Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5097
Peer-review started: February 19, 2017
First decision: March 3, 2017
Revised: May 12, 2017
Accepted: July 4, 2017
Article in press: July 4, 2017
Published online: July 28, 2017
Processing time: 162 Days and 3.8 Hours
The inflammatory process plays a central role in the development and progression of numerous pathological situations, such as inflammatory bowel disease (IBD), autoimmune and neurodegenerative diseases, metabolic syndrome, and cardiovascular disorders. IBDs involve inflammation of the gastrointestinal area and mainly comprise Crohn’s disease (CD) and ulcerative colitis (UC). Both pathological situations usually involve recurring or bloody diarrhea, pain, fatigue and weight loss. There is at present no pharmacological cure for CD or UC. However, surgery may be curative for UC patients. The prescribed treatment aims to ameliorate the symptoms and prevent and/or delay new painful episodes. Flavonoid compounds are a large family of hydroxylated polyphenolic molecules abundant in plants, including vegetables and fruits which are the major dietary sources of these compounds for humans, together with wine and tea. Flavonoids are becoming very popular because they have many health-promoting and disease-preventive effects. Most interest has been directed towards the antioxidant activity of flavonoids, evidencing a remarkable free-radical scavenging capacity. However, accumulating evidence suggests that flavonoids have many other biological properties, including anti-inflammatory, antiviral, anticancer, and neuroprotective activities through different mechanisms of action. The present review analyzes the available data about the different types of flavonoids and their potential effectiveness as adjuvant therapy of IBDs.
Core tip: Inflammatory bowel diseases (IBDs) involve inflammation of the gastrointestinal tract and primarily comprise Crohn's disease and ulcerative colitis. Currently, there is no cure for most of the IBDs. Emerging evidence suggests that flavonoids have many biological and pharmacological properties, including anti-inflammatory, antiviral, anticancer, and neuroprotective activities through different mechanisms of action. The present review critically analyzes the current experimental evidence on the therapeutic potential of flavonoids in IBD.
- Citation: Salaritabar A, Darvishi B, Hadjiakhoondi F, Manayi A, Sureda A, Nabavi SF, Fitzpatrick LR, Nabavi SM, Bishayee A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J Gastroenterol 2017; 23(28): 5097-5114
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5097.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5097
Inflammation is a protective and complex process consisting of a set of molecular, cellular and vascular defensive responses against any injury, including chemical, physical, or biological attacks, and focused on restoring tissue function[1]. Inflammatory diseases comprise a group of illnesses characterized by a long-term pro-inflammatory state[2]. A large number of pathologies are considered as inflammatory diseases, such as autoimmune and cardiovascular disorders, chronic obstructive pulmonary diseases, neurodegenerative diseases, and chronic inflammatory bowel disease (IBD). This inflammatory response is associated with changes in vascular permeability, increases in blood flow, leukocyte mobilization and rise in the production of inflammatory mediators[3,4]. Some of the produced mediators, mainly cytokines, are able to activate signaling by nuclear factor-kappa B (NF-κB), a transcription factor which also mediates the inflammatory response[5-7].
IBDs include a group of pathologies characterized by chronic and uncontrolled inflammation associated with deregulation of both adaptive and innate immunity that affects the gastrointestinal tract[8,9]. The bowel inflammation results in symptoms, such as abdominal pain, bleeding, recurrent diarrhea and weight loss[8,9]. The two main pathologies are Crohn’s disease (CD) and ulcerative colitis (UC). CD can affect any area of the gastrointestinal tract, from the mouth to the anus, although the ileum is the most affected section. In contrast, UC primarily affects the colon and the rectum. The exact cause of IBD is not fully known, although there is an interaction between diverse factors, such as an immune system disturbance, genetic predisposition, and environmental factors, which activates the damaging immune response in the intestines. Today, there is no effective pharmacological treatment that allows for cure of the disease. Medical therapy is focused on non-specific immunosuppressive therapies, including thiopurines and methotrexate[10,11]. The occurrence and prevalence of IBDs are progressively growing in all areas around the world, suggesting its appearance as a global disease in the near future[12].
The term flavonoid derives from the Latin word “flavus”, meaning yellow, and comprises a group of secondary metabolic compounds widely found in plants well known for the distinctive blue, red, and purple anthocyanin pigments of their different structures. Although they are not stated as nutrients and regardless of their physiological functions in plants, flavonoids are key ingredients of the human diet[13]. Based on epidemiological studies, diets rich in flavonoids are in direct correlation with increased longevity and decreased cardiovascular disease incidence, despite consuming diets with high fat content[14-17]. Many biological effects have been attributed to the flavonoids, in addition to their antioxidant properties, some of which include anti-inflammatory, antimicrobial, vasodilatory, anti-ischemia and anticancer effects[16,18-20].
Recently, owing to their significant antioxidant and free radical scavenging properties observed in vitro, interest towards investigating new possible health benefits has significantly increased. In fact, flavonoids are of great nutritional value in inflammatory diseases because they can block many pro-inflammatory proteins and can be considered natural inhibitors of inflammation, ameliorating the intensity of inflammation[21]. In addition to the direct antioxidant activity, flavonoids are capable of activating diverse antioxidant and protective genes via nuclear transcription factors and also of inhibiting inflammatory pathways[22]. Flavonoids influence the composition of the microbial flora, favoring the growth of bifidum and lactobacilli bacteria and stimulating an anti-inflammatory environment[23-26].
As described earlier, since the precise etiology of IBD has not been identified clearly yet and no specific causal treatment has been established thus far, based on the aforementioned biological effects, flavonoids may be of great utility in managing IBD. It should be noted that another review paper broadly covered this topic during the past year[27]. In this review, we discuss the different subclasses of flavonoids and existing data about their effectiveness in preclinical models of IBD, which could translate to a future role in human IBD therapy.
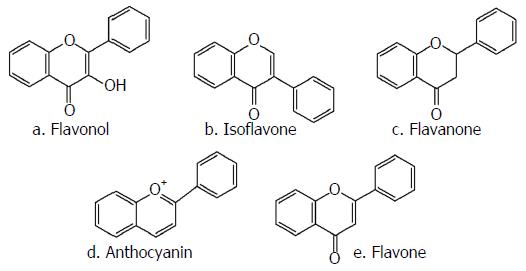
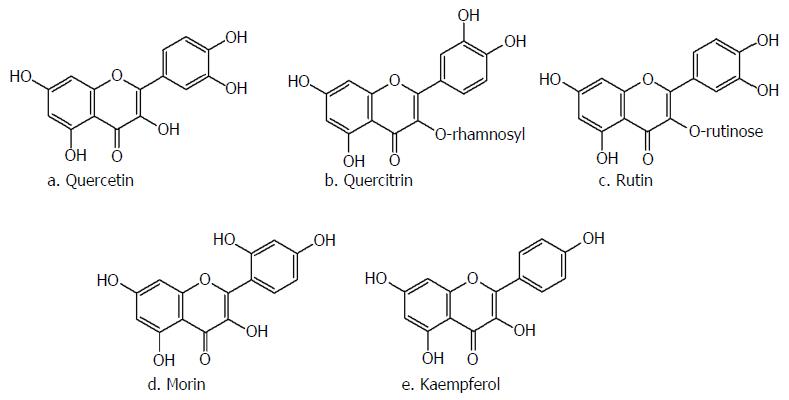
Flavonols are one of the main subclasses of flavonoids, with the specific 3-hydroxyflavone structural backbone depicted in Figure 1A. Multiple flavonols have been extracted from leaves, flowers and the outer part of plants and their pharmacological effects have been evaluated through several studies. The most well-known members of this group of flavonoids are quercetin (3,3’,4’,5,7-pentahydroxyflavone), rutin (quercetin 3-rutinoside), morin and kaempferol (Figure 2)[28].
Quercetin and its glycosylated derivatives, such as rutin and quercitrin (quercetin 3-rhamnoside), are the foremost representatives of flavonols, demonstrating remarkable effects on attenuation of pharmacological models of colitis[29-32]. Although many in vitro studies have determined that quercetin is more effective than its glycosylated derivatives in reducing the inflammatory response, the majority of in vivo studies did not observe this same efficacy. In this way, it was reported that a diet with 0.1% rutin in its composition supplied during 2 wk, but not quecetin, ameliorated dextran sulfate sodium (DSS)-induced colitis in a mouse model via lessening of pro-inflammatory cytokine production[29].
Poor stomach[33] and intestinal absorption[34,35] of these compounds are the primary obstacles against reaching an adequate concentration in colon. Many studies investigating the gastrointestinal absorption of flavonols have suggested that the hydrophilic structure of quercitrin and rutin is the main cause of their poor absorption. Present in colon, glycosylated flavonols are cleaved by colon microflora forming the aglycon shape of these compounds[36-38]. Therefore, it is suggested that quercitrin and rutin can act as pro-drugs of quercetin, preserving the aglycon moiety from absorption and assuring an intact nature in the colon and ability to reach where it will be further hydrolyzed and yield quercetin[34,39,40]. It seems that colon-specific drug delivery systems are a necessary strategy to preserve quercetin from degradation and absorption through the gastrointestinal tract and to increase its availability. Castangia et al[41] demonstrated that chitosan/nutriose-coated vesicles represent a promising strategy to improve quercetin concentration in the colon. Additionally, Guazelli et al[30] depicted that quercetin-encapsulated microcapsules are more effective in pharmacological animal models of colitis compared to intact quercetin.
In a study performed on acetic acid-induced colitis in mice, treatment with quercetin (100 mg/kg) loaded pectin/casein polymer microcapsules significantly prevented the depletion of glutathione (GSH) reservoirs in the colon. Although the results did not evidence significant statistical differences between treatment and control groups, at least a significant tendency for preserving GSH reservoirs was observed[30]. According to Dodda et al[42,43] administration of quercetin (50 and 100 mg/kg, intra-rectal) in tri-nitrobenzene sulfonic acid (TNBS)- and (50 and 100 mg/kg, p.o.) in acetic acid-induced colitis rat models resulted in a considerable elevation in the GSH levels when compared with the control group. A main limitation of these two studies is the use of high concentrations of quercetin that cannot be achieved with a normal diet. With regard to quercitrin, the oral administration of 1 and 5 mg/kg at 2 h before TNBS-induced colitis in rats thwarted GSH depletion[44]. Also, in two separate studies, Sánchez de Medina et al[44] and Cruz et al[45] showed that oral pretreatment of rats with 1 and 5 mg/kg quercitrin and 5, 10 and 25 mg/kg of rutin increased GSH levels in both acute and chronic phase of TNBS-induced colitis.
Other mechanisms for ameliorating flavonols effects on pharmacological models of colitis include the suppression of nitric oxide (NO) production and/or inducible nitric oxide synthase (iNOS) expression. Camuesco et al[46] proposed that the histological and biochemical anti-inflammatory effects of quercitrin might be related to a decrease in iNOS expression through down-regulation of NF-κB in colonic tissue. They also demonstrated that oral administration of quercitrin (1 and 5 mg/kg) significantly reduced the iNOS expression, contributing to the inhibition of iNOS activity. This outcome had been supported by subsequent studies.
A down-regulation of the inflammatory response of macrophages derived from bone marrow, inhibition of cytokine and NO synthase expression through inhibiting the NF-κB pathway in the presence of quercetin and quercitrin (1 mg/kg/d, 15 d) was reported in an experimental rat model of colitis provoked by DSS. This study also suggested that the effects of quercitrin observed in vivo might originate from the release of quercetin by intestinal microflora[31]. In two distinct studies, the in vitro inhibitory effects of quercetin on NO production were demonstrated in lipopolysaccharide (LPS)-induced macrophages. In both studies, the expression of mRNA and protein of NOS was attenuated in cell cultures and this effect was attributed to suppression of the NF-κB pathway[47,48].
In another study, a diet containing 0.1% rutin mitigated the DSS-induced weight loss and improved colitis histological scores in mice probably through inhibition of interleukin (IL)-1β and subsequent inhibition of the induction of iNOS in enterocytes[29]. Also, oral administration to rats with TNBS-induced colitis of rutin (10 mg/kg) for 6 d had significant ameliorating effects on inflammation of the colon, with similar effectiveness as sulfasalazine (30 mg/kg), and also decreased myeloperoxidase (MPO) activity[40]. Finally, rutin (28.5 and 57 mg/kg per day by gavage) also demonstrated anticolitis activity, when examined in a mouse T-cell transfer model of IBD[49].
The main limitation of quercitrin and rutin in IBD is the fact that the compounds represent only a small fraction of the flavonoids usually ingested in the diet, being probably insufficient to exert a significant pharmacological effect. For that reason, the development of pharmacological formulations containing concentrations that can be quantitatively active and therapeutic is required.
As a flavonols family member, morin is present in a variety of fruits, vegetables and beverages[50]. In several studies, antioxidant, anti-inflammatory, anticancer, antidiabetic, and cytoprotective effects of this compound were assessed[51]. The effects of morin were evaluated in acute and chronic stages of the TNBS-induced colitis. In the acute model of colitis, pretreatment with morin (25 mg/kg) significantly alleviated the intestinal inflammation via inhibition of colonic leukotriene B4 (LTB4) production and due to its antioxidant properties[52]. In the chronic phase of colitis, the administration of morin (25 mg/kg) exhibited significant anti-inflammatory effect via attenuating production of inflammatory mediators, such as free radicals, LTB4, NO and IL-1β[53]. Although the authors confirmed that morin showed inhibitory effects against colonic NO synthase activity in an in vitro assay, the specific pathway mediating the anti-inflammatory effects has been not investigated. Finally, the authors stated, similar to quercitrin and rutin, that the amounts of morin employed in this study were notably higher than that attained through dietary consumption.
Kaempferol is abundant in plants of the genera Delphinium, Camellia, Berberis, Citrus, Brassica, Allium, Malus, etc.[54], and similar to other flavonoids is naturally bond to different sugars[55]. This flavonol possesses different biological activities, such as anticancer[56-61], antimicrobial[62,63], antioxidant[64-68], and anti-inflammatory[69-71]. According to Calderón-Montaño et al[55], kaempferol’s anti-inflammatory property is mostly derived from its ability to inhibit NF-κB, activator of transcription 1 and activator protein 1 pathways that regulate a wide spectrum of genes, including cytokines, growth factors, stress-response proteins[55,71]. Inhibition of these pathways is associated with a decrease in tumor necrosis factor-alpha (TNF-α) levels, IL-1β and IL-8 expression, cyclooxygenase-2 (COX-2), lipoxygenase and iNOS activation and with a reduction of cellular levels of reactive oxygen species[55]. Also, Park et al[72] reported that 0.3% kaempferol administered pre- and post-feeding diminished DSS-induced colitis in mice through down-regulation of TNF-α, IL-6, IL-1β, NOS, and COX-2 at the mRNA expression level. In addition, the compound reduced LTB4, prostaglandin E2 (PGE2) and NO levels and MPO activity. Furthermore, in the prefer group, kaempferol preserved the goblet cell function, which was indicated via up-regulation of trefoil factor family 2 mRNA expression in the distal colon mucosa[72].
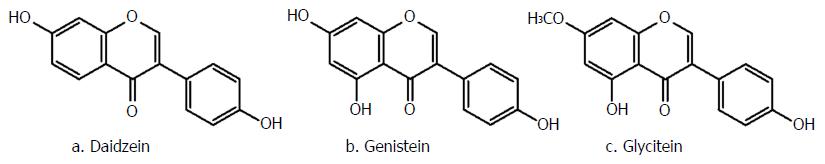
One of the main subclasses of flavonoids, mostly found in soybeans, nuts and whole grains are isoflavones. These naturally occurring compounds are glycoside conjugates, predominantly malonyl-glycosides, and have a common backbone of 3-phenylchromen-4-one (Figure 1B)[73]. During different stages of food processing, including fermentation or hot water extraction, the glycosidic groups of genistin, diadzein and glycitinis (Figure 3) are removed[74]. After ingestion, intestinal enzymes and/or intestinal microflora hydrolyze the conjugated isoflavones and produce more bioactive and bioavailable genistein, diadzein and glycitein[74,75]. Afterwards, these unconjugated aglycones are either passively absorbed through the small intestine or metabolized to other metabolites, such as equol, P-ethyl phenol and di-hydroglycitein, consistent with diadzein, genistein and glycitein, respectively[76,77].
Along with antimicrobial, antioxidant, anti-inflammatory and anticancer activities, isoflavones can also reduce the risk of cardiovascular diseases and osteoporosis[75]. Additionally, the structural similarity of these compounds with estrogens, specifically 17-β estradiol, allows them to act as partial estrogen receptors (ERs)[77,78].
Some studies have demonstrated that estrogen receptors, especially ER subtype beta (ER-β), play a key role in improving the epithelial intestinal barrier[79,80]. Moussa et al[80] reported that daily treatment with 0.45 mg of fermented soy germ ingredient (FSG), mainly consisting of isoflavones and Bowman-Birk inhibitors (BBI, a serine protease inhibitor), significantly suppressed TNBS-induced colitis in rats via two different mechanisms. First through ER-signaling of isoflavones, which is able to reduce inflammatory cytokines, and second through protease-activated receptor - mediated pathway ascribed to BBI. In a recent study, FSG suppressed macrophage migration inhibitory factor production which in turn down-regulated the IL-1β. In addition, FSG can elevate the level of IL-10 and limit gut permeability in colitis. The FSG effects have been antagonized by administration of ER antagonist, proposing that the FSG effects are mainly mediated by ER-β[80].
Activation of the ER pathway is associated with an increased expression of membrane tight junction proteins, which can improve the intestinal barrier integrity, and decreased pro-inflammatory cytokine release. This anti-inflammatory response can be mediated by increased release on the anti-inflammatory IL-10, which in turn inhibits pro-inflammatory cytokine release. Finally, it is interesting to note that these evidenced effects took place when using doses of isoflavones near to the daily ingestion authorized in humans (1 mg/kg body weight/d).
Similar results were obtained using fermented Peuraria lobata extract, rich in isoflavones, in ameliorating the gastrointestinal barrier function in colitis induced by DSS. A reduction of inflammatory cytokines’ mRNA expression and recovery of construction and expression of tight junction proteins was observed in vitro. Additionally, restoration of goblet cells and improvement of epithelial structure in the colonic mucosa was reported in vivo[81]. Also, the isoflavone rich fraction of soybean extract inhibited IL-8 production through TNF-α suppression in the Caco-2 cell line in a dose-dependent manner[82]. In this way, IL-8 release induced by hydrogen peroxide or by IL-1β was not ameliorated when cells were treated with the extract, indicating the implication of TNF-α.
As mentioned above, genistein is another well-characterized aglycon isoflavone. The efficacy of this compound in experimental IBD models has been studied in various experiments. Similar to other isoflavones, genistein can bind to ERs, especially ER-β, the most abundantly expressed subtype in the gastrointestinal tract mimicking estrogenic action[83]. Various studies have demonstrated that ER ligands possess the ability to attenuate IBD symptoms[84,85]. Inflammation-mediated mechanisms have been widely described for protective effects of genistein.
In the study performed by Seibel et al[86] it was demonstrated that genistein oral administration (100 mg/kg body weight) in TNBS-induced colitis in rats resulted in a significant reduction in MPO activity and COX-2 mRNA expression[83]. The authors hypothesized that the treatment with genistein can inhibit the expression of MPO and COX-2 by means of an ER-dependent mechanism, inhibiting the activation of the NF-κβ signaling pathway. Contrarily, Seibel et al[86] informed that in uterus and postnatal rats, exposure to a phytoestrogen-rich diet (including high and low dose of genistein and daidzein) not only did not protect the offspring from TNBS-induced colitis but also enhanced the extent of acute inflammation through increasing neutrophil infiltration and COX-2 protein expression. These surprising results may be attributed to the fact that COX-2 expression is partly repressed by phytoestrogens in the acute phase of inflammation, exacerbating colon inflammation. The authors suggest as a possibility that most of the positive results were obtained in chronic models of IBD and, consequently, it may be an initial magnification of the inflammatory response followed by an enhanced anti-inflammatory process.
Furthermore, based on another in vitro study, genistein significantly prevented the xanthine oxidase/xanthine-induced oxidative stress-mediated alteration in paracellular junctional protein complexes, such as tyrosine phosphorylation and their disassembly from the junctional complex, thereby protecting tight junctions from malfunction in Caco-2 cells[87]. Also, the administration of genistein significantly inhibited the disruption of tight junction by acetaldehyde through deterring tyrosine phosphorylation in Caco-2 cells[88]. The mechanism of action is related to genistein activity as tyrosine kinase inhibitor, which is capable of reducing the tyrosine phosphorylation of functional proteins and also of protecting against dissociation of these proteins from the cytoskeleton[87,88]. Moreover, these protective effects of genistein have also been observed against intestinal tight junction barrier damage triggered by inflammatory mediators, including TNF-α and enteric bacteria, such as Escherichia coli, Proteus mirabilis, Listeria monocytogenes or Salmonella typhimurium[89,90].
Sergent et al[91] revealed an inhibitory effect of genistein on IL-6 and monocyte chemoattractant protein-1 (MCP-1) overproduction in a model of inflamed human intestinal epithelium when investigating the anti-inflammatory activity of some phenolic molecules. In the same study, genistein also down-regulated the levels of NOS and 14 different inflammatory genes[91]. In another study, genistein supplied in dietary concentration thwarted the overproduction of TNF-α and IL-6 in RAW 264.7 macrophages treated with LPS[92]. The ameliorated inflammatory response seems to be mediated by inhibition of NF-κβ activation following AMPK phosphorylation.
Diadzein is another important bioavailable isoflavone exhibiting protective effects in mice with DSS-induced colitis via suppressing the expression of IL-6, IL-8, IL-12, p40 and interferon-gamma (INF-γ) and triggering IL-10 secretion from mesenteric lymph node cells[93]. The authors also reported that diadzein inhibited cytokine production in human monocytic cell lines after Toll-like receptor (TLR)-2 and TLR-4 stimulation, suggesting that the TLR signaling pathway could be a target for isoflavones effects[93]. However, in contrast, equol, the metabolite of diadzein, perpetuates colitis in the DSS-induced model via an unknown mechanism[94]. The treatment timing could be responsible for the negative results since isoflavones can potentiate inflammation in acute colitis, as was mentioned above. In this study, biochemical analysis was performed on day 5 after initiating the colitis treatment, whereas most of the studies reporting beneficial effects lasted more than 14 d since the colitis induction. This possibility is reinforced by the fact that genistein did not improve the severity of colitis[94].
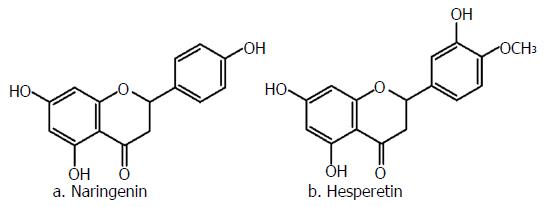
Hesperidin and naringin (Figure 4), like other flavonoids found naturally as glycosides[95], are common constituents of citrus fruits[96]; their skeleton is represented in Figure 1C. Naringin (naringenin-7-O-neohesperidoside) and hesperidin (hesperetin-7-rutinoside) are mainly hydrolyzed by the microflora of the distal part of the small intestine and colon into their aglycones, naringenin and hesperetin, respectively[97].
Various in vivo and in vitro investigations have evidenced the therapeutic activity of naringin, naringenin, hesperidin and hesperetin in several pathological conditions, including cancer, cardiovascular and neurological disorders, and diabetes mellitus[96,98,99] that might be attributed to the anti-inflammatory and antioxidant effects of these constituents.
Kumar et al[100] evaluated the efficacy of naringin in acetic acid-induced colitis models and reported that administration of naringin in different concentrations significantly attenuated inflammatory responses, which in turn prevented further DNA damage. This study also demonstrated that the malondialdehyde (MDA), MPO, NO, xanthine oxidase and alkaline phosphatase concentrations were significantly decreased after naringin treatment, with respect to the control group. Furthermore, this study also reported a reduction of ulcer lesions by naringin, suggesting a potential protection of colonic microflora from the corrosive effect of acetic acid.
Other studies have also demonstrated that naringenin can ameliorate colitis in different animal models of colitis. In a remarkable study conducted by Dou et al[101], it was demonstrated that naringenin treatment significantly improved colitis through down-regulating the mRNA expression of several proinflammatory mediators, including iNOS, MCP-1, intercellular adhesion molecule-1, COX-2, IL-6 and TNF-α. Additionally, they found that the administration of naringenin significantly inhibited up-regulation of TLR-4 expression, which in turn reduced pro-inflammatory cytokines, especially IL-6 and TNF-α. Moreover, phospho-NF-κB p65 protein levels were also decreased, correlating with decrease in phospho-IκBα protein concentrations. These data were consistent with in vitro results obtained by the same investigators[101].
Still other studies have demonstrated the effects of naringenin in different concentrations on colitis models via inhibition of INF-γ, macrophage inflammatory protein 2 (MIP-2), PGE2, NO, IL-6, IL-17A and IL-1β expression[102,103]. Furthermore, naringenin can protect the tight junction barrier[102]. Altogether, these results seem to indicate that targeting of the TLR-4/NF-κB signaling pathway might be one of the underlying mechanisms implicated in the protective effects of naringenin against IBD.
Xu et al[104] demonstrated that hesperidin, just like naringenin, exhibited its beneficiary effects in DSS-induced colitis via decreasing MDA contents, MPO activity, and IL-6 expression levels. In addition, hesperidin reduced iNOS activity and production of PGE2 and NO in a mouse macrophage cell line[104]. However, based on the present data, the explanation of the possible mechanism by which hesperidin acts is difficult to clarify.
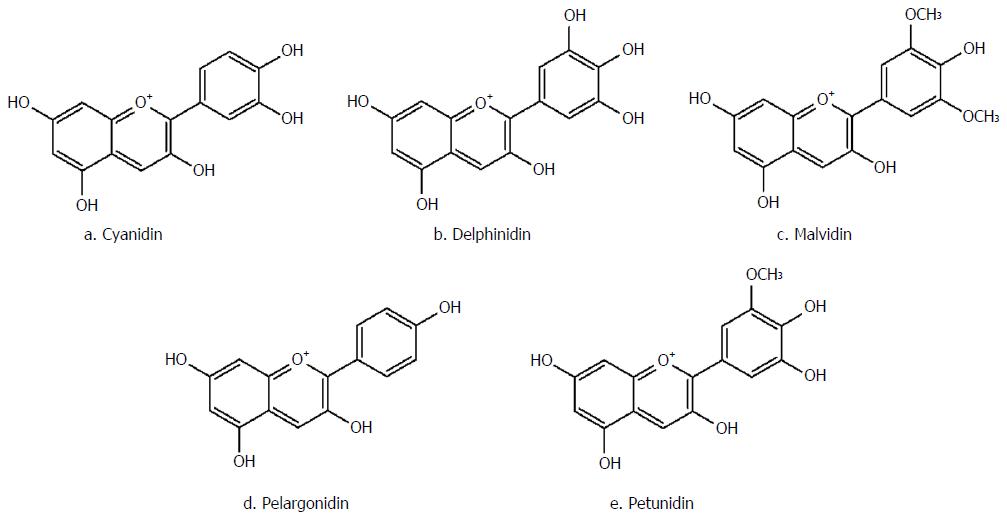
As the red pigment of different berries, currants and grapes, anthocyanins are composed of an anthocyanidin [malvidin, cyanidin, pelargonidin, petunidin, delphinidin, etc. (Figure 5)] conjugated with one to three sugar molecules, including glucose, galactose, xylose, arabinose and rhamnose[105]. In most cases, anthocyanins absorption is dependent on the structure of their aglycone moiety. This group of natural compounds is not regularly metabolized; however, in acidic media, they become rearranged and more stable[106]. Overall, anthocyanins are only partially absorbed and have demonstrated limited biological activity on enterocytes.
Various investigations have been centered on the antioxidant activity of anthocyanins; however, the anti-inflammatory effect has also been extensively observed in other non-intestinal tissues[107-109]. Based on a randomized trial with human participants on a dietary regimen rich in purple-flesh potatoes, containing high amounts of anthocyanins, a significant rise in serum antioxidant parameters levels and a decrease in pro-inflammatory markers, such as IL-6 and C-reactive protein, was reported[110]. Other studies have also reported radical scavenging and anti-inflammatory activities of anthocyanin-containing natural compounds, including bilberry juice, press cake and soybean seed[111,112].
The main limitation in studies testing anthocyanins is the fact that fruit extracts rich in these compounds were used, but not the isolated compounds themselves. All these extracts are an excellent source of vitamin C and other antioxidant compounds, in addition to anthocyanins such as non-flavonoid condensed tannins[113]. As the composition of these extracts was not carried out in those studies, it cannot be specifically concluded that anthocyanins are solely responsible for the protective effects against IBD.
Strawberry as a member of the Rosaceae family, and is a fruit rich in anthocyanin compounds derived from pelargonidin and cyanidin aglycones[114,115]. The anti-IBD effects of strawberry anthocyanins have been mostly attributed to the free-radical scavenging and anti-inflammatory properties of these compounds[116,117]. Based on an in vivo study on acetic acid-induced colitis in rat, oral or rectal administration of strawberry significantly decreased the infiltration of polymorphonuclear cells to the inflammatory site and lessened epithelial necrosis and lesions[118].
As a member of the Ericaceae family, anthocyanins found in blueberry are derivatives of malvidin, delphinidin, cyanidin and petunidin aglycones[119]. Anthocyanins present in blueberry mainly decrease colony number of Clostridium perfringens and Enterococcus Spp, increase butyric acid concentrations, reduce the amounts of succinic acid, decrease IL-6, TNF-α and IFN-γ, increase IL-10 plasma concentrations, and suppress mucosal congestions and colon wall thickening[120,121].
Cranberry is another member of the Ericaceae family, which is full of anthocyanin compounds. Based on a study performed by Xiao et al[122], the administration of cranberry polyphenols was associated with a reduction in colon length, MPO activity, disease progression, infiltration of inflammatory cells and structural damage to the mucosa in an experimental animal model of colitis induced by DSS[122].
Belonging to the Vitaceae family, grape is rich in anthocyanins, with glycoside forms of malvidin, delphinidin, petunidin, petonidin and pelargonidin[123]. Oral administration of grape juice has been shown to significantly reduce the concentrations of TNF-α and iNOS, and COX-2 enzyme activities. Furthermore, it can reduce peripheral blood genotoxicity and morphological signs of cell damage in colitis[124].
As an Ericaceae family member, among other berries, bilberry fruit possesses the highest amounts of anthocyanins (about 300 to 700 mg/100 g fresh fruit). The main anthocyanins in this fruit include delphinidin, malvidin, cyanidin and petonidin[125]. The anti-IBD mechanisms of this fruit have been proposed to involve suppression of TNF-α, IL-6 and INF-γ secretion, resulting in colon shortening and decreased histological scores, causing intestinal inflammation and ileum mucosal injury[126-128].
Barberry is a member of the Berberidaceae family, which is full of anthocyanins, with capabilities of decreasing macroscopic ulcer index and ulcer area, wet weight/length ratio of colon and infiltration of inflammation-inducing cells[129].
Phaseolus vulgaris or black bean is another natural dietary food with high levels of anthocyanins which can significantly reduce inflammatory processes in IBD through suppressing the expression of IL-6, IL-9, IL-17a and IFN-γ. Furthermore, these compounds can also decrease IL-1β, IL-17a, TNF-α and IFN-γ serum levels, contributing to a more effective suppression of inflammation in experimental IBD[130].
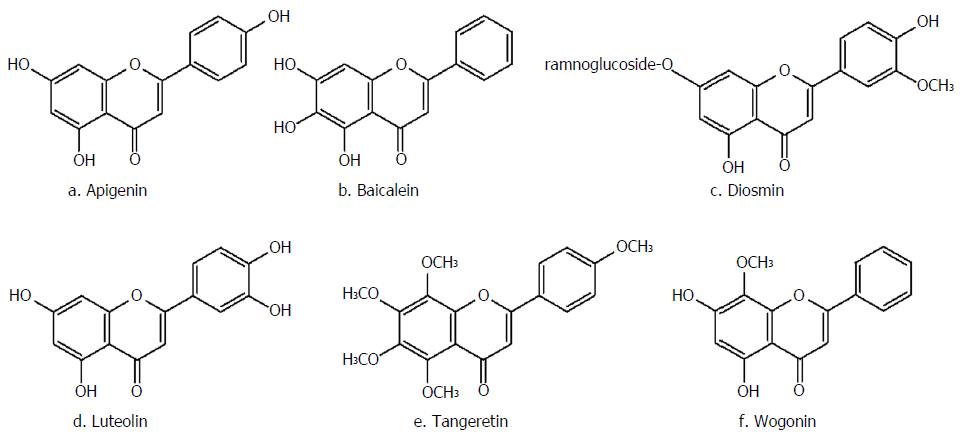
Flavones are an important subclass of flavonoids, possessing the backbone of phenylchromen-4-one. The natural compounds present in this subclass, consisting of apigenin, baicalein, luteolin, diosmin, wogonin and tangeretin (Figure 6), are mainly found in foods, medicinal herbs and cereals. Flavones are mainly found as 7-O-glycososides; however, C-glycosides (in which the sugar is linked to an aromatic carbon atom) have also been identified. Nevertheless, despite presence of these compounds in teas and cereals, few data exist in regards to the flavone-C-glycosides form of these compounds[131].
Apigenin, present in chamomile, parsley and celery, is the main ingredient of wheatgrass juice. It has been shown to be mostly effective in treatment of UC. This naturally-occurring compound is believed to possess both antioxidative and anti-inflammatory effects, which may be beneficial in the case of IBD therapy. Furthermore, this natural compound has the potency to prevent the transactivation induced by TNF-α[132,133]. Of note, apigenin showed efficacy in a murine DSS colitis model by inhibiting inflammasome pathways and therefore production of IL-1β and down-regulation of iNOS and COX-2, and to reduce serum levels of matrix metalloproteinase-3[134]. However, the underlying mechanism of action was not investigated and additional investigations are required in order to provide the basis for the anti-IBD effects. A randomized controlled trial on managing UC through administration of wheatgrass juice demonstrated that although sigmoidoscopic evaluation did not demonstrate any significant differences among the treatment and control group, other symptomatic indicators of disease activity, such as rectal bleeding, were significantly improved[133].
As the main component of Suctellaria baicalensis, baicalein is another member of the flavones group, which is also found in vegetables and fruits. Multiple beneficial activities, including anti-inflammatory, antioxidant and anti-allergic responses, have been reported after administration of this compound in different disorders[135-137]. The plant has also been shown to be significantly effective against experimental IBD. Based on results of a study using a murine model of colitis, investigators found that baicalein could significantly improve colitis inflammatory symptoms, including blood hemoglobin content, rectal bleeding and weight loss, with similar effects to that of sulfasalazine, the reference drug[138]. These effects were also comparable with those observed with wogonin and baicalin[139].
Luteolin, found abundantly in Salvia tomentosa, has been shown to significantly lessen shortening of colon length and reduce the histological score of colitis. As reported by Nishitani et al[140], luteolin significantly suppressed macrophage and IFN-γ producing CD4+ T cell infiltration into the colon mucosa. Furthermore, the treatment with luteolin significantly improved the mRNA expression of IFN-γ in colon. Additionally, co-culture of intestinal epithelial Caco-2 cells and macrophage RAW264.7 cells submitted to luteolin treatment led to the suppression of IL-8 gene expression in the intestinal cells without disrupting the epithelial monolayer. Furthermore, TNF-α and pro-inflammatory cytokines’ expression, including that of TNF-α, IL-6 and IL-1β, were also significantly reduced in RAW264.7 cells by this compound[140]. The authors proposed that luteolin aglycones are liberated by the Caco-2 epithelial cells inhibiting NF-κβ translocation into the nucleus of RAW264.7 macrophages. This action is followed by suppression of TNF-α gene expression in and release from RAW264.7 cells, leading to reduction in the IL-8 expression in Caco-2 cells.
Diosmin, the main active component found in Scrophularia nodosa, was initially isolated in 1925 and applied as therapeutic agent in 1969. Similar to the other members of this subfamily, diosmin has demonstrated several anti-inflammatory, antimutagenic and free radical scavenging effects. Crespo et al[141] investigated the anti-inflammatory activity of this compound on the acute phase immune response in a rat model of TNBS colitis and found that pretreatment with diosmin could significantly reduce colonic damage through suppressing MPO enzyme activity, increasing colonic GSH levels and preventing further production of leukotriene B4 and MDA. Additionally, this compound could also inhibit the inflammation and oxidative damage in colitis[141]. The reduction of leukotriene B4 levels is an interesting mechanism of action because this compound is implicated in the pathogenesis of IBD since it strongly promotes neutrophil chemotaxis and activation favoring the inflammatory process.
As an O-methylated flavone, wogonin is another flavonoid-like chemical agent found in Scutellaria baicalensis[142]. Oroxindin is also the glucuronide form of wogonin isolated from Oroxylum indicum[143]. Wogonin has been shown to possess several pharmacological effects, most importantly the anti-inflammatory ones. Based on Wang et al[144], Caco-2 cells exposure to wogonin significantly diminished LPS-induced alterations in trans-epithelial electrical resistance and fluorescent markers of transportation. Furthermore, this phytochemical could significantly suppress LPS-induced changes in tight junction proteins, mostly claudin-1 and zonula occludens-1 (ZO-1). Additionally, the expression of IL-6, IL-8, IL-1β, iNOS and COX-2 was also suppressed by pretreatment with wogonin. The expression of different molecules, including TLR4, MyD88 and TAK1, were significantly suppressed by wogonin. The more interesting finding was that the translocation of NF-κB and its capacity to bind with DNA in LPS-induced Caco-2 cells was also significantly reduced. Consequently, it can be concluded that wogonin, mostly through diminishing the TLR4-mediated inflammatory response and preserving intentional barrier function, could be a potent treatment for IBD[144].
As the main component of Citrus Spp pericarp, tangeretin can effectively inhibit the expression of TNF-α, IL-23 and IL-12 through restricting the activation of NF-κB in LPS-treated dendritic cells. Furthermore, oral administration of this compound was able to suppress the inflammatory process by preventing the activation NF-κB and mitogen-activated protein kinases (MAPK) pathways and lessening MPO activity in mice with TNBS-induced colitis. Tangeretin could also increase the altered TNBS-suppressed expression of several tight junction proteins, such as claudin-1, ocluadin-1 and ZO-1. Additionally, this compound also prevented TNBS-induced type-1 T helper (Th1) and type-17 T helper (Th17) cells’ differentiation. Also, tangeretin could inhibit T-bet, RAR-related orphan receptor-γ, IL-12, IL-17 and TNF-α expression. According to the aforementioned results, it can be concluded that oral administration of tangeretin through suppression of IL-12 and TNF-α expression as well as NF-κB activation results in the attenuation of UC[145].
Fisetin is a flavonoid, which can be found in many fruits and vegetables. Recently, this compound showed efficacy against DSS-induced colitis in a mouse model. The mechanism of anti-colitis activity was multifactorial through inhibition of diverse signaling pathways including Akt, p38 MAPK and NF-κB in the murine colon[146]. This study evidenced that fisetin is capable of reducing the LPS-induced phosphorylation of IκBα and NF-κβ (p65) binding activity to DNA. This inhibition was associated with inhibition of upstream proteins related to NF-κβ activation. Specifically, the study reported an attenuation in the phosphorylation of Akt and, therefore, the activation of p38 MAPK and NF-κB in the colon[147].
Flavanols are characterized by the presence of flavan-3-ol as a monomeric unit. Flavanols are usually divided in monomers (or catechins) and condensed tannins (dimers, trimers, oligomers, and polymers)[148]. The group of catechins is composed by flavanols with catechin, epicatechin, gallocatechin, epigallocatechin, and diverse specific gallic acid esters at the 3-OH position. This type of flavonoid is present in notable amounts in a diversity of fruits and beverages, including grapes, lychees, strawberries, cacao and green tea[149]. The bioavailability of flavanols depends on each compound, although absorbed flavanols generally present a short half-life in plasma and undergo an extensive phase II metabolism[150,151]. A portion of ingested flavanols is absorbed intact, whereas the remaining fraction is metabolized by the gut microflora, and the resulting metabolites absorbed. Flavanols have a remarkable direct antioxidant activity, but a capacity to stimulate antioxidant enzymes has also been demonstrated. In addition, flavanols exert anti-inflammatory activities by inhibiting/lowering pro-inflammatory enzymes[152].
Many studies have focused on investigating the potential anti-inflammatory effects of flavanols, mainly epigallocatechin and proanthocyanidins from grape seeds, in animal models of IBD. In a first approach, an experimental diet containing catechin significantly decreased colonic damage and MPO activity in TNBS-induced UC in rats compared with a group fed a basal diet[153]. In an in vitro study, epicatechin was capable of inhibiting the permeabilization of Caco-2 cell monolayers induced after TNF-α treatment[154]. The preventive effects were mediated, at least in part, via inhibition of NADPH oxidase and NF-κB activation by reducing IκBα phosphorylation and subsequent nuclear transport and DNA binding. Similar protective results were obtained using catechin-7-O-β-D-glucopyranoside from Phaseolus calcaratus Roxburgh (fabaceae) seeds in a rat model of experimental colitis[155]. The treatment increased GSH levels and reduced MPO activity and protein levels as well as the mRNA and protein levels of lipid mediators (COX-2, iNOS, TNF-α, IL-1β) via inhibition the NF-κB pathway. Moreover, increased mRNA levels of the mucins MUC2 and MUC3, main components of the mucosal layer in the colon, were also evidenced.
Epigallocatechin-3-gallate (EGCG), a main constituent of green tea, has been extensively investigated as an anti-inflammatory agent in IBD. EGCG inhibited the gene expression and release of IL-8, PGE2 and MIP-3α in human colon adenocarcinoma cell lines stimulated with TNF-α[156]. In diverse animal models of colitis, EGCG was evidenced to inhibit MPO activity and histamine levels in colon mucosa, to reduce macrophage chemotaxis and neutrophil infiltration, and to increase the activities of antioxidant enzymes and reduce the production of pro-inflammatory cytokines[157-159]. However, together with these beneficial anti-inflammatory effects, EGCG treatment has been reported to induce a macronutrient malabsorption which can represent a dose-limiting adverse effect that should be taken into account if it is to be translated to the clinic for treatment of IBD[160]. In this way, the co-administration of 1-piperoylpiperidin (piperine), an alkaloid with the capability enhancing EGCG availability, resulted in significantly higher anti-inflammatory effects, with respect to EGCG alone, allowing the use of lower doses of EGCG (6.9 mg/kg body weight)[158]. Finally, the administration of peracetylated epigallocatechin-3-gallate (AcEGCG) was more active in preventing colon damage than EGCG[161]. The authors found that AcEGCG reduced inflammatory mediators by down-regulating the PI3K/Akt/NFκB pathway and increased the expression of heme-oxygenase-1 (HO-1) through induction of extracellular signal-regulated protein kinase (ERK)1/2 signaling together with acetylation of NF-E2-related factor 2 (Nrf2). Unfortunately, the mechanism of action was only investigated in AcEGCG and not in EGCG, making it difficult to know the cause of the different degree of activity between both compounds.
Proanthocyanidins from grape seeds were also investigated as therapeutic agents against UC in a rat model. Treatment with the proanthocyanidins significantly improved the colonic damage and decreased the pro-inflammatory mediators, such as MPO, as well as iNOS activity and levels of IL-1β and TNF-α, increased synthesis of the anti-inflammatory cytokines IL-2 and IL-4, reduced inflammatory cell infiltration, and increased antioxidant enzyme activities[162-164]. The reduction in mucosal inflammation was proposed to be mediated by inhibition of the NF-κB signal transduction pathway. The treatment with proanthocyanidins resulted in a significant reduction in IκB kinase (IκK) activation, leading to suppression in the phosphorylation-induced degradation of IκBα and nuclear translocation[163,165].
Protective effects of thearubigin and theaflavin-3,3’-digallate (TFDG), present in black tea, were evidenced as they ameliorated the disruption of colonic architecture and inflammation[166,167]. Similar to the other flavanols, the anti-inflammatory effects of thearubigin and TFDG seem to be mediated through down-regulation of the NF-κβ pathway by inhibiting the degradation of its endogenous inhibitor Iκβα.
Some complex mixtures rich in flavanols, such as polyphenol-enriched cocoa extract (containing catechin, epicatechin, procyanidin B1 and B2) and oligonol (containing 17.6% of catechin-type monomers and 18.6% of proanthocyanidins) were also effective against animal models of colitis[168,169]. Both treatments significantly reduced colon damage, inflammation, leukocyte infiltration and oxidative stress markers, whereas antioxidant enzyme activities were increased. The mechanism of action seems to involve the inhibition of transcription factors STAT1 and STAT3, which are associated with cytokine and growth factor receptors’ synthesis and with innate and acquired immune cells’ regulation, respectively. In addition, NF-κB activity was also inhibited, which indicates its participation in the anti-inflammatory effects of the extract.
Although there are multiple sources of evidence to support the anti-inflammatory effects of flavonoids, their therapeutic use in IBD has been almost exclusively studied in in vitro studies or animal models. To date, clinical studies are scarce, and further research with well-controlled procedures and higher number of patients is essential to establish the potential therapeutic use of flavonoids. In a first approach, an open pilot trial investigated the effect of anthocyanin-rich bilberry mixture in 13 subjects suffering from UC[128]. After 6 wk of intervention, elevated rates of clinical improvement were observed with a significant decrease in mucosal inflammation and a reduction in the levels of fecal calprotectin. However, after completion of the intervention, a raise in calprotectin levels and disease symptoms were reported following the 4-wk follow-up.
Another pilot study investigated the activity of (-)-EGCG (400 mg or 800 mg) that was supplied to 15 individuals with mild to moderate UC, whereas another 4 were assigned to a placebo control[170]. The response rate to the treatment after 56 d of therapy was 66.7% and the active treatment remission rate was 53.3%; on the contrary, none of the control subjects showed signs of improvement. Koláček et al[171] conducted a pilot study to determine the relation between oxidative stress in pediatric CD patients in remission and the influence of a polyphenol extract (Pycnogenol®, 70% ± 5% procyanidins). Patients reported reduced antioxidant defenses and increased oxidative damage markers when compared with healthy controls. A 10-wk course of polyphenol extract administration positively influenced the oxidative parameters in patients suffering from CD. A randomized, placebo-controlled clinical trial was designed to investigate the effects of silymarin (42 intervention vs 38 placebo) in UC patients[172]. Silymarin (140 mg) was given to the patients for 6 mo, together with the standard therapy. Silymarin was well tolerated by the patients and silymarin improved hemoglobin levels, ESR and disease activity index with respect to the placebo group.
It is well known that IBD causes prolonged inflammation of the gastrointestinal tract. However, the main cause of the IBD is unknown and currently there is no effective treatment to cure this disease. Anti-inflammatory agents are the first course of clinical action in IBD treatment, reducing inflammation of the digestive tract but also eliciting many side effects. Flavonoids are potent anti-inflammatory compounds that could be an interesting alternative in the IBD management. Diverse studies have reported significant beneficial effects of specific flavonoids or foods rich in these compounds against inflammation in IBD using animal models and cell culture.
Tables 1 and 2 summarize the effects of flavonoids in animal models of UC and CD. The immense majority of these investigations have been conducted in DSS and TNBS colitis models. More studies in other chronic models of IBD (e.g., T cell transfer, IL-10 knockout, chronic oxazolone models of colitis) would be beneficial. Moreover, such studies could be conducted in conjunction with currently used IBD drugs (e.g., mesalamine, corticosteroids), in order to probe for additive/synergistic pharmacological effects. Such studies may provide an impetus for designing/conducting future clinical trials with flavonoids as adjunct therapeutic agents.
| Flavonoid | IBD model | Ref. |
| Quercetin | Acetic acid (mice) | [30] |
| Quercetin | DSS (rats) | [29] |
| Rutin | DSS (mice) | [29] |
| Kaempferol | DSS (mice) | [72] |
| Diadzein | DSS (mice) | [94] |
| Naringenin | DSS (mice) | [101] |
| Hesperidin | DSS (mice) | [104] |
| Anthocyanin (strawberry) | Acetic acid (rats) | [118] |
| Anthocyanin (blueberry) | Mdr1a-/- (mice) | [120] |
| Anthocyanin (cranberry) | DSS (mice) | [122] |
| Apigenin | DSS (mice) | [134] |
| Baicalein | DSS (mice) | [138] |
| Luteolin | DSS (mice) | [140] |
| Fisetin | DSS (mice) | [146] |
| Epigallocatechin-3-gallate | DSS (mice) | [158-161] |
| Oligonol | DSS (mice) | [169] |
| Flavonoid | IBD model | Ref. |
| Quercetin | TNBS (rats) | [42] |
| Rutin | TNBS (rats) | [147] |
| Rutin | T cell transfer (mice) | [49] |
| Morin | TNBS (rats) | [52,53] |
| Genistein | TNBS (rats) | [83] |
| Diosmin | TNBS (rats) | [41] |
| Tangeretin | TNBS (mice) | [142] |
| Catechin | TNBS (rats) | [153] |
| Epigallocatechin-3-gallate | TNBS (rats) | [157] |
| Proanthocyanidins (grape) | TNBS (rats) | [162-165] |
| Thearubigin | TNBS (mice) | [167] |
Clinical trials analyzing the efficacy of flavonoids against IBD are still lacking and greater efforts should focus on studies with human patients. Further in-depth clinical studies are mandatory for confirming the therapeutic effects of flavonoids in IBD. More research within the fields of genetics, immunology, biochemistry and microbiology will provide a better knowledge of the underlying mechanisms involved in IBD, and are expected to increase treatment possibilities and their efficacies. Flavonoids could be a useful adjunct therapy in order to reduce or to ameliorate the symptoms of IBD and to potentiate the effects of future therapies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hoensch P, Lakatos PL, Liu F, Sipahi AM S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 2. | Cicchitti L, Martelli M, Cerritelli F. Chronic inflammatory disease and osteopathy: a systematic review. PLoS One. 2015;10:e0121327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12-d26. [PubMed] |
| 4. | Koj A. Initiation of acute phase response and synthesis of cytokines. Biochim Biophys Acta. 1996;1317:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 217] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853-6866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3116] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 6. | Katori M, Majima M. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm Res. 2000;49:367-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Fitzpatrick LR. Novel Pharmacological Approaches for Inflammatory Bowel Disease: Targeting Key Intracellular Pathways and the IL-23/IL-17 Axis. Int J Inflam. 2012;2012:389404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Wéra O, Lancellotti P, Oury C. The Dual Role of Neutrophils in Inflammatory Bowel Diseases. J Clin Med. 2016;5:pii: E118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 9. | Corridoni D, Arseneau KO, Cominelli F. Inflammatory bowel disease. Immunol Lett. 2014;161:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Deepak P, Loftus EV Jr. Ustekinumab in treatment of Crohn’s disease: design, development, and potential place in therapy. Drug Des Devel Ther. 2016;10:3685-3698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 609] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 12. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3526] [Article Influence: 271.2] [Reference Citation Analysis (5)] |
| 13. | Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 1996] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 14. | de Lange DW, Verhoef S, Gorter G, Kraaijenhagen RJ, van de Wiel A, Akkerman JW. Polyphenolic grape extract inhibits platelet activation through PECAM-1: an explanation for the French paradox. Alcohol Clin Exp Res. 2007;31:1308-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Rosenkranz S, Knirel D, Dietrich H, Flesch M, Erdmann E, Böhm M. Inhibition of the PDGF receptor by red wine flavonoids provides a molecular explanation for the “French paradox”. FASEB J. 2002;16:1958-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1112] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 17. | Burr ML. Explaining the French paradox. J R Soc Health. 1995;115:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Alvesalo J, Vuorela H, Tammela P, Leinonen M, Saikku P, Vuorela P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem Pharmacol. 2006;71:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Subarnas A, Wagner H. Analgesic and anti-inflammatory activity of the proanthocyanidin shellegueain A from Polypodium feei METT. Phytomedicine. 2000;7:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Calderone V, Chericoni S, Martinelli C, Testai L, Nardi A, Morelli I, Breschi MC, Martinotti E. Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part II. modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004;70:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 614] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 23. | Lu MF, Xiao ZT, Zhang HY. Where do health benefits of flavonoids come from? Insights from flavonoid targets and their evolutionary history. Biochem Biophys Res Commun. 2013;434:701-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. 2015;54:325-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 25. | Parkar SG, Trower TM, Stevenson DE. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe. 2013;23:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 26. | Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1044] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 27. | Vezza T, Rodríguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients. 2016;8:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 28. | Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 29. | Kwon KH, Murakami A, Tanaka T, Ohigashi H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem Pharmacol. 2005;69:395-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Guazelli CF, Fattori V, Colombo BB, Georgetti SR, Vicentini FT, Casagrande R, Baracat MM, Verri WA Jr. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J Nat Prod. 2013;76:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 443] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 32. | Caddeo C, Nácher A, Díez-Sales O, Merino-Sanjuán M, Fadda AM, Manconi M. Chitosan-xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J Microencapsul. 2014;31:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Crespy V, Morand C, Besson C, Manach C, Demigne C, Remesy C. Quercetin, but not its glycosides, is absorbed from the rat stomach. J Agric Food Chem. 2002;50:618-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727-747. [PubMed] |
| 35. | Crespy V, Morand C, Manach C, Besson C, Demigne C, Remesy C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am J Physiol. 1999;277:G120-G126. [PubMed] |
| 36. | Macdonald IA, Mader JA, Bussard RG. The role of rutin and quercitrin in stimulating flavonol glycosidase activity by cultured cell-free microbial preparations of human feces and saliva. Mutat Res. 1983;122:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276-1282. [PubMed] |
| 38. | van der Sluis AA, Dekker M, Jongen WM. Flavonoids as bioactive components in apple products. Cancer Lett. 1997;114:107-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Hollman PC, Bijsman MN, van Gameren Y, Cnossen EP, de Vries JH, Katan MB. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic Res. 1999;31:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 40. | Kim H, Kong H, Choi B, Yang Y, Kim Y, Lim MJ, Neckers L, Jung Y. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm Res. 2005;22:1499-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Castangia I, Nácher A, Caddeo C, Merino V, Díez-Sales O, Catalán-Latorre A, Fernàndez-Busquets X, Fadda AM, Manconi M. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats. Acta Biomater. 2015;13:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Dodda D, Chhajed R, Mishra J, Padhy M. Targeting oxidative stress attenuates trinitrobenzene sulphonic acid induced inflammatory bowel disease like symptoms in rats: role of quercetin. Indian J Pharmacol. 2014;46:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Dodda D, Chhajed R, Mishra J. Protective effect of quercetin against acetic acid induced inflammatory bowel disease (IBD) like symptoms in rats: possible morphological and biochemical alterations. Pharmacol Rep. 2014;66:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Sánchez de Medina F, Gálvez J, Romero JA, Zarzuelo A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J Pharmacol Exp Ther. 1996;278:771-779. [PubMed] |
| 45. | Cruz T, Gálvez J, Ocete MA, Crespo ME, Sánchez de Medina L-H F, Zarzuelo A. Oral administration of rutoside can ameliorate inflammatory bowel disease in rats. Life Sci. 1998;62:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Camuesco D, Comalada M, Rodríguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Gálvez J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol. 2004;143:908-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 47. | Liu SH, Lu TH, Su CC, Lay IS, Lin HY, Fang KM, Ho TJ, Chen KL, Su YC, Chiang WC. Lotus leaf (Nelumbo nucifera) and its active constituents prevent inflammatory responses in macrophages via JNK/NF-κB signaling pathway. Am J Chin Med. 2014;42:869-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 48. | Kim BH, Cho SM, Reddy AM, Kim YS, Min KR, Kim Y. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Biochem Pharmacol. 2005;69:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Mascaraque C, Aranda C, Ocón B, Monte MJ, Suárez MD, Zarzuelo A, Marín JJ, Martínez-Augustin O, de Medina FS. Rutin has intestinal antiinflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol Res. 2014;90:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Caselli A, Cirri P, Santi A, Paoli P. Morin: A Promising Natural Drug. Curr Med Chem. 2016;23:774-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 51. | Dhanasekar C, Kalaiselvan S, Rasool M. Morin, a Bioflavonoid Suppresses Monosodium Urate Crystal-Induced Inflammatory Immune Response in RAW 264.7 Macrophages through the Inhibition of Inflammatory Mediators, Intracellular ROS Levels and NF-κB Activation. PLoS One. 2015;10:e0145093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Ocete MA, Gálvez J, Crespo ME, Cruz T, González M, Torres MI, Zarzuelo A. Effects of morin on an experimental model of acute colitis in rats. Pharmacology. 1998;57:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Gálvez J, Coelho G, Crespo ME, Cruz T, Rodríguez-Cabezas ME, Concha A, Gonzalez M, Zarzuelo A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment Pharmacol Ther. 2001;15:2027-2039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Devi KP, Malar DS, Nabavi SF, Sureda A, Xiao J, Nabavi SM, Daglia M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol Res. 2015;99:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 395] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 55. | Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011;11:298-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 778] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 56. | Lee J, Kim JH. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway In Vitro. PLoS One. 2016;11:e0155264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 57. | Lee GA, Choi KC, Hwang KA. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ Toxicol Pharmacol. 2017;49:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Kim SH, Hwang KA, Choi KC. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J Nutr Biochem. 2016;28:70-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 59. | Song H, Bao J, Wei Y, Chen Y, Mao X, Li J, Yang Z, Xue Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol Rep. 2015;33:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Dang Q, Song W, Xu D, Ma Y, Li F, Zeng J, Zhu G, Wang X, Chang LS, He D. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol Carcinog. 2015;54:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Kim SH, Choi KC. Anti-cancer Effect and Underlying Mechanism(s) of Kaempferol, a Phytoestrogen, on the Regulation of Apoptosis in Diverse Cancer Cell Models. Toxicol Res. 2013;29:229-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Tatsimo SJ, Tamokou Jde D, Havyarimana L, Csupor D, Forgo P, Hohmann J, Kuiate JR, Tane P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res Notes. 2012;5:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 63. | Bloor SJ. An antimicrobial kaempferol-diacyl-rhamnoside from Pentachondra pumila. Phytochemistry. 1995;38:1033-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Huang YB, Lin MW, Chao Y, Huang CT, Tsai YH, Wu PC. Anti-oxidant activity and attenuation of bladder hyperactivity by the flavonoid compound kaempferol. Int J Urol. 2014;21:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 65. | Nirmala P, Ramanathan M. Effect of kaempferol on lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur J Pharmacol. 2011;654:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Kampkötter A, Gombitang Nkwonkam C, Zurawski RF, Timpel C, Chovolou Y, Wätjen W, Kahl R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch Toxicol. 2007;81:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem. 2006;54:9798-9804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 68. | Sanz MJ, Ferrandiz ML, Cejudo M, Terencio MC, Gil B, Bustos G, Ubeda A, Gunasegaran R, Alcaraz MJ. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica. 1994;24:689-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Parveen Z, Deng Y, Saeed MK, Dai R, Ahamad W, Yu YH. Antiinflammatory and analgesic activities of Thesium chinense Turcz extracts and its major flavonoids, kaempferol and kaempferol-3-O-glucoside. Yakugaku Zasshi. 2007;127:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 70. | Goel RK, Pandey VB, Dwivedi SP, Rao YV. Antiinflammatory and antiulcer effects of kaempferol, a flavone, isolated from Rhamnus procumbens. Indian J Exp Biol. 1988;26:121-124. [PubMed] |
| 71. | Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 612] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 72. | Park MY, Ji GE, Sung MK. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig Dis Sci. 2012;57:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Prasad S, Phromnoi K, Yadav VR, Chaturvedi MM, Aggarwal BB. Targeting inflammatory pathways by flavonoids for prevention and treatment of cancer. Planta Med. 2010;76:1044-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. 2015;5:56-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 75. | Ko KP. Isoflavones: chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev. 2014;15:7001-7010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 76. | Sepehr E, Cooke GM, Robertson P, Gilani GS. Effect of glycosidation of isoflavones on their bioavailability and pharmacokinetics in aged male rats. Mol Nutr Food Res. 2009;53 Suppl 1:S16-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 78. | Marzocchella L, Fantini M, Benvenuto M, Masuelli L, Tresoldi I, Modesti A, Bei R. Dietary flavonoids: molecular mechanisms of action as anti- inflammatory agents. Recent Pat Inflamm Allergy Drug Discov. 2011;5:200-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587:3317-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 80. | Moussa L, Bézirard V, Salvador-Cartier C, Bacquié V, Lencina C, Lévêque M, Braniste V, Ménard S, Théodorou V, Houdeau E. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS One. 2012;7:e49547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Choi S, Woo JK, Jang YS, Kang JH, Jang JE, Yi TH, Park SY, Kim SY, Yoon YS, Oh SH. Fermented Pueraria Lobata extract ameliorates dextran sulfate sodium-induced colitis by reducing pro-inflammatory cytokines and recovering intestinal barrier function. Lab Anim Res. 2016;32:151-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Satsu H, Hyun JS, Shin HS, Shimizu M. Suppressive effect of an isoflavone fraction on tumor necrosis factor-alpha-induced interleukin-8 production in human intestinal epithelial Caco-2 cells. J Nutr Sci Vitaminol (Tokyo). 2009;55:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 84. | Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC Jr. Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G118-G125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 86. | Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Degen GH, Diel P. In utero and postnatal exposure to a phytoestrogen-enriched diet increases parameters of acute inflammation in a rat model of TNBS-induced colitis. Arch Toxicol. 2008;82:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Rao RK, Basuroy S, Rao VU, Karnaky Jr KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 88. | Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280-G1288. [PubMed] |
| 89. | Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 90. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. [PubMed] |
| 91. | Sergent T, Piront N, Meurice J, Toussaint O, Schneider YJ. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem Biol Interact. 2010;188:659-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 92. | Ji G, Zhang Y, Yang Q, Cheng S, Hao J, Zhao X, Jiang Z. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS One. 2012;7:e53101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 93. | Morimoto M, Watanabe T, Yamori M, Takebe M, Wakatsuki Y. Isoflavones regulate innate immunity and inhibit experimental colitis. J Gastroenterol Hepatol. 2009;24:1123-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 94. | Sakai T, Furoku S, Nakamoto M, Shuto E, Hosaka T, Nishioka Y, Sone S. Soy isoflavone equol perpetuates dextran sulfate sodium-induced acute colitis in mice. Biosci Biotechnol Biochem. 2011;75:593-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5980] [Cited by in RCA: 5213] [Article Influence: 179.8] [Reference Citation Analysis (0)] |
| 96. | Li C, Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Crit Rev Food Sci Nutr. 2017;57:613-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 97. | Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61:472-477. [PubMed] |
| 98. | Rani N, Bharti S, Krishnamurthy B, Bhatia J, Sharma C, Kamal MA, Ojha S, Arya DS. Pharmacological Properties and Therapeutic Potential of Naringenin: A Citrus Flavonoid of Pharmaceutical Promise. Curr Pharm Des. 2016;22:4341-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 99. | Mir IA, Tiku AB. Chemopreventive and therapeutic potential of “naringenin,” a flavanone present in citrus fruits. Nutr Cancer. 2015;67:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 100. | Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132-145. [PubMed] |
| 101. | Dou W, Zhang J, Sun A, Zhang E, Ding L, Mukherjee S, Wei X, Chou G, Wang ZT, Mani S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br J Nutr. 2013;110:599-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 102. | Azuma T, Shigeshiro M, Kodama M, Tanabe S, Suzuki T. Supplemental naringenin prevents intestinal barrier defects and inflammation in colitic mice. J Nutr. 2013;143:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 103. | Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013;19:5633-5644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 165] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 104. | Xu L, Yang ZL, Li P, Zhou YQ. Modulating effect of Hesperidin on experimental murine colitis induced by dextran sulfate sodium. Phytomedicine. 2009;16:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Prior RL, Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006;40:1014-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 318] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 106. | Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Aspects Med. 2010;31:446-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 107. | Shin DY, Lee WS, Kim SH, Kim MJ, Yun JW, Lu JN, Lee SJ, Tsoy I, Kim HJ, Ryu CH. Anti-invasive activity of anthocyanins isolated from Vitis coignetiae in human hepatocarcinoma cells. J Med Food. 2009;12:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Speciale A, Canali R, Chirafisi J, Saija A, Virgili F, Cimino F. Cyanidin-3-O-glucoside protection against TNF-α-induced endothelial dysfunction: involvement of nuclear factor-κB signaling. J Agric Food Chem. 2010;58:12048-12054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 109. | Zhang Y, Lian F, Zhu Y, Xia M, Wang Q, Ling W, Wang XD. Cyanidin-3-O-beta-glucoside inhibits LPS-induced expression of inflammatory mediators through decreasing IkappaBalpha phosphorylation in THP-1 cells. Inflamm Res. 2010;59:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 110. | Kaspar KL, Park JS, Brown CR, Mathison BD, Navarre DA, Chew BP. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J Nutr. 2011;141:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 111. | Puupponen-Pimiä R, Nohynek L, Ammann S, Oksman-Caldentey KM, Buchert J. Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J Agric Food Chem. 2008;56:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Kim JM, Kim JS, Yoo H, Choung MG, Sung MK. Effects of black soybean [Glycine max (L.) Merr.] seed coats and its anthocyanidins on colonic inflammation and cell proliferation in vitro and in vivo. J Agric Food Chem. 2008;56:8427-8433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Pinto Mda S, de Carvalho JE, Lajolo FM, Genovese MI, Shetty K. Evaluation of antiproliferative, anti-type 2 diabetes, and antihypertension potentials of ellagitannins from strawberries (Fragaria × ananassa Duch.) using in vitro models. J Med Food. 2010;13:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 114. | Häkkinen SH, Törrönen AR. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: influence of cultivar, cultivation site and technique. Food Res Int. 2000;33:517-524. [RCA] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 253] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 115. | Versari A, Biesenbruch S, Barbanti D, Farnell P, Galassi S. Effects of pectolytic enzymes on selected phenolic compounds in strawberry and raspberry juices. Food Res Int. 1997;30:811-817. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 116. | Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 815] [Cited by in RCA: 674] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 117. | Hannum SM. Potential impact of strawberries on human health: a review of the science. Crit Rev Food Sci Nutr. 2004;44:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 118. | Tanideh N, Akbari Baseri F, Jamshidzadeh A, Ashraf M, Kuhi O, Mehrabani D. The healing effect of strawberry extract on acetic acid-induced ulcerative colitis in rat. World Appl Sci J. 2014;31:281-288. |
| 119. | Mazza G, Miniati E. Anthocyanins in fruits, vegetables, and grains: CRC press, 1993. . |
| 120. | Paturi G, Mandimika T, Butts CA, Zhu S, Roy NC, McNabb WC, Ansell J. Influence of dietary blueberry and broccoli on cecal microbiota activity and colon morphology in mdr1a(-/-) mice, a model of inflammatory bowel diseases. Nutrition. 2012;28:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 121. | Kalkan Yildirim H. Evaluation of colour parameters and antioxidant activities of fruit wines. Int J Food Sci Nutr. 2006;57:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 122. | Xiao X, Kim J, Sun Q, Kim D, Park CS, Lu TS, Park Y. Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem. 2015;167:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Sodagari HR, Farzaei MH, Bahramsoltani R, Abdolghaffari AH, Mahmoudi M, Rezaei N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2015;9:807-820. [PubMed] |
| 124. | Marchi P, Paiotti AP, Artigiani Neto R, Oshima CT, Ribeiro DA. Concentrated grape juice (G8000™) reduces immunoexpression of iNOS, TNF-alpha, COX-2 and DNA damage on 2,4,6-trinitrobenzene sulfonic acid-induced-colitis. Environ Toxicol Pharmacol. 2014;37:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 125. | Chu WK, Cheung SC, Lau RA, Benzie IF. 4 Bilberry (Vaccinium myrtillus L.). Lester Packer, PhD 2011: 55. . |
| 126. | Jakesevic M, Xu J, Aaby K, Jeppsson B, Ahrné S, Molin G. Effects of bilberry (Vaccinium myrtillus) in combination with lactic acid bacteria on intestinal oxidative stress induced by ischemia-reperfusion in mouse. J Agric Food Chem. 2013;61:3468-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Piberger H, Oehme A, Hofmann C, Dreiseitel A, Sand PG, Obermeier F, Schoelmerich J, Schreier P, Krammer G, Rogler G. Bilberries and their anthocyanins ameliorate experimental colitis. Mol Nutr Food Res. 2011;55:1724-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | Biedermann L, Mwinyi J, Scharl M, Frei P, Zeitz J, Kullak-Ublick GA, Vavricka SR, Fried M, Weber A, Humpf HU. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis - an open pilot study. J Crohns Colitis. 2013;7:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 129. | Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative Study of Berberis vulgaris Fruit Extract and Berberine Chloride Effects on Acetic Acid-Induced Colitis in Rats. Iran J Pharm Res. 2011;10:97-104. [PubMed] |
| 130. | Zhang C, Monk JM, Lu JT, Zarepoor L, Wu W, Liu R, Pauls KP, Wood GA, Robinson L, Tsao R. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br J Nutr. 2014;111:1549-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 131. | Hollman PCH, Arts ICW. Flavonols, flavones and flavanols-nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1081-1093. [DOI] [Full Text] |
| 132. | Padalia S, Drabu S, Raheja I, Gupta A, Dhamija M. Multitude potential of wheatgrass juice (Green Blood): An overview. Chron Young Scientists. 2010;1:23. |
| 133. | Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double-blind placebo-controlled trial. Scand J Gastroenterol. 2002;37:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 134. | Márquez-Flores YK, Villegas I, Cárdeno A, Rosillo MÁ, Alarcón-de-la-Lastra C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem. 2016;30:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 135. | Wang YH, Yu HT, Pu XP, Du GH. Baicalein prevents 6-hydroxydopamine-induced mitochondrial dysfunction in SH-SY5Y cells via inhibition of mitochondrial oxidation and up-regulation of DJ-1 protein expression. Molecules. 2013;18:14726-14738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 136. | Liu A, Wang W, Fang H, Yang Y, Jiang X, Liu S, Hu J, Hu Q, Dahmen U, Dirsch O. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur J Pharmacol. 2015;748:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 137. | Mabalirajan U, Ahmad T, Rehman R, Leishangthem GD, Dinda AK, Agrawal A, Ghosh B, Sharma SK. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PLoS One. 2013;8:e62916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 138. | Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 139. | Bayless TM, Harris ML. Inflammatory bowel disease and irritable bowel syndrome. Med Clin North Am. 1990;74:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 140. | Nishitani Y, Yamamoto K, Yoshida M, Azuma T, Kanazawa K, Hashimoto T, Mizuno M. Intestinal anti-inflammatory activity of luteolin: role of the aglycone in NF-κB inactivation in macrophages co-cultured with intestinal epithelial cells. Biofactors. 2013;39:522-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 141. | Crespo ME, Gálvez J, Cruz T, Ocete MA, Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999;65:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 142. | Hui KM, Huen MS, Wang HY, Zheng H, Sigel E, Baur R, Ren H, Li ZW, Wong JT, Xue H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem Pharmacol. 2002;64:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 173] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 143. | Nair AR, Joshi B. Oroxindin - A new flavone glucuronide fromOroxylum indicum Vent. 1979;323-327. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 144. | Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res. 2015;64:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 145. | Eun SH, Woo JT, Kim DH. Tangeretin Inhibits IL-12 Expression and NF-κB Activation in Dendritic Cells and Attenuates Colitis in Mice. Planta Med. 2017;83:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 146. | Sahu BD, Kumar JM, Sistla R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-κB signaling. J Nutr Biochem. 2016;28:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 147. | Mascaraque C, López-Posadas R, Monte MJ, Romero-Calvo I, Daddaoua A, González M, Martínez-Plata E, Suárez MD, González R, Marín JJ. The small intestinal mucosa acts as a rutin reservoir to extend flavonoid anti-inflammatory activity in experimental ileitis and colitis. J Funct Foods. 2015;13:117-125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 148. | Whiting DA. Natural phenolic compounds 1900-2000: a bird’s eye view of a century’s chemistry. Nat Prod Rep. 2001;18:583-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Hackman RM, Polagruto JA, Zhu QY, Sun B, Fujii H, Keen CL. Flavanols: digestion, absorption and bioactivity. Phytochem Rev. 2008;7:195. [DOI] [Full Text] |
| 150. | Thilakarathna SH, Rupasinghe HP. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367-3387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 506] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 151. | Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S-242S. [PubMed] |
| 152. | Stevenson DE, Hurst RD. Polyphenolic phytochemicals--just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900-2916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 153. | Sato K, Kanazawa A, Ota N, Nakamura T, Fujimoto K. Dietary supplementation of catechins and alpha-tocopherol accelerates the healing of trinitrobenzene sulfonic acid-induced ulcerative colitis in rats. J Nutr Sci Vitaminol (Tokyo). 1998;44:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 154. | Contreras TC, Ricciardi E, Cremonini E, Oteiza PI. (-)-Epicatechin in the prevention of tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Arch Biochem Biophys. 2015;573:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 155. | Kook SH, Choi KC, Cho SW, Cho HK, Lee KD, Lee JC. Catechin-7-O-β-D-glucopyranoside isolated from the seed of Phaseolus calcaratus Roxburgh ameliorates experimental colitis in rats. Int Immunopharmacol. 2015;29:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 156. | Porath D, Riegger C, Drewe J, Schwager J. Epigallocatechin-3-gallate impairs chemokine production in human colon epithelial cell lines. J Pharmacol Exp Ther. 2005;315:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 157. | Mochizuki M, Hasegawa N. Protective effect of (-)-epigallocatechin gallate on acute experimental colitis. J Health Sci. 2005;51:362-364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Brückner M, Westphal S, Domschke W, Kucharzik T, Lügering A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J Crohns Colitis. 2012;6:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 159. | Yeoh BS, Aguilera Olvera R, Singh V, Xiao X, Kennett MJ, Joe B, Lambert JD, Vijay-Kumar M. Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am J Pathol. 2016;186:912-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 160. | Bitzer ZT, Elias RJ, Vijay-Kumar M, Lambert JD. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol Nutr Food Res. 2016;60:2267-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 161. | Chiou YS, Ma NJ, Sang S, Ho CT, Wang YJ, Pan MH. Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J Agric Food Chem. 2012;60:3441-3451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 162. | Li XL, Cai YQ, Qin H, Wu YJ. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol. 2008;86:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 163. | Wang YH, Ge B, Yang XL, Zhai J, Yang LN, Wang XX, Liu X, Shi JC, Wu YJ. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int Immunopharmacol. 2011;11:1620-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 164. | Wang YH, Yang XL, Wang L, Cui MX, Cai YQ, Li XL, Wu YJ. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can J Physiol Pharmacol. 2010;88:888-898. [PubMed] |
| 165. | Li X, Yang X, Cai Y, Qin H, Wang L, Wang Y, Huang Y, Wang X, Yan S, Wang L. Proanthocyanidins from Grape Seeds Modulate the NF-κB Signal Transduction Pathways in Rats with TNBS-Induced Ulcerative Colitis. Molecules. 2011;16:6721-6731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 166. | Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol. 2003;139:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 167. | Maity S, Ukil A, Karmakar S, Datta N, Chaudhuri T, Vedasiromoni JR, Ganguly DK, Das PK. Thearubigin, the major polyphenol of black tea, ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol. 2003;470:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 168. | Andújar I, Recio MC, Giner RM, Cienfuegos-Jovellanos E, Laghi S, Muguerza B, Ríos JL. Inhibition of ulcerative colitis in mice after oral administration of a polyphenol-enriched cocoa extract is mediated by the inhibition of STAT1 and STAT3 phosphorylation in colon cells. J Agric Food Chem. 2011;59:6474-6483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 169. | Yum HW, Zhong X, Park J, Na HK, Kim N, Lee HS, Surh YJ. Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid Redox Signal. 2013;19:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 170. | Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. 2013;19:1904-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 171. | Koláček M, Muchová J, Dvořáková M, Paduchová Z, Žitňanová I, Čierna I, Országhová Z, Székyová D, Jajcaiová-Zedníčková N, Kovács L. Effect of natural polyphenols (Pycnogenol) on oxidative stress markers in children suffering from Crohn’s disease-a pilot study. Free Radic Res. 2013;47:624-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 172. | Rastegarpanah M, Malekzadeh R, Vahedi H, Mohammadi M, Elahi E, Chaharmahali M, Safarnavadeh T, Abdollahi M. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin J Integr Med. 2015;21:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |