Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5127
Peer-review started: February 8, 2017
First decision: April 5, 2017
Revised: May 6, 2017
Accepted: July 12, 2017
Article in press: July 12, 2017
Published online: July 28, 2017
Processing time: 178 Days and 12.6 Hours
To determine the role of corticotropin releasing factor receptor (CRF2) in epithelial permeability and enterocyte cell differentiation.
For this purpose, we used rat Sprague Dawley and various colon carcinoma cell lines (SW620, HCT8R, HT-29 and Caco-2 cell lines). Expression of CRF2 protein was analyzed by fluorescent immunolabeling in normal rat colon and then by western blot in dissociated colonic epithelial cells and in the lysates of colon carcinoma cell lines or during the early differentiation of HT-29 cells (ten first days). To assess the impact of CRF2 signaling on colonic cell differentiation, HT-29 and Caco-2 cells were exposed to Urocortin 3 recombinant proteins (Ucn3, 100 nmol/L). In some experiments, cells were pre-exposed to the astressin 2b (A2b) a CRF2 antagonist in order to inhibit the action of Ucn3. Intestinal cell differentiation was first analyzed by functional assays: the trans-cellular permeability and the para-cellular permeability were determined by Dextran-FITC intake and measure of the transepithelial electrical resistance respectively. Morphological modifications associated to epithelial dysfunction were analyzed by confocal microscopy after fluorescent labeling of actin (phaloidin-TRITC) and intercellular adhesion proteins such as E-cadherin, p120ctn, occludin and ZO-1. The establishment of mature adherens junctions (AJ) was monitored by following the distribution of AJ proteins in lipid raft fractions, after separation of cell lysates on sucrose gradients. Finally, the mRNA and the protein expression levels of characteristic markers of intestinal epithelial cell (IEC) differentiation such as the transcriptional factor krüppel-like factor 4 (KLF4) or the dipeptidyl peptidase IV (DPPIV) were performed by RT-PCR and western blot respectively. The specific activities of DPPIV and alkaline phosphatase (AP) enzymes were determined by a colorimetric method.
CRF2 protein is preferentially expressed in undifferentiated epithelial cells from the crypts of colon and in human colon carcinoma cell lines. Furthermore, CRF2 expression is down regulated according to the kinetic of HT-29 cell differentiation. By performing functional assays, we found that Ucn3-induced CRF2 signaling alters both para- and trans-cellular permeability of differentiated HT-29 and Caco-2 cells. These effects are partly mediated by Ucn3-induced morphological changes associated with the disruption of mature AJ in HT-29 cells and tight junctions (TJ) in Caco-2 cells. Ucn3-mediated activation of CRF2 decreases mRNA and protein expression levels of KLF4 a transcription factor involved in IEC differentiation. This signaling is correlated to a down-regulation of key IEC markers such as DPPIV and AP, at both transcriptional and post-transcriptional levels.
Our findings suggest that CRF2 signaling could modulate IEC differentiation. These mechanisms could be relevant to the stress induced epithelial alterations found in inflammatory bowel diseases.
Core tip: Stress is recognized to participate in the development and/or aggravation of gastrointestinal (GI) disorders mainly by altering intestinal epithelial functions. This occurs partly through the deregulation of neuromediator secretion, but their activity on enterocyte differentiation remains not totally understood. We found that expression of corticotropin releasing factor receptor 2 (CRF2), a stress ligand receptor is inversely correlated to the differentiation status of colon cell lines. We thus investigated the effect of CRF2 signaling on intestinal epithelial differentiation using Ucn3 agonist treatments applied to different cancer cell lines that mimic this process. We identified that CRF2 activation affect both the early steps of differentiation and an established differentiated state, by down-regulating transcription factors such as krüppel-like factor 4. This effect is correlated with alterations of epithelial permeability and cellular adhesion junctions. Our results argue that CFR2-induced alterations of enterocyte differentiation may contribute to stress-mediated barrier dysfunction and the development of GI disorders.
- Citation: Ducarouge B, Pelissier-Rota M, Powell R, Buisson A, Bonaz B, Jacquier-Sarlin M. Involvement of CRF2 signaling in enterocyte differentiation. World J Gastroenterol 2017; 23(28): 5127-5145
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5127
The gut is composed of various cell populations distributed in different layers. These cells contribute to the intestinal epithelial homeostasis and function by controlling epithelial permeability, intestinal secretory, motor and immune functions. The intestinal epithelium consists of a monolayer of cells, which borders the lumen of the digestive tract and acts as a barrier against antigenic stimuli of food, bacterial, viral and parasitic origin. This organ is therefore the target of permanent aggressions whose accumulations can lead to a chronic inflammatory response favoring the deregulation of the morphology and functions of the intestine as it was observed in pathological disorders such as inflammatory bowel disease (IBD)[1]. In the long run, IBD can induce further complications and increase the risk of developing other pathologies such as colorectal cancer (CRC)[2]. Enterocytes, which represent 80% of the cells forming this monolayer, are specialized cells characterized by structural features including polarized morphology and a complex set of cell-cell and cell-extracellular matrix (ECM) junctions. Three types of junctional complexes mainly ensure cell-cell adhesion: (1) tight junctions (TJ) composed of transmembrane proteins (such as claudin, occludin), which are linked to actin cytoskeleton (CSK) via scaffold proteins like zona occludens (ZO); (2) adherens junctions (AJ) which comprise E-cadherin connected to actin CSK via catenin and regulated by p120 catenins (ctn); and (3) desmosomes[3,4] and p120ctn regulate AJ by controlling cadherin clustering, endocytosis and stability as well as actin CSK anchorage[5].
In epithelial cells, assembly of adhesion complexes occurs at the plasma membrane, where individual proteins and lipids are known to be restricted to apical and basolateral domains. Others and we have shown that lipid rafts (LR) are specialized subdomains, highly enriched in cholesterol and sphingolipids, which play a role in the spatial organization and function of AJ and TJ[6,7]. As well as having a structural role, adhesion complexes are also preferential sites for signal transduction which control multiple aspects of the cell’s behavior, mainly proliferation and differentiation[8-10]. Thus alterations of these signaling platforms may alter the differentiation process during intestinal epithelial renewal as well as during tumor development (review by[11]). This has been particularly highlighted in the intestinal epithelium by manipulating E-cadherin function[12]. The expression of E-cadherin protein is decreased in invasive CRC, a process that correlates with the acquisition of a mesenchymal phenotype[13]. Although each adhesion complex has its own particular mechanism of formation, regulation and function, theyall interact with one another through an extensive communication and mutually influence each other’s dynamics and signaling properties.
In the last decade, stress (from psychological or environmental origins) has been recognized to participate in the development and/or aggravation of gastrointestinal (GI) disorders such as IBD or CRC[14,15-19]. The effects of stress are mediated through the secretion of specific stress neuromediators, such as corticotropin releasing factor (CRF) or its analogs Urocortin 2 and 3 (Ucn2/3)[19]. These peptides act through the activation of corticotropin releasing factor receptors 1 and 2 (CRF1/CRF2), two class II G protein coupled receptors (GPCR) with different affinities[17]. Ucn3 binds exclusively to CRF2[20]. The expression of CRF receptors and ligands in the GI tract has been investigated in rodents and humans (for review[21]). In the colon, all the cells that compose the different layers of the intestinal mucosa mostly express these molecules indicating that the intestine is a target for stress signaling. CRF receptors are primarily coupled to Gαs and trigger cAMP formation via adenylyl cyclase activation[18]. This signaling pathway could participate in the dissociation of intercellular adhesion complexes in intestinal epithelial cells (IEC)[22]. CRF receptors are also able to activate the Src kinase by promoting its auto-phosphorylation on Y418[23]. Activation of src kinase could contribute to the opening of the intestinal barrier by modulating the phosphorylation status of intercellular junction proteins[24]. We previously demonstrated that CRF2 activation signals through the Src/ERK pathway to modulate cell-cell junctions in CRC cell lines[25].
The digestive epithelium is a very dynamic tissue that is constantly renewed. Indeed, it is fully regenerated within 3-5 d under normal homeostasis and this process is even faster after injury. This renewal is carried out by the stem cells located at the bottom of the crypts[26]. They first divide and give rise to progenitors (transiently amplified cells), which occupy most of the crypt, and then undergo a final division before starting a maturation and terminal differentiation program into either absorptive enterocytes or the secretory lineages (goblet, enteroendocrine and paneth cells). Differentiation takes place as the cells migrate in cohort along the crypt-villus axis before dying by anoïkosis and finally exfoliated at the tip of the villi in the small intestine. The mechanisms that regulate cell proliferation in the crypt, migration and differentiation of progenitor cells are partially understood. It is recognized that these mechanisms are based on fine spatio-temporal regulation of many genes along the crypt-villus axis. This regulation involves transcription factors (Cdx2, Hox, HNF, GATA4, KLF4...) expressed under the control of growth factors, hormones, cytokines but also by cell-cell or cell-ECM interactions[27,28]. Similarly, reciprocal interactions between the epithelium and the mesenchyme are necessary for the morphogenetic and differentiation processes that occur during organogenesis and migration along the crypt-villus axis[29-31]. Furthermore, IEC cell renewal and differentiation may also respond to environmental conditions including luminal nutriments, GI hormones and more recently psychological stress such as maternal deprivation (MD)[32-34]. Indeed, the CRF receptor signaling induced by MD markedly altered the quantitative distribution of secretary cells (paneth and goblet cells) of the intestinal epithelium, which may contribute to the development of epithelial barrier defects.
To date, the role of stress and its mediators on enterocyte differentiation has not been investigated. In the present study, our aim was first to characterize the expression pattern of CRF2 in normal rat colon epithelial cells and in human colon carcinoma cell lines. This distribution led us to determine the role of CRF2 signaling in the modulation of epithelial cell permeability and enterocyte-like differentiation.
All cell lines used in this study are human colon cancer cells. SW620, HCT8, HT-29 and Caco-2 cells obtained from the American Type Culture Collection (ATCC, Manassas, VA, United States) were cultured at 37 °C in a 5% CO2 atmosphere in DMEM media containing 25 mmol/L glucose (Invitrogen, Cergy Pontoise, France) and supplemented with 10% fetal bovine serum (Invitrogen), 5% penicillin and streptomycin. The cells were harvested in phosphate-buffered saline (PBS) supplemented with 1 mmol/L EDTA and 0.05% trypsin.
Cell differentiation: HT-29 cell differentiation was initiated by replacing standard medium of 90% confluent adherent cells by glucose-free DMEM (InVitroGen) supplemented with 10% dialyzed fetal calf serum, 5 mmol/L galactose, 15 mmol/L HEPES pH 7, selenous acid (10-2 g/mL), penicillin, and streptomycin. This medium (Gal medium or differentiation medium) was changed every day. Caco-2 cells differentiate spontaneously when they reach confluence. The first day of confluence was determined by phase contrast microscopy and designated “day 0”. The medium was changed every day.
Male Sprague Dawley rats, 250-300 g, were purchased from Charles River France. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimated to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for on week prior to experimentation. They were sacrificed in agreement with local ethic committee policies, by 40 mg/kg IP injection of pentobarbital sodium and submitted to 200 mL intra-cardiac perfusion of NaCl 0.9%. Epithelial cells were isolated from proximal colon section as previously described by[35]. Briefly, the tissue section was tied up at one end, reverted and filled to distension with PBS prior to closing the remaining open end. The section is then incubated with shaking at 37 °C for 45 min in 2 mL of dissociating buffer (PBS containing 1 mmol/L EDTA, 1 mmol/L dithiothreitol, pH 7.3) containing protease inhibitor cocktail (Roche, Meylan, France). Dissociated epithelial cells were collected after a sedimentation step of 60 min and then harvested by centrifugation at 1500 rpm at 4 °C for 5 min, snap frozen in liquid nitrogen, and stored at -80 °C.
Rat proximal colons were collected, fixed overnight in PFA 4%, sucrose 3% and cryoprotected 12 h in sucrose 30% until freezing in isopentane. Samples of 3 different rats were embedded in Tissue-Teck OCT compound, cryosectioned at 30 μm thickness and placed on Superfrost plus slides for immunofluorescence (same protocol as for cells - see below).
Cells grown on glass coverslips were fixed with 3% (wt:vol) paraformaldehyde, 2% sucrose for 10 min at 37 °C, and further permeabilized with 0.5% Triton X-100 in PBS for 15 min at room temperature (RT). Cells were washed twice in PBS containing 0.05% Tween 20, and blocked with 3% bovin serum albumin diluted in washing buffer for 1 h at RT. Then cells were stained for 1 h at 37 °C with primary antibody diluted in blocking solution and then incubated with secondary antibody coupled to Alexa 488 (1:500) for 45 min at 37 °C. Cells were washed three times in washing buffer after each incubation with antibodies. Before the last wash, nuclei were labelled during 5 min with TO-PRO3 (Invitrogen). Coverslips were permanently mounted with Mowiol (Calbiochem, France). Fluorescence photomicrographs were taken using a confocal laser-scanning microscope (Leica TCS SPE). Primary antibodies were used at the following dilutions: anti-CRF2 (1/200; Abcam 12964, Paris, France), anti- human E-cadherin (1:500; HECD1, Takara Biochemicals, Paris, France), anti-p120ctn (1:200, Beckton Dickinson, France), and phalloidin-TRITC (25 ng/mL) (Sigma-Aldrich, L’Isle d’Abeau, France).
For total protein extraction, cells were lysed with RIPA buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 2 mmol/L CaCl2 and MgCl2) supplemented with a mixture of protease inhibitors (1:100, Roche, Meylan, France). Protein concentrations in lysates were determined using the copper reduction/bicinchoninic acid (BCA) assay (Pierce Chemical Co) according to the manufacturer’s instructions. Proteins (75 μg in Laemli buffer containing 2-mercaptoethanol) were resolved on 10% acrylamide gels, transferred onto activated PVDF membranes (Ge HealthCare, Dutscher, France). Membranes were first blocked in 5% fat-free dry milk, 0.1% Tween 20 in PBS for 2h at RT. After overnight incubation at 4 °C with primary antibodies diluted in the blocking solution, blots were washed 3 times in PBS, 0.1% Tween 20, and then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (dilution of 1:30000, Jackson Immunoresearch, Immunotech, Marseille, France) for 1 h at RT before extensive washes. Immunoblottings were visualized by chemiluminescence (Amersham ECL reagents) and revealed on hyperfilm ECL (Ge Healthcare) and quantified with Image J software from NIH. Primary antibodies were used at the following dilutions: anti-CRF2 (1:1000), anti-villin (1:2000, gift of Dr. Robin from Institute Curie, Paris, France), anti-human E-cadherin (1:1000), anti-p120ctn (1:1000), anti-actin (1:100, Sigma-Aldrich), anti-DPPIV (1/1000, BML SA-451 Enzo Life Science) and anti-KLF4 (1/500, GENETEX).
Immunoblots shown are representative of at least three independent experiments. All graphs represent the mean value ± SEM of protein expression levels measured by densitometric analysis with “Image J” software (NIH). For quantification, the expression level of each protein was normalized to actin for each sample. In order to display a fold increase over control, the relative expression of proteins in control conditions was indexed to 1 for each of three repeated experiments.
Paracellular permeability (FITC-dextran intake): The paracellular permeability of early differentiated HT-29 or differentiated Caco-2 cell monolayers was assessed by determining FITC-dextran cell intake. Cells were seeded in 24-MultiWell dishes and allowed to differentiate 10 d in Gal medium. At day 10, 2 mL of medium containing FITC-dextran of 4 kDa (2 mg/mL, Sigma-Aldrich) and 10 mmol/L HEPES were put on the cultures. Subsequently, wells were treated with or without 100 nmol/L Ucn3 (Sigma-Aldrich) and cells were replaced in a 37 °C, 5% CO2 incubator. At scheduled times (10, 20, 30 and 40 min), FITC-dextran containing medium was removed and cells were washed twice in cold PBS before lysis in RIPA buffer. Measurements of fluorescence were performed using the microplate reader PHERAstar Plus (BMG LABTECH). Excitation wavelength: 485 nm and emission wavelength: 520 nm. The background is obtained by measurement of MH medium fluorescent. These experiments were realized at least three times.
Measurement of transepithelial electrical resistance (TEER): Cells were seeded in the top compartment of a 24-multiwell insert plate (Corning PET) with 0.4 μm pore size and cultured until they reached their differentiation state. In some experiments cells were pretreated overnight with 1 mmol/L Astressin 2b (A2b, Sigma-Alrich) before exposure or not to 100 nmol/L Ucn3. TEER was measured as described previously[36] using a Millicell Electrical Resistance System (Millipore, Bedford, MA, United States). TEER was calculated as ohm times centimeter squared (Ω. cm2) by multiplying it by the surface area of the monolayer. The TEER of the polyester membrane in Transwells (approximately 30 Ω. cm2) was subtracted from all readings.
Briefly, post-nuclear supernatant from HT-29 cells was solubilized in 1.5 mL of buffer A (25 mmol/L HEPES pH 7.4, 150 mmol/L NaCl, 5 mmol/L EGTA, protease inhibitors mixture) containing 1% Triton × 100 for 1 h at 4 °C. Lysates were ultracentrifuged after mixing with an equal volume of 80% sucrose in buffer A and laid down at the bottom of a ultracentrifuge tube, which was then overlaid with 6 mL of 38% sucrose followed by 3 mL of 5% sucrose, all diluted in buffer A. After centrifugation at 38000 rpm for 16 h in a SW41 rotor (Beckman Coulter) at 4 °C, 1 mL fractions were harvested from the top.
Total RNA extractions were performed using the Nucleospin RNA kit (Macherey-Nagel, France) according to the manufacturer’s instructions. Two micrograms of total RNA was denaturized and subsequently processed for reverse transcription using the M-MLV (Euromedex, France) according to the manufacturer’s instructions and then run on a thermocycler (Eppendorf). Primer sequences and probes are: KLF4 (Tm 56 °C, 35 cycles), forward: TGCTGATTGTCTATTTTTGCGTTTA, reverse: GAGAAGAAACGAAGCCAAAACC; DPPIV(Tm 60 °C, 35 cycles), forward: CCCGCGGCCTTTATAC, reverse: GTGGTAAGACGGAGCCTGAC; AP (Tm 67 °C, 35 cycles), forward: GCAACCCTGCAACCCACCCAAGGAG, reverse: CCAGCATCCAGATGTCCCGGGAG; GAPDH (Tm 60 °C, 25 cycles), forward: TCCTCCTGCGACAGTCA and reverse: CACCACCTTCTTGATGTCATC. PCR conditions were: 5 min at 92 °C followed by numbers of cycles depending on the primers (40 s at 92 °C, 40 s at Tm and 1 min at 72 °C) and 10 min at 72 °C. PCR were analyzed on 1% (w/v) agarose gel. Quantification was performed using Image J (NIH software). GAPDH was used as housekeeping gene.
Cells were suspended in lysis buffer (5 mmol/L Na2SO4, 1 mmol/L Tris/HCl, pH 7.6, supplemented with protease inhibitor cocktail) and sonicated for 30 s at 4 °C (400 J/W s). Then the homogenate was centrifuged for 10 min at 1060 g. The supernatant was collected for the enzymatic assays performed in microplate.
Alkaline phosphatase (AP) activity was measured with 1, 5 or 10 μL of lysates containing approximately 2.5 μg/μL of proteins adjusted to 100 μL with reaction buffer (50 mmol/L glycine pH 10.5, 0.5 mmol/L MgCl2, 5 mmol/L CaCl2 and 2 mmol/L ZnCl2). Then 100 μL of substrate was added to each well (10 mmol/L p-nitrophenylphosphate disodium salt (Sigma-Aldrich) in reaction buffer). The plate was incubated for 2 h at 37 °C and stopped by the addition of 50 μL of 1 mol/L NaOH. The production of p-nitrophenol was estimated by measuring the optical density at 420 nm using the microplate reader PHERAstar Plus (BMG LABTECH).
Dipeptidyl peptidase activity was determined by the digestion of 50 μL of 3 mmol/L GlyPro-p-nitroanilide (Sigma-Aldrich) prepared in previously described reaction buffer by 50 μL of cell lysates for 30 min at 37 °C. The reaction was stopped with 50 μL of 0.1 mol/L sodium acetate and reaction products were measured at 420 nm. Results are expressed as enzyme activity in international units per milligram of protein previously estimated by BCA assays (Pierce). All experiments were performed in triplicates.
The statistical methods of this study were reviewed by Jacques Brocard from the Grenoble Institute of Neurosciences (INSERMU1216). Statistics were performed using the Prism 5.0 software (GraphPad Software, CA, United States). Throughout the study, parametric 1way ANOVA tests followed by Bonferroni’s multiple comparison tests for selected data have been performed as described. Note that for each kind of experiment (Functional permeability assays; densitometric analyses and enzymatic activity assays), normality of distribution of all the measures was verified with a D’Agostino-Pearson omnibus normality test (not shown).
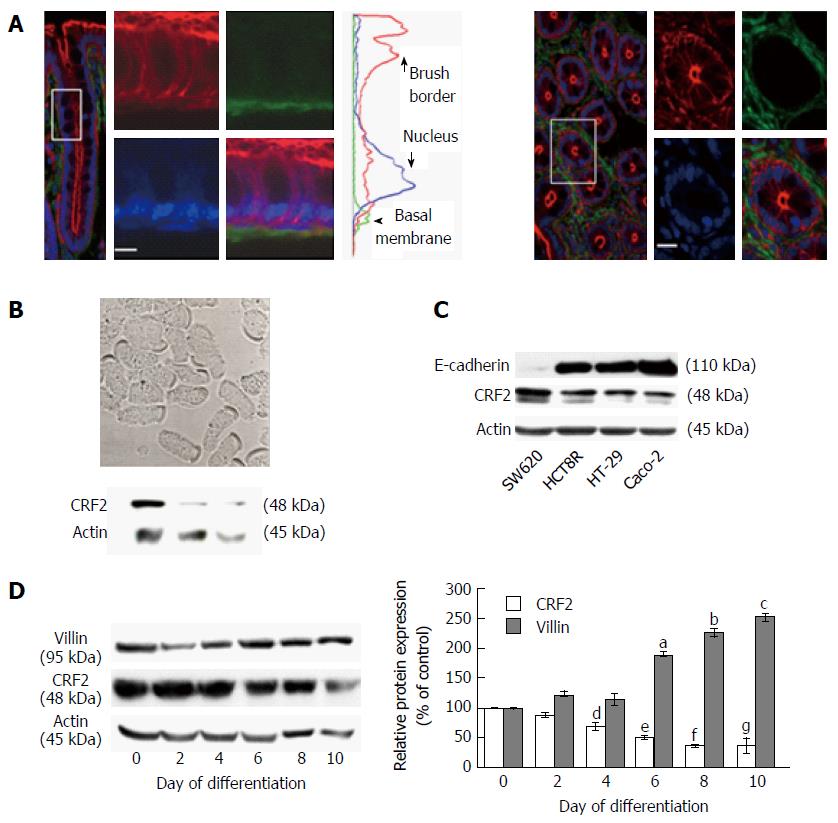
CRF2 expression and localization in IEC is quite controversial (for review[21]). Confocal microscopy analyses performed on colonic section of Sprague-Dawley rats indicated that CRF2 is expressed at the basal membrane of epithelial cells present in the crypts (Figure 1A). CRF2 protein expression was further confirmed by immunoblotting on dissociated IEC (Figure 1B). CRF2 protein expression was also observed in various epithelial derived carcinoma cell lines (Figure 1C). However, our data indicated that CRF2 expression seems to depend on the differentiation status of the cell lines: CRF2 expression is higher in less differentiated cell lines such as SW620 or HCT8 compared to more differentiated cell lines such as HT-29 or Caco-2. Furthermore, the CRF2 expression is also inversely correlated to E-cadherin expression in these cell lines. These data have been confirmed by a correlation taking all values independently (r2 = 0.8748, P < 0.0001). HT-29 and Caco-2 were able to differentiate under specific culture conditions. HT-29 cell differentiation is achieved by switching glucose with galactose in culture medium (Gal medium)[37]. During the first ten days of culture in Gal medium, which correspond to the initial step of HT-29 cell differentiation, cells undergo structural changes (polarity and development of mature AJ) and start to express some epithelial differentiation markers such as digestive enzymes [AP, dipeptidyl peptidase IV (DPP IV)…][38]. Acquisition of the epithelial phenotype takes several days, and the highest degree of differentiation has been found to be optimum after 50 d of culture in Gal medium. To further confirm the correlation between CRF2 expression and cell differentiation status, we investigated the level of CRF2 expression during the first ten days of HT-29 cell differentiation (Figure 1D). Changes in the expression of the villin protein (a calcium-regulated actin binding protein of the brush border of IEC) were used as control of HT-29 cell differentiation. Western blot analysis indicated that villin protein expression increased according to the kinetic of culture in the differentiating medium, while CRF2 protein expression decreased accordingly.
Taken together, our data suggest that CRF2 expression is associated to a poor differentiated status of IEC.
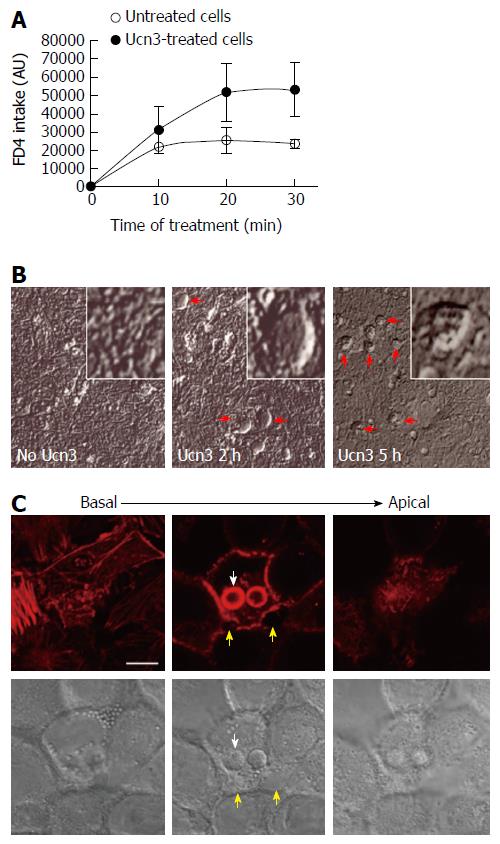
The presence of CRF2 at the basal membrane of colonic IEC and its expression regulation during differentiation of colonic cancer cell lines suggest that CRF2 agonist such as Ucn3 could act directly on IEC to regulate their differentiation status and their function. One important function of IEC such as enterocytes is to perform an effective barrier to harmful macromolecules and microorganisms while maintaining a selective permeability for nutriments. To face this challenge, IEC have developed two mechanisms: the management of ion selectivity, nutriments and solutes occurs via the para-cellular route crossing between the epithelial cells, while large molecules such as antigens and immunoglobulins pass through epithelial cells via the trans-cellular route[39]. We first analyzed the effect of CRF2 signaling on the trans-cellular permeability by measuring dextran-FITC (4 kDa) intake of early-differentiated HT-29 cells treated or not with 100 nmol/L Ucn3 during 5 h (Figure 2A). In control conditions the diffusion of dextran-FITC was stable during the time course of the experiment. In contrast, treatment with Ucn3 induced a two-fold increase in the release of dextran-FITC intake indicating that CRF2 signaling could increase the trans-cellular permeability (Two-way ANOVA, P < 0.01). Phase contrast microscopy indicated the presence of refractile structures (diameter comprised between 3 and 20 μm) in the cell monolayer following Ucn3 treatment (Figure 2B). These structures could be involved in the trans-cellular transport. To further investigate this hypothesis we labeled fibrillar actin with phalloidin-TRITC as a marker of intracellular trafficking vesicles (vacuoles). As shown in Figure 2C, treatment of early-differentiated HT-29 cells with Ucn3 (100 nmol/L, 2 h) favored the organization of intracellular spheres (their membranes were evidenced with phalloidin-TRITC) that co-localized with Differential Interference Contrast (DIC) signal.
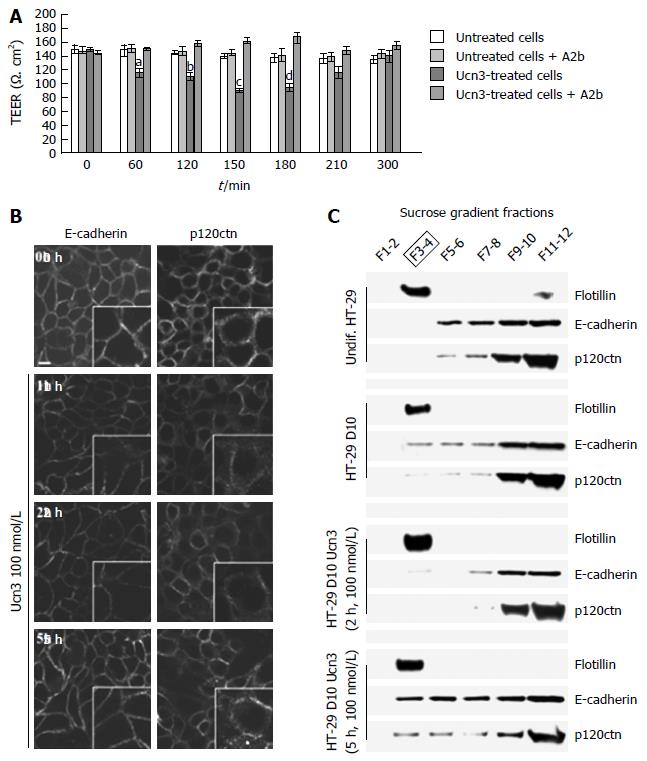
We next investigated the impact of CRF2 signaling on para-cellular transport by measuring the TEER of ten days confluent HT-29 monolayers (Figure 3A). While HT-29 cells did not form mature TJ until a complete differentiation process (e.g., at day 50 in Gal medium), we observed an increase of 70% in the TEER between undifferentiated and early-differentiated HT-29 cells suggesting that intercellular adhesion complexes involved in the permeability are functional (data not shown). At this stage, mature AJ complexes participate both in cell permeability and in the acquisition of TJ complexes since they are the first adhesion complexes to be formed[40]. In ten days confluent HT-29 monolayers, treatment by Ucn3 (100 nmol/L) induced a transient and statistically significant TEER decrease after 60 min that reverted within 300 min. This effect was strongly inhibited by Astressin 2b (A2b), a selective inhibitor of CRF2. As CRF2 signaling can modulate cell adhesion in adenocarcinoma cell lines[25], and since intercellular junctions are responsible of permeability, we investigated the effect of Ucn3 on the localization of E-cadherin and p120ctn, two major proteins of AJ complexes. Confocal microscopy analysis showed that E-cadherin staining appears as a honeycomb-like pattern at the membrane cortex (Figure 3B, left panel). Membrane E-cadherin staining is thickened and altered after 1 h and 2 h of treatment with Ucn3, while after 5 h the E-cadherin labeling seems to be restored. Similar results were observed with p120ctn staining, which is in part at the cell-cell contacts and some in the cytoplasm (Figure 3B, right panel). By stabilizing E-cadherin at the cell membrane, p120ctn participates in the regulation of E-cadherin function[41,42]. We have previously described that mature AJ required the recruitment of proteins in specific membrane domains called LR[6]. We thus examined the distribution of E-cadherin and p120ctn in LR identified by flotillin marker, after protein separation on sucrose gradient (Figure 3C). LR were purified from undifferentiated HT-29 cells or HT-29 cells differentiated ten days in Gal medium and then treated for 0, 2 h or 5 h with 100 nmol/L Ucn3. In all conditions tested, flotillin was found exclusively in fractions 3 and 4 (F3-4), which confirmed the presence of LR in those fractions. A small amount of flotillin was found in fractions 11-12 of undifferentiated HT-29 cells. In these cells, E-cadherin and p120ctn proteins are not expressed in LR, but distributed in fractions 5 to 12 with a stronger expression in fractions 9 to 12 (Figure 3C, upper panel). In ten days differentiated HT-29 cells, we found E-cadherin and p120ctn expressed in LR fractions at a level similar to fractions 5 to 8. The expression of these proteins was greater in fractions 9 to 12. After 2 h of treatment of differentiated HT-29 cells with Ucn3, we observed a decrease of E-cadherin and a loss of p120ctn expression in LR and fractions 5-6, while no changes were observed in fractions 9 to 12. Treatment for 5 h with Ucn3 induced a recruitment of E-cadherin and p120ctn proteins in LR compared to differentiated cells (fold increase of protein expression to flotillin in LR: 5.7 and 3.5 respectively) as well as in fractions 5 to 8. No differences in protein expression for E-cadherin was observed in fractions 9 to 12 while there was a slight decrease of p120ctn. The expression of TJ proteins (ZO-1, claudins, occludins…) had also been investigated in these gradients: their expression was restricted to fractions 9-12 and no modifications were observed in the different conditions studied (data not shown).
Interestingly, the recruitment of E-cadherin and p120ctn in LR after 5 h of treatment with Ucn3 correlated with the re-expression of AJ proteins at cell-cell contacts and the restoration of permeability efficiency. Taken together these results argue for a role of CRF2 signaling in the disorganization of AJ in early differentiated HT-29 cells which is probably responsible for the damage of para- and trans- cellular permeability.
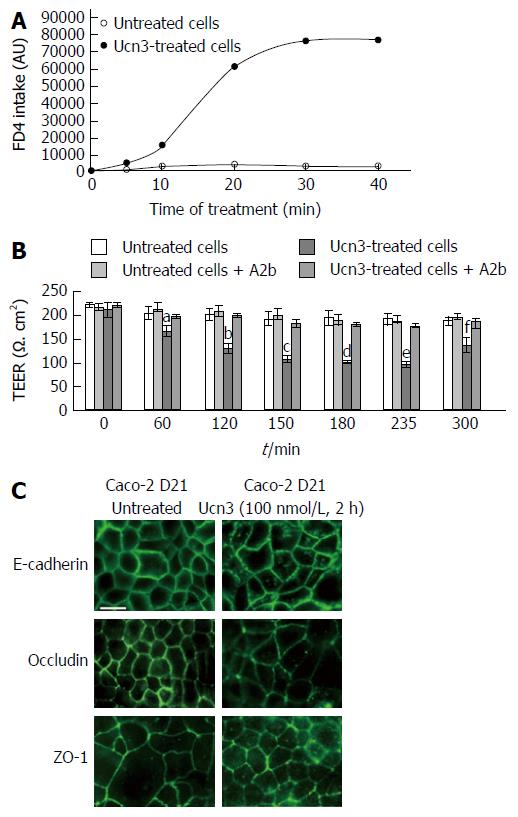
Since Caco-2 cells differentiate spontaneously into enterocyte-like cells after reaching confluence, we used these cells to evaluate the effect of CRF2 signaling on IEC differentiation[43]. At 21 d post-confluence, Caco-2 cells are maximally differentiated into mature absorptive epithelial cells, both phenotypically and functionally. They form a polarized monolayer of cells that: express microvilli at their apical pole, contain digestive enzymes (such as disaccharidases or peptidases) and establish intercellular contacts with both mature AJ and TJ[44-46]. Therefore they constitute a good model for observation of passive para-cellular permeability[47] and analysis of IEC differentiation. Prior to exploring the effect of CRF2 signaling on cell differentiation we first checked the regulation of cell permeability by Ucn3 in differentiated Caco-2 cells (Figure 4A and B). Regarding the trans-cellular permeability, we found that as for HT-29 cells, the diffusion of dextran-FITC was stable during the time course of the experiment in untreated cells. However the barrier formed by Caco-2 cells was more stringent since a lesser intake of dextran-FITC is observed compare to HT-29 cells in the same conditions. Treatment with Ucn3 induced a 35-fold increase in the intake of dextran-FITC at 30 min indicating that CRF2 signaling could increase the trans-cellular permeability (Figure 4A, two-way ANOVA, P < 0.001). Compared to HT-29 cells, in absence of Ucn3, TEER is also higher in Caco-2 cells suggesting more efficient intercellular complexes. However, unlike HT-29 cells, Ucn3 treatment induced a transient decrease of TEER after 120 min that was not completely reverted within 300 min (Figure 4B). Nevertheless, A2b treatment totally abolished the Ucn3-mediated decrease of TEER confirming that CRF2 signaling alters intercellular junctions even in more differentiated epithelial cells such as Caco-2 cells. To confirm this hypothesis we examined the distribution of intercellular junction proteins by confocal microscopy (Figure 4C). We found that in 21-d differentiated Caco-2 cells, E-cadherin (AJ marker), occludin and ZO-1 (TJ markers) labeling was mostly at the cell membrane, at intercellular contacts. Following Ucn3 treatment (100 nmol/L, 2 h), the membrane labeling became punctuate and sometimes disappeared (occludin) whereas cytoplasmic labeling increased (E-cadherin and ZO-1).
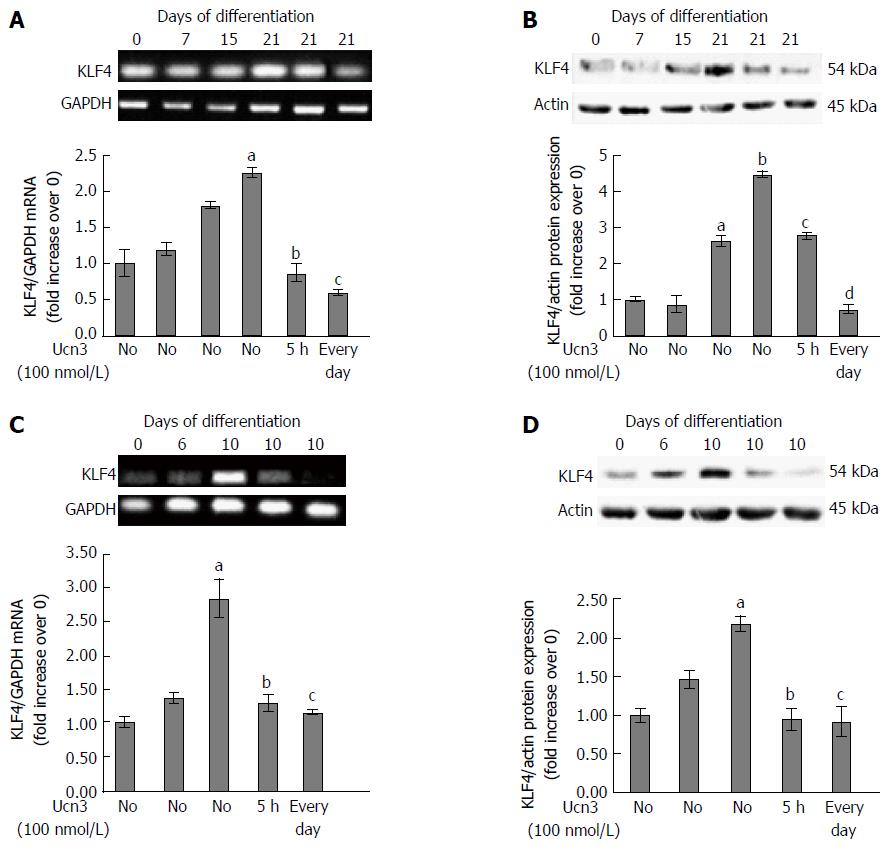
Among the criteria of epithelial differentiation, cell polarization and changes in gene expression are mostly reported. It has been previously described that intercellular complexes constitute a signaling platform involved in the regulation of cell differentiation by activating transcription factors responsible for cell growth arrest and differentiation marker expression[8,9,48,49]. Various transcription factors have been identified as regulators of IEC differentiation such as Cdx2, HNF4, GATA4 or Sox9. We focused our experiment on the Krüppel-like factor 4 (KLF4), a zinc-finger transcription factor that regulates a range of biological processes, including cell growth, differentiation and tumorigenesis[50,51]. Furthermore, KLF4 expression appears to be antagonist of CRF2 expression in normal intestinal epithelium as well as in CRC. Indeed, in normal intestine, expression of KLF4 is associated with the terminally differentiated state of epithelial cells near the luminal surface and of goblet cells in the crypts, whereas KLF4 is down-regulated in CRC[52-54]. It has also been shown to regulate IEC morphology and polarity and has been identified as a tumor suppressor[53,55-57]. To determine whether in vitro model of differentiation recapitulate the pattern of KLF4 expression suggesting a role in cell differentiation, we analyzed KLF4 mRNA and protein levels at different time points during 21 d of culture of Caco-2 cells (Figure 5A and B) or 10 d of culture of HT-29 cells (Figure 5C and D). KLF4 mRNA transcript levels increased according to the kinetic of differentiation and the maximal level of KLF4 mRNA transcript was detected at 21 d (2 fold increase compared to day 0) in Caco-2 cells and 10 d (3 fold increase compared to day 0) in HT-29 cells. However the basal level of KLF4 mRNA transcripts was greater in Caco-2 cells compared to HT-29 cells. We then analyzed the effect of Ucn3 on KLF4 mRNA transcripts in 21 d differentiated Caco-2 cells or 10 d differentiated HT-29 cells either exposed for 5 h at 100 nmol/L Ucn3 (acute treatment) or each day of differentiation with 100 nmol/L Ucn3 (chronic treatment). As shown in Figure 5A and C, Ucn3 completely abolished the differentiation mediated up-regulation of KLF4 mRNA transcripts following acute or chronic treatment. Regarding KLF4 protein levels, we found that KLF4 protein expression increased according to the kinetic of differentiation (Figure 5B and C); the maximal level of KLF4 protein was detected at 21 d of culture for Caco-2 cells (4.5 fold increase compared to day 0) and 10 d of culture for HT-29 cells (2 fold increase compared to day 0). Furthermore, in Caco-2 cells, Ucn3 reduced KLF4 protein enrichment at day 21 by 30% following acute treatment and totally abolished KLF4 protein enrichment following chronic treatment (Figure 5B). In HT-29 cells, Ucn3 totally abolished KLF4 protein enrichment at day 10 following acute and chronic treatments (Figure 5D).
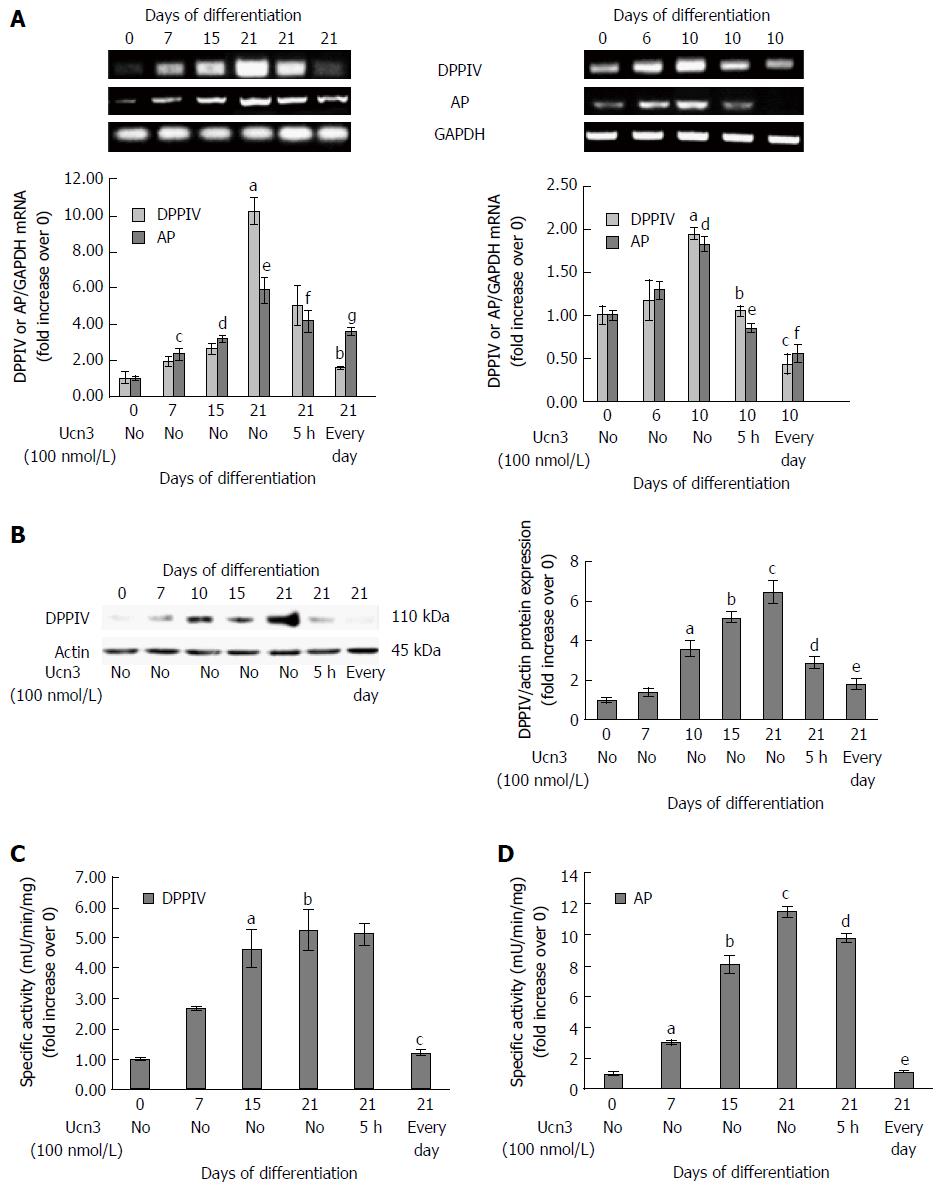
Regulation of intestinal transcription factors has been correlated with the expression of several markers of mature epithelium at both the mRNA and protein levels. We previously observed that CRF2 expression is inversely correlated with villin during HT-29 cell differentiation (Figure 1E). We next tested the effect of CRF2 signaling on other characteristic markers of differentiated enterocytes, including dipeptidyl peptidase 4 (DPPIV) and the brush border enzyme AP. At the transcriptional level, we found that DPPIV and AP mRNA transcript levels increased according to the kinetic of differentiation of the both cell lines. The maximal level of DPPIV and AP mRNA transcript was detected at 21 d in Caco-2 cells (respectively: 10 fold and 6 fold increase compared to day 0) (Figure 6A, left panel). In HT-29 cells, the maximal level of DPPIV and AP mRNA transcripts was detected at 10 d (2 fold increase compared to day 0 for each transcripts) (Figure 6A, right panel). In Caco-2 cells, Ucn3 reduced DPPIV mRNA enrichment at day 21 by 50% following acute treatment and totally following chronic treatment. Acute treatment has very little effect on AP mRNA transcripts while chronic treatment reduced by 40% the level of AP mRNA transcripts. Once more, Ucn3 completely abolished the differentiation-mediated up-regulation of DPPIV and AP mRNA transcripts following acute or chronic treatment in HT-29 cells (Figure 6A, right panel). We next analyzed the effect of CRF2 signaling at the protein level in Caco-2 cells. We observed a marked increase of DPPIV protein expression, which coincided, with the kinetic of Caco-2 differentiation with a maximum at 21 d (6 fold increase compared to day 0) (Figure 6B). The expression of DPPIV protein in 21 d differentiated Caco-2 cells was highly inhibited with both acute (56%) and chronic (71%) exposure to Ucn3. Additionally, we looked at the specific enzymatic activities of DPPIV and AP. In line with the increase of DPPIV protein expression, we found an increase in the specific enzymatic activities of both DPPIV and AP during the time course of Caco-2 cell differentiation (Figure 6C and D). However, we observed that only chronic exposure to Ucn3 reduced both enzyme activities to their day 0 level, whereas acute treatment with Ucn3 had only a little effect on AP activity (Figure 6D, right panel).
Taken together these data indicate that CRF2 signaling may regulate IEC differentiation by modulating the expression of transcriptional factors involved in the regulation of characteristic markers of differentiated enterocytes.
In this study, we showed for the first time that CRF2 signaling might delay enterocyte differentiation either by affecting intercellular complexes but also by regulating gene and protein expression.
The CRFergic system is a central element of stress response. The expression and regulation of CRF2 have been mainly described at the level of the enteric nervous system (ENS), the enteric blood vessels and the immune cells of the mucosa[58]. Nevertheless, studies have demonstrated its expression in the IEC, particularly those localized in the upper region of the crypts in the colon[59]. However, the CRF2 receptor localization remains unclear. The receptor may have either apical[59] or basolateral location[60]. Our confocal microscopy experiments, carried out on tissue sections but also on carcinoma cell lines, are in favor of a distribution of CRF2 at the basal membrane of IEC in the colon of Sprague Dawley rats and at the baso-lateral membrane of early differentiated HT-29 cells (data not shown) supporting the hypothesis of a localization of CRF2 oriented to the mucosa. Such localization of CRF2 could allow: (1) paracrine stimulation by the ENS or by ligands produced by the immune cells; and (2) autocrine stimulation by ligands produced by IEC themselves.
The role of the components of the CRF system in cancer is correlated with their level of expression and also with their cellular distribution. Indeed, CRF components are expressed and secreted by various cancer cells but the cytoplasmic distribution of CRF2 observed in de-differentiated or metastatic tumors was associated with diseases at a more advanced stage[61-63]. Accordingly, our results describe aberrant expression of CRF2 and ligands in both CRC tumors and cell lines, depending on their grade and/or differentiation status suggesting a role for CRF2 in the loss of cellular organization and tumor progression[25].
Stress causes disruption of intestinal epithelial barrier homeostasis, leading to worsening of GI disorders such as IBD and irritable bowel syndrome (IBS)[18,64,65]. Changes in intestinal mucosa permeability have been attributed to an alteration of junctional molecules, whose expression is affected by the actively inflamed status in IBD or IBS patients, in particular the expression of ZO-1, occludin, E-cadherin and desmoglein-2[66]. To understand the role of the CRFergic system in the regulation of intestinal homeostasis, approaches have been developed based either on the inhibition of ligands or the inhibition of receptors, via genetic or pharmacological extinction or via administration of peripheral CRF or various CRF antagonists[19,67-72]. Stress-induced modulation of colonic permeability seems to be either CRF1- or CRF2- dependent. This modulation has been attributed to eosinophils or ENS-derived CRF which activate mast cells that in turn induce TNFα and protease release as well as finally disruption of TJ[73-75]. Therefore, very few studies have investigated the activation of CRF2 in IEC, whose expression is increased under inflammatory conditions in patients with IBD[60,76] or under stressful conditions (personal data). Our results show that the increase in intestinal permeability induced by Ucn3 is due to CRF2 signaling since the effect was abolished by a pre-treatment with Astressin 2B, a CRF2 antagonist. The increase in both para- and trans-cellular permeabilities is correlated with an alteration of intercellular adhesion complexes such as AJ and TJ in more differentiated cells. Indeed, CRF2 signaling modifies the membrane distribution of AJ and TJ proteins. According to the increase of both E-cadherin and p120ctn in LR of HT-29 cells during their early differentiation (from day 0 to 10) our data are consistent with the previously described role of LR in intercellular complex maturation[6,7]. Treatment of these cells with Ucn3 (2 h) induced a decrease of E-cadherin and p120ctn in LR. These changes coincide with the decrease in TEER observed in differentiated HT-29 cells after 2 h of treatment with Ucn3, suggesting that the disorganization of AJ following activation of CRF2 could be responsible for an increase in intestinal permeability. Such alterations in the distribution of proteins of intercellular junctions are found in inflammatory models. Indeed, the presence of TJ proteins is decreased in LR of IEC of rats subjected to TNBS-induced colitis[77]. The stimulation of CRF2 could promote the activation of Src, a kinase that is strongly involved in the regulation of AJ[25]. Src kinase allows insertion of AJ by phosphorylation of PI3K[78]. Conversely, if AJ are already in place, phosphorylation of Src leads to AJ destabilization[79] by phosphorylation of p120ctn[80], leading to endocytosis of E-Cadherin which will then be ubiquitinylated and degraded by the proteasome[81]. These elements are consistent with the disappearance of p120ctn and E-cadherin from LR under Ucn3 treatment (2 h). At 5 h of treatment with Ucn3, the expression profile of E-cadherin and p120ctn in the different fractions of the gradient is intermediate between that of the undifferentiated cells (D0) vs the differentiated cells (D10). We suppose that there is a membrane enrichment of E-cadherin that could result from more active recycling, restoring the AJ. Furthermore, the increase in the expression of E-cadherin and p120ctn in fractions 5-6 and 7-8 could illustrate newly formed junctions whose maturation state is intermediate between untreated HT-29 cells at D0 and D10. These results are also consistent with the partial return of TEER to the level of HT-29 cells treated with Ucn3.
The phenomena of pinocytosis and autophagy involved in the internalization of the extracellular apical medium, forming the vacuoles, could correspond to the refringent zone observed in phase contrast microscopy. These results are consistent with the increase in HRP flux observed in different stress models in animals[69,82,83]. The appearance of intracellular vacuoles in the monolayer of differentiated HT-29 cells treated with Ucn3 could also contribute to the increase in permeability. Furthermore, the persistence of these structures after 5 h of treatment with Ucn3 while E-cadherin molecules accumulate in LR suggests that independent mechanisms could contribute to the increase in permeability. A similar phenotype was observed in presence of forskolin, an activator of adenylate cyclase (data not shown). This suggests that AMPc production is involved in this mechanism. CRF2 activation in IEC could also lead to an AMPc-mediated increase in trans-cellular permeability.
Gland atrophy and mucin depletion have been observed during chronic colitis[84,85]. Knowing the protective function of mucins in the epithelial barrier, it seems likely that in response to the inflammation induced by crypt epithelial damage and ulceration, the epithelium responds by increasing proliferation and thus, reducing differentiation[86]. Estienne et al[34] showed that activation of CRF1 and CRF2 induced by MD markedly induce alterations in the differentiation of IEC resulting in a hyperplasia of enteroendocrine cells and depletion of Paneth and Goblet cells, which may lead to the development of an epithelial barrier defect. The decrease does not exceed the duration of the cell population renewal of the epithelium suggesting that in order to induce a long-term effect, CRF signaling must affect stem cells. Analyzing various characteristic markers of IEC differentiation, we demonstrate that CRF2 signaling could also affect enterocyte-like differentiation of human adenocarcinoma cell lines. AJ-mediated signaling is linked to activation of Wnt, PI3K/Akt and FGF pathways that are particularly important in intestinal cell proliferation and differentiation[87-89]. Recurrent alteration of AJ may decrease the activation of the signaling pathways necessary for the progression of enterocyte differentiation. Indeed, chronic administration of Ucn3 during differentiation delays the increase in DPPIV and PA activity found in differentiated Caco-2 cells. Regulation of DPPIV activity is correlated with a down-regulation of DPPIV protein expression following Ucn3 exposure. As it might be expected, the exposure to chronic Ucn3 compared to a single exposure (acute stress) has more severe consequences on enzyme activities. In vivo, the alteration generated by an acute stress does not exceed 5 d or the time of the cellular renewal of the intestinal epithelium. In these experiments, the colonic epithelial barrier is morphologically altered, the expression of mRNAs coding for the TJ proteins is reduced and the differentiation of the colon cells is modified[68]. The use of chronic stress (5-10 d of repeated exposure to stressors) is thought to reflect more accurately the daily stressors of humans. Indeed, the exposure to chronic water avoidance stress (WAS) leads to enhanced ultrastructural abnormalities in the epithelium, characterized by reduced crypt length (caused by increased apoptosis) and increased cell proliferation, in an attempt to replace damaged cells and decrease cell differentiation. The presence of undifferentiated cells within the epithelium may be responsible for the lackiness of the epithelial barrier[90].
Another way by which Ucn3 could affect enterocyte differentiation is by modulating ECM proteins. Indeed, we found that exposure of HT-29 cells to Ucn3 induced remodeling of ECM components by regulating both metalloprotease secretion and laminin 332 deposit ([25] and unpublished data). The role of ECM in the reinforcement of E-cadherin-dependent signaling has been previously described in Caco-2 cells[91]. Activation of integrins, which occurs after Caco-2 cells are cultured on complex ECM stimulates the expression of apolipoprotein A IV, a marker of IEC differentiation. Similarly, laminin 111 isoform induces the expression of the sucrase-isomaltase in Caco-2 cells, together with the nuclear shuttling of nucleolin, a transcriptional regulator[92]. This process requires the activation of the p38/MAPK signaling pathway, a cascade known to activate the Caudal type homeobox 2 (Cdx2) protein, a key intestinal transcription factor involved in intestinal epithelial differentiation[93]. In contrast, laminin 332 expression is more associated with inflammation processes such as the restitution of inflamed epithelium but also tumor invasion[94].
KLF4 is a zinc finger transcription factor enriched in the intestinal epithelium[95]. Based on in situ and immunohistochemical experiments, KLF4 localizes to the upper region of the colonic crypt and the villi of small intestine, its expression increases during differentiation along the crypt-villus axis and during intestinal cell maturation in vitro of either the absorptive or the goblet cell lineage[54]. Furthermore, KLF4 is down-regulated in CRC and has been proposed as a tumor suppressor[53,56,73,96-99]. KLF4 exhibits an expression pattern similar to APC, a negative regulator of the Wnt pathway[100]. We confirmed an increased expression of KLF4 in colonic tumor epithelial cells during their differentiation at both transcriptional and post-transcriptional levels. However, we found that CRF2 activation by Ucn3 decreases the mRNA and protein expression of KLF4 in differentiated HT-29 and Caco-2 cells, suggesting that stress may regulate intestinal homeostasis by controlling transcription factor expression. Along these lines, it has been demonstrated that WAS reduces goblet cell number and mucin 2 synthesis via decreased Cdx2 expression[101]. Since KLF4 expression is dependent of Cdx2 in human colon cancer cells, our findings are consistent with these reports[73]. KLF4 regulates both differentiation and growth which is likely fundamental for maintenance of intestinal homeostasis and for its tumor suppressor activity[102]. In this regard, KLF4 transcriptional targets are involved in cell differentiation such as genes coding for laminin 111, AP and villin[103,104]. The Ucn3-mediated down-regulation of KLF4 in differentiated Caco-2 cells may result in the decrease of DPPIV and AP activities. The mechanism by which CRF2 activation regulates intestinal homeostasis remains unknown. Many observations are in favor of an indirect effect of CRF2 action on KLF4 expression: (1) KLF4 expression increases during the process of cell differentiation whereas CRF2 expression decreases; (2) KLF4 expression is transcriptionally regulated during cell differentiation in both cell lines; and (3) KLF4 expression is increased with the establishment of mature intercellular junctions. One possible mechanism is that by dissociating intercellular junctions Ucn3-mediated activation of CRF2 signaling could indirectly regulate KLF4 expression at both transcriptional and post-transcriptional levels. Indeed, we have found that CRF2 signaling induces an alteration of AJ, a process associated with the delocalization of AJ proteins. Release of β-ctn from AJ complexes leads to the transcriptional activity of β-ctn/Tcf signaling which plays a crucial role in homeostasis and transformation of the intestinal mucosa[10,105]. Furthermore, it has been proposed that elevated β-ctn/Tcf signaling reduces levels of KLF4[54]. We observed that Ucn3-mediated cell dissociation is associated with nuclear translocation of β-ctn (data not shown). The decrease in expression of KLF4 following activation of CRF2 could therefore induce: (1) an increase in proliferation; (2) an altered intestinal epithelial differentiation; (3) a loss of mucus cells causing a large decrease in mucus and thus leading to mechanical (by chyme) and chemical (by digestive juices) changes in the epithelium; (4) an impairment of the release of defenses promoting bacterial proliferation; and (5) an epithelio-mesenchymal transition at the origin of tumor development.
In conclusion, we showed that CRF2 signaling induces alterations in both the epithelium permeability and the differentiation of colonic carcinoma cell lines. To our knowledge, this is the first report showing that CRF2 signaling modifies the enterocyte-like differentiation process. On one hand, by altering the differentiation of enterocyte cells, stress could lead to the development of epithelial barrier defects and alterations of mucosal function, contributing to the enhancement of GI disorders. On the other hand, by altering the differentiation status of cancer cells, stress may contribute to tumor development. CRF2 could therefore play a role in tumor progression by loss of cellular contacts, increased cell permeability and decreased KLF4 expression.
The authors would like to thank Maximin Detrait and Anna Garnier for their technical support. The authors also would like to thank Jacques Brocard for his precious help in biostatistics.
In the last decade, the influence of stress (psychological and environmental) on pathogenesis received increased awareness. This is particularly true for gastrointestinal (GI) disorders occurring in inflammatory bowel disease (IBD) and colorectal cancers (CRC). In human as well as in animal models, stress has been described to alter intestinal barrier integrity and function. Furthermore stress neuromediators, such as urocortins (Ucn 2/3) and their receptor the Corticotropin Releasing Factor 2 (CRF2) were upregulated in poorly differentiated CRC and promote metastatic potential through an epithelial-mesenchymal transition-like process. These observations led us to investigate the role of CRF2 signaling in the modulation of epithelial permeability and enterocyte-like cell differentiation.
Patients with IBD often suffer from intestinal inflammatory flares that favor the development of colitis associated cancer. Stress could favor the development and/or aggravation of GI disorders by inducing flares. However the mechanisms involved in this process are still poorly understood, but are mainly associated with epithelial barrier dysfunction.
The authors’ results reinforce the role of stress in the development and/or aggravation of GI disorders. While stress has been described to modulate the fate of secretory epithelial cells, its role on enterocyte differentiation remains unknown. New findings from our work indicate that: (1) CRF2 protein is preferentially expressed in undifferentiated epithelial cells from the crypts of colon and in human cell lines; (2) Ucn3-mediated CRF2 signaling alters enterocyte differentiation by down-regulating KLF4 transcription factor expression; (3) this effect relies on alterations of cell permeability and cellular adhesion junctions; and (4) the effect on cell differentiation is greater following chronic exposure to Ucn3 rather than acute exposure. The impact of stress on enterocyte differentiation may contribute to barrier dysfunction and development of GI disorders.
To our knowledge, this is the first report showing that CRF2 signaling modifies the enterocyte-like differentiation process. On one hand, by altering the differentiation of enterocyte cells, stress could lead to the development of epithelial barrier defects and alterations of mucosal function, contributing to the enhancement of GI disorders. On the other hand, stress-induced loss of cellular differentiation favors tumor initiation and progression. Thus a better understanding of the underlying mechanisms associated with stress will propose new therapeutic targets.
The CRFergic system is a central element of the stress response constituted of specific stress neuromediators, such as corticotropin releasing factor or its analogs urocortins (Ucn 1, 2 and 3) and their receptors CRF1 and CRF2.
This manuscript demonstrated that Ucn3-induced CRF2 signaling could modulate intestinal epithelial cell differentiation and epithelial cell permeability. The authors found CRF2 was associated with a poor differentiated status of IEC. Then, they proved CRF2 signaling altered the trans- and para-cellular permeability, and delayed colonic cell differentiation. In general, the work would be potentially useful to reveal the roles of CRF2 signaling in tumor progression.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Xiao Y S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 360] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 2. | Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313-3323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 515] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 4. | Shahbazi MN, Megias D, Epifano C, Akhmanova A, Gundersen GG, Fuchs E, Perez-Moreno M. CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions. J Cell Biol. 2013;203:1043-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279:9512-9521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Chartier NT, Lainé MG, Ducarouge B, Oddou C, Bonaz B, Albiges-Rizo C, Jacquier-Sarlin MR. Enterocytic differentiation is modulated by lipid rafts-dependent assembly of adherens junctions. Exp Cell Res. 2011;317:1422-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771-1781. [PubMed] |
| 8. | Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226-8234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Peignon G, Thenet S, Schreider C, Fouquet S, Ribeiro A, Dussaulx E, Chambaz J, Cardot P, Pinçon-Raymond M, Le Beyec J. E-cadherin-dependent transcriptional control of apolipoprotein A-IV gene expression in intestinal epithelial cells: a role for the hepatic nuclear factor 4. J Biol Chem. 2006;281:3560-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mariadason JM, Bordonaro M, Aslam F, Shi L, Kuraguchi M, Velcich A, Augenlicht LH. Down-regulation of beta-catenin TCF signaling is linked to colonic epithelial cell differentiation. Cancer Res. 2001;61:3465-3471. [PubMed] |
| 11. | Pelissier-Rota MA, Chartier NT, Jacquier-Sarlin MR. Dynamic Regulation of Adherens Junctions: Implication in Cell Differentiation and Tumor Development. Intercellular Communication in Cancer. Springer Netherlands, 2015: 53-149. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Schneider MR, Dahlhoff M, Horst D, Hirschi B, Trülzsch K, Müller-Höcker J, Vogelmann R, Allgäuer M, Gerhard M, Steininger S. A key role for E-cadherin in intestinal homeostasis and Paneth cell maturation. PLoS One. 2010;5:e14325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K. The E-cadherin adhesion molecule and colorectal cancer. A global literature approach. Anticancer Res. 2008;28:3815-3826. [PubMed] |
| 14. | Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 15. | Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med. 2008;8:247-252. [PubMed] |
| 16. | Mawdsley JE, Rampton DS. The role of psychological stress in inflammatory bowel disease. Neuroimmunomodulation. 2006;13:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Hisamatsu T, Inoue N, Yajima T, Izumiya M, Ichikawa H, Hibi T. Psychological aspects of inflammatory bowel disease. J Gastroenterol. 2007;42 Suppl 17:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Chang J, Adams MR, Clifton MS, Liao M, Brooks JH, Hasdemir B, Bhargava A. Urocortin 1 modulates immunosignaling in a rat model of colitis via corticotropin-releasing factor receptor 2. Am J Physiol Gastrointest Liver Physiol. 2011;300:G884-G894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Santos J, Yates D, Guilarte M, Vicario M, Alonso C, Perdue MH. Stress neuropeptides evoke epithelial responses via mast cell activation in the rat colon. Psychoneuroendocrinology. 2008;33:1248-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 266] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Ducarouge , Jacquier-sarlin M. Stress neuromediators are key regulators of the intestinal barrier: Link to inflammation and cancer: Stress neuromediators regulate the intestinal barrier. Trends Cell Mol Biol. 2011;6:59-88. |
| 22. | Boucher MJ, Laprise P, Rivard N. Cyclic AMP-dependent protein kinase A negatively modulates adherens junction integrity and differentiation of intestinal epithelial cells. J Cell Physiol. 2005;202:178-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Yuan Z, McCauley R, Chen-Scarabelli C, Abounit K, Stephanou A, Barry SP, Knight R, Saravolatz SF, Saravolatz LD, Ulgen BO. Activation of Src protein tyrosine kinase plays an essential role in urocortin-mediated cardioprotection. Mol Cell Endocrinol. 2010;325:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399-404. [PubMed] |
| 25. | Ducarouge B, Pelissier-Rota M, Lainé M, Cristina N, Vachez Y, Scoazec JY, Bonaz B, Jacquier-Sarlin M. CRF2 signaling is a novel regulator of cellular adhesion and migration in colorectal cancer cells. PLoS One. 2013;8:e79335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 552] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 27. | Teller IC, Beaulieu JF. Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev Mol Med. 2001;3:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci. 2010;96:207-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Kedinger M, Lefebvre O, Duluc I, Freund JN, Simon-Assmann P. Cellular and molecular partners involved in gut morphogenesis and differentiation. Philos Trans R Soc Lond B Biol Sci. 1998;353:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Simon-Assmann P, Kedinger M. Heterotypic cellular cooperation in gut morphogenesis and differentiation. Semin Cell Biol. 1993;4:221-230. [PubMed] |
| 31. | Yasugi S, Mizuno T. Molecular analysis of endoderm regionalization. Dev Growth Differ. 2008;50 Suppl 1:S79-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 188] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 347] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 34. | Estienne M, Claustre J, Clain-Gardechaux G, Paquet A, Taché Y, Fioramonti J, Plaisancié P. Maternal deprivation alters epithelial secretory cell lineages in rat duodenum: role of CRF-related peptides. Gut. 2010;59:744-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Ménard D, Pothier P, Gallo-Payet N. Epidermal growth factor receptors during postnatal development of the mouse colon. Endocrinology. 1987;121:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Rao R, Baker RD, Baker SS. Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem Pharmacol. 1999;57:685-695. [PubMed] |
| 37. | Huet C, Sahuquillo-Merino C, Coudrier E, Louvard D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J Cell Biol. 1987;105:345-357. [PubMed] |
| 38. | Gout S, Marie C, Lainé M, Tavernier G, Block MR, Jacquier-Sarlin M. Early enterocytic differentiation of HT-29 cells: biochemical changes and strength increases of adherens junctions. Exp Cell Res. 2004;299:498-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Shao L, Serrano D, Mayer L. The role of epithelial cells in immune regulation in the gut. Semin Immunol. 2001;13:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol. 2002;4:E101-E108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 450] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 41. | Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol. 1995;15:4819-4824. [PubMed] |
| 42. | Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189-202. [PubMed] |
| 43. | Pinto M. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323-330. |
| 44. | Beaulieu JF, Quaroni A. Clonal analysis of sucrase-isomaltase expression in the human colon adenocarcinoma Caco-2 cells. Biochem J. 1991;280:599-608. [PubMed] |
| 45. | Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology. 1992;103:414-423. [PubMed] |
| 46. | Hauri HP, Roth J, Sterchi EE, Lentze MJ. Transport to cell surface of intestinal sucrase-isomaltase is blocked in the Golgi apparatus in a patient with congenital sucrase-isomaltase deficiency. Proc Natl Acad Sci USA. 1985;82:4423-4427. [PubMed] |
| 47. | Balimane PV, Chong S. Cell culture-based models for intestinal permeability: a critique. Drug Discov Today. 2005;10:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 48. | Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 334] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 49. | Lili LN, Farkas AE, Gerner-Smidt C, Overgaard CE, Moreno CS, Parkos CA, Capaldo CT, Nusrat A. Claudin-based barrier differentiation in the colonic epithelial crypt niche involves Hopx/Klf4 and Tcf7l2/Hnf4-α cascades. Tissue Barriers. 2016;4:e1214038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 315] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 51. | Black AR, Black JD, Azizkhan-Clifford J. Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 850] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 52. | Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619-2628. [PubMed] |
| 53. | McConnell BB, Ghaleb AM, Nandan MO, Yang VW. The diverse functions of Krüppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007;29:549-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 54. | Flandez M, Guilmeau S, Blache P, Augenlicht LH. KLF4 regulation in intestinal epithelial cell maturation. Exp Cell Res. 2008;314:3712-3723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 55. | Yu T, Chen X, Zhang W, Li J, Xu R, Wang TC, Ai W, Liu C. Krüppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS One. 2012;7:e32492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Zhao W, Hisamuddin IM, Nandan MO, Babbin BA, Lamb NE, Yang VW. Identification of Krüppel-like factor 4 as a potential tumor suppressor gene in colorectal cancer. Oncogene. 2004;23:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 57. | Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60 Suppl 7:33-46. [PubMed] |
| 59. | Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Moss AC, Anton P, Savidge T, Newman P, Cheifetz AS, Gay J, Paraschos S, Winter MW, Moyer MP, Karalis K. Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2alpha. Gut. 2007;56:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Kaprara A, Pazaitou-Panayiotou K, Kortsaris A, Chatzaki E. The corticotropin releasing factor system in cancer: expression and pathophysiological implications. Cell Mol Life Sci. 2010;67:1293-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Miceli F, Ranelletti FO, Martinelli E, Petrillo M, Scambia G, Navarra P, Ferrandina G. Expression and subcellular localization of CRH and its receptors in human endometrial cancer. Mol Cell Endocrinol. 2009;305:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Florio P, De Falco G, Leucci E, Torricelli M, Torres PB, Toti P, Dell’Anna A, Tiso E, Santopietro R, Leoncini L. Urocortin expression is downregulated in human endometrial carcinoma. J Endocrinol. 2006;190:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 65. | Scharl M, Rogler G. Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterol. 2012;28:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216-G228. [PubMed] |
| 67. | Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 68. | Demaude J, Levêque M, Chaumaz G, Eutamène H, Fioramonti J, Bueno L, Ferrier L. Acute stress increases colonic paracellular permeability in mice through a mast cell-independent mechanism: involvement of pancreatic trypsin. Life Sci. 2009;84:847-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Kiliaan AJ, Saunders PR, Bijlsma PB, Berin MC, Taminiau JA, Groot JA, Perdue MH. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998;275:G1037-G1044. [PubMed] |
| 70. | Teitelbaum AA, Gareau MG, Jury J, Yang PC, Perdue MH. Chronic peripheral administration of corticotropin-releasing factor causes colonic barrier dysfunction similar to psychological stress. Am J Physiol Gastrointest Liver Physiol. 2008;295:G452-G459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008;8:274-281. [PubMed] |
| 72. | Yang PC, Jury J, Söderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104-114; quiz 363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 73. | Dang DT, Mahatan CS, Dang LH, Agboola IA, Yang VW. Expression of the gut-enriched Krüppel-like factor (Krüppel-like factor 4) gene in the human colon cancer cell line RKO is dependent on CDX2. Oncogene. 2001;20:4884-4890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Zheng PY, Feng BS, Oluwole C, Struiksma S, Chen X, Li P, Tang SG, Yang PC. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 75. | Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. 2012;7:e39935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 76. | Buckinx R, Adriaensen D, Nassauw LV, Timmermans JP. Corticotrophin-releasing factor, related peptides, and receptors in the normal and inflamed gastrointestinal tract. Front Neurosci. 2011;5:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Li Q, Zhang Q, Zhang M, Wang C, Zhu Z, Li N, Li J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008;275:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Pang JH, Kraemer A, Stehbens SJ, Frame MC, Yap AS. Recruitment of phosphoinositide 3-kinase defines a positive contribution of tyrosine kinase signaling to E-cadherin function. J Biol Chem. 2005;280:3043-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113:1319-1334. [PubMed] |
| 80. | Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 81. | Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 658] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 82. | Santos J, Saunders PR, Hanssen NP, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am J Physiol. 1999;277:G391-G399. [PubMed] |
| 83. | Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198-G203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 84. | McCormick DA, Horton LW, Mee AS. Mucin depletion in inflammatory bowel disease. J Clin Pathol. 1990;43:143-146. [PubMed] |
| 85. | Morson BC. Rectal biopsy in inflammatory bowel disease. N Engl J Med. 1972;287:1337-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1222] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 87. | Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301-314. [PubMed] |
| 88. | Tran NL, Adams DG, Vaillancourt RR, Heimark RL. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905-32914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963-2971. [PubMed] |
| 90. | Boudry G, Jury J, Yang PC, Perdue MH. Chronic psychological stress alters epithelial cell turn-over in rat ileum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1228-G1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 91. | Schreider C, Peignon G, Thenet S, Chambaz J, Pinçon-Raymond M. Integrin-mediated functional polarization of Caco-2 cells through E-cadherin--actin complexes. J Cell Sci. 2002;115:543-552. [PubMed] |
| 92. | Turck N, Lefebvre O, Gross I, Gendry P, Kedinger M, Simon-Assmann P, Launay JF. Effect of laminin-1 on intestinal cell differentiation involves inhibition of nuclear nucleolin. J Cell Physiol. 2006;206:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Houde M, Laprise P, Jean D, Blais M, Asselin C, Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001;276:21885-21894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Pyke C, Salo S, Ralfkiaer E, Rømer J, Danø K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132-4139. [PubMed] |
| 95. | Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009-20017. [PubMed] |
| 96. | Choi BJ, Cho YG, Song JW, Kim CJ, Kim SY, Nam SW, Yoo NJ, Lee JY, Park WS. Altered expression of the KLF4 in colorectal cancers. Pathol Res Pract. 2006;202:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Kanai M, Wei D, Li Q, Jia Z, Ajani J, Le X, Yao J, Xie K. Loss of Krüppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12:6395-6402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN, Wu M. Down-regulation of gut-enriched Kruppel-like factor expression in esophageal cancer. World J Gastroenterol. 2002;8:966-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | Wei D, Kanai M, Huang S, Xie K. Emerging role of KLF4 in human gastrointestinal cancer. Carcinogenesis. 2006;27:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 100. | Smith KJ, Johnson KA, Bryan TM, Hill DE, Markowitz S, Willson JK, Paraskeva C, Petersen GM, Hamilton SR, Vogelstein B. The APC gene product in normal and tumor cells. Proc Natl Acad Sci USA. 1993;90:2846-2850. [PubMed] |
| 101. | Shigeshiro M, Tanabe S, Suzuki T. Repeated exposure to water immersion stress reduces the Muc2 gene level in the rat colon via two distinct mechanisms. Brain Behav Immun. 2012;26:1061-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 102. | Chen X, Whitney EM, Gao SY, Yang VW. Transcriptional profiling of Krüppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326:665-677. [PubMed] |
| 103. | Hinnebusch BF, Siddique A, Henderson JW, Malo MS, Zhang W, Athaide CP, Abedrapo MA, Chen X, Yang VW, Hodin RA. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Kruppel-like factor. Am J Physiol Gastrointest Liver Physiol. 2004;286:G23-G30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 104. | Piccinni SA, Bolcato-Bellemin AL, Klein A, Yang VW, Kedinger M, Simon-Assmann P, Lefebvre O. Kruppel-like factors regulate the Lama1 gene encoding the laminin alpha1 chain. J Biol Chem. 2004;279:9103-9114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |