 homologue has not been identified[16,17], limiting the experimental opportunities to study the phenotypic consequences of IL-26 gene knockout and IL-26-mediated functions in murine models in vivo.
Although IL-19, IL-20, and IL-24, members of IL-10 cytokine family, are located in proximity to the IL-10 gene on human chromosome 1q32, IL-26 is located on chromosome 12q15 between the genes encoding IFN-γ and IL-22[18]. For its signaling, IL-26 requires the heterodimeric receptors composed of IL-20R1 and IL-10R2[19]. The transmembrane protein IL-20R1 has been shown to possess the specific ligand-binding site for IL-26, whereas the IL-10R2 subunit acts as an essential second chain that completes the assembly of its active receptor complex and signaling[20]. IL-22, IL-26, IL-28A/B and IL-29 also use IL-10R2 for their signaling. IL-10R2 is ubiquitously expressed in various tissues, but the expression of IL-20R1 is absent in hematopoietic cells and is restricted within non-hematopoietic cells[19,21].
Previous reports have suggested that intracellular signaling of IL-26 is mediated by the Janus kinase-signal transducer and activator of transcription (STAT) pathway[16,20]. Additionally, IL-26 has been reported to activate extracellular signal-related kinase (ERK)-1/2, stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), p38 mitogen-activated protein kinase and phosphoinositide 3-kinase (PI3K) in various kinds of cells[7,16,20,22].
Human colonic subepithelial myofibroblasts (SEMFs) are located immediately subjacent to the basement membrane in the normal intestinal mucosa, juxtaposed against the bottom of the epithelial cells[23-25]. These cells are considered to play a role in the regulation of a number of epithelial cell functions, such as epithelial proliferation and differentiation. Isolated SEMFs retain their representative and differentiated phenotypes[23].
Recent studies have demonstrated that IL-26 is involved in the pathophysiology of chronic inflammatory disorders, such as rheumatoid arthritis[15] and chronic hepatitis C infection[26]. Concerning IBD, the pathological role of IL-26 has been reported in CD, but remains unclear in UC[16]. In addition, functional analysis of IL-26 has been studied using a transformed cell line, but there are no reports using primary culture cells. In the present study, to explore the potential role of IL-26 in IBD, we investigated the expression of IL-26 in the inflamed mucosa of UC and CD patients. Furthermore, the biological functions and the intra-cellular signal pathways activated by IL-26 were investigated in human colonic SEMFs.
All reagents and antibiotics used in this study were commercially purchased as shown in Supplementary Table 1.
| Gene name | Accession number | Primers | |
| IL-26 | NM_018402.1 | Sense | 5’-GGCAGAAATTGAGCCACTGT-3’ |
| Antisense | 5’-TCCAGTTCACTGATGGCTTTG-3’ | ||
| IL-10R2 | NM_000628.4 | Sense | 5’-GGCTGAATTTGCAGATGAGCA-3’ |
| Antisense | 5’-GAAGACCGAGGCCATGAGG-3’ | ||
| IL-20R1 | NM_014432.3 | Sense | 5’-TACACCCCTCAGCTCCAAGACT-3’ |
| Antisense | 5’-GAAGGAATTACACAGCCTGCCAG-3’ | ||
| IL-6 | NM_000600.4 | Sense | 5’-GGTACATCCTCGACGGCATCT-3’ |
| Antisense | 5’-GTGCCTCTTTGCTGCTTTCAC-3’ | ||
| IL-8 | NM_000584.3 | Sense | 5’-ATGACTTCCAAGCTGGCCGTGGCT-3’ |
| Antisense | 5’-TCTCAGCCCTCTTCAAAAACTTCTC-3’ | ||
| β-actin | NM_001101.3 | Sense | 5’-TGACCCAGATCATGTTTGAGACCT-3’ |
| Antisense | 5’-CCACGTCACACTTCATGATGGAG-3’ | ||
| STAT1 | NM_139266 | Sense | 5’-GGAAGGGGCCATCACATTCA-3’ |
| Antisense | 5’-GTAGGGTTCAACCGCATGGA-3’ | ||
| STAT3 | NM_003150.3 | Sense | 5’-GGAGGAGTTGCAGCAAAAAG-3’ |
| Antisense | 5’-GGAGGAGTTGCAGCAAAAAG-3’ | ||
| c-jun | NM_002228.3 | Sense | 5’-CAGGTGGCACAGCTTAAACA-3’ |
| Antisense | 5’-GTTTGCAACTGCTGCGTTAG-3’ | ||
| NF-κBp65 | NM_003998.3 | Sense | 5’-CGCATCCAGACCAACAACAA-3’ |
| Antisense | 5’-GCATTCAGGTCGTAGTCCCC-3’ |
The diagnosis of IBD was based on conventional clinical and endoscopic criteria. Surgically-resected specimens and biopsy specimens from 49 patients with UC and 19 patients with CD were used after obtaining written informed consent. All experiments were approved by the local ethics committee of the Shiga University of Medical Science (Permit number: 27-27).
The clinical activity of IBD was determined according to the colitis activity index for UC[27] and CD activity index[28]. Normal colonic tissues were obtained from the distal part of the surgically resected sample of colon cancer (n = 3) and using colonoscopy (n = 17).
Immunohistochemical analyses were performed according to the method described in our previous report[29]. Briefly, goat anti-IL-26, goat anti-IL-20R1 and rabbit anti-IL-10R2 were used as the primary antibodies. After incubation with the primary antibodies, the sections were treated with HRP-labeled anti-goat or anti-rabbit antibodies. Diaminobenzidine was used as a substrate for color development.
For double-staining procedures, anti-IL-20R1 or anti-IL-10R2 antibodies were applied and incubated overnight in a humidified chamber. Subsequently, anti-α-smooth muscle actin (SMA) antibodies were applied and incubated overnight. Dylight488-labeled anti-goat IgG, Dylight549-labeled anti-mouse IgG, or Dylight549-labeled anti-mouse IgG were used as secondary antibodies. Images were obtained with a digital confocal laser scanning microscope LSM510 version 3.0 (Carl Zeiss Microscopy, Tokyo, Japan).
Primary cultures of colonic SEMFs were prepared according to the method reported by Mahida et al[30]. The cellular characteristics and culture conditions have also been described in our previous report[31]. The studies were performed on passages 3-6 of SEMFs.
The expression of mRNA in the samples was assessed by reverse transcription polymerase chain reaction (RT-PCR) and real-time PCR analysis. RT-PCR was performed according to the methods described in our previous report[32]. Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and was reverse transcribed using SuperScript II (Invitrogen). Subsequently, cDNAs were generated using SYBR Premix Ex Taq (TAKARA, Shiga, Japan), and real-time PCR was performed using a LightCycler480 System II (Roche Diagnostics, Basel, Switzerland) with specific primers for target genes. The PCR primers used in this study are shown in Table 1.
Concentrations of IL-6 and IL-8 in cell culture supernatants were determined using ELISA kits (R&D systems, Minneapolis, MN, United States).
Human colonic SEMFs were transfected with siRNA specific for STAT1, STAT3, nuclear factor (NF)-κBp65, and c-Jun according to the instructions for Lipofectamine RNAiMAX (Invitrogen). Briefly, human colonic SEMFs were cultured in complete medium without antibiotics in the presence of a mixture of an RNAi duplex and Lipofectamine RNAiMAX for 24 h, and were then stimulated with or without IL-26 for 3 h.
Nuclear proteins were extracted using the CelLytic NuCLEAR Extraction Kit (Sigma-Aldrich, St. Louis, MO, United States). Extracted nuclear proteins were subjected to immunoblotting with rabbit anti-NF-κBp65 (C-20) antibody or mouse anti-phospho (P)-c-Jun (KM-1) antibody, followed by incubation with HRP-labeled anti-rabbit antibody or HRP-labeled anti-mouse antibody. Immunoblots were performed according to a method previously described[33,34]. Signal detection was performed using the enhanced chemiluminescence Western blot system (GE Healthcare, Little Chalfont, United Kingdom).
Cytoplasmic protein was extracted using a lysis buffer [50 mmol/L Tris pH 8.0, 0.5% Nonidet P-40, 1 mmol/L EDTA, 150 mmol/L NaCl, 2 mmol/L Na3VO4, 1 mmol/L NaF, 20 mmol/L Na4P2O7, 1 mmol/L PMSF, 10% glycerol and complete Mini Protease Inhibitor Cocktail (Roche Diagnostics)]. Extracted protein was subjected to immunoblotting with antibodies against phospho-p44/42 MAPK (ERK1/2), p38 MAPK, or SAPK/JNK, Akt, STAT1, or STAT3 followed by incubation with HRP-labeled anti-rabbit antibody or HRP-labeled anti-mouse antibody. After detection as described above, the membrane was stripped using Restore Western Blot Stripping Buffer (Thermo Scientific Inc., Waltham, MA) and was then incubated with antibodies against total-p44/42 MAPK (ERK1/2), p38 MAPK, SAPK/JNK, Akt, STAT1, or STAT3.
Single comparisons were analyzed using the nonparametric Mann-Whitney U test. Differences resulting in P values of less than 0.05 were considered to be statistically significant. The statistical methods of this study were reviewed by a biomedical statistician from Shiga University of Medical Science.
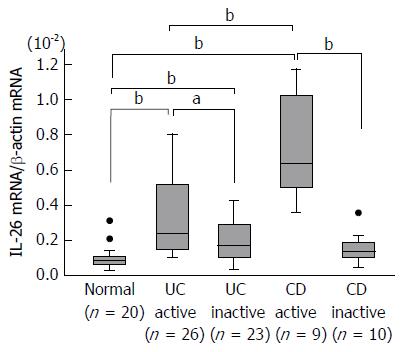
The mRNA expression of IL-26 in the inflamed mucosa of IBD patients was evaluated using real-time PCR. As shown in Figure 1, IL-26 mRNA expression was faintly detected in normal mucosa. The mucosal mRNA expression of IL-26 was significantly higher in active UC patients than in the inactive UC mucosa and normal mucosa. Similar findings were also observed in the inflamed mucosa of CD patients. The average level of IL-26 mRNA expression was significantly higher in active CD mucosa than in active UC mucosa.
IL-26 protein expression was evaluated by immunohistochemical analysis. IL-26 positive cells were not detected in normal mucosa, but the number of IL-26 positive cells markedly increased in the inflamed mucosa of UC and CD patients (Figure 2A). Moreover, the number of IL-26 positive cells more increased in the inflamed mucosa of CD patients as compared to in the inflamed mucosa of UC patients (Figure 2A). This result was confirmed by the results from Figure 1. Furthermore, to identify the cellular source of IL-26, double staining was performed. As shown in Figure 2B, double staining studies indicated that IL-26-positive cells were positive for CD4 (T cell), CD56 (NCAM; NK cell), or CD68 (macrophage). These findings indicated that CD4+ T cells, NK cells, and macrophages were the cellular sources of IL-26 in the inflamed mucosa of UC and CD patients.
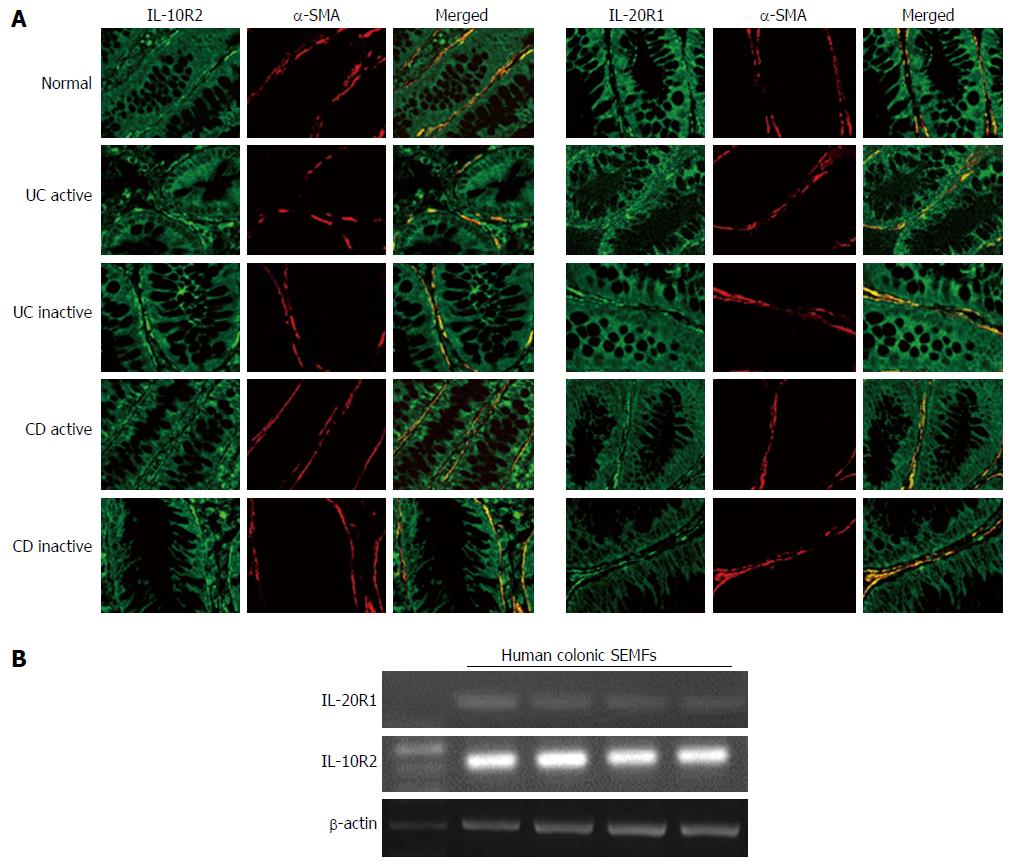
We looked for the presence of either IL-20R1 or IL-10R2 in the inflamed mucosa of IBD patients. It has been reported that the expression of IL-20R1 is restricted in non-hematopoietic cells, while IL-10R2 is ubiquitously expressed[19,35]. The tissue samples from the normal, the active IBD and inactive IBD were double stained with anti-α-SMA, a marker for myofibroblasts, and IL-20R1 or IL-10R2. As shown in Figure 3A, IL-20R1 was expressed in epithelial cells and in some of the other cells in the submucosa, and the cells in the subepithelial region also stained with α-SMA. IL-10R2 expression was detected in various cells including epithelial cells and leukocytes, and the cells at the subepithelial region also stained with α-SMA. The expression levels of IL-20R1 and IL-10R2 were not different between in the mucosa from the normal and in the active or inactive mucosa from IBD patients. These results suggested that human colonic SEMFs are expressing the IL-26 receptor.
We also confirmed the IL-20R1 and IL-10R2 expression in isolated human colonic SEMFs. As shown in Figure 3B, isolated human colonic SEMFs expressed IL-20R1 and IL-10R2 mRNAs.
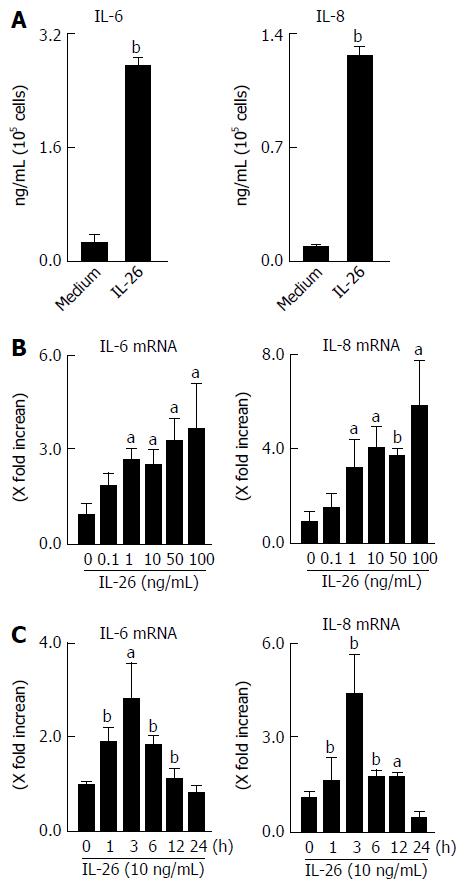
Based on the expression of IL-26 receptor in colonic SEMFs, we examined the biological effect of IL-26 on human colonic SEMFs in vitro. The cells were incubated with IL-26 (100 ng/mL) for 12 h, and then IL-6 and IL-8 levels in supernatants were evaluated using ELISA. As shown Figure 4A, IL-26 induced a significant increase in the secretion of IL-6 and IL-8. These responses were also confirmed at the mRNA levels. IL-26 dose- and time-dependently induced the mRNA expression of IL-6 and IL-8 (Figure 4B and C).
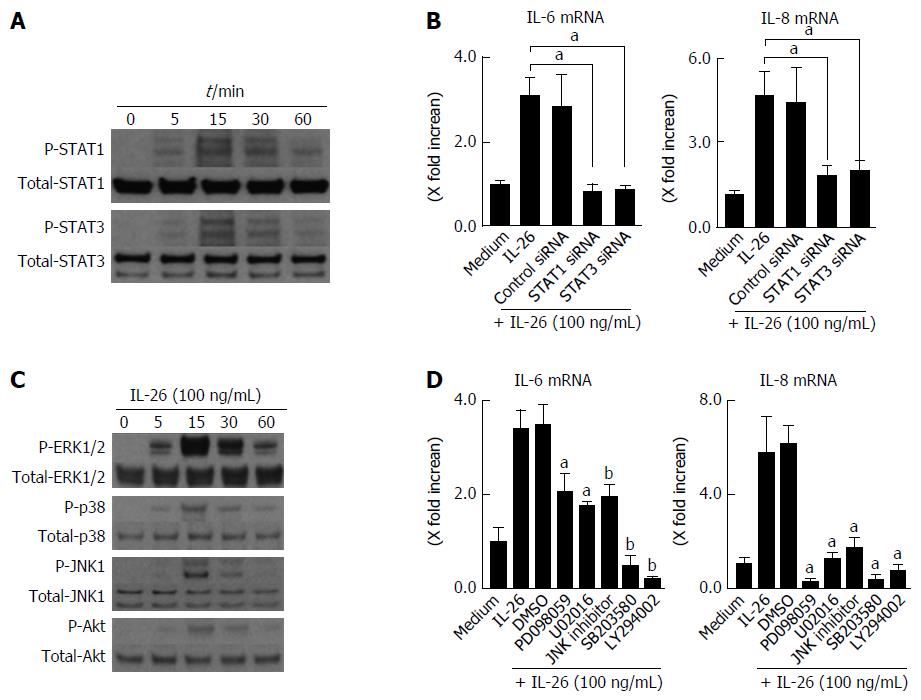
It has been previously reported that the activation of STAT1 and STAT3 is induced by IL-26[16,22]. Therefore, we examined whether IL-26 induced the phosphorylation of STAT1 and STAT3 in human colonic SEMFs. IL-26 induced the phosphorylation of STAT1 and STAT3 as early as 5 min after stimulation with IL-26 (Figure 5A).
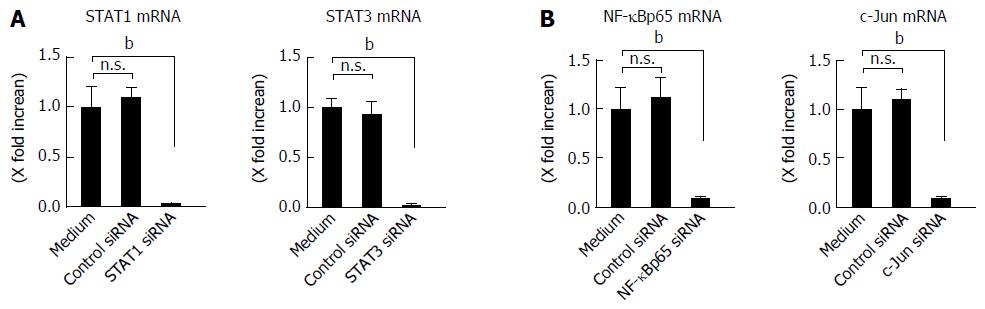
Involvement of STAT1 and STAT3 activation in IL-26-induced IL-6 and IL-8 was tested using siRNA specific for STAT1 and STAT3. As shown in Figure 5B, the siRNA specific for STAT1 and STAT3 significantly suppressed IL-26-induced mRNA expression of IL-6 and IL-8 effectiveness of siRNA for STAT1 and STAT3 is presented in Figure 6A. These findings indicated that the activation of STAT1 and STAT3 is involved in the induction of IL-6 and IL-8 by the stimulation of IL-26 in human colonic SEMFs.
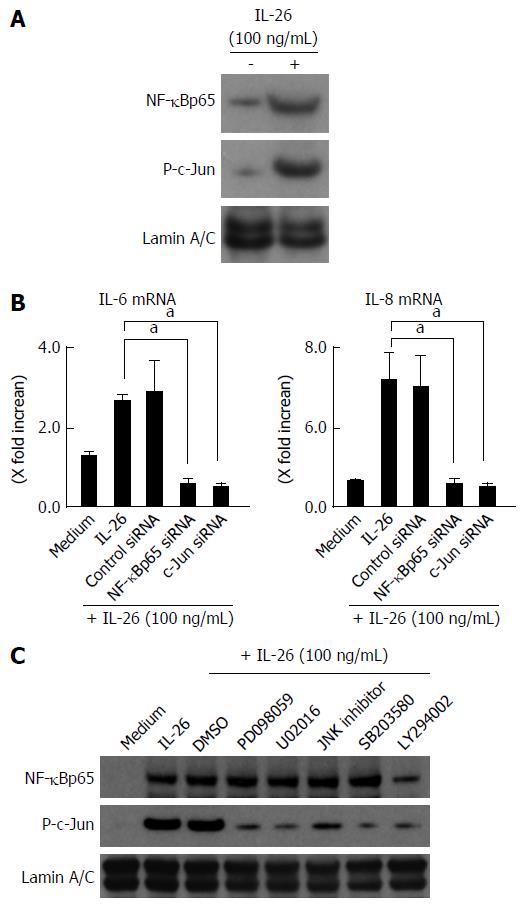
The MAPKs and Akt are involved in the cytokine signaling in various kinds of cells. We examined whether IL-26 activates MAPKs and Akt using immunoblot analysis. As shown in Figure 5C, IL-26 induced a phosphorylation of MAPKs, including p42/44MAPK, SAPK/JNK, and p38MAPK, and Akt as early as 5 min after stimulation with IL-26. Moreover, MEK1/2 inhibitors (PD098059 and U0216), a p38 MAPK inhibitor (SB203580), a JNK inhibitor (JNK inhibitor 1) and a PI3K inhibitor (LY294002) significantly suppressed IL-26-induced IL-6 and IL-8 mRNA expression (Figure 5D). These findings indicate that the activation of MAPKs and the PI3K/Akt pathway is involved in the IL-26-induced IL-6 and IL-8 expression in human colonic SEMFs.
The expression of a number of inflammatory genes is regulated by the activation of transcription factors such as NF-κB and AP-1[36]. In the nuclear proteins, the expression of NF-κB and phosphorylated c-Jun was clearly detected after IL-26 stimulation (Figure 7A). The siRNAs specific for NF-κBp65 and c-Jun (AP-1) significantly suppressed the mRNA expression of IL-6 and IL-8 effectiveness of siRNA for NF-κBp65 and c-Jun (AP-1) is presented in Figure 6B, (Figure 7B). These findings indicate an involvement of NF-κB and AP-1 activation in IL-26-induced IL-6 and IL-8 expression. As shown in Figure 7C, IL-26-induced NF-κBp65 phosphorylation was suppressed by PI3K inhibitor (LY294002), but was not affected by MAPKs. On the other hand, IL-26-induced AP-1 (c-Jun) phosphorylation was suppressed by both MAPKs and PI3K inhibitor. These findings indicate that PI3K, but not MAPKs, plays a role in IL-26-induced NF-κB activation, but that both MAPKs and PI3K activation are involved in IL-26-induced AP-1 activation.
In the present study, we demonstrated that: (1) the expression of IL-26 is enhanced in the inflamed mucosa of UC and CD patients; (2) human colonic SEMFs express IL-26 receptor complex IL-10R2/IL-20R1 in vivo and in vitro; (3) human colonic SEMFs secrete inflammatory mediators in response to IL-26 stimulation; and (4) IL-26 induced inflammatory mediators via the activation of STAT1/3 and MAPKs/PI3K followed by the activation of NF-κB/AP-1. These observations suggest that IL-26 plays a role in the pathophysiology of IBD.
In this study, we found that IL-26 mRNA expression was enhanced in the inflamed mucosa of UC and CD patients. Its expression was higher in the active mucosa of CD patients than in the active mucosa of UC patients. These findings are similar to our previous observation that mucosal mRNA expression of Th17 cytokines, such as IL-17 and IL-22, was enhanced in the inflamed mucosa of UC and CD patients[29,37]. The mRNA expression of either IL-17 or IL-22 was higher in CD patients than UC patients[29,37]. Th17 cells are now recognized as one of the cellular sources of IL-26[15]. These findings suggest that the IL-26-expressing CD4+ T cells in this study are probably Th17 cells and that Th17 cells are more closely associated with the pathophysiology of CD patients than UC patients.
In the intestinal mucosa, CD56+NCR+ type 3 innate lymphoid cells (ILC3s), a subclass of CD56+ NKp44+ NK cells, have been reported to concomitantly express IL-22 and IL-26, especially following stimulation with IL-23[11]. This suggests that the IL-26-producing CD56+ cells in our study may be NCR+ ILC3s. Our observation of IL-26 expression by CD68+ macrophages is supported by the recent report that CD68+ macrophages are the main IL-26-producing cells in joints with rheumatoid arthritis[15]. Thus, our observations in this study indicate that various types of immune cells are producing IL-26 in the inflamed mucosa of IBD. Further investigation using more precise cellular markers should be performed to more clearly identify the cellular source of IL-26 in the inflamed mucosa of IBD.
There are some reports concerning the in vivo expression of IL-26 under normal and pathological conditions. Corvaisier et al[15] demonstrated a pathogenic role of IL-26 in rheumatoid arthritis on the basis of its capacity to induce pro-inflammatory cytokines. IL-23-induced IL-26 plays a role in the pathophysiology of psoriasis[8]. Moreover, Dambacher et al[16] have reported that IL-26 modulated proliferation and pro-inflammatory gene expression in colon cancer cells and that IL-26 expression was upregulated in active CD, suggesting a role of IL-26 in the innate host cell response during intestinal inflammation. In addition, a genome-wide association study identified IL-26 as one of the susceptibility genes associated with UC[38], suggesting a pathophysiological role of IL-26 in patients with UC. However, previous studies of the clinical role of IL-26 mainly focused on CD rather than UC[16]. In this study, we found that IL-26 was enhanced in the inflamed mucosa of patients with UC, as well as patients with CD. In addition, human colonic SEMFs were expressing functionally-active IL-10R2 and IL-20R1 and secreted inflammatory cytokines in response to IL-26. This phenomenon is supported by previous reports that the expression of IL-20R1 is restricted within non-hematopoietic cells[19,21]. These observations suggest an interaction between IL-26 and colonic SEMFs in inflammatory responses in the colonic mucosa. IL-26 may stimulate the induction of inflammatory mediators from colonic SEMFs and possibly contributes to the inflammatory responses in IBD mucosa. However, recent studies have revealed that Th17-derived cytokines, such as IL-17 and IL-22, possess conflicting (pro-inflammatory and protective) roles in the mucosa[39]. So, further investigations to determine whether IL-26 production is ultimately tissue protective or a significant source of tissue damage in IBD mucosa are required in the future.
There are a few reports concerning the signaling pathway of IL-26 in the colonic tissue. A previous report using transformed epithelial cell lines suggested that IL-26 induces activation of STAT1/3, ERK1/2, SAPK/JNK1/2 and Akt[16]. To our knowledge, this is the first report to state that IL-26 activates STAT1/3 and leads to the induction of IL-6 and IL-8 expression in non-transformed cells derived from human colon. We have also revealed that IL-26 induced the activation of MAPKs, including ERK1/2, p38MAPK and SAPK/JNKL1/2, and PI3K/Akt. Furthermore, we found that PI3K, but not MAPKs, plays a role in IL-26-induced NF-κB activation, but that both MAPKs and PI3K activation were required in IL-26-induced AP-1 activation. These results indicate that IL-26-induced inflammatory responses in the colonic mucosa are mediated by various signaling pathways including STAT1/3, MAPKs and PI3K/Akt, followed by the activation of NF-κB and AP-1.
In conclusion, we demonstrated that the expression of IL-26 is increased in the inflamed mucosa of IBD patients. In human SEMFs, IL-26 induced an activation of STAT1/3 and MAPKs/PI3K, leading to an activation of NF-κB and AP-1. Since the IL-26 gene has not been identified in rodents, the experiments using human colonic SEMFs will contribute to the investigation of the true role of IL-26 in gut inflammation.
Recent studies have reported that interleukin (IL)-26 is involved in the pathophysiology of chronic inflammatory disorders. Concerning inflammatory bowel disease (IBD), the pathological role of IL-26 has been reported in Crohn’s disease (CD), but remains unclear in ulcerative colitis. Moreover, functional analysis of IL-26 has been studies using a transformed cell line, but there are no reports using primary culture cells.
The authors found that the expression of IL-26 was enhanced in the inflamed mucosa of IBD as compared to in the normal mucosa. The cellular source of IL-26 in the inflamed mucosa are CD4+ T cells, NK cells, and macrophages. Human colonic subepithelial myofibroblasts (SEMFs) are target cells of IL-26 in the mucosa of IBD.
This study revealed that IL-26 enhanced the induction of inflammatory mediators, IL-6 and IL-8, in the human colonic SEMFs. The inhibitors of IL-26 signaling pathway significantly suppressed the induction of IL-6 and IL-8. The inhibition of IL-26 signaling may lead to the suppression of intestinal inflammation.
The results of this study indicated that IL-26 may an important role in the pathogenesis of IBD. The authors suggested that IL-26 can a therapeutic candidate for IBD.
IL-26 is a member of the IL-10 cytokine family. IL-26 is located on chromosome 12q5. IL-26 requires the heterodimeric receptors composed of IL-20R1 and IL-10R2.
The authors demonstrated that an increased expression of IL-26 in the inflamed mucosa of IBD patients and explored its possible pathway in IBD pathology. The study is well designed and the demonstration seems sufficient.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Marzaban R, Pastorelli R, Reyes VE, Wu ZQ, Zimmer V S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Goldsmith JR, Sartor RB. The role of diet on intestinal microbiota metabolism: downstream impacts on host immune function and health, and therapeutic implications. J Gastroenterol. 2014;49:785-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 2. | Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 3. | Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 699] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 4. | Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 5. | Fuyuno Y, Yamazaki K, Takahashi A, Esaki M, Kawaguchi T, Takazoe M, Matsumoto T, Matsui T, Tanaka H, Motoya S. Genetic characteristics of inflammatory bowel disease in a Japanese population. J Gastroenterol. 2016;51:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Mizoguchi A, Takeuchi T, Himuro H, Okada T, Mizoguchi E. Genetically engineered mouse models for studying inflammatory bowel disease. J Pathol. 2016;238:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Knappe A, Hör S, Wittmann S, Fickenscher H. Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J Virol. 2000;74:3881-3887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1566] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 9. | Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, Ghannam S, Molès JP, Danger Y, Ravon E, Lesaux S. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423-7430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 415] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 10. | Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 1061] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 11. | Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1031] [Cited by in RCA: 1050] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 12. | Hughes T, Becknell B, McClory S, Briercheck E, Freud AG, Zhang X, Mao H, Nuovo G, Yu J, Caligiuri MA. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008-4010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Che KF, Tengvall S, Levänen B, Silverpil E, Smith ME, Awad M, Vikström M, Palmberg L, Qvarfordt I, Sköld M. Interleukin-26 in antibacterial host defense of human lungs. Effects on neutrophil mobilization. Am J Respir Crit Care Med. 2014;190:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Sziksz E, Pap D, Lippai R, Béres NJ, Fekete A, Szabó AJ, Vannay Á. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediators Inflamm. 2015;2015:764641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 15. | Corvaisier M, Delneste Y, Jeanvoine H, Preisser L, Blanchard S, Garo E, Hoppe E, Barré B, Audran M, Bouvard B. IL-26 is overexpressed in rheumatoid arthritis and induces proinflammatory cytokine production and Th17 cell generation. PLoS Biol. 2012;10:e1001395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Dambacher J, Beigel F, Zitzmann K, De Toni EN, Göke B, Diepolder HM, Auernhammer CJ, Brand S. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Wang T, Díaz-Rosales P, Martin SA, Secombes CJ. Cloning of a novel interleukin (IL)-20-like gene in rainbow trout Oncorhynchus mykiss gives an insight into the evolution of the IL-10 family. Dev Comp Immunol. 2010;34:158-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Jones EA, Flavell RA. Distal enhancer elements transcribe intergenic RNA in the IL-10 family gene cluster. J Immunol. 2005;175:7437-7446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 847] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 20. | Sheikh F, Baurin VV, Lewis-Antes A, Shah NK, Smirnov SV, Anantha S, Dickensheets H, Dumoutier L, Renauld JC, Zdanov A. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol. 2004;172:2006-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Blumberg H, Conklin D, Xu WF, Grossmann A, Brender T, Carollo S, Eagan M, Foster D, Haldeman BA, Hammond A. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 432] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 22. | Hör S, Pirzer H, Dumoutier L, Bauer F, Wittmann S, Sticht H, Renauld JC, de Waal Malefyt R, Fickenscher H. The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J Biol Chem. 2004;279:33343-33351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007;114:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1-C9. [PubMed] |
| 25. | Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183-C201. [PubMed] |
| 26. | Miot C, Beaumont E, Duluc D, Le Guillou-Guillemette H, Preisser L, Garo E, Blanchard S, Hubert Fouchard I, Créminon C, Lamourette P. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut. 2015;64:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [PubMed] |
| 28. | Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology. 1979;77:843-846. [PubMed] |
| 29. | Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1356] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 30. | Mahida YR, Beltinger J, Makh S, Göke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341-G1348. [PubMed] |
| 31. | Andoh A, Fujino S, Bamba S, Araki Y, Okuno T, Bamba T, Fujiyama Y. IL-17 selectively down-regulates TNF-alpha-induced RANTES gene expression in human colonic subepithelial myofibroblasts. J Immunol. 2002;169:1683-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Nishida A, Andoh A, Imaeda H, Inatomi O, Shiomi H, Fujiyama Y. Expression of interleukin 1-like cytokine interleukin 33 and its receptor complex (ST2L and IL1RAcP) in human pancreatic myofibroblasts. Gut. 2010;59:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Nishida A, Nagahama K, Imaeda H, Ogawa A, Lau CW, Kobayashi T, Hisamatsu T, Preffer FI, Mizoguchi E, Ikeuchi H. Inducible colitis-associated glycome capable of stimulating the proliferation of memory CD4+ T cells. J Exp Med. 2012;209:2383-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Shimada M, Andoh A, Hata K, Tasaki K, Araki Y, Fujiyama Y, Bamba T. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, Fujiyama Y. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol. 2009;183:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1266] [Cited by in RCA: 1264] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 37. | Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 38. | Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 39. | Dige A, Støy S, Rasmussen TK, Kelsen J, Hvas CL, Sandahl TD, Dahlerup JF, Deleuran B, Agnholt J. Increased levels of circulating Th17 cells in quiescent versus active Crohn’s disease. J Crohns Colitis. 2013;7:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |