Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5167
Peer-review started: February 14, 2017
First decision: March 30, 2017
Revised: April 13, 2017
Accepted: May 9, 2017
Article in press: May 9, 2017
Published online: July 28, 2017
Processing time: 168 Days and 12.1 Hours
To investigate the underlying mechanism by which CXCL12 and CXCL6 influences the metastatic potential of colon cancer and internal relation of colon cancer and stromal cells.
Western blotting was used to detect the expression of CXCL12 and CXCL6 in colon cancer cells and stromal cells. The co-operative effects of CXCL12 and CXCL6 on proliferation and invasion of colon cancer cells and human umbilical vein endothelial cells (HUVECs) were determined by enzyme-linked immunosorbent assay, and proliferation and invasion assays. The angiogenesis of HUVECs through interaction with cancer cells and stromal cells was examined by angiogenesis assay. We eventually investigated activation of PI3K/Akt/mTOR signaling by CXCL12 involved in the metastatic process of colon cancer.
CXCL12 was expressed in DLD-1 cancer cells and fibroblasts. The secretion level of CXCL6 by colon cancer cells and HUVECs were significantly promoted by fibroblasts derived from CXCL12. CXCL6 and CXCL2 could significantly enhance HUVEC proliferation and migration (P < 0.01). CXCL6 and CXCL2 enhanced angiogenesis by HUVECs when cultured with fibroblast cells and colon cancer cells (P < 0.01). CXCL12 also enhanced the invasion of colon cancer cells. Stromal cell-derived CXCL12 promoted the secretion level of CXCL6 and co-operatively promoted metastasis of colon carcinoma through activation of the PI3K/Akt/mTOR pathway.
Fibroblast-derived CXCL12 enhanced the CXCL6 secretion of colon cancer cells, and both CXCL12 and CXCL6 co-operatively regulated the metastasis via the PI3K/Akt/mTOR signaling pathway. Blocking this pathway may be a potential anti-metastatic therapeutic target for patients with colon cancer.
Core tip: This study has provided the first report of fibroblast-derived CXCL12 enhancement of CXCL6 secretion in colon cancer cells, and of both CXCL12 and CXCL6 co-operatively regulating metastasis through the PI3K/Akt/mTOR signaling pathway. Blockage of this pathway may be a potential anti-metastatic therapeutic target for patients with colon cancer. Our work might encourage further investigation into more potent angiogenesis modulating agents to improve the effectiveness of colon cancer therapies.
- Citation: Ma JC, Sun XW, Su H, Chen Q, Guo TK, Li Y, Chen XC, Guo J, Gong ZQ, Zhao XD, Qi JB. Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J Gastroenterol 2017; 23(28): 5167-5178
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5167
Colon cancer is the fourth most frequently diagnosed cancer in the United States. In 2015, an estimated 93090 new cases of colon cancer occurred in the United States. During that same year, it was estimated that 49700 patients died from colon and rectal cancers[1]. The poor prognosis of colon cancer is attributable to its tendency of metastases. However, the precise mechanisms that determine the directional proliferation and invasion of cancer cells into specific organs remain to be established[2,3]. Therefore, exploring the fundamental mechanism of invasion, proliferation, metastasis and tumor biological behaviors at the level of cellular or molecular microenvironments is needed in clinical diagnosis and therapy.
Chemokines (chemotactic cytokines) form a complex family of small, secreted proteins that play an important role in innate and adaptive immunity, homeostatic processes, angiogenesis and tumorigenesis[4,5]. Based upon the position of conserved cysteine residues, chemokines are classified into four subfamilies (C, CC, CXC, CX3C)[6]. CXC chemokines have been proven to modulate tumor behaviors, especially in regulation of angiogenesis, activation of a tumor-specific immune response and stimulation of tumor cell proliferation in an autocrine or paracrine fashion[7]. However, updated research has shed new light on this subfamily of cytokines, indicating that its members have multifaceted roles in the microenvironment that consists of the tumor cells themselves and/or stromal cells, including infiltrating leukocytes, endothelial cells (ECs) and fibroblasts.
The functions of CXC chemokines in the tumor microenvironment depend considerably on the chemokine type and tumor and stromal cells’ characteristics. In addition, there are cases in which chemokines have been implicated as having tumor-inhibiting gene activities, and there are many more examples of CXC chemokines with tumor-promoting roles[8-11]. Two of the most famous members are the stromal cell-derived factor-1 (SDF-1/CXCL12/IL12) and chemokine ligand 6 (CXCL6). Numerous studies have shown that their activities would increase the establishment of tumorigenesis, invasion, proliferation and metastases.
Recent analysis has shown that CXCL12 supports the survival or growth of a variety of normal or malignant cell types, including hematopoietic progenitors, germ cells, leukemia B cells and breast carcinoma cells[12-15]. Other studies have shown that the CXCL12/CXCR4 and related axis are involved in tumor metastasis to sites which are characterized by high production of CXCL12, such as liver, lung and bone marrow[16,17]. Activation of the CXCL12/CXCR4 signaling axis leads to chemotaxis, cell survival, and/or proliferation; however, the downstream signaling cascades are tissue-specific and not well characterized in EC[18].
CXCL6, a small cytokine belonging to the CXC chemokine family, is also known as granulocyte chemotactic protein 2. As its former name suggests, CXCL6 is a chemoattractant for neutrophilic granulocytes[13-14]. It elicits its chemotactic effects by interacting with the chemokine receptors CXCR1 and CXCR2. This tumor progression may occur as a function of the regulation of angiogenesis, cell motility, immune cell infiltration, cell growth and survival in the microenvironment, and modulation of local anti-tumor immune responses[19]. As evidenced by various experiments, CXCL6 is over expressed in colorectal, breast, lung and thyroid cancers. Actions of tumor cells in the microenvironment were also regulated by complicated molecular mechanisms[20-23].
Different chemokines played their specific roles. Both the angiogenesis-promoting effect of CXCL6 and chemotactic effect of CXCL12 play important roles in tumorigenesis and metastasis[24,25]. However, the molecular mechanisms of the active signaling pathway by which CXCL12 and CXCL6 co-operatively regulate metastasis of colon cancer remain to be clarified.
The purpose of this study was to investigate the co-operative promotion of metastatic potential and the underlying mechanism of CXCL12 and CXCL6 in order to better understand the interaction between colon cancer cells and stromal cells. Furthermore, our study provided data to demonstrate that phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway plays an important role in CXCL12 simulation and that this process is involved in the development and metastasis of colon cancer. Understanding the biologic mechanisms responsible for regulation of chemokines may enable better molecular targeted therapies to treat patients with metastatic colon cancer.
Recombinant human CXCL6 and CXCL12 were purchased from R&D Systems (Minneapolis, MN, United States). Neutralizing monoclonal anti-human CXCL12 (anti-CXCL12 Ab), anti-human CXCL6 (anti-CXCL6 Ab), anti-human CXCR4 (anti-CXCR4 Ab) were obtained from Carbiochem (San Diego, CA, United States).
The human colon cancer cell lines HT29, WiDr, CaCo-2, DLD-1 and Colo320 were obtained from the American Type Culture Collection (Rockville, MD, United States). DLD-1, WiDr and CaCo-2 were maintained in minimum essential medium (Eagle’s; (Sigma Chemical Co., St. Louis, MO, United States) with high glucose and 10% fetal bovine serum (FBS). HT-29 and Colo320 were maintained in RPMI-1640 medium (Sigma Chemical Co.) supplemented with 10% FBS. HUVECs were obtained from Kurubo Co. (Osaka, Japan) and maintained in HuMedia-EG2 medium supplemented with 2% FBS, 5 ng/mL basic fibroblast growth factor, 10 μg/mL heparin, 10 ng/mL epidermal growth factor, and 1 μg/mL of hydrocortisone, according to the supplier’s instruction (Kurubo Co.). All cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air.
Cells were cultured in the media supplemented with 1% FBS for 1 d. After the indicated treatments, the cells were lysed in lysis buffer [25 mmol/L Tris (pH 7.8) with H3PO, 2 mmol/L CDTA, 10 mmol/L DTT, 10% glycerol, 1% Triton® X-100, 2 mmol/L PMSF, 1 mmol/L sodium orthovanadate, and 10 μmol/L leupeptin]. The protein concentrations were measured with a BCA protein assay kit (Pierce, Rockford, IL, United States). The amounts of samples were 30 μg per lane. The lysates were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene membrane (Immobilo PVDF; Nihon Millipore Ltd, Tokyo, Japan). The membrane was incubated in the blocking buffer for 60 min at room temperature. The blocking buffer consisted of 5% nonfat dry milk dissolved into Tris-buffered saline containing 0.1% Tween 20 (TBS-T). After washing the membrane with TBS-T, the membrane was immunoblotted with each primary antibody (Ab) diluted into 1:1000-2000 overnight at 4 °C. Afterward, membranes were washed with TBS-T three times, and subjected to HRP-conjugated secondary Ab for 60 min at room temperature. Protein Ab complexes were visualized with an ECL western blotting detection and analysis system (Amersham Biosciences, Buckinghamshire, United Kingdom). β-actin western blots served as controls.
All cancer cell lines and fibroblasts were separately seeded at a density of 3 × 105 cells/mL into 12-well plates containing medium with 10% FBS and allowed to adhere overnight. The medium was exchanged, and cells were cultured for an additional 48 h. The medium was collected and microcentrifuged at 1500 rpm for 5 min to remove particles, and the supernatants were frozen at -80 °C until performance of enzyme-linked immunosorbent assay (ELISA). Concentration of CXCL6 was measured by ELISA kit (R&D Systems) according to the manufacturer’s instructions.
In order to further investigate the synergistic effect of the tumor-stromal interaction, we tested the effect of fibroblast-derived CXCL12 on cancer cell CXCL6 production using a double-chamber method in 24-well plates. Fibroblasts were seeded at a density of 1 × 105 cells/well into 24-well plates, and allowed to adhere overnight. The medium was exchanged with or without CXCL12 Ab, and then co-cultured with 5 × 104 HT-29, WiDr, CaCo-2, DLD-1 and fibroblasts cells that had been placed into inserts with 0.45-mm2 pores (Kurabo Co.). The co-culture systems were incubated for an additional 48 h, and CXCL6 concentration was subsequently measured as described above. Each condition was assessed using 5 independent samples.
To confirm the effect of chemokines on HUVEC proliferation, we performed the proliferation assay according to the manufacturer’s instructions. HUVECs were seeded at a density of 5 × 103 cells/100 μL in 96-well plates and allowed to adhere overnight. Then, cultures were re-fed with fresh media containing various concentrations of CXCL6 or CXCL12. After 72 h incubation, 10 μL WST-1 reagent was added to each well and cells were incubated for another 4 h at 37 °C, then the cell proliferation was measured by the WST-1 Cell Proliferation Assay System (Takara Bio Inc., Shiga, Japan). The absorbance was determined using a microplate reader (Molecular Devices, Sunnyvale, CA, United States) at a test wavelength of 450 nm and reference wavelength of 690 nm.
The effects of CXCL12, CXCL6 and co-cultures with fibroblasts or colon cancer cells (DLD-1) on invasive capability of HUVECs were determined by Matrigel-coated invasion chambers (Becton Dickinson, Bedford, MA, United States) according to the manufacturer’s instructions. This system is separated by a PET membrane coated with Matrigel Matrix such that only invasive cells can migrate through the membrane to the reverse side. HUVECs (5 × 104 cells/mL) were suspended in medium containing 2% FBS and seeded into the Matrigel pre-coated transwell chambers consisting of polycarbonate membranes with 8-μm pores, and fibroblasts or DLD-1 cells were seeded at a density of 2 × 105 cells/well into the inner chambers in 24-well plates, then the transwell chambers were then placed into 24-well plates, into which we added basal medium only or basal medium containing gradient concentrations of CXCL6 (0 ng/mL, 0.1 ng/mL, 1 ng/mL, 10 ng/mL, 10 ng/mL + 10 μg/mL CXCL6 Ab) or CXCL12 (0 ng/mL, 0.1 ng/mL, 1 ng/mL, 10 ng/mL, 10 ng/mL + 10 μg/mL CXCL12 Ab). After incubating for 24 h and HUVECs for 16 h, the upper surface of the transwell chambers was wiped with a cotton swab and the invading cells were fixed and stained with Diff-Quick stain. The number of invading cells was counted in five random microscopic fields of the low filter surface under a microscope at 200 × magnification. Each condition was assessed in triplicate.
To investigate the influence of CXCL6 on tubule formation by HUVECs, HUVECs and fibroblasts were co-culture in the basal medium using an angiogenesis kit (Kurabo Co.) according to the manufacturer’s protocol. First, HUVECs and fibroblasts were co-cultured in 24-well plates with basal medium. The media were exchanged every 2 d, with co-incubation continuing for a total of 11 d. The co-culturing system was stained with anti-CD31 Ab. The areas of angiogenesis were measured quantitatively over ten different microscopic fields for each well using an image analyzer (Kurabo Co.).
To further investigate the influence of colon cancer cell-derived CXCL12 on tubular formation by HUVECs, the colon cancer cells (DLD-1 secreting CXCL12 or CaCo-2 and HT-29 not secreting CXCL12), HUVECs, and fibroblasts were co-cultured using a double-chamber method in 24-well plates. DLD-1, CaCo-2 or HT-29 cells (5 × 104 cells) were seeded into transwell chambers, consisting of polycarbonate membrane with 0.45-m pores and allowed to adhere overnight. Transwell chambers were then placed in the HUVECs/fibroblasts co-culture system with or without 10 ng/mL of CXCL12 or CXCL12 Ab and exchanged on the sixth day. All cells were cultured for a total of 11 d. HUVEC tubular formation was described as above. This assay allowed us to evaluate angiogenesis quantitatively and examine tumor-stromal interactions through soluble cytokines.
Data are presented as mean ± SD. Differences in the mean of two groups were analyzed by an unpaired t-test. Multiple group comparisons were performed by one-way ANOVA with a post hoc test for subsequent individual group comparisons. P < 0.05 was considered statistically significant. Mean values and SD were calculated for experiments performed in triplicate (or more).
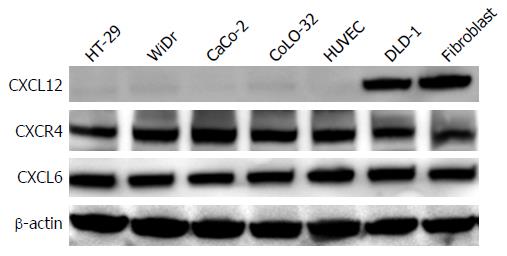
Western blotting results revealed that CXCL12 protein was only expressed in fibroblasts and DLD-1, but not in HT29, WiDr, CaCo-2, Colo320 and HUVECs. CXCR4 and CXCL6 were expressed in all colon cancer cell lines, fibroblasts and HUVECs (Figure 1).
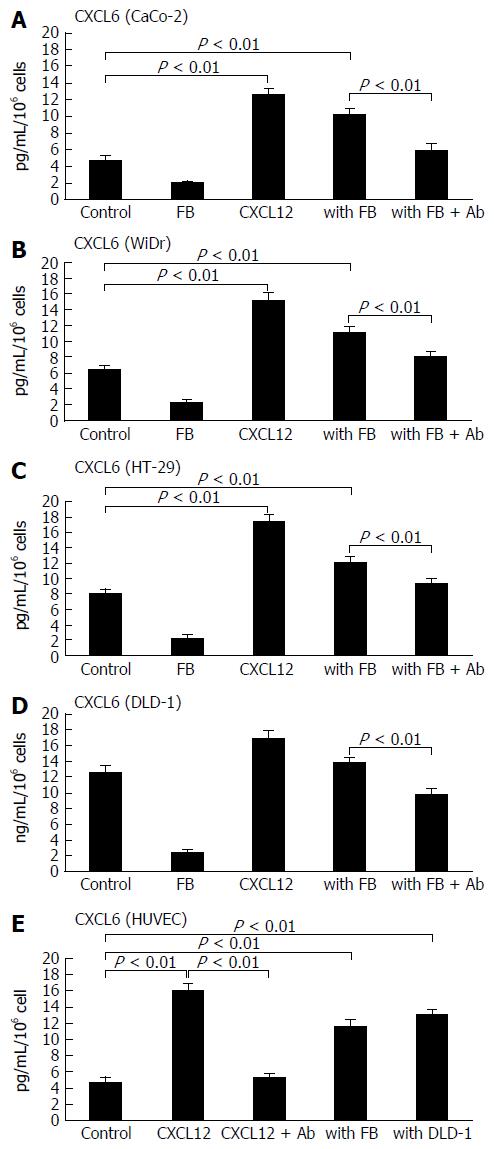
The secreted CXCL6 level was measured by ELISA assay in colon cancer cell lines and stromal cells. On the basis of this assay, secretion of CXCL6 was higher in DLD-1 and HT-29 cell supernatants than in supernatants from CaCo-2, WiDr and HUVECs. The addition of recombinant CXCL12 significantly enhanced CXCL6 production in CaCo-2 (2.54-fold vs control, P < 0.01; Figure 2A), WiDr (2.07-fold vs control, P < 0.01; Figure 2B), HT-29 (1.87-fold vs control, P < 0.01; Figure 2C) and HUVEC (2.79-fold vs control, P < 0.01; Figure 2E). Likewise, co-culture with fibroblast cells also significantly enhanced CaCo-2 (1.89-fold vs control, P < 0.01), WiDr (1.67-fold vs control, P < 0.01), HT-29 (1.62-fold vs control, P < 0.01) and HUVEC (2.15-fold, vs control, P < 0.01) cells’ secretion of CXCL6. On the other hand, recombinant CXCL12 and co-culture with fibroblasts did not promote the CXCL6 secretion in DLD-1 culture supernatants (Figure 2D). Co-culture with DLD-1 cells significant enhanced CXCL6 secretion level in the HUVEC culture supernatants as well (P < 0.01), because fibroblasts could secrete CXCL12 protein. Furthermore, the enhanced CXCL6 production elicited by co-culturing with fibroblast cells and recombinant CXCL12 were significantly inhibited in the presence of CXCL12 Ab (P < 0.01).
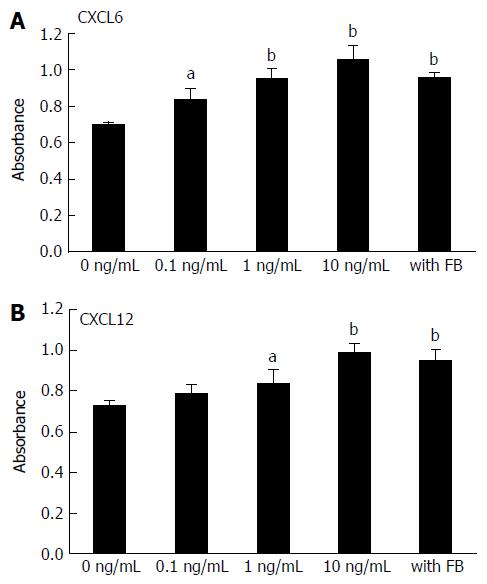
To create stromal cell supernatants, fibroblast cells were seeded to a final number of 5 × 106 cells/5 mL into 100-mm dishes containing medium with 10% FBS, and were cultured overnight. Cells were then cultured in medium containing 2% FBS for 48 h. The culture media were collected and microfuged at 1500 rpm for 5 min to remove any particles, and the supernatants were used in proliferation assays. Recombinant CXCL6 elicited enhanced proliferation of HUVECs in a dose-dependent manner, and co-culture with fibroblasts caused significantly enhanced HUVEC proliferation (P < 0.05, P < 0.01; Figure 3A). Recombinant CXCL6 also promoted the proliferation of HUVECs in a concentration-dependent manner (P < 0.05, P < 0.01; Figure 3B).
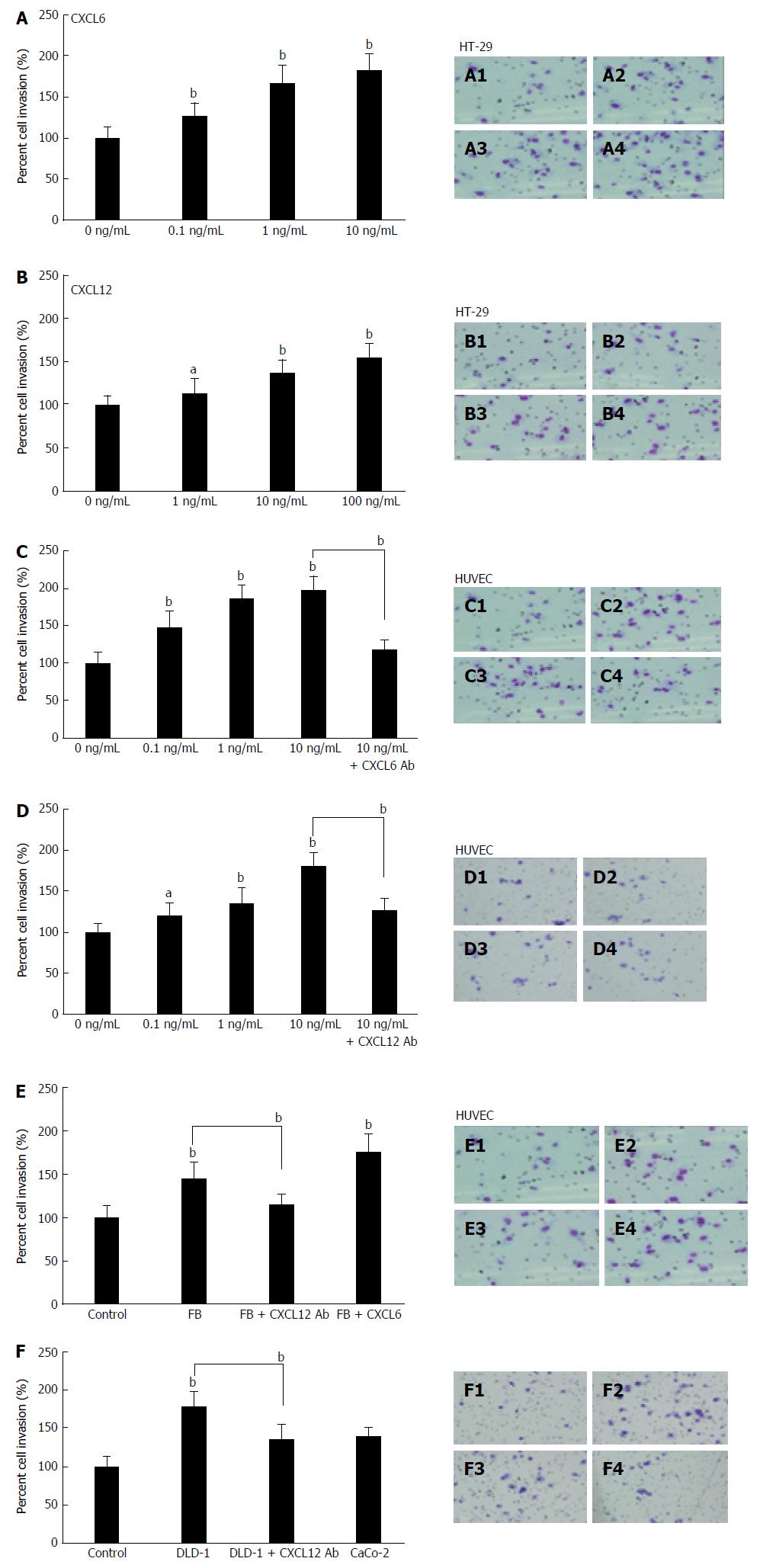
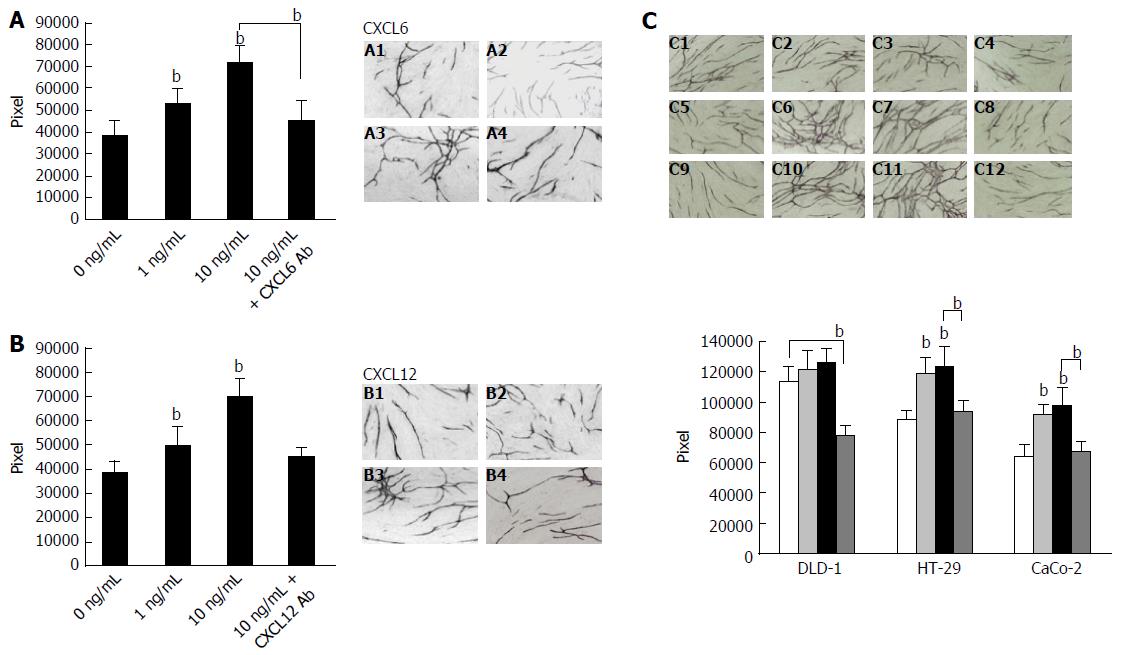
The invasion assay was used to investigate whether CXCL12 and CXCL6 influence invasiveness of colon cancer cell lines. The invasive capacity of HT-29 cells was promoted by stimulation using recombinant CXCL6 (Figure 4A) and CXCL12 (Figure 4B) in a concentration-dependent manner (P < 0.05, P < 0.01), and 10 ng/mL of CXCL6 and CXCL12 significantly promoted cancer cell invasion (P < 0.01). Interestingly, CXCL6 (Figure 4C) and CXCL12 (Figure 4D) also significantly enhanced the invasion of HUVECS in a dose-dependent manner (P < 0.05, P < 0.01). However, the invasive behavior of HUVECs upon CXCL6 stimulation was more significant than upon CXCL12 stimulation. Enhancement of invasive ability of HUVECs by CXCL6 and CXCL12 stimulation were blocked by pre-incubating HUVECs with neutralizing anti-CXCL6 and anti-CXCL12 Ab, respectively (P < 0.05, P < 0.01; Figure 4C).
To investigate the interaction between colon cancer and stromal cell-derived CXCL12 in the tumor microenvironment, we next examined the role of cell-derived CXCL12 on HUVECs’ invasiveness using the Matrigel double culturing chamber invasion assay. The invasive capability of HUVECs were enhanced by co-cultivation with fibroblasts (P < 0.01; Figure 4E) and DLD-1 (P < 0.01; Figure 4F); meanwhile, the enhancement of HUVEC invasive behavior was inhibited by neutralizing anti-CXCL12 Ab (P < 0.01), and the addition of recombinant CXCL6 significantly enhanced HUVECs invasiveness in co-cultivation with fibroblasts system as well (P < 0.01; Figure 4E). At the same time, co-cultivation with CaCo-2 cells did not significantly increase the invasion of HUVECs.
To further determine the role of CXCL12 and CXCL6 in the living cell microenvironment, we focused on the interaction between tumor cells and stromal cells by characterizing angiogenic activity in co-cultured fibroblasts and vascular ECs, and the effect of CXCL6 and CXC12 in this system. Initially, we measured the influence of CXCL6 and CXCL12 on tube formation by HUVECs. HUVEC tube formation was significantly enhanced in a dose-dependent manner following treatment CXCL6 (P < 0.01; Figure 5A) and CXCl12 (P < 0.01; Figure 5B). The enhanced angiogenesis of HUVECs was inhibited by the addition of neutralizing anti-CXCL6 and anti-CXCL12 Ab (P < 0.01).
In order to explore the different secreted CXCL2 of colon cancer cells influence on tube formation by HUVECs, we cultured three cell lines using double chamber methods to determine the interaction among them. The tubular formation was significantly enhanced by co-culture with DLD-1 cells compared with control (HUVECs and fibroblasts only) or co-culture with HT-29 and CaCo-2 cells, respectively (P < 0.01; Figure 5C). Moreover, the CXCL12 and CXCL6 could significantly promote the tubular formation in co-culture with HT-29 and CaCo-2 cells system (P < 0.01). In contrast, the enhanced tubular formation by HUVECs was significantly inhibited by addition of anti CXCL12 Ab in co-culture with DLD-1 cells (P < 0.01).
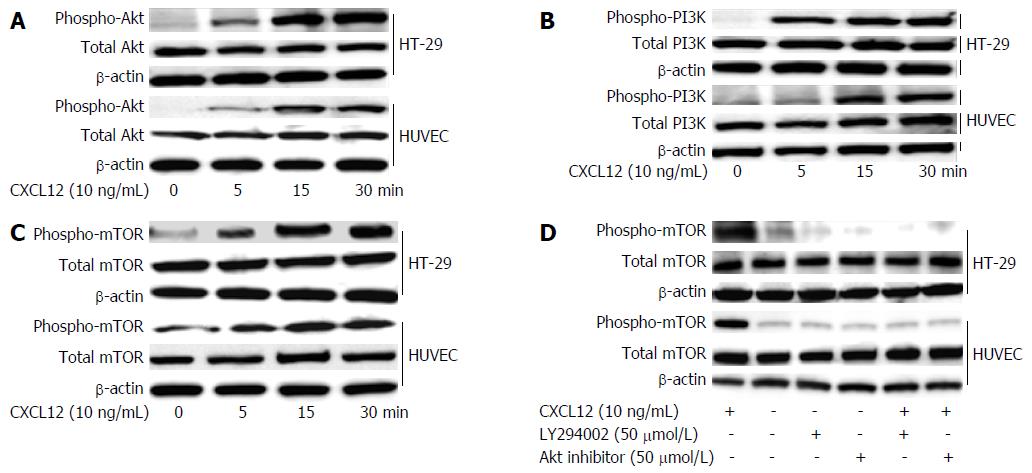
We used the colon cancer cell HT-29 and stromal cell HUVECs to examine activation of the PI3K/Akt/mTOR signaling pathway, a downstream target of CXCL12. The stimulation by 10 ng/mL of CXCL12 could increase Akt (Figure 6A), PI3K (Figure 6B) and mTOR (Figure 6C) phosphorylation in a time-dependent manner in HT-29 cells and HUVECs. To determine the role of mTOR, we investigated the effect of CXL12 and PI3K/Akt inhibitor on the activation of mTOR in colon cancer cells and HUVECs; we looked at the effects of IGF-1 and/or PI3/Akt kinase inhibitors on the activation of mTOR in these cells. HT-29 and HUVECs were pre-treated for 60 min with PI3K/Akt inhibitors and then stimulated overnight with CXCL12 (100 ng/mL). The extracted proteins were separated by SDS-PAGE, transferred to membranes, and the membranes probed with Ab directed against phospho-mTOR and total mTOR. We found that by CXCL12-mediated increase phospho-mTOR was inhibited by 50 μmol/L PI3K inhibitor (LY294002) and 50 μmol/L Akt kinase inhibitor. These data indicate that CXCC12 regulates the PI3K/Akt/mTOR signaling pathway activity and suggest that the PI3K/Akt/mTOR signaling pathway could participate in the regulation of metastatic behavior by colon cancer cells (Figure 6D).
Many tumors produce chemokines, which may explain the presence of the tumor-associated microenvironment. However, the role of these chemokines in tumor biology is still unclear. Chemokines form a complex family of small, secreted proteins that play important roles in innate and adaptive immunity, homeostatic processes, angiogenesis and tumorigenesis[4]. Recent exploration of the tumor microenvironment has become the crux of research aimed at explaining tumor behaviors, especially those involving metastasis of solid tumors as in colon, stomach, liver, lung and breast cancers.
The tumor microenvironment consists of tumor, stromal, immune and inflammatory cells, all of which produce cytokines, growth factors and adhesion molecules[26,27], and the abnormal expression of cytokines has been shown to have great effect on tumor behaviors, such as tumor progression and metastasis[28,29]. The CXC chemokine family of cytokines, which are founded in the microenvironment, represent a significant difference between tumors and normal tissues[30]. The tumor microenvironment contains secreted chemokines representing distinctive profiles, the components of each having specific target cells. The chemokine CXCL12, through its receptor CXCR4, positively regulates angiogenesis by promoting EC migration and tube formation. However, the relevant downstream signaling pathways in EC have not been defined.
Our previous studies elucidated that IL-1α is one of the most important inflammatory cytokines involved in the metastatic process of colon cancer. IL-1α contributed to the regulation of tumor growth, progression, and liver metastasis in primary gastric carcinoma and pancreatic cancer. Pancreatic cancer cell-derived IL-1α increases fibroblast-derived hepatocyte growth factor (HGF) secretion in a paracrine manner, and that enhanced HGF expression promotes invasion, proliferation and angiogenesis of cancer cells. In the living microenvironment of the tumor, the chemokines act as couriers or guides for promoting tumor development and the metastasis process[31-33].
As a structural component of tumor tissue, fibroblasts have been shown to be deeply involved in tumor proliferation and the mitogenic processes. Fibroblasts produce certain cytokines that influence neighboring cells, including malignant cells[4]. The precise role of chemokines in neovascularization during inflammation or tumor growth is not yet fully understood. We investigated here whether cancer cell stromal cell-derived CXCL12 influences colon cancer CXCL6 secretion, thereby co-regulating the metastatic potential of colon cancer. Our results revealed that CXCL12 was expressed in DLD-1 and fibroblasts, while CXCL6 and CXCR4 were expressed in all cell lines.
The most salient observations of our study were that the secreted CXCL6 levels by colon cancer cells and HUVECs were significantly promoted by cancer cell (DLD-1)- and stromal cell (fibroblast)-derived CXCL12 in the co-culturing system, and that the enhanced CXCL6 production could be significantly inhibited by CXCL12 Ab. Similar results were reported for other effects through the up-regulation of MMP-9, providing a possible mechanism mediating the effect of CXCL6 on metastasis[34]. In our study, CXCL6 and CXCL12 not only co-operatively enhanced proliferation and invasion of HUVECs, but also promoted the invasion of colon cancer cells. Similarly, CXCL6 has been reported to be up-regulated in colon cancer, and plays key roles in the induction and maintenance of gut inflammation, enhancing the development and growth of colitis-associated colorectal cancer[35].
To further investigate the inaction between CXC chemokines and cancer cell living microenvironment, we focused on the interaction between tumor cells and stromal cells by characterizing angiogenic activity in co-cultured fibroblasts and vascular ECs, and the effect of CXCL6 and CXC12 in this system. HUVEC tube formation was significantly enhanced by CXCL6. We aimed to explore the influence of different secreted CXCL2 from colon cancer cells on tube formation by HUVECs. The tubular formation was significantly enhanced by co-culture with DLD-1 cells, as compared with colon cancer cells, and this is related to the produced CXCL12. In contrast, the enhanced tubular formation by HUVECs was significantly inhibited by addition of anti-CXCL12 Ab in co-culture with DLD-1 cells (Figure 5). CXCL12 should be the initial factor secreted by fibroblasts, and the target colon cancer cells enhanced the secretion of CXCL6 after CXCL12 combined with its receptor CXCR4. The proliferation and invasion of colon cancer cells and HUVECs have been activated and enhanced after a series of complicated biochemical reactions.
Breakthroughs of insights into the tumor microenvironment have made great contributions towards clinical treatment. All kinds of anti-carcinoma chemo-therapeutics have been based upon this mechanism, and there is no exception among the newly targeted cancer therapies or the gene therapies; proof of effects on critical pathways in proliferation or differentiation are sought. Chemokines are chemo-attractant cytokines that regulate genetic activity of leukocytes and other cell types, including tumor and stromal cells[36]. It is already known that mTOR is an atypical intracellular serine/threonine protein kinase regulated by PI3K. The activated mTOR pathway has been identified in several human malignancies, thus being an attractive target for anticancer therapy[37].
Our results showed that CXCL12-enhanced secretion level of CXCL6 co-regulation of invasion, proliferation and angiogenesis were dependent on PI3K-Akt-mTOR signaling activation. However, both of these factors up-regulation of PI3K-Akt-mTOR survival signaling were decreased by selective inhibitors of PI3K and Akt. All these results suggest that both the CXCL12 factor and the enhancement of CXCL6 expression serve to co-operatively promote metastatic potential in colon cancer cells. CXCL12-induced activation of this signaling pathway could be inhibited by PI3K/Akt inhibitor, consistent with the inhibition of metastatic capabilities of colon cancer cells. This cascade may be a key pathway for colon cancer cells to metastasize.
Crosstalk between CXCR4, CXCL12 and PI3K/mTOR has been previously described in peritoneal disseminated gastric cancer and pancreatic cancer. The solid tumors indicate an interconnection between CXCL12 and mTOR signaling. Interfering with mTOR signaling has abolished chemotaxis towards CXCL12[38]. The mTOR will enhance cell growth and proliferation via promotion of the ribosome S6 protein kinase (p70S6K) and inhibition of the eIF4E-binding proteins (4E-BP1), and can even enhance the secretion of vascular endothelial growth factor and angiogenesis by promoting expression of the transcription factor hypoxia-inducible factor 1 and its downstream target genes. Under a series of exterior and interior factors, cancer cell proliferation and invasion can be induced and cell apoptosis can be avoided by initiating the PI3K/Akt/mTOR pathway[39].
In conclusion, this is the first report on the concomitant involvement of CXC12 and CXCL6 both transducing the mTOR pathway, affecting progression and spreading of human colon cancer cells, ultimately suggesting that targeting of CXCR4 and mTOR may improve therapeutic efficacy and prevent mTOR-targeting agents’ resistance. Our work should encourage further investigation into more potent angiogenesis modulating agents to improve the effectiveness of colon cancer therapies.
Colon cancer is the fourth most frequently diagnosed cancer worldwide. The poor prognosis of colon cancer is attributable to its tendency of metastases. However, the precise mechanisms of metastasis are still unknown. The target of this study was to investigate the underlying mechanism by which CXCL12 and CXCL6 influences the metastatic potential of colon cancer and the internal relation of colon cancer and stromal cell, as well as to investigate the interaction with CXCL12/CXCL6/PI3K/Akt/mTOR signaling in the metastatic process.
The functions of CXC chemokines in the tumor microenvironment depend considerably on the chemokine type and tumor and stromal cell characteristics. Both the angiogenesis-promoting effect of CXCL6 and chemotactic effect of CXCL12 play important roles in tumorigenesis and metastasis. However, the molecular mechanisms of the activity signaling pathway by which CXCL12 and CXCL6 co-operatively regulate metastasis of colon cancer remain to be clarified.
This research provides the first demonstrations of fibroblast-derived CXCL12 enhancing CXCL6 secretion of colon cancer cells. CXCL6 and CXCL12 not only co-operative enhanced proliferation and invasion of HUVECs, but also promoted the invasion of colon cancer cells via the PI3K/Akt/mTOR signaling pathway. Blocking this pathway may be a potential anti-metastatic therapeutic target for patients with colon cancer. This work might encourage further investigation into more potent angiogenesis modulating agents to improve the effectiveness of colon cancer therapies.
The concomitant involvement of CXC12 and CXCL6 transduces the mTOR pathway, affecting progression and spread of human colon cancer cells. The authors suggest that targeting CXCR4 and mTOR may improve therapeutic efficacy and prevent mTOR- targeting agents’ resistance. The authors’ work should encourage further investigation into more potent angiogenesis modulating agents to improve the effectiveness of colon cancer therapies.
The CXC chemokine family of cytokines, found in the microenvironment, represent the significantly distinctive profiles of tumors and normal tissues. The tumor microenvironment contains secreted chemokines representing distinctive profiles, the components of each having specific target cells. The chemokine CXCL12, through its receptor CXCR4, positively regulates angiogenesis by promoting endothelial cell (EC) migration and tube formation.
The results of this study for the relationship between CXCL6 and CXCL12 in colorectal cancer and ECs seem to be of interest to many readers, and the experiment is well planned. But, before publication, several issues have to be considered.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jung YD S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D, Jemal A. Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin. 2015;65:457-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 2. | Cotte E, Villeneuve L, Passot G, Boschetti G, Bin-Dorel S, Francois Y, Glehen O; French Research Group of Rectal Cancer Surgery (GRECCAR). GRECCAR 8: impact on survival of the primary tumor resection in rectal cancer with unresectable synchronous metastasis: a randomized multicentre study. BMC Cancer. 2015;15:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Kim GR, Ha GH, Bae JH, Oh SO, Kim SH, Kang CD. Metastatic colon cancer cell populations contain more cancer stem-like cells with a higher susceptibility to natural killer cell-mediated lysis compared with primary colon cancer cells. Oncol Lett. 2015;9:1641-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 944] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 5. | Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 517] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2814] [Cited by in RCA: 2818] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 7. | Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 453] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 8. | Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16:133-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 534] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 10. | Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1747] [Cited by in RCA: 1801] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 12. | Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2625] [Cited by in RCA: 2886] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 13. | Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, Sunshine MJ, O’Brien W, Raz E, Littman D, Wylie C. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development. 2003;130:4279-4286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Lataillade JJ, Clay D, Bourin P, Hérodin F, Dupuy C, Jasmin C, Le Bousse-Kerdilès MC. Stromal cell-derived factor 1 regulates primitive hematopoiesis by suppressing apoptosis and by promoting G(0)/G(1) transition in CD34(+) cells: evidence for an autocrine/paracrine mechanism. Blood. 2002;99:1117-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655-2663. [PubMed] |
| 16. | Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3966] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 18. | Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1113] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 19. | Ohtani H, Jin Z, Takegawa S, Nakayama T, Yoshie O. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte-rich gastric carcinoma. J Pathol. 2009;217:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Costantini S, Romano G, Rusolo F, Capone F, Guerriero E, Colonna G, Ianora A, Ciliberto G, Costantini M. Anti-Inflammatory Effects of a Methanol Extract from the Marine Sponge Geodia cydonium on the Human Breast Cancer MCF-7 Cell Line. Mediators Inflamm. 2015;2015:204975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Dabkeviciene D, Jonusiene V, Zitkute V, Zalyte E, Grigaitis P, Kirveliene V, Sasnauskiene A. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med Oncol. 2015;32:258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 571] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 23. | Ben-Baruch A. Site-specific metastasis formation: chemokines as regulators of tumor cell adhesion, motility and invasion. Cell Adh Migr. 2009;3:328-333. [PubMed] |
| 24. | Staiano RI, Loffredo S, Borriello F, Iannotti FA, Piscitelli F, Orlando P, Secondo A, Granata F, Lepore MT, Fiorelli A. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J Leukoc Biol. 2016;99:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Coperchini F, Pignatti P, Leporati P, Carbone A, Croce L, Magri F, Chiovato L, Rotondi M. Normal human thyroid cells, BCPAP, and TPC-1 thyroid tumor cell lines display different profile in both basal and TNF-α-induced CXCL8 secretion. Endocrine. 2016;54:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Poutahidis T, Erdman SE. Commensal bacteria modulate the tumor microenvironment. Cancer Lett. 2016;380:356-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Semenza GL, Ruvolo PP. Introduction to tumor microenvironment regulation of cancer cell survival, metastasis, inflammation, and immune surveillance. Biochim Biophys Acta. 2016;1863:379-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Karachaliou N, Pilotto S, Bria E, Rosell R. Platelets and their role in cancer evolution and immune system. Transl Lung Cancer Res. 2015;4:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 29. | Ou Z, Wang Y, Liu L, Li L, Yeh S, Qi L, Chang C. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget. 2015;6:26065-26078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Han TT, Fan L, Li JY, Xu W. Role of chemokines and their receptors in chronic lymphocytic leukemia: function in microenvironment and targeted therapy. Cancer Biol Ther. 2014;15:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, Wakasugi T, Funahashi H, Sato M, Okada Y. Interleukin-1alpha enhances angiogenesis and is associated with liver metastatic potential in human gastric cancer cell lines. J Surg Res. 2008;148:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Xu D, Matsuo Y, Ma J, Koide S, Ochi N, Yasuda A, Funahashi H, Okada Y, Takeyama H. Cancer cell-derived IL-1α promotes HGF secretion by stromal cells and enhances metastatic potential in pancreatic cancer cells. J Surg Oncol. 2010;102:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, Wakasugi T, Funahashi H, Sato M, Takeyama H. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Bernardini G, Ribatti D, Spinetti G, Morbidelli L, Ziche M, Santoni A, Capogrossi MC, Napolitano M. Analysis of the role of chemokines in angiogenesis. J Immunol Methods. 2003;273:83-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Waldner MJ, Neurath MF. Cytokines in colitis associated cancer: potential drug targets? Inflamm Allergy Drug Targets. 2008;7:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Ruffini PA, Morandi P, Cabioglu N, Altundag K, Cristofanilli M. Manipulating the chemokine-chemokine receptor network to treat cancer. Cancer. 2007;109:2392-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5794] [Cited by in RCA: 6609] [Article Influence: 508.4] [Reference Citation Analysis (1)] |
| 38. | Dillenburg-Pilla P, Patel V, Mikelis CM, Zárate-Bladés CR, Doçi CL, Amornphimoltham P, Wang Z, Martin D, Leelahavanichkul K, Dorsam RT. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/mTORC1 axis. FASEB J. 2015;29:1056-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |