Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5196
Peer-review started: April 20, 2017
First decision: May 12, 2017
Revised: May 16, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: July 28, 2017
Processing time: 110 Days and 22.1 Hours
To assess factors associated with the higher effect of metformin on mortality in diabetic colorectal cancer (CRC) patients, since the factors related to the effectiveness of metformin have not been identified yet.
Between January 2000 and December 2010, 413 patients diagnosed with both stage 3/4 CRC and diabetes mellitus were identified. Patients’ demographics and clinical characteristics were analyzed. The effect of metformin on CRC-specific mortality and the interactions between metformin and each adjusted factor were evaluated.
Total follow-up duration was median 50 mo (range: 1-218 mo). There were 85 deaths (45.9%) and 72 CRC-specific deaths (38.9%) among 185 patients who used metformin, compared to 130 total deaths (57.0%) and 107 CRC-specific deaths (46.9%) among 228 patients who did not use metformin. In multivariate analysis, survival benefit associated with metformin administration was identified (HR = 0.985, 95%CI: 0.974-0.997, P = 0.012). Interaction test between metformin and sex after adjustment for relevant factors revealed that female CRC patients taking metformin exhibited a significantly lower CRC-specific mortality rate than male CRC patients taking metformin (HR = 0.369, 95%CI: 0.155-0.881, P = 0.025). Furthermore, subgroup analysis revealed significant differences in CRC-specific mortality between the metformin and non-metformin groups in female patients (HR = 0.501, 95%CI: 0.286-0.879, P = 0.013) but not male patients (HR = 0.848, 95%CI: 0.594-1.211, P = 0.365). There were no significant interactions between metformin and other adjusted factors on CRC-specific mortality.
We showed a strong sex-dependent difference in the effect of metformin on CRC-specific mortality in advanced stage CRC patients with diabetes.
Core tip: Evidence from previous studies has identified the anti-tumor effect of metformin; however, the factors related to effectiveness of metformin in diabetic colorectal cancer (CRC) patients have not been identified yet. Identifying subgroup patients who benefit from metformin treatment is important for future clinical application of metformin, and a strong sex-dependent difference of metformin effect in advanced CRC patients has been identified in this present study.
- Citation: Park JW, Lee JH, Park YH, Park SJ, Cheon JH, Kim WH, Kim TI. Sex-dependent difference in the effect of metformin on colorectal cancer-specific mortality of diabetic colorectal cancer patients. World J Gastroenterol 2017; 23(28): 5196-5205
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5196.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5196
Although the survival rate for colorectal cancer (CRC) has increased because of early detection and intervention at earlier stages, CRC is still the 3rd most common cancer and the 4th leading cause of cancer death in the western and Asian countries[1-3]. Cancer and diabetes, especially type 2 diabetes mellitus (DM), are two of the most prevalent diseases and major causes of morbidity and mortality worldwide[4]. Despite some argument concerning the influence of diabetes on CRC, studies, including meta-analyses, have consistently demonstrated that type 2 DM is an independent risk factor for CRC and that diabetic patients with CRC have worse outcomes than non-diabetics[4,5]. A possible role for anti-hyperglycemic medications in progression and prognosis of CRC has been suggested, based on the hypothesis that tumor growth is promoted by the trophic action of insulin[4,6].
Metformin is broadly used for the treatment of type 2 DM, which successfully decreases circulating levels of glucose and insulin mainly by improving insulin resistance. Evidence from preclinical studies has identified the anti-tumor effect of metformin, showing inhibition of tumor growth and induction of apoptosis in cell lines and animal models of various cancers[7-9]. Several clinical studies, including our previous study[10-18], have shown the ability of metformin to reduce the incidence of CRC and improve survival of CRC patients. As stated in other studies, one of the potential mechanisms of the anti-tumor effect of metformin is via activation of AMP-activated protein kinase (AMPK). AMPK activation has an inhibitory effect on cancer cell growth and new blood vessel formation by prohibiting activation of the mammalian target of rapamycin (mTOR)[19-21]. With these direct cellular effects of metformin, the indirect or systemic effect of metformin is relief of insulin resistance-associated hyperinsulinemia and hyperglycemia, which counteracts the dependence of cancer cells on glucose as predominant source of energy[20,22].
Despite substantial evidence from in vivo and in vitro research supporting the possible efficacy of metformin as an anti-cancer agent and numerous clinical studies investigating the effect of metformin on CRC, particular factors or specific groups of patients associated with the effectiveness of metformin have not been identified. Our study assessed factors that may affect the efficacy of the anti-cancer action of metformin on CRC-specific mortality in diabetic CRC patients. Herein, we selected particular factors that might be associated with the “more effective” group (those who benefit from metformin for improving CRC-specific survival) and verified these assumptions using interaction analysis.
The electronic records of 9472 consecutive patients with a diagnostic code of colon or rectal cancer seen at a single institution (Severance Hospital, Yonsei University, Seoul, Korea) between January 1, 2000 and December 31, 2010 were identified. A manual retrospective review was conducted for all patients to identify those with a prior history of DM. Among those identified, 1584 had the type 2 diabetes diagnostic code during follow-up, of which 790 were excluded based on the following exclusion criteria: type I diabetes (n = 38), diabetes diagnosed after CRC diagnosis (n = 521), incomplete records (including medication records) (n = 77), metformin use for less than 6 mo (n = 105), and any cancer previous to CRC diagnosis (n = 49). According to our previous studies, only stage 3 CRC patients[12] and resectable stage 4 CRC patients[23] showed a survival benefit from metformin. Considering these results, advanced stage CRC patients in the latter two groups, denoting stage 3 and 4 patients, were selected and analyzed; this group included 185 DM patients treated with metformin and 228 DM patients not taking metformin. There were 135 female patients (32.7%) in the study population.
Patient demographics and clinical characteristics, including age at diagnosis, sex, total follow-up duration, duration of diabetes, body mass index (BMI), family history of colorectal malignancy, smoking history and drinking history were obtained from medical records. BMI was stratified into “underweight” (BMI < 18.5), “normal” (BMI range: 18.5-24.9), “overweight” (BMI range: 25.0-29.9) and “obese” (BMI ≥ 30.0), based on World Health Organization BMI classification[24]. Laboratory findings included plasma glucose levels, glycated hemoglobin (HbA1C) levels, and pretreatment carcinoembryonic antigen (CEA) levels. Information relevant to the CRC diagnosis, such as stage, site, histology, differentiation, resection margin, lymphovascular invasion, microsatellite instability (MSI) status, and treatment modality were reviewed via the medical records as well. The use of other diabetes medications (sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, insulin, etc.) and the use of aspirin were also explored. The date of diagnosis of CRC was defined as the day of pathologic diagnosis. Every enrolled patient had undergone colonoscopy. We identified deaths through medical records, and determined the cause of death in all cases.
The institutional review board of Severance Hospital, Yonsei University, Seoul, Korea approved this study.
All patients were diagnosed with pathologically confirmed CRC and were evaluated during their baseline visit to Severance Hospital for appropriate staging according to the 7th version of the AJCC Tumor/Node/Metastatic staging system. Treatment modality was determined by extent and location of the tumor. Based on the National Comprehensive Cancer Network guideline, locally advanced tumors or advanced tumors with resectable metastatic lesions were treated by surgery followed by adjuvant chemotherapy with or without radiotherapy, or by neoadjuvant chemotherapy, or chemoradiation therapy followed by surgery. Advanced CRC with distant metastasis was treated by palliative chemotherapy or conservative care.
Differences between the metformin and non-metformin groups with regard to covariates were determined using Pearson’s χ2 test or Student’s t-test when the data were categorical or continuous, respectively. In the primary analyses, the odds of overall and CRC-specific death for patients with diabetes treated with metformin and not treated with metformin were calculated using univariate logistic regression analysis. The multivariate Cox proportional hazards regression method was used to estimate HRs and 95%CIs after adjustment for patient-related variables, including age at diagnosis, sex, stage of cancer, BMI, diabetes duration, smoking history, cancer site, and use of insulin, aspirin, sulfonylurea and thiazolidinedione.
Survival curves were generated using the Kaplan-Meier method and were compared using log-rank statistics. In the secondary analyses, interaction analyses of Cox regression results were performed to reveal the factors associated with metformin use. These variables included age at diagnosis (≥ 50 or < 50), sex (male or female), smoking history (yes or no), tumor stage (III or IV), site (colon or rectum), sulfonylurea use (yes or no), insulin use (yes or no), and DM duration (years).
All P values were two sided, with a P < 0.05 considered significant. Most of the statistical analyses were performed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, United States). SAS version 9.2 (SAS Inc., Cary, NC, United States) was used when identifying the cut-off value of metformin duration that provided the best fit of the log-rank test statistics of overall and CRC-specific survival.
The metformin and non-metformin groups had similar patient demographics and clinical characteristics (Table 1). The median age of patients was 64 years (range: 33-91 years). Baseline characteristics, including age at diagnosis, sex, BMI, familial history of cancer, smoking and drinking history, were not significantly different between the metformin group and the non-metformin group. Factors associated with cancer, including tumor stage, tumor site (colon or rectum), tumor differentiation, resection margin positivity, MSI status, and pretreatment CEA level were not significantly different between the two groups. Clinical characteristics associated with diabetic severity status, such as HbA1C levels and duration of diabetes, were similar between the two groups; however, serum fasting glucose levels were lower in the non-metformin group compared to the metformin group (143.8 mg/dL vs 132.4 mg/dL, P = 0.012).
| Metformin group, n = 185 | Non-metformin group, n = 228 | P value | |
| Age at diagnosis in yr, mean ± SD | 63.5 ± 8.789 | 63.49 ± 10.218 | 0.991 |
| < 50 | 12 (6.5) | 27 (11.8) | 0.064 |
| ≥ 50 | 173 (93.5) | 201 (88.2) | |
| Sex | 0.921 | ||
| Male | 125 (67.6) | 153 (67.1) | |
| Female | 60 (32.4) | 75 (32.9) | |
| DM duration in yr, median (range) | 8 (1-120) | 6 (1-40) | 0.068 |
| Family history of CRC | 8 (4.3) | 13 (5.7) | 0.526 |
| BMI in kg/m2, mean ± SD | 23.6 ± 3.0 | 23.5 ± 3.0 | 0.596 |
| Normal < 25 | 148 (80.0) | 173 (76.5) | 0.300 |
| Overweight 25-30 | 33 (17.8) | 51 (22.6) | |
| Obese ≥ 30 | 4 (2.2) | 2 (0.9) | |
| Smoking | 0.371 | ||
| Never-smoker | 89 (48.1) | 123 (53.9) | |
| Ex-smoker | 42 (22.7) | 40 (17.5) | |
| Current smoker | 54 (29.2) | 65 (28.5) | |
| Alcohol | 0.556 | ||
| None | 82 (44.3) | 112 (49.1) | |
| < 1 drink/d | 42 (22.7) | 51 (22.4) | |
| ≥ 1 drink/d | 61 (33.0) | 65 (28.5) | |
| Aspirin use | 50 (27.0) | 39 (17.1) | 0.015 |
| Insulin use | 17 (9.2) | 27 (16.2) | 0.035 |
| Sulfonylurea use | 116 (62.7) | 153 (67.1) | 0.350 |
| Thiazolidinedione use | 18 (9.7) | 12 (5.3) | 0.082 |
| CEA in ng/mL, median (range) | 4.7 (0.2-9100.0) | 6.4 (0.1-5946.0) | 0.359 |
| HbA1c, mean ± SD | 8.7 ± 16.7 | 7.3 ± 1.4 | 0.349 |
| Glucose in mg/dL, AC ± SD | 143.8 ± 46.1 | 132.4 ± 41.1 | 0.012 |
| Cholesterol in mg/dL, total ± SD | 167.9 ± 47.8 | 164.6 ± 39.6 | 0.483 |
| Tumor stage | 0.110 | ||
| III | 136 (73.5) | 151 (66.2) | |
| IV | 49 (26.5) | 77 (33.8) | |
| Tumor site | 0.940 | ||
| Colon | 106 (57.9) | 130 (58.3) | |
| Rectum | 77 (42.1) | 93 (41.7) | |
| Histology | 0.001 | ||
| Adenocarcinoma | 177 (97.8) | 199 (89.6) | |
| Mucinous carcinoma | 4 (2.2) | 23 (10.4) | |
| Differentiation | 0.155 | ||
| Well differentiated | 13 (7.4) | 16 (7.8) | |
| Moderately differentiated | 148 (84.6) | 174 (84.5) | |
| Poorly differentiated | 14 (8.0) | 12 (5.8) | |
| Resection margin + | 3 (1.7) | 1 (0.6) | 0.371 |
| Lymphovascular invasion | 56 (25.9) | 76 (50.0) | 0.031 |
| MSI state | 0.670 | ||
| MSi | 78 (89.7) | 68 (90.7) | |
| MSI-low | 6 (6.9) | 6 (8.0) | |
| MSI-high | 3 (3.4) | 1 (1.3) | |
| Treatment modality | 0.160 | ||
| Resection only | 9 (4.9) | 20 (8.8) | |
| Resection + adjuvant chemotherapy | 115 (63.2) | 126 (55.3) | |
| Resection + chemoradiotherapy | 22 (12.1) | 24 (10.5) | |
| Neoadjuvant chemotherapy + resection | 17 (9.3) | 17 (7.5) | |
| Chemotherapy only | 17 (9.3) | 35 (15.4) | |
| Conservative care | 2 (1.1) | 6 (2.6) |
The use of other diabetes medications, including insulin, sulfonylurea and thiazolidinedione, was also investigated, because the status of individuals taking these medications could reflect later stage diabetes, and these medications could be associated with tumorigenesis and prognosis. The use of these medications was not significantly different between the two groups, with the exception of insulin use, which was lower in the metformin group than in the non-metformin group (9.2% vs 16.2%, P = 0.035). Aspirin, a drug known to have beneficial effects in cancer survival, was also evaluated and its use was statistically different between two groups (27.0% vs 17.1%, P = 0.015). Meanwhile, there was no difference in the treatment modality used for CRC between the two groups.
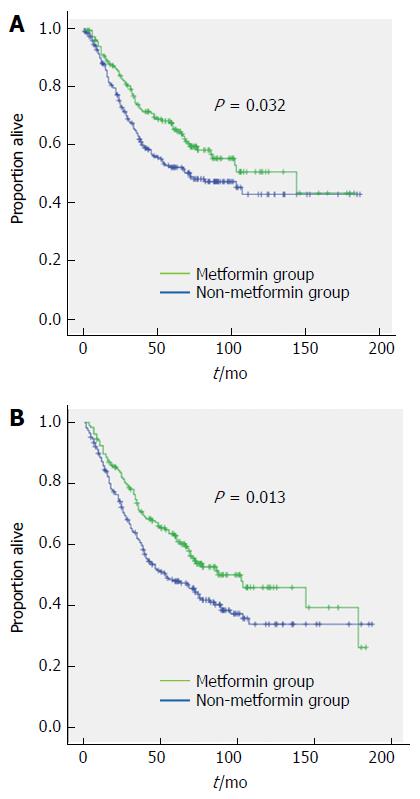
The median follow-up duration was 50 mo (range: 1-180 mo). With respect to the entire cohort, there were 129 (31.3%) recurrences, 215 (52.0%) total deaths, and 179 (43.3%) CRC-specific deaths. With respect to metformin use, there were 85 (45.9%) total deaths and 72 (38.9%) CRC-specific deaths among 185 patients who used metformin, compared with 130 (57.0%) total deaths and 107 (46.9%) CRC-specific deaths among 228 patients who did not use metformin. The estimated 5-year CRC-specific survival rates were 65.4% and 52.4% for the metformin and non-metformin groups, respectively, and 10-year CRC-specific survival rates were 50.8% and 43.1%, respectively. These results were significantly different (HR = 0.724, 95%CI: 0.537-0.976, P = 0.032) (Figure 1A). For the metformin and non-metformin groups, the estimated 5-year overall survival rates were 60.8% and 47.9% respectively, and the 10-year overall survival rates were 45.8% and 33.8% respectively, also showing significant differences (HR = 0.706, 95%CI: 0.537-0.929, P = 0.013) (Figure 1B).
In addition, we used the duration of metformin treatment in multivariate survival analysis, and showed this factor to be an independent predictor for CRC-specific mortality in diabetic patients with advanced CRC after adjustment of clinically relevant factors (HR = 0.985; 95%CI: 0.974-0.992, P = 0.012). BMI (HR = 0.514, 95%CI: 0.287-0.919, P = 0.025), tumor stage (HR = 8.401; 95%CI: 5.285-13.355, P < 0.001), and HbA1C level (HR = 1.015, 95%CI: 1.004-1.027, P = 0.01) were also revealed as independent predictive factors (Table 2).
| HR | 95%CI | P value | |
| Age at diagnosis of ≥ 50 or < 50 | 0.723 | 0.312-1.675 | 0.449 |
| Sex, female or male | 0.592 | 0.357-0.982 | 0.042 |
| BMI of ≥ 25 or < 25 | 0.514 | 0.287-0.919 | 0.025 |
| Smoking history as yes or no | 0.681 | 0.359-1.291 | 0.239 |
| Aspirin use as yes or no | 0.802 | 0.455-1.415 | 0.446 |
| Metformin treatment duration in mo | 0.985 | 0.974-0.997 | 0.012 |
| Sulfonylurea use as yes or no | 1.300 | 0.798-2.120 | 0.292 |
| Insulin use as yes or no | 1.041 | 0.511-2.121 | 0.912 |
| Stage IV or III | 8.401 | 5.285-13.355 | < 0.001 |
| Site as rectum or colon | 0.823 | 0.521-1.299 | 0.403 |
| Pathology | 0.801 | 0.238-2.701 | 0.721 |
| Diabetes duration | 0.968 | 0.935-1.001 | 0.059 |
| HbA1C | 1.015 | 1.004-1.027 | 0.010 |
We performed another analysis with the metformin group only, using total duration of metformin treatment. The results showed that improvement of CRC-specific (HR = 0.976, 95%CI: 0.948-0.995, P = 0.012) and overall survival rates (HR = 0.982, 95%CI: 0.967-0.997, P = 0.019) was associated with longer duration of metformin treatment, after adjustment of clinically relevant factors, including age at diagnosis, sex, medication history, tumor stage, tumor site, diabetes duration, and HbA1C. Analysis using Contal and O’Quigley’s method[25] revealed that the cut-off value for metformin treatment duration that fit the CRC-specific survival statistics was 22 mo.
To determine the subgroup with the greater metformin effect, interaction tests between metformin and each clinical factor were performed, after adjustment for other covariates including age at diagnosis, sex, BMI, medication use, stage, site, diabetes duration, and HbA1C. Interaction tests between metformin and sex with adjustment for relevant factors revealed that female CRC patients treated with metformin exhibited a significantly lower CRC-specific mortality rate compared to male CRC patients treated with metformin (HR = 0.369, 95%CI: 0.155-0.881, P = 0.025) (Table 3).
| HR | 95%CI | P value | |
| Metformin-Sex | 0.369 | 0.155-0.881 | 0.025 |
| Metformin-BMI | 1.000 | 0.974-1.026 | 0.972 |
| Metformin-Site | 0.941 | 0.441-2.006 | 0.875 |
| Metformin-HbA1c | 0.999 | 0.926-1.078 | 0.979 |
Subgroup analysis based on sex was performed and showed a significant difference in CRC-specific mortality between the metformin and non-metformin groups for females (HR = 0.013, 95%CI: 0.286-0.879, P = 0.013) (Figure 2B), while there was no significant difference between the two groups for males (HR = 0.365, 95%CI: 0.594-1.211, P = 0.365) (Figure 2A). Interaction analysis of metformin with other adjusted factors did not show any significant difference in CRC-specific mortality (Table 3). Intriguingly, as documented earlier, the duration of metformin treatment affected both CRC-specific mortality and overall mortality; however, the mean duration of metformin treatment between males and females was not significantly different (33.76 ± 24.45 mo for males and 28.05 ± 20.59 for females, log rank P = 1.06). Subgroup analysis based on metformin treatment showed that female patients had significantly lower CRC-specific mortality than males in the metformin group (HR = 0.332, 95%CI: 0.144-0.764, P = 0.009), while the non-metformin group showed no significant difference in CRC-specific mortality between male and female patients (HR = 0.73, 95%CI: 0.38-1.402, P = 0.345).
Two of the most common diseases worldwide, DM and CRC, share numerous risk factors. Previous studies, including meta-analyses, demonstrated the association between DM and increased risk of CRC; moreover, metformin, one of the most commonly prescribed anti-diabetes agents, improved survival of CRC patients[11,12,14,16,18]. We previously showed that CRC patients with diabetes treated with metformin had lower mortality than those not treated with metformin, and that metformin treatment was associated with a decreased incidence of colorectal adenomas in diabetic patients with previous CRC[12,26]. Furthermore, we showed an association between the metformin treatment in stage IV CRC patients with diabetes and lower risk of tumor recurrence after curative resection[23]. However, there has been no study that investigated the specific subgroup within CRC patients with diabetes who obtained a survival benefit from metformin use. In the present study, we aimed to determine the particular subgroup among diabetic CRC patients that could benefit from the anti-cancer effect of metformin and discovered that sex was the single clinical factor that predicted improved survival related to metformin treatment. In addition, by including the duration of metformin treatment as a factor, we showed that longer duration of metformin treatment was associated with improved CRC-specific and overall survival.
Metformin treatment has been associated with decreased risk and improved survival of DM patients with various types of cancer, including colorectal, pancreatic, liver, ovarian, breast, and endometrial[11,18]. The mechanism of action of metformin as an anti-cancer drug has not been clearly identified, although a shared pathogenesis for DM and some cancers is possible, e.g., beta oxidation of fatty acids or mitochondrial function[22]. One of the most well-known mechanisms of metformin is the stimulation of peripheral AMPK with decreased hepatic gluconeogenesis, increased insulin sensitivity, and hepatic fatty acid oxidation[20]. Under physiological conditions, AMPK is an intracellular energy sensor and is activated when the cellular AMP/ATP ratio increases. AMPK activation leads to inhibition of mTOR signaling. mTOR phosphorylation is mainly involved in cell growth, cell cycle progression, and angiogenesis. Inhibition of mTOR signaling can be an excellent cancer therapy target, as the mTOR pathway is commonly decontrolled in numerous types of cancer, and activation of this pathway is associated with poor prognosis and resistance to chemotherapy[21,27,28]. Other suggested anti-cancer mechanisms of metformin include reduced insulin growth factor-1, inhibition of angiogenesis, apoptosis, and induction of cell cycle arrest[6,29,30].
In our study population, females had a higher survival rate associated with metformin treatment after adjustment of other clinically significant factors. No other studies have reported the interaction between sex and survival benefit from metformin in diabetics with CRC. However, the study by Lee et al[18] of a cohort of 800000 Taiwanese showed that metformin effectively reduced the incidence of CRC in diabetic women and liver cancer in diabetic men, which suggested that sex could be an important interaction factor. Numerous explanations for this phenomenon can be suggested, and the higher survival rate of females compared to males among patients with CRC should initiate additional studies.
A Japanese study of 82402 patients with invasive CRC who had undergone surgery between 1985 and 2004 revealed a reduced risk of CRC-specific death for females relative to males that persisted over time[31]. McArdle et al[32] reported that overall survival and CRC-specific survival was significantly higher in females among patients who underwent elective surgery, after adjustment of clinical covariates. One study conducted in Israel by Purim et al[33] also reported sex-age interactions with the incidence of CRC and survival of CRC patients showed lower incidence and better prognosis for females. The answer for this superior CRC survival in females compared to males is usually related to female sex hormone status, particularly serum estrogen levels[31,34,35].
Circulating levels of 17b-estradiol (E2), the main estrogenic compound, are exceedingly higher in females compared to males and decrease with increasing age. While females are exposed to relatively high levels of endogenous E2 between adolescence and the 4th or 5th decade of life, in males, E2 levels remain low and steady, and drop minimally with aging. However, after menopause, serum E2 levels of females decline to levels similar to those of males. Moreover, the effect of estrogen on the gastrointestinal tract is well known, and in esophageal, gastric, and colon cancers, which have higher incidence and mortality rates among males, the role of estrogen has been investigated[36,37]. Wang et al[38] reported that people at risk of esophageal cancer have low levels of estrogen compared to healthy subjects. This finding was supported by experimental studies showing that estrogen regulates growth, cell differentiation, and cell function in the gastrointestinal tract. The possible role of estrogen in CRC development has been suggested by several lines of epidemiological, clinical and experimental evidence; however, the effect of estrogen in the progression of CRC has not been clearly identified[37,39].
With respect to metformin and female hormones, we hypothesized that metformin acts on the estrogen pathway to affect progression of CRC. This can be inferred from another result of Cossor et al[40], showing no significant survival benefit of metformin in post-menopausal females. Reports of the anti-cancer effect of metformin in estrogen receptor (ER)-positive breast cancer and the anti-estrogenic effect of metformin in control of abnormal endometrial proliferative disorders support this hypothesis[41,42]. The decrease in ER expression in tumors from females with endometrial cancer and DM treated with metformin compared to women treated with insulin also supports this hypothesis[42]. In addition, metformin repressed protein and mRNA expression of E2/ERα-regulated genes to a greater degree than tamoxifen, which resulted in inhibition of cell proliferation of ERα-positive breast cancer cells[41].
Interestingly, estrogen (E2) primarily prevented the development of CRC; however, in CRC patients, E2 promoted cancer progression[43]. Proliferation of CRC cells is known to be mediated by ERα, while the level of ERα expression is usually low in normal colon tissue and CRC tissue[43]. However, when the expression of ERβ in cancer cells decreases and the ratio of ERα/ERβ rises, ERα expression becomes dominant and results in cell proliferation and inhibition of apoptosis[43,44]. Interestingly, studies have demonstrated sex differences in ER expression in CRC[44,45]. Nüssler et al[44] reported a significant increase in ERα protein expression in males but not in females, while there was no significant difference in ERα and ERβ protein in normal colon mucosa between males and females. In the same study, ERβ protein expression in CRC cells was significantly decreased in both males and females, but far more in males[44].
Another study conducted by Press et al[45] reported the correlation between ERβ protein expression in CRC cells, overall survival and sex. Higher ERβ protein expression was associated with better overall survival in females but worse survival in males[45]. From these reports, we inferred that ER status in CRC tissue might have a role in cancer progression that could be different between males and females. The effect of metformin might be related to estrogen, regulation of ERα or ERβ expression, or, possibly, E2/ERα ratio as well. Although these relationships have not been elucidated thus far, we postulate that our findings provide the basis for future studies.
As confounding factors, DM severity and treatment with other drugs could affect the survival benefit conferred by metformin. Severity and duration of DM are important factors for cancer progression, considering that persistent hyperglycemia and hyperinsulinemia might alter the immune system and cause a chronic pro-inflammatory condition[4,5]. This pathologic state is due to the metabolic abnormalities that characterize diabetes, especially under conditions of poor metabolic control. In the present study, we measured glycated hemoglobin to represent the severity of DM, and total duration since diagnosis of DM. In addition, other anti-hyperglycemic agents may conceal or diminish this metformin-related cancer protection.
Therefore, we adjusted DM severity and duration, along with other anti-hyperglycemic agents, to assess the dose-dependent survival benefit of metformin. Several studies have investigated the relationship between metformin duration or dosage-related numerical values and the incidence of CRC. While there are some discrepancies between the study results, one study showed that patients treated with metformin for over 3 years showed a significantly reduced relative risk of CRC (HR = 0.643, 95%CI: 0.490-0.845) compared to patients not treated with metformin[46]. Interestingly, Lee et al[18] demonstrated that total cancer incidence was significantly associated with mean daily dose of metformin. Furthermore, subgroup analysis of males and females showed other intriguing results; only the hazard ratio of liver cancer incidence was significantly associated with mean daily dose of metformin in males, while CRC incidence was significantly associated with mean daily dose in females[18]. Our study results showed the relationship between the cumulative effect of metformin and CRC-specific survival. In addition, duration of DM since diagnosis, duration of metformin treatment, and level of glycated hemoglobin were not significantly different between males and females, which showed that the severity of DM or months of metformin treatment had no effect on the sex-related interaction.
While this study provided notable associations between metformin treatment and sex in the survival of CRC patients with DM, there were some limitations. First, we could not capture metformin treatment non-compliance, which could have resulted in exposure misclassification and biased the results toward the null hypothesis. Additional study limitations included a small sample size, which reduced the power to detect significant differences in survival, even though our findings were similar to previous studies of metformin treatment and CRC outcomes. Data regarding the specific cancer location, such as right or left sided, were not available for this study. Location of cancer is an important difference between males and females, where females develop more proximal, and males more distal colon and rectal cancers[47,48]. Finally, because the data analyzed in this study population were collected from a tertiary medical care unit, results may not be generalizable to the general population. Further studies with a larger and more diverse population should be conducted to strengthen the relationship between sex and the anti-cancer effect of metformin in CRC patients with DM. Moreover, future prospective studies should consider this sex-specific difference when performing clinical trials using metformin as an additive therapeutic agent for diabetic and non-diabetic CRC patients.
Previous studies showed metformin use was associated with decreased colorectal cancer (CRC) mortality. The identification of factors associated with the effect of metformin on mortality in diabetic CRC patients will provide useful information when applying metformin in cancer treatment.
Despite substantial evidence from in vivo and in vitro research supporting the possible efficacy of metformin as an anti-cancer agent and numerous clinical studies investigating the effect of metformin on CRC, particular factors or specific groups of patients associated with the effectiveness of metformin have not been identified. Herein, authors selected particular factors that might be associated with the “more effective” group, i.e., those who benefit from metformin for improving CRC-specific survival, and verified these assumptions using interaction analysis.
The authors discovered that sex was the single clinical factor that predicted improved survival related to metformin treatment. This is also the first study to report the interaction between sex and survival benefit from metformin in diabetics with CRC. Furthermore, results of this study showed the relationship between the cumulative effect of metformin and CRC-specific survival.
The results from this study showing sex-related effectiveness of metformin in survival of diabetic CRC patients can be applied to the additional usage of metformin in conventional adjuvant chemotherapy. These future prospective studies should consider this sex-specific difference when performing clinical trials using metformin as an additive therapeutic agent for diabetic and non-diabetic CRC patients.
Metformin is an oral medication which is broadly used for the treatment of type 2 diabetes mellitus by decreasing circulating levels of glucose and insulin and mainly by improving insulin resistance. AMP-activated protein kinase and mammalian target of rapamycin are intracellular molecules associated with cell metabolism and growth.
This is a very good work, the authors addressed the factors associated with the effect of metformin on mortality in diabetic CRC patients. Interestingly, the results showed that female CRC patients taking metformin exhibited a significantly lower CRC-specific mortality rate than male CRC patients taking metformin. Identifying subgroup patients who benefit from metformin treatment is important for further study in this field and this manuscript provided interesting and valuable findings.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abdel-Rahman WM, Guo XZ, Salvadori M, Zhu X S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9956] [Article Influence: 995.6] [Reference Citation Analysis (0)] |
| 2. | Lim D, Ha M, Song I. Trends in major cancer mortality in Korea, 1983-2012, with a joinpoint analysis. Cancer Epidemiol. 2015;39:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Sung JJ, Lau JY, Goh KL, Leung WK; Asia Pacific Working Group on Colorectal Cancer. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 598] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 4. | Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 765] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 5. | Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 590] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 6. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 7. | Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer. 2010;17:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Bojkova B, Orendas P, Garajova M, Kassayova M, Kutna V, Ahlersova E, Ahlers I. Metformin in chemically-induced mammary carcinogenesis in rats. Neoplasma. 2009;56:269-274. [PubMed] |
| 9. | Hosono K, Endo H, Takahashi H, Sugiyama M, Uchiyama T, Suzuki K, Nozaki Y, Yoneda K, Fujita K, Yoneda M. Metformin suppresses azoxymethane-induced colorectal aberrant crypt foci by activating AMP-activated protein kinase. Mol Carcinog. 2010;49:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Singh S, Singh H, Singh PP, Murad MH, Limburg PJ. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:2258-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Zhang P, Li H, Tan X, Chen L, Wang S. Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol. 2013;37:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Lee JH, Kim TI, Jeon SM, Hong SP, Cheon JH, Kim WH. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131:752-759. [RCA] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Fransgaard T, Thygesen LC, Gögenur I. Metformin Increases Overall Survival in Patients with Diabetes Undergoing Surgery for Colorectal Cancer. Ann Surg Oncol. 2016;23:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323-2328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, Eng C, Hassan MM. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Lin JJ, Gallagher EJ, Sigel K, Mhango G, Galsky MD, Smith CB, LeRoith D, Wisnivesky JP. Survival of patients with stage IV lung cancer with diabetes treated with metformin. Am J Respir Crit Care Med. 2015;191:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 19. | El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223-228. [PubMed] |
| 20. | Cusi K, Consoli A, DeFronzo RA. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1996;81:4059-4067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 123] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3802] [Cited by in RCA: 4202] [Article Influence: 175.1] [Reference Citation Analysis (0)] |
| 22. | Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci. 2013;34:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 23. | Lee DJ, Kim B, Lee JH, Park SJ, Hong SP, Cheon JH, Kim TI, Kim WH. [The effect of metformin on responses to chemotherapy and survival in stage IV colorectal cancer with diabetes]. Korean J Gastroenterol. 2012;60:355-361. [PubMed] |
| 24. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8251] [Article Influence: 392.9] [Reference Citation Analysis (0)] |
| 25. | Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Statistics Data Anal. 1999;30:253-270. [RCA] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 478] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 26. | Lee JH, Jeon SM, Hong SP, Cheon JH, Kim TI, Kim WH. Metformin use is associated with a decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer. Dig Liver Dis. 2012;44:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Wang XW, Zhang YJ. Targeting mTOR network in colorectal cancer therapy. World J Gastroenterol. 2014;20:4178-4188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 28. | Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med. 2011;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 29. | Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505-2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 429] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 31. | Kotake K, Asano M, Ozawa H, Kobayashi H, Sugihara K. Gender differences in colorectal cancer survival in Japan. Int J Clin Oncol. 2016;21:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | McArdle CS, McMillan DC, Hole DJ. Male gender adversely affects survival following surgery for colorectal cancer. Br J Surg. 2003;90:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Purim O, Gordon N, Brenner B. Cancer of the colon and rectum: potential effects of sex-age interactions on incidence and outcome. Med Sci Monit. 2013;19:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Wichmann MW, Müller C, Hornung HM, Lau-Werner U, Schildberg FW; Colorectal Cancer Study Group. Gender differences in long-term survival of patients with colorectal cancer. Br J Surg. 2001;88:1092-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Kim HM, Kim HS. Gender-specific Colorectal Cancer: Epidemiologic Difference and Role of Estrogen. Korean J Gastroenterol. 2014;63:201. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Singh S, Langman MJ. Oestrogen and colonic epithelial cell growth. Gut. 1995;37:737-739. [PubMed] |
| 38. | Wang QM, Yuan L, Qi YJ, Ma ZY, Wang LD. Estrogen analogues: promising target for prevention and treatment of esophageal squamous cell carcinoma in high risk areas. Med Sci Monit. 2010;16:HY19-HY22. [PubMed] |
| 39. | Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106:574-582. [PubMed] |
| 40. | Cossor FI, Adams-Campbell LL, Chlebowski RT, Gunter MJ, Johnson K, Martell RE, McTiernan A, Simon MS, Rohan T, Wallace RB. Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer Epidemiology. 2013;37:742-749. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Kim J, Lee J, Jang SY, Kim C, Choi Y, Kim A. Anticancer effect of metformin on estrogen receptor-positive and tamoxifen-resistant breast cancer cell lines. Oncol Rep. 2016;35:2553-2560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Markowska A, Pawałowska M, Filas V, Korski K, Gryboś M, Sajdak S, Olejek A, Bednarek W, Spiewankiewicz B, Lubin J. Does Metformin affect ER, PR, IGF-1R, β-catenin and PAX-2 expression in women with diabetes mellitus and endometrial cancer? Diabetol Metab Syndr. 2013;5:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: Estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19:5842-5848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Nüssler NC, Reinbacher K, Shanny N, Schirmeier A, Glanemann M, Neuhaus P, Nussler AK, Kirschner M. Sex-specific differences in the expression levels of estrogen receptor subtypes in colorectal cancer. Gend Med. 2008;5:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Press OA, Zhang W, Gordon MA, Yang D, Haiman CA, Azuma M, Iqbal S, Lenz HJ. Gender-related survival differences associated with polymorphic variants of estrogen receptor-β (ERβ) in patients with metastatic colon cancer. Pharmacogenomics J. 2011;11:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 47. | Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167-5175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 274] [Cited by in RCA: 334] [Article Influence: 33.4] [Reference Citation Analysis (6)] |
| 48. | Koo JH, Leong RW. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |