Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5395
Peer-review started: January 16, 2016
First decision: February 9, 2017
Revised: February 25, 2017
Accepted: March 4, 2017
Article in press: March 6, 2017
Published online: August 7, 2017
Processing time: 203 Days and 10.7 Hours
To assess the efficacy and safety of a new treatment modality, cellular immune therapy based on personalized peptide vaccination (PPV-DC-CTL) combined with radiotherapy, for treating advanced hepatocellular carcinoma (HCC).
A total of nine patients with advanced HCC were enrolled. Multidisciplinary consultation confirmed that all the patients definitely had no opportunity of surgery, because four patients had multiple liver metastases (the number of liver lesions > 3), one patient had liver metastases and portal vein tumor thrombosis, one patient had lung and bone metastases, two patients had liver and lung metastases and one patient had liver metastasis and peritoneal metastasis. Patients with metastasis were treated with precise radiotherapy combined with PPV-DC-CTL.
Following radiotherapy and one to three cycles of PPV-DC-CTL treatment, AFP levels were significantly decreased in six patients and imaging assessment of the lesions showed a partial response (PR) in three patients and stable disease in the other three patients. The response rate was 33% and disease control rate was 66%. This regimen was found to be safe and well tolerated. None of the patients developed liver or kidney side effects. Only one patient developed grade II bone marrow suppression and the remaining patients had no significant hematological side effects.
Radiotherapy combined with PPV-DC-CTL provides a new therapeutic strategy for patients with advanced HCC, which is well tolerated, safe, feasible and effective.
Core tip: Advanced hepatocellular carcinoma (HCC) is a challenging disease to treat because of its advanced stage at diagnosis and rapid progression. We developed a new treatment modality, cellular immune therapy based on personalized peptide vaccination combined with radiotherapy, to treat advanced HCC. It integrates personalized peptide vaccination in tumor immunotherapy, takes full advantages of the immune modulation of radiotherapy, promotes tumor cells to release antigens and results in a more effective therapeutic strategy with regard to local and systemic control.
- Citation: Shen J, Wang LF, Zou ZY, Kong WW, Yan J, Meng FY, Chen FJ, Du J, Shao J, Xu QP, Ren HZ, Li RT, Wei J, Qian XP, Liu BR. Phase I clinical study of personalized peptide vaccination combined with radiotherapy for advanced hepatocellular carcinoma. World J Gastroenterol 2017; 23(29): 5395-5404
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5395.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5395
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide[1]. The resection rate for HCC is approximately 10%-30% and the overall prognosis is very poor with a 5-year survival rate of 5%-6%[2]. Even worse, the recurrence rate is high after radical resection. In addition to surgery, radiofrequency ablation, transcatheter arterial chemoembolization (TACE), microwave ablation, cryoablation, radioactive seed implantation, high-intensity focused ultrasound, radiation therapy, chemotherapy and targeted drugs are available for patients with unresectable tumors; however, the efficacy of these treatments is limited and the long-term prognosis in these patients is still poor[3]. Moreover, serious side effects induced by treatments such as TACE, chemotherapy and targeted drugs make it less likely for patients to receive long-term treatment with these therapies.
Due to the success of immunotherapy in other tumor types, especially melanoma, it has been used with high expectation in HCC treatment[4]. The liver, as a metabolizing organ and immune organ, has unique characteristics and patients with HCC present with special anti- and pro-tumor responses during the development of this malignancy[4-6]. Currently, based on the tumor-associated antigens (TAAs) identified in different tumors, many cancer vaccination strategies have been investigated[7,8]. However, therapeutic vaccines for HCC are still unavailable, although the application of prophylactic vaccines, including the hepatitis B virus vaccine, has been reported to decrease the prevalence of HCC. Numerous factors hinder tumor vaccine research, which are mainly associated with the way the host immune system is stimulated to kill cancer cells. Shortage of TAAs or tumor specific antigens is the most important among these factors[9].
Recently, personalized peptide vaccination (PPV), a novel immunotherapeutic approach based on a specific pool of peptides, was introduced. The pool of peptides includes all information on the personal human leukocyte antigen class 1A type (HLA class 1A) and the pre-existing immunity of the host before vaccination.host before vaccination (Figure 1). A maximum of four HLA class 1A-matched peptides were selected from this pool and used for the PPV[7]. Compared with other methods of immunotherapy, the PPV has several advantages. First, it increases the possibility of avoiding both tumor heterogeneity and immunological diversity. Second, the vaccine contains ‘‘personalized’’ antigens with pre-existing immunity which can trigger antigen specific memory T cells to produce rapid and strong secondary immune responses. Moreover, an important characteristic of PPV is that it activates cytotoxic lymphocytes (CTL), which have stronger antitumor cytotoxicity, higher proliferative ability and more cytolytic activity than lymphokine-activated killer cells in vitro and in vivo. Recent studies have suggested that immunotherapy with cytotoxic lymphocytes plays an important role in preventing HCC recurrence and metastasis[4-6].
In order to improve the efficacy and reduce the side effects of such treatment, we conducted a phase I clinical trial and treated unresectable HCC patients with radiation therapy combined with immunotherapy consisting of PPV peptides, dendritic cells (DC) and CTL (PPV-DC-CTL). We successfully developed a new PPV-based immunotherapeutic approach using a maximum of four HLA class 1A-matched peptides selected from the pooled peptides of the host.
A total of nine patients with advanced HCC were enrolled. Multidisciplinary consultation confirmed that all the patients had no opportunity of surgery, because four patients had multiple liver metastases ( the number of liver lesions > 3), one patient had liver metastases and portal vein tumor thrombosis, one patient had lung and bone metastases, two patients had liver and lung metastases and one patient had liver metastasis and peritoneal metastasis (Table 1). Every patient has been informed of the study protocol and signed an informed consent form prior to the study. This clinical trial was approved by the Drum Tower Hospital ethical review board.
| Patients | Age | Sex | BCLC stage | HK | Tumor site(s) | Radiotherapy target | Dose of radiotherapy | Cycle(s) of PPV-DC-CTL |
| P1 | 66 | Male | B | IIIB | Liver | Partial liver mass | PGTV 5 Gy*10f; PTV 2.5 Gy*10f | 3 |
| P2 | 56 | Female | B | IIIB | Liver | Partial liver mass | PGTV 5 Gy*10f; PTV 2.5 Gy*10f | 3 |
| P3 | 54 | Male | C | IVa | Liver and portal vein tumor thrombosis | Portal vein tumor thrombosis | PGTV 5 Gy*10f; PTV 2.5 Gy*10f | 2 |
| P4 | 37 | Male | C | IVa | Bone and lung | Bone metastasis | PGTV 4 Gy*10f; PTV 3 Gy*10f | 3 |
| P5 | 56 | Male | C | IVa | Liver and peritoneal metastases | Peritoneal metastasis | PTV 0.5 Gy BID *2f | 2 |
| P6 | 45 | Male | B | IIIB | Liver | - | - | 2 |
| P7 | 43 | Male | B | IIIB | Liver | Partial liver mass | PGTV 5 Gy*10f; PTV 2.5 Gy*10f | 3 |
| P8 | 59 | Male | C | IVa | Liver and lung | Partial lung mass | PGTV 5 Gy*10f; PTV 2.5 Gy*10f | 1 |
| P9 | 72 | Male | C | IVa | Lung | - | - | 2 |
Radiotherapy: Patients with liver metastasis, portal vein tumor thrombosis and pulmonary metastasis were treated with precise radiotherapy [planning gross target volume (PGTV), ten fractions at 5 Gy each; planning target volume (PTV), ten fractions at 2.5 Gy each] combined with PPV-DC-CTL; patients with bone metastasis were treated with precise radiotherapy of bone (PGTV, ten fractions at 4 Gy each; PTV, ten fractions at 3 Gy each) combined with PPV-DC-CTL; patients with peritoneal metastasis were treated with precise TomoTherapy of the peritoneum (PTV, two fractions at 0.5 Gy each, BID) combined with PPV-DC-CTL.
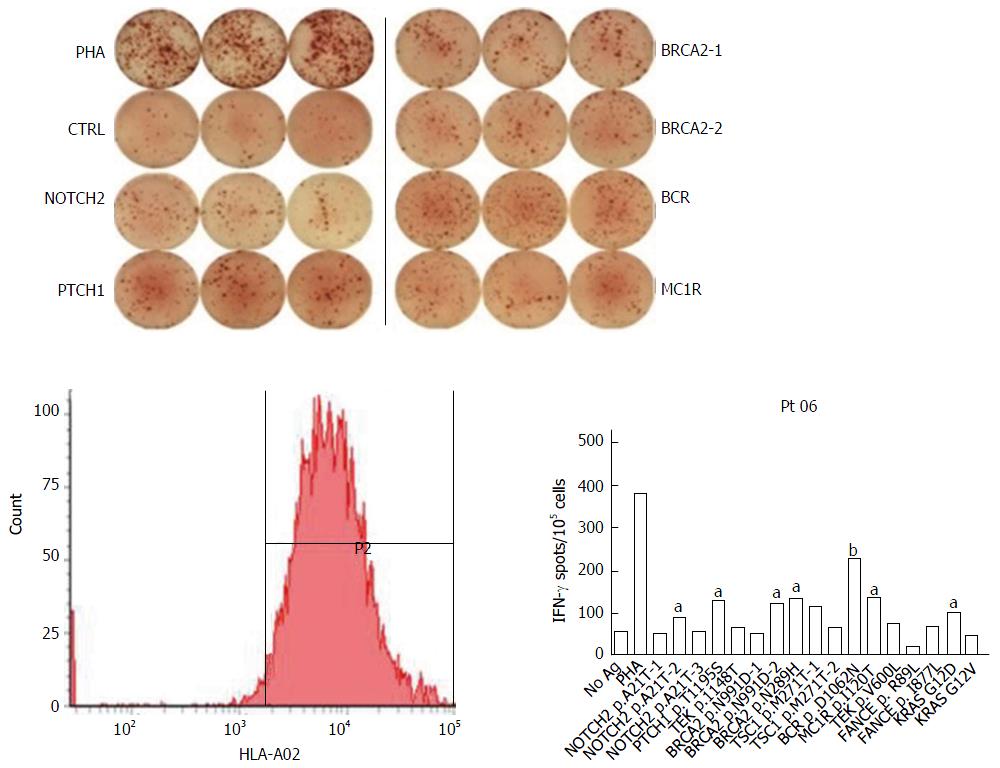
Selection of PPV peptides: First, peptide candidate library, including mutated peptides and highly expressed peptides, was established according to the gene mutation and expression spectra of HCC and previous studies about PPV. Peptides for vaccination of every specific patient were selected from the peptide candidate library with the consideration of the preexisting immunity of the host before vaccination (Figure 1). The detailed procedure was described in references[7,9] and the personalized peptides for these nine patients are as follows:
P1: CORE-18, MUC-12, KRAS-A02-G13D1, PSCA-76
P2: PI3KCA-A02-H1047L-1, CORE-35, WTP53-149, AFP-137
P3: EGFR-800, KRAS-A11-G13D, CYPB-84, CTNNB1-A11-S45F
P4: KRAS11-12C, EGFR-54, AFP-403, Survivin28-80
P5: AFP-357, VEGFR2-169, KRAS-A11-12C, MRP3-1293
P6: KRAS-A11-12D, CTNNB1-A11-41A, CTNNB1-A11-S45F, KRAS-A11-12R
P7: SART3-109, CORE-18, PSCA-7, hTERT-540
P8: AFP-357, KRAS-A11-12D, VEGFR2-169, PSCA-776
P9: CTNNB1-A11-S45F, CTNNB11-41A, CTNNB11-45P, EGFR-54
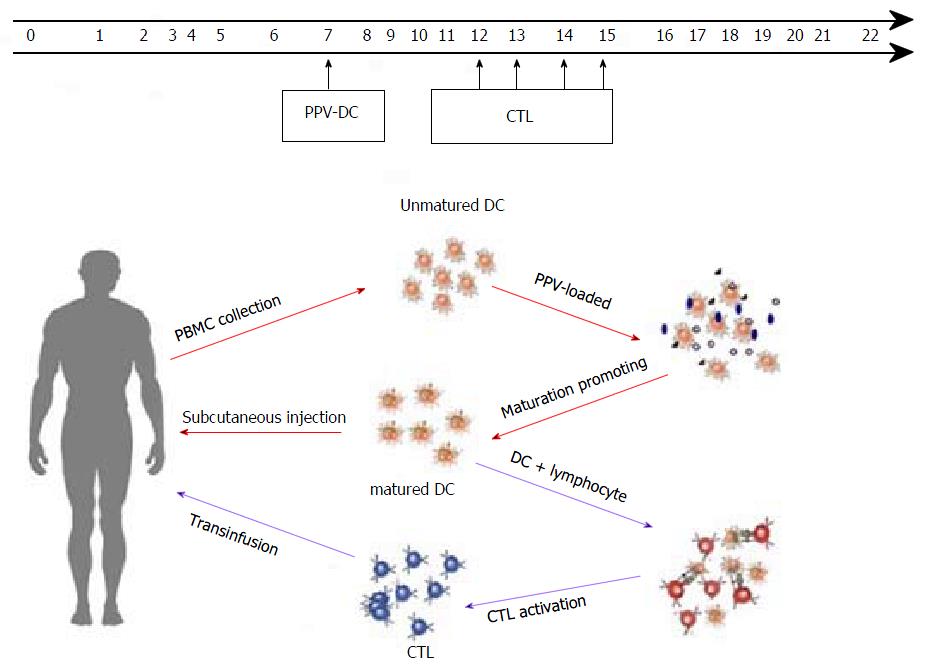
Collection of peripheral blood mononuclear cells (PBMC) and transfusion of DC-CTL: PPV peptides were load on DC (D0), which were sorted from PBMC and then these DC were infused back to patients after culture for 7 d in vitro (D7). To obtain the CTL, we used PPV and PPV-DC to stimulate the T cells. In 12 to 15 days, these CTL were transfused to patients (D12-15) (Figure 2). The above steps were defined as one cycle and it was repeated every 21 d.
To assess the immune responses to and other effects of PPV, the levels of CD3+, CD8+, CD4+ T lymphocytes, natural killer (NK) cells and B lymphocytes were examined prior to blood collection and after CTL transfusion. Alpha-fetoprotein (AFP) test was performed once a cycle after vaccinations. Routine blood tests and liver and kidney function tests were performed once a cycle. Other side effects such as rash, fever and diarrhea were also monitored.
Clinical response was evaluated by computed tomography scans and abdominal magnetic resonance imaging prior to vaccination and once every two cycles of therapy. The RESIST-based clinical response levels were assessed by partial response (PR), stable disease (SD) and progressive disease (PD). The percentage of patients with PR was defined as the response rate (RR) to treatment while PR plus SD was defined as the disease control rate (DCR).
Paired-samples t-test was used to compare the levels of CD3+, CD8+, CD4+ T lymphocytes, NK cells and B lymphocytes between prior to blood collection and after CTL transfusion. Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS, version 19.0.
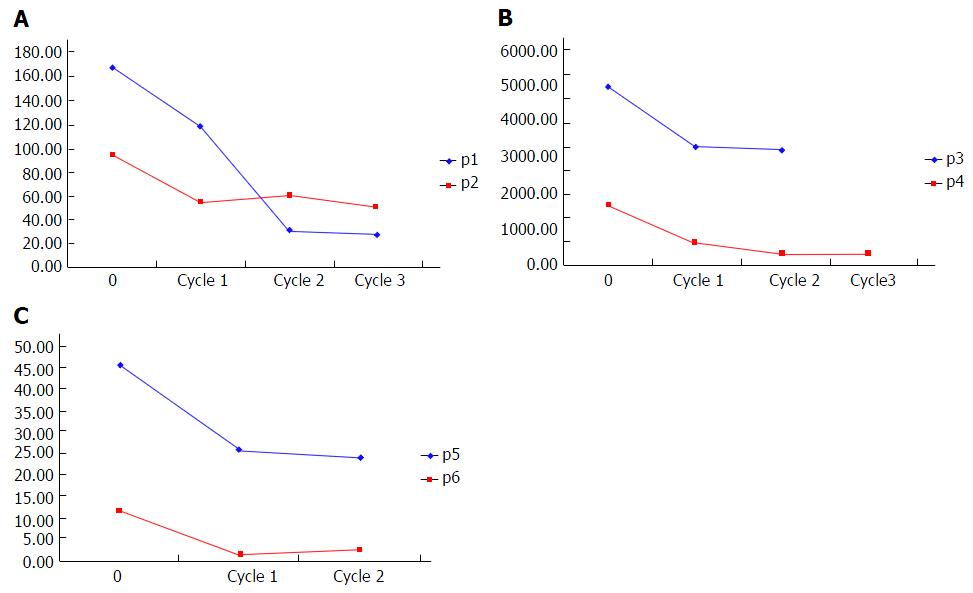
Following radiotherapy and 1-3 cycles of PPV-DC-CTL treatment, AFP levels were significantly decreased in six patients and imaging assessment of the lesions showed a PR in three patients and SD in the other three patients. RR was 33% and DCR was 66% (Table 2). This regimen was found to be safe and well tolerated. None of the patients developed liver or kidney side effects. Only one patient developed grade 2 bone marrow suppression and the remaining patients had no significant hematological side effects. Only two patients developed grade I rash and the remaining patients had no skin side effects. Six patients had low-grade fever (37 °C-38 °C) and none of the patients developed diarrhea (Table 3).
| Patient | Change of AFP | AFP before treatment | AFP after first cycle | AFP after second cycle | AFP after third cycle | Radiological evaluation |
| P1 | ↓ | 168.5 | 118.0 | 29.7 | 27.3 | PR |
| P2 | ↓ | 94.9 | 53.8 | 59.8 | 50.2 | PR |
| P3 | ↓ | 4942.3 | 3297.1 | 3180.0 | - | PR |
| P4 | ↓ | 1691.6 | 619.7 | 312.2 | 302.0 | SD |
| P5 | ↓ | 45.7 | 25.9 | 24.1 | - | SD |
| P6 | ↓ | 11.6 | 1.7 | 2.9 | - | SD |
| P7 | ↑ | 1029.7 | 1700.0 | 3800.0 | 3818.3 | PD |
| P8 | ↑ | 737.8 | 2005.6 | - | - | PD |
| P9 | ↑ | 157.5 | 294.1 | 248.0 | - | PD |
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Constitutional symptom | ||||
| Fever | 6 | 0 | 0 | 0 |
| Tumor pain | 0 | 0 | 0 | 0 |
| Rash | 2 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 |
| Respiratory | ||||
| Dyspnea | 0 | 0 | 0 | 0 |
| Hypoxia | 0 | 0 | 0 | 0 |
| Neurological | ||||
| CNS cerebrovascular ischemia | 0 | 0 | 0 | 0 |
| Blood/bone marrow | ||||
| Anemia | 2 | 0 | 0 | 0 |
| Neutropenia | 0 | 1 | 0 | 0 |
| Lymphocytopenia | 0 | 0 | 0 | 0 |
| Thrombocytopenia | 3 | 0 | 0 | 0 |
| Metabolic and laboratory | ||||
| AST elevation | 0 | 0 | 0 | 0 |
| ALT elevation | 0 | 0 | 0 | 0 |
| Scr elevation | 0 | 0 | 0 | 0 |
| BUN elevation | 0 | 0 | 0 | 0 |
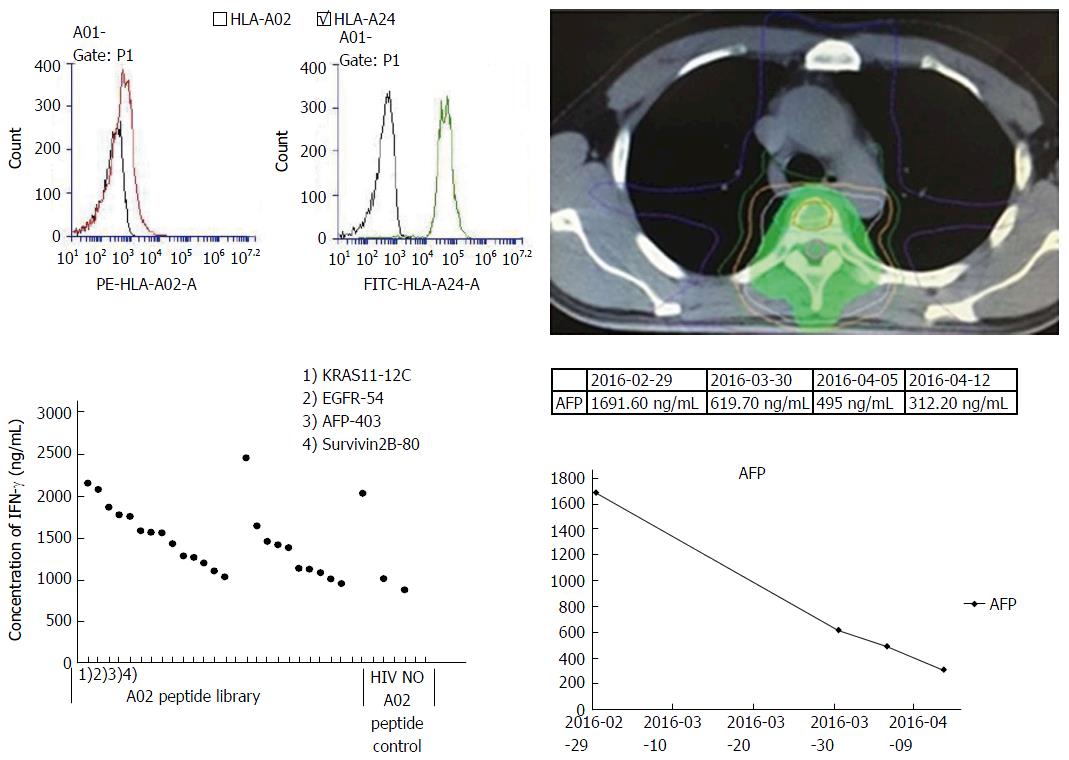
As indicated in Figure 3, the levels of AFP were significantly decreased in patients 1 to 6 and radiological evaluation showed PR or SD. Radiation treatment had a partial effect in patient 1 for certain tumor lesions. However, due to their large size, radiation may not reach all tumor metastases in the liver. The liver masses within and out of the radiation field were both significantly reduced in size after combined radiotherapy and PPV-DC-CTL treatment (Figure 4). This result suggested that CTL-based immune therapy and radiotherapy together reduced tumor progression even in those patients who did not receive radiotherapy. Patient 4 had tumor metastases in T4 vertebra and the lung. After treatment with radiotherapy and PPV-DC-CTL, AFP significantly declined and chest pain was alleviated. This provided further evidence that the synergistic combination of radiotherapy and PPV-DC-CTL effectively controlled tumor lesions, even in the lung where radiotherapy was not administered (Figure 5).
Four of nine patients completed three cycles of PPV-DC-CTL treatment, four patients completed two cycles and the remaining patient only received one cycle of treatment. Patient 3 did not continue the 3rd cycle of PPV-DC-CTL treatment due to carcinoma emboli in the portal vein which was controlled following treatment. The patient was treated with sorafenib instead with improvement. This patient was diagnosed in April 2015 with the last follow-up in June 2016. Compared with the reported mean overall survival (OS) of patients with carcinoma emboli in the portal vein in previous studies, the survival time of this patient was much longer (14 mo vs 3 mo). Due to a decrease in tumor markers, patients 5 and 6 both completed the 2nd cycle of treatment. Patient 5 received sorafenib instead and patient 6 received traditional Chinese medicine. To date, these two patients are still alive and their OS is over 10 mo. Patient 8 dropped out of the clinical trial due to a rise in tumor markers after the 1st cycle. Patient 9 did not continue treatment due to the development of rash. However, compared with pretreatment, patients 8 and 9 improved after PPV-DC-CTL treatment.
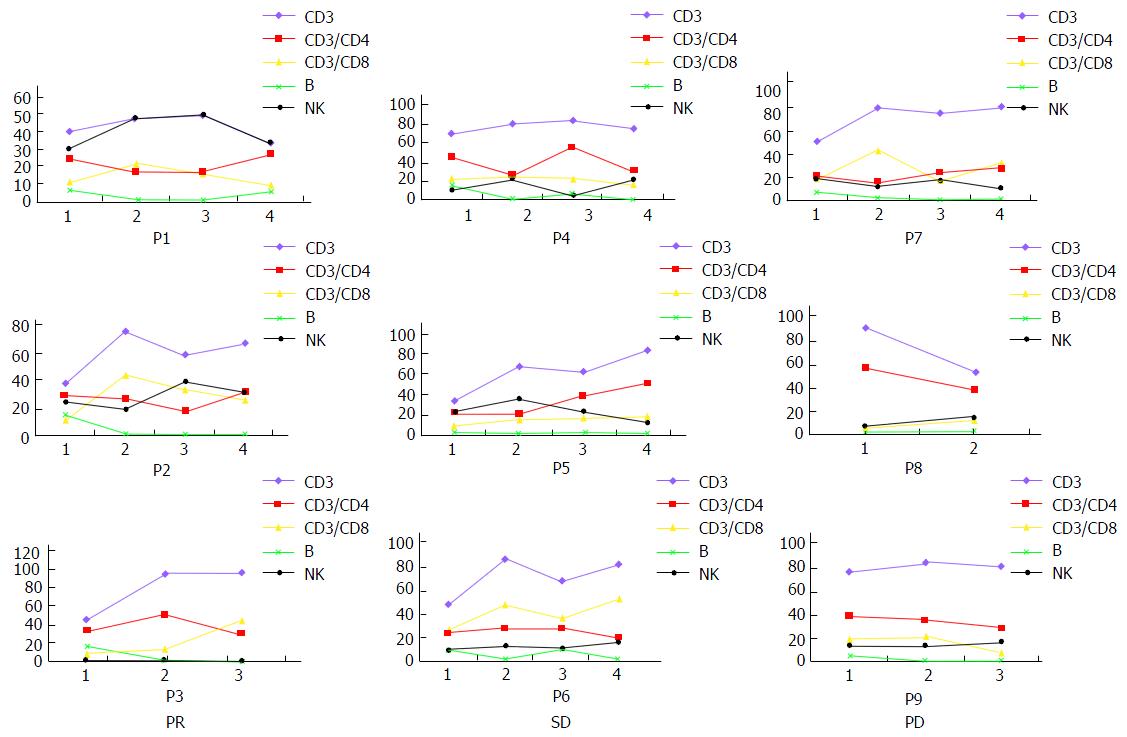
In addition, cellular immune responses specific to the treatment, including the levels of CD3+, CD8+, CD4+, NK cells and B lymphocytes, were analyzed in blood samples before and after CTL transfusion at each cycle. It was found that CD3+, CD8+ cytotoxic T lymphocytes and NK cells increased after CTL transfusion (P < 0.05), suggesting the possibility of immune activation (Table 4, Figure 6). However, B lymphocytes were decreased and CD4+ cytotoxic T lymphocytes showed no significant change.
To the best of our knowledge, this is the first report of the application and outcome of radiotherapy combined with PPV-DC-CTL for the treatment of advanced HCC. In the nine patients included in this study, this regimen was well tolerated without serious side effects and achieved good disease control, with an RR of 33% and a DCR of 66%, which was significantly better than those of TACE, sorafenib and chemotherapy[10].
It has been hypothesized that radiotherapy may successfully immunize some patients against cancer, converting the irradiated tissue into an in situ vaccine and endowing the host with a set of new and powerful tools to control systemic disease[11]. Radiotherapy can be used as a more general “immune response modifier”, a novel tool to add to the arsenal of immunotherapy agents, but the response varies with the dose per fraction[12-16]. It is still unclear how the host-tumor relationship is affected by radiation, but it has been proved that when switching from a conventional schedule (2 Gy/fraction, 5 fractions a week) to a 5-10 Gy/fraction schedule, the immune effect is more significant[17]. This may be due to more rapid cell killing, more vascular damage and stronger inflammatory cytokine induction at higher radiation doses. Therefore, in this context, the radiotherapy regimen we chose for liver and lung metastases was 5 Gy/fraction, 5 fractions a week. This schedule improves local control and can enhance the immune effect. However, we chose 4 Gy/fraction, 5 fractions a week for bone metastasis in order to protect the spinal cord. For peritoneal metastasis, we chose 0.5 Gy/fraction, 2-fractions, BID, with the purpose of reducing side effects in the colon and increasing the immune effect.
Based on pre-existing host immunity, a number of peptide antigens selected and screened from vaccine candidates are appropriate for this treatment regimen. In earlier studies on PPV, the assay of peptide specific IFN-γ production with an average cut-off level of 1 in 10000 cells was used to define pre-existing immunity. It was discovered that the enlargement and magnitude of CTL activation partly depend on the frequency of peptide specific CTL precursors from PBMC[7,18]. When CTL precursors are tested in PBMC before vaccination followed by the administration of specific peptides, a strong and rapid stimulation of CTL with potent clinical benefit is induced in specific patients as reported in some clinical trials of advanced cancer[19,20].
To date, a number of phase I and II clinical trials on PPV have been carried out[7]. All the trials mentioned above have indicated that PPV is well tolerated and safe without serious adverse effects and can stimulate a much stronger immune response in certain patients. Assessed using the response evaluation criteria, some patients who received PPV exhibited objective clinical responses with boosted immune responses[20]. In the current study, we also designed and conducted a phase I clinical trial involving patients with advanced HCC. First, we developed a new immunotherapeutic strategy of personalized peptide vaccination, according to the mutation spectrum of liver cancer and previous literature on vaccine peptides. We then stimulated strong activation of CTL with PPV peptide-loaded DC, which were transfused into patients to enhance the effect of immunotherapy. In addition, radiotherapy was administered to release vaccination in situ and to control the disease. Undoubtedly, PPV-DC-CTL and radiotherapy showed synergetic effects, especially in patients 1 and 4, as the tumors both within and out of the radiation field decreased in size or remained stable after treatment. Other advantages of this combined treatment were the avoidance of chemotherapy-induced side effects and overcoming the limitation of radiation therapy for extensive metastases.
In conclusion, we have shown the potential of PPV as a novel treatment strategy for advanced HCC patients. Further randomized clinical trials are essential to verify the clinical benefit of PPV in HCC patients. In addition, accurate biomarkers for predicting patients who would benefit most from PPV-based treatment remain to be identified.
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide. The resection rate for HCC is approximately 10%-30% and the overall prognosis is very poor with a 5-year survival rate of 5%-6%. Even worse, the recurrence rate is high after radical resection. In addition to surgery, radiofrequency ablation, transcatheter arterial chemoembolization (TACE), microwave ablation, cryoablation, radioactive seed implantation, high-intensity focused ultrasound, radiation therapy, chemotherapy and targeted drugs are available for patients with unresectable tumors; however, the efficacy of these treatments is limited and the long-term prognosis in these patients is still poor. Moreover, serious side effects induced by treatments such as TACE, chemotherapy and targeted drugs make it less likely for patients to receive long-term treatment with these therapies. The authors herein successfully developed a new PPV-based immunotherapeutic approach combined with radiotherapy, which was proved to be more effective and safe in HCC.
Immunotherapy has been used with high expectation in HCC treatment. The liver, as a metabolizing organ and immune organ, has unique characteristics and patients with HCC present with special anti- and pro-tumor responses during the development of this malignancy. Currently, based on the tumor-associated antigens (TAA) identified in different tumors, many cancer vaccination strategies have been investigated. However, therapeutic vaccines for HCC are still unavailable, although the application of prophylactic vaccines, including the hepatitis B virus vaccine, has been reported to decrease the prevalence of HCC. Numerous factors hinder tumor vaccine research, which are mainly associated with the way the host immune system is stimulated to kill cancer cells. In the current study, the authors developed a new PPV-based immunotherapeutic approach combined with radiotherapy, which was proved to be more effective and safe in HCC.
To the best of our knowledge, this is the first report of the application and outcome of radiotherapy combined with PPV-DC-CTL for the treatment of advanced HCC. In the current study, the authors have shown the potential of PPV as a novel treatment strategy for advanced HCC patients. Further randomized clinical trials are essential to verify the clinical benefit of PPV in HCC patients. In addition, accurate biomarkers for predicting patients who would benefit most from PPV-based treatment remain to be identified.
Radiotherapy combined with PPV-DC-CTL provides a new therapeutic strategy for patients with advanced HCC, which is well tolerated, safe, feasible and effective.
PPV-DC-CTL refers to personalized peptide vaccination. It is a novel immunotherapeutic approach based on a specific pool of peptides. The pool of peptides includes all information on the personal human leukocyte antigen class 1A type (HLA class 1A) and the pre-existing immunity of the host before vaccination. A maximum of four HLA class 1A-matched peptides were selected from this pool and used for the PPV. Compared with other methods of immunotherapy, the PPV has several advantages. First, it increases the possibility of avoiding both tumor heterogeneity and immunological diversity. Second, the vaccine contains ‘‘personalized’’ antigens with pre-existing immunity which can trigger antigen specific memory T cells to produce rapid and strong secondary immune responses. Moreover, an important characteristic of PPV is that it activates cytotoxic lymphocytes (CTL), which have stronger antitumor cytotoxicity, higher proliferative ability and more cytolytic activity than lymphokine-activated killer cells in vitro and in vivo.
This is an interesting study. The issue proposed by the authors is an important and potential method in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Facciorusso A, Kao JT, Streba LAM S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang S
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12182] [Article Influence: 1522.8] [Reference Citation Analysis (3)] |
| 2. | Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol. 2013;59:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Dhir M, Melin AA, Douaiher J, Lin C, Zhen WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK, Are C. A Review and Update of Treatment Options and Controversies in the Management of Hepatocellular Carcinoma. Ann Surg. 2016;263:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 4. | Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12:681-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 450] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 5. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-1391.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 6. | Hong YP, Li ZD, Prasoon P, Zhang Q. Immunotherapy for hepatocellular carcinoma: From basic research to clinical use. World J Hepatol. 2015;7:980-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Noguchi M, Sasada T, Itoh K. Personalized peptide vaccination: a new approach for advanced cancer as therapeutic cancer vaccine. Cancer Immunol Immunother. 2013;62:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Yamada A, Sasada T, Noguchi M, Itoh K. Next-generation peptide vaccines for advanced cancer. Cancer Sci. 2013;104:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Yamada T, Terazaki Y, Sakamoto S, Yoshiyama K, Matsueda S, Komatsu N, Waki K, Yamada A, Kawahara A, Kage M. Feasibility study of personalized peptide vaccination for advanced non-small cell lung cancer patients who failed two or more treatment regimens. Int J Oncol. 2015;46:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 11. | Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84:879-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 807] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 13. | Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099-3107. [PubMed] |
| 14. | Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, Friedman K, Ponzo F, Babb JS, Goldberg J. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 526] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 15. | Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 834] [Article Influence: 69.5] [Reference Citation Analysis (1)] |
| 16. | Yang G, Kong Q, Wang G, Jin H, Zhou L, Yu D, Niu C, Han W, Li W, Cui J. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother Radiopharm. 2014;29:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 433] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 18. | Hida N, Maeda Y, Katagiri K, Takasu H, Harada M, Itoh K. A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunol Immunother. 2002;51:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Mine T, Gouhara R, Hida N, Imai N, Azuma K, Rikimaru T, Katagiri K, Nishikori M, Sukehiro A, Nakagawa M. Immunological evaluation of CTL precursor-oriented vaccines for advanced lung cancer patients. Cancer Sci. 2003;94:548-556. [PubMed] |
| 20. | Tsuda N, Mochizuki K, Harada M, Sukehiro A, Kawano K, Yamada A, Ushijima K, Sugiyama T, Nishida T, Yamana H. Vaccination with predesignated or evidence-based peptides for patients with recurrent gynecologic cancers. J Immunother. 2004;27:60-72. [PubMed] |