Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4796
Peer-review started: February 10, 2017
First decision: March 3, 2017
Revised: April 7, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: July 14, 2017
Processing time: 164 Days and 6.4 Hours
To evaluate the levels of von Willebrand factor (VWF) and metalloproteinase with thrombospondin type-1 motif, number 13 (ADAMTS13) in inflammatory bowel disease (IBD) and correlate them with the disease activity.
Consecutive patients with IBD aged 18 years or older were enrolled in the study. Forty-seven patients with ulcerative colitis (UC), 38 with Crohn’s disease (CD), and 50 healthy controls were included. The white blood cell count, haematocrit, platelet count, fibrinogen, partial activated thromboplastin time, C-reactive protein, albumin, VWF antigen level (VWF:Ag), VWF ristocetin cofactor activity (VWF:RCo), VWF collagen-binding activity (VWF:CB), and ADAMTS13 antigen level (ADAMTS13:Ag) and activity (ADAMTS13act) were measured. The following ratios were assessed: VWF:RCo/VWF:Ag, VWF:CB/VWF:Ag, VWF:Ag/ADAMTS13act, and ADAMTS13act/ADAMTS13:Ag.
Compared to controls, the odds ratio (OR) of an elevated VWF: Ag > 150% was 8.7 (95%CI: 2.7-28.1) in the UC group and 16.2 (95%CI: 4.8-54.0) in the CD group. VWF:CB was lower in UC patients, and active CD was associated with a higher VWF: RCo (+38%). The ORs of VWF:CB/VWF:Ag < 0.7 (a marker of acquired von Willebrand syndrome) in the UC and CD groups were 11.9 (95%CI: 4.4-32.4) and 13.3 (95%CI: 4.6-38.1), respectively. Active UC was associated with lower ADAMTS13:Ag (-23%) and ADAMTS13act (-20%) compared to UC in remission. Patients with active CD had a 15% lower ADAMTS13act than controls. The activity of UC, but not that of CD, was inversely correlated with ADAMTS13:Ag (r = -0.76) and ADAMTS13act (r = -0.81).
Complex VWF-ADAMTS13-mediated mechanisms disturb haemostasis in IBD. A reduced WVF:CB is a risk factor for bleeding, while a lower ADAMTS13 level combined with an elevated VWF:Ag could predispose one to thrombosis.
Core tip: A tightly regulated balance between von Willebrand factor and metalloproteinase with thrombospondin type-1 motif, number 13 (ADAMTS13) is important for haemostasis, and its dysregulation might predispose one to either thrombotic events or bleeding. We demonstrated a decrease in ADAMTS13act levels in Crohn’s disease (CD) patients, and in ADAMTS13:Ag and ADAMTS13act in ulcerative colitis (UC) patients, in whom these parameters were negatively correlated with disease activity and inflammatory markers. We report for the first time the presence of abnormalities typical of type A2 acquired von Willebrand syndrome in inflammatory bowel disease patients. These findings provide insight into the elevated risk for thromboembolic events and bleeding observed in UC and less frequently in CD.
- Citation: Cibor D, Owczarek D, Butenas S, Salapa K, Mach T, Undas A. Levels and activities of von Willebrand factor and metalloproteinase with thrombospondin type-1 motif, number 13 in inflammatory bowel diseases. World J Gastroenterol 2017; 23(26): 4796-4805
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4796.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4796
In inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), a prothrombotic state leads to increased risk for thrombosis[1-3]. The estimated risk of venous thromboembolism is threefold higher in IBD patients than in the general population[4]. A role for microvascular thrombi in the pathogenesis of IBD is supported by reports of a lower incidence of IBD in patients with inherited bleeding disorders, such as haemophilia A and B, and von Willebrand’s disease (VWD)[5].
Inflammation and blood coagulation are closely related. An inflammatory state disturbs the balance between procoagulant and anticoagulant mechanisms, leading to enhanced blood coagulation, thrombin generation, and deposition of poorly lysable fibrin[6-9]. Von Willebrand factor (VWF) is an acute-phase protein and a marker of endothelial damage[10-12]. Activation of endothelial cells by inflammation impairs their anticoagulant function[13-16]. VWF is crucial for platelet adhesion and aggregation. High-molecular-weight multimers (HMWMs) are involved in platelet aggregation under high shear stress but present solely in the subendothelium and released into the bloodstream following activation of endothelial cells under normal conditions[17].
To assess VWF activity, two assays are commonly used: VWF ristocetin co-factor activity (VWF:RCo), which evaluates the ability of VWF to interact with platelet glycoprotein Ib, and VWF binding to collagen (VWF:CB), which evaluates platelet adhesion to collagen exposed following damage to the endothelium[18].
Type 2A acquired von Willebrand syndrome (AVWS) is characterised by an acquired qualitative VWF deficit associated with HMWM depletion, which increases the risk of mucocutaneous bleeding. VWF:CB is used to detect the qualitative abnormalities associated with HMWM depletion in type 2A VWD[18]. In the absence of shear stress, HMWMs are highly resistant to proteolysis by metalloproteinase with thrombospondin type-1 motif, number 13 (ADAMTS13)[19]. Regulation of the size of VWF multimers by ADAMTS13 is critical for normal haemostasis[20].
ADAMTS13 is a glycoprotein synthesised in endothelial cells and in hepatic stellate cells; it cleaves large VWF multimers to smaller, less-active multimers[17]. Impaired cleavage of large VWF multimers due to inadequate ADAMTS13 levels and/or impaired ADAMTS13 activity (ADAMTS13act) leads to thrombotic microangiopathies (TMA), which occur in several diseases such as thrombotic thrombocytopenic purpura (TTP), sepsis, malaria, malignancy, liver diseases, myocardial infarction, and ischaemic stroke[11,12,21]. In severe inflammatory states, in which the VWF level is markedly elevated, a normal or slightly decreased ADAMTS13 level may be insufficient to control ultra-large VWF (UL-VWF) multimers[22].
A tightly regulated balance between VWF and ADAMTS13 is important for haemostasis, and its dysregulation might predispose one to either thrombotic events or bleeding. For example, patients undergoing elective coronary artery bypass grafting with lower preoperative VWF:RCo and high ADAMTS13 antigen (ADAMTS13:Ag) levels have a higher risk of high postoperative bleeding[23].
To the best of our knowledge, the association between ADAMTS13:Ag or ADAMTS13act and VWF antigen level (VWF:Ag) and activity in IBD has not been investigated in connection with the disease clinical activity. Therefore, we evaluated the plasma levels of these proteins and assessed their associations with the clinical characteristics and levels of inflammatory markers in patients with IBD.
Consecutive patients with IBD aged 18 years or older were enrolled; the study population included 38 subjects with CD and 47 with UC. Disease was diagnosed based on classic histological, endoscopic, and radiological criteria[24]. The patients were followed-up at the Department of Gastroenterology, Hepatology and Infectious Diseases, Jagiellonian University Medical College, Krakow, Poland. The controls were 50 healthy volunteers recruited from the hospital staff and their acquaintances. The exclusion criteria were pregnancy, concomitant inflammatory disorders, and severe diseases including myocardial infarction, stroke, thromboembolism, known haemorrhagic diathesis, cancer, renal insufficiency, liver injury and diabetes. None of the patients or volunteers had been treated with aspirin, heparin, oral anticoagulants, or oral contraceptives.
The subjects provided informed written consent to participate in the study, which was approved by the Jagiellonian University Bioethics Committee (no KBET/287/B/2014). Participants gave also informed consent for data sharing. The study was performed in accordance with the ethical principles of the Helsinki Declaration of 2008.
The clinical assessment included the presence of comorbidities, cigarette-smoking habits, and medications. Body mass index was calculated. In patients with CD and UC, the following parameters were evaluated: disease duration, disease location, disease activity, complications, and past surgical procedures. Complications were defined as abscesses, fistulae, and stenoses resulting in post-obstructive symptoms. To determine the location of lesions in UC and CD patients, the Montreal classification was employed[25]. To assess CD and UC activity, the CD activity index[26] and disease activity index were used[27].
Fasting blood samples were collected from the antecubital vein in the morning. On the same day, using routine laboratory techniques, the following laboratory parameters were determined: WBC, haematocrit, platelet count, fibrinogen, partial activated thromboplastin time, C-reactive protein (CRP), and albumin.
VWF:Ag was measured by latex immunoassay using an STAR coagulation instrument (Diagnostica Stago, Asnieres, France), with a detection limit of 2 IU/dL. The intra- and inter-assay coefficients of variation were 5.2% and 5.5%, respectively. VWF:RCo was assessed turbidimetrically (Siemens, Erlangen, Germany); the intra- and inter-assay coefficients of variation were 6.9% and 7.5%. The detection threshold for the assay was 5 IU/dL. VWF:CB was determined by ELISA using type III collagen (Sigma) diluted in acetic acid, with a limit of detection of 4 IU/dL; the intra- and inter-assay coefficients of variation were 6.1% and 6.7%. ADAMTS13:Ag and ADAMTS13act were quantified by means of fluorogenic assays (Technoclone, Wien, Austria). The intra- and inter-assay coefficients of variation were 5.8% and 6.5% for the level and 7.3% and 7.8% for the activity. The lower limit of detection of both tests was 5 IU/dL. The normal reference ranges for VWF and ADAMTS13 parameters were 50-150 IU/dL.
The following ratios were assessed: VWF:RCo/VWF:Ag, VWF:CB/VWF:Ag, VWF:Ag/ADAMTS13act, and ADAMTS13act/ADAMTS13:Ag.
The study was powered to have an 80% likelihood of detecting a 30% effect size among patients with UC, CD, and healthy controls in ADAMTS13act using a significance level α = 0.05 and one-way analysis of variance (ANOVA; fixed effects). To demonstrate an effect size or greater in this variable, 37 patients were required in each group.
Continuous data with a skewed distribution are presented as means with standard deviations or medians with interquartile ranges. Discrete data are presented as numbers and percentages. To compare mean values, a one-way ANOVA was performed if all assumptions were met; otherwise a Kruskal-Wallis test or Welch test was used. Normality was verified using the Shapiro-Wilk test, and the Levene test was applied to investigate the heterogeneity of variance. The χ2 test or Fisher’s exact test was performed to examine associations between categorical data. Odds ratios were calculated for selected features. Correlations between continuous variables were assessed by calculating the Pearson or Spearman coefficient. A multivariate linear regression was employed to verify the impact of factors on ADAMTS13:Ag and ADAMTS13act.
Calculations were performed using the R statistical package version 3.2.2 (The R Foundation for Statistical Computing, http://www.r-project.org). The G*Power 3 software version 3.1.9.2 was used for the power calculation[28]. The statistical review of the study was performed by a biomedical statistician, Kinga Salapa from the Department of Bioinformatics and Telemedicine, Jagiellonian University Medical College.
The characteristics of the IBD patients and controls are presented in Table 1. Patients with UC were treated with mesalamine 2-4 g/d and 10 of them were on maintenance therapy with thiopurines. All patients with CD were on maintenance therapy with thiopurines and the subgroup with inflammatory lesions in the large intestine and the ileocecal region was treated with mesalamine (2 g/d). Data for the active and inactive UC and CD groups are presented in Tables 2 and 3, respectively.
| Ulcerative colitis | Crohn’s disease | Control group | |
| n = 47 | n = 38 | n = 50 | |
| Men | 22 (46.81) | 20 (52.63) | 35 (70) |
| Age, yr | 37 (26-47)b | 29.5 (22-34)a | 36 (29-46) |
| BMI, kg/m2 | 22.1 (20.5-25) | 20.43 (18.38-22.9) | 23.9 (22.2-25.3) |
| Smoking | 9 (19.15) | 15 (39.47) | 17 (34) |
| Disease duration, yr | 5 (2.5-9) | 3.5 (2-6) | NA |
| Surgery | 0 (0)b | 11 (28.9) | NA |
| Complications | 3 (6.4)b | 11 (28.9) | NA |
| Location | |||
| Rectum | 7 (14.9) | NA | NA |
| Left-sided | 25 (53.2) | NA | NA |
| Extensive colitis | 15 (31.9) | NA | NA |
| Ileal | NA | 12 (31.6) | NA |
| Colonic | NA | 3 (7.9) | NA |
| Ileocolonic | NA | 23 (60.5) | NA |
| Pregnancies, n | 17 | 12 | 13 |
| Miscarriages, n | 0 | 1 | 1 |
| Ulcerative colitis inactive | Ulcerative colitis active | Control group | |
| n = 21 | n = 26 | n = 50 | |
| Disease activity index (points) | 2.33 ± 1.68b | 7.96 ± 1.91 | NA |
| White blood cells, × 103/μL | 6.89 (4.91-7.74) | 8.22 (5.54-9.65) | 6.75 (6.1-7.9) |
| Platelet coun, × 103/μL | 286.02 (216-303)b | 326 (252-379)a | 229.5 (199-268) |
| Haematocrit (%) | 43.4 (39.95-44.55)b | 39.85 (36.8-42.2) | 41.1 (39.5-43) |
| Haemoglobin, g/dL | 13.5 ± 1.67 | 12.7 ± 1.5a | 14.3 ± 1.1 |
| Fibrinogen, g/L | 2.52 (2.19-3.92)b | 4.97 (3.83-6.99)a | 3.05 (2.48-4.16) |
| CRP, mg/L | 1.24 (0.79-1.89)b | 10.7 (7.22-20.8)a | 1.68 (.98-2.24) |
| Crohn’s disease inactive | Crohn’s disease active | Control group | |
| n = 16 | n = 22 | n = 50 | |
| CDAI (points) | 83.38 ± 33.30b | 236.05 ± 63.46 | NA |
| White blood cells, × 103/μL | 6.74 (5.47-7.08) | 6.71 (4.91-9.02) | 6.75 (6.1-7.9) |
| Platelet count, × 103/μL | 291.5 (209-336)b | 324.5 (289-386)a | 229.5 (199-268) |
| Haematocrit, % | 42.7 (39.75-45.1)b | 37.9 (35.8-39.8)a | 41.1 (39.5-43) |
| Haemoglobin, g/dL | 13.99 ± 1.36b | 12.15 ± 1.4a | 14.3 ± 1.1 |
| Fibrinogen, g/L | 3.81 (2.63-4.7)b | 6.52 (4.76-8.26)a | 3.05 (2.48-4.16) |
| CRP, mg/L | 2.95 (1.19-5.72)b | 33.8 (16.4-72.3)a | 1.68 (0.98-2.24) |
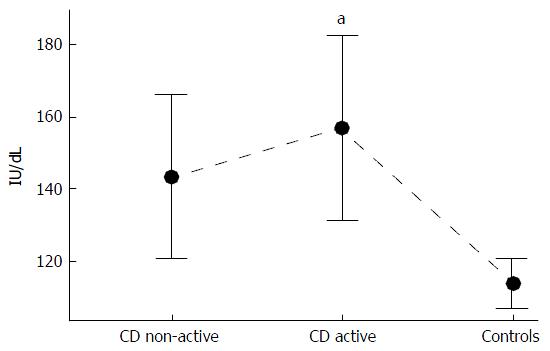
VWF:Ag was higher in patients with active and inactive UC (Table 4) and CD (Table 5) as compared to controls. VWF activity, as assessed by the VWF:CB coefficient, was lower in patients with active and inactive UC compared to controls (Table 4). No such difference was evident in CD patients (Table 5). Patients with active CD had 38% higher VWF: RCo activity than the controls (Figure 1). Approximately 50% of the IBD patients (n = 42) had a VWF:Ag of greater than 150%, compared to 8% (n = 4) of the controls. In the UC group, the OR of a VWF; Ag greater than 150% was 8.7 (95%CI: 2.7-28.1), while in the CD group the OR was 16.2 (95%CI: 4.8-54.0) vs controls (Figure 2). None of the subjects had a deficiency in VWF; no cases of VWD were observed.
| Ulcerative colitis inactive | Ulcerative colitis active | Control group | |
| n = 21 | n = 26 | n = 50 | |
| VWF:RCo, IU/dL | 120.61 ± 36.33 | 134.2 ± 46.7 | 114.03 ± 24.67 |
| VWF:Ag, IU/dL | 139.6 (110.3-166.5)a | 144.95 (120.8-180.5)a | 106.65 (90.7-122.5) |
| VWF:CB, IU/dL | 84.93 ± 13.88a | 78.87 ± 14.99a | 97.99 ± 10.3 |
| ADAMTS13: Ag (IU/dL) | 86.15 ± 10.17a,b | 66.39 ± 11.45a | 103.86 ± 10.84 |
| ADAMTS13act (IU/dL) | 81.2 (79.4-92.9)b | 65.05 (55.7-75.1)a | 111.0 (97.8-122.8) |
| VWF:RCo/VWF:Ag | 0.96 (0.92-0.97) | 0.97 (0.87-1.01) | 0.97 (0.91-1.15) |
| VWF:CB/VWF:Ag | 0.68 (0.50-0.79)a | 0.5 (0.45-0.70)a | 0.91 (0.80-1.05) |
| VWF:Ag/ADAMTS13act | 1.54 (1.34-2.07)a | 2.22 (1.65-2.84)a | 0.98 (0.79-1.20) |
| ADAMTS13act/ADAMTS13:Ag | 1.01 (0.97-1.02) | 1.0 (0.97-1.03) | 1.02 (0.94-1.18) |
| Crohn’s disease inactive | Crohn’s disease active | Control group | |
| n = 16 | n = 22 | n = 50 | |
| VWF:RCo, IU/dL | 143.47 ± 46.12 | 157.04 ± 61.03a | 114.03 ± 24.67 |
| VWF:Ag, IU/dL | 135.4 (116.85-175.65)a | 182.98 (130.4-219.8)a | 106.65 (90.7-122.5) |
| VWF:CB, IU/dL | 98.85 (86.15-102.25) | 89.3 (80.6-99.7) | 98.05 (91.9-101.8) |
| ADAMTS13:Ag, IU/dL | 104.44 ± 9.96 | 99.29 ± 15.33 | 103.86 ± 10.84 |
| ADAMTS13act, IU/dL | 104.82 ± 8.61 | 94.48 ± 15.74a | 111.05 ± 17.69 |
| VWFRCo/VWF:Ag | 0.96 (0.95-1.00) | 0.96 (0.79-1.01) | 0.97 (0.91-1.15) |
| VWF:CB/VWF:Ag | 0.65 (0.45-0.87)a | 0.54 (0.45-0.65)a | 0.91 (0.80-1.05) |
| VWF:Ag/ADAMTS13act | 1.26 (1.12-1.62)a | 1.88 (1.48-2.56)a | 0.98 (0.79-1.20) |
| ADAMTS13act/ADAMTS13:Ag | 0.99 (0.94-1.05) | 0.96 (0.92-1.03) | 1.02 (0.94-1.18) |
Patients with active UC had a 23% lower ADAMTS13: Ag and ADAMTS13act compared to those in remission (Table 4). No such differences were noted in the CD group, in which patients with active CD had a 15% lower ADAMTS13act than the controls (Table 5).
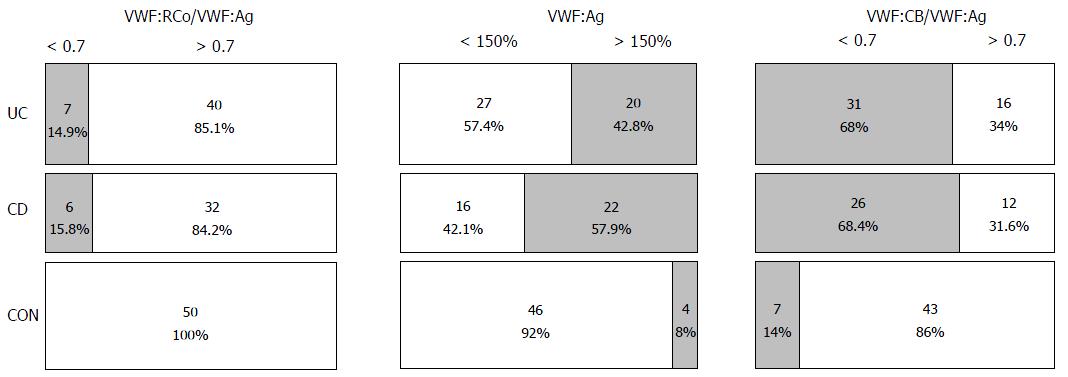
Both the UC and CD patients had lower VWF:CB/VWF:Ag coefficients than the controls. The mean VWF:CB/VWF:Ag values tended to be lower in patients with exacerbated disease compared to subjects in remission (P > 0.05). Importantly, the OR of a VWF:CB/VWF:Ag < 0.7 in the UC group was 11.9 (95%CI: 4.4-32.4) vs controls, while that in the CD patients was 13.3 (95%CI: 4.6-38.1). The OR of a VWF:RCo/VWF:Ag of less than 0.7 in the UC group was 18.7 (95%CI: 1.0-337.4) and that in the CD group was 20.2 (95%CI: 1.1-370.8) vs control subjects.
The VWF:Ag/ADAMTS13act coefficients were higher in the two disease groups than in the control group. In the UC group, there were inverse correlations between ADAMTS13: Ag and VWF:RCo (r = -0.35, P = 0.0168) and between ADAMTS13:Ag and VWF:Ag (r = -0.33, P = 0.0245). In the CD group there were inverse associations between ADAMTS13:Ag and VWF:Ag (r = -0.39, P = 0.0158) and between ADAMTS13:Ag and VWF:CB (r = - 0.33, P = 0.0388). In both groups, ADAMTS13:Ag was closely correlated with ADAMTS13act (UC: r = 0.98; CD: r = 0.85; P < 0.0001 for both).
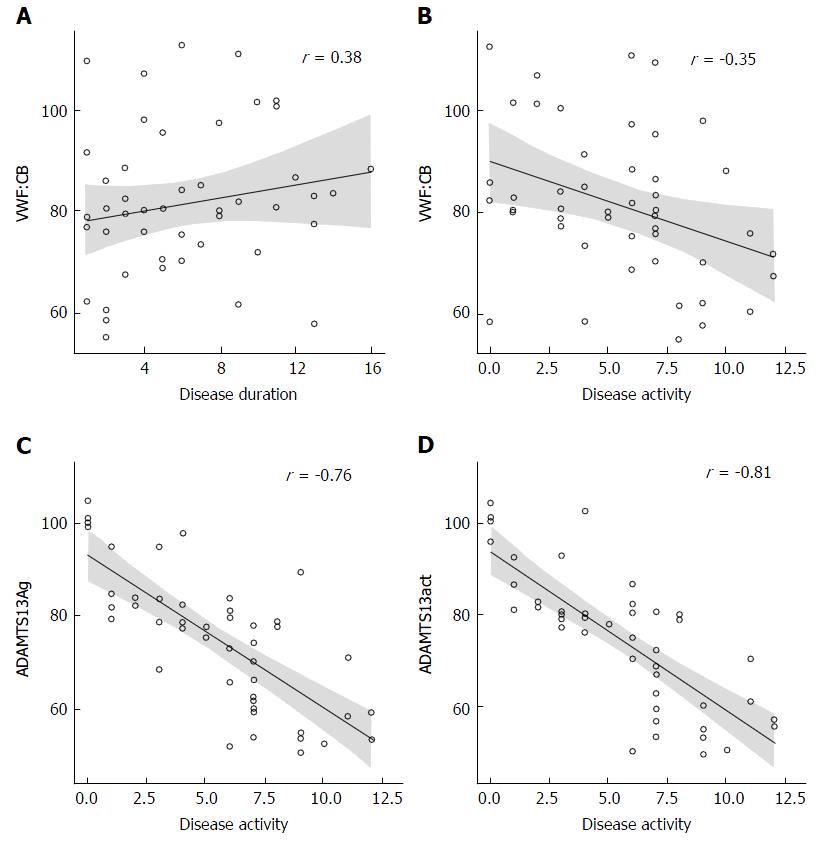
VWF:CB was negatively correlated with disease activity (r = -0.35, P = 0.0157) and positively correlated with disease duration (r = 0.38, P = 0.0116) in the UC group. UC patients also showed inverse correlations between disease activity and ADAMTS13: Ag (r = -0.76, P < 0.0001) and ADAMTS13act (r = -0.81, P < 0.0001) (Figure 3).
The multivariate linear regression model showed that in the UC patients 63.5% of the ADAMTS13Ag variation was associated with three predictors: disease activity, CRP, and platelets, with the first having the greatest effect (Table 6). Moreover, disease activity explained approximately 61.4% of the variation in ADAMTS13act in UC patients.
| ADAMTS13:Ag | ADAMTS13act | |
| Platelets | β = -0.03 (95%CI: -0.07; -0.01) | - |
| P = 0.044 | ||
| C-reactive protein | β = -0.19 (95%CI: -0.33; -0.05) | β = -0.25 (95%CI: -0.38; -0.12) |
| P = 0.103 | P < 0.001 | |
| Disease activity | β = -2.27 (95%CI: -3.18; -1.35) | β = -0.2.27 (95%CI: -3.48; -1.86) |
| P < 0.001 | P < 0.001 | |
| adj.R2 | 65.3% | 69.5% |
In the UC group, ADAMTS13: Ag was inversely correlated with platelet counts (r = - 0.57, P < 0.0001), fibrinogen (r = -0.62, P < 0.0001), and CRP levels (r =-0.67, P < 0.0001). Similar associations were observed for ADAMTS13act in this group (r = -0.55, r = -0.7, and r = -0.72, all P < 0.0001, respectively).
In the CD group, there were no associations between ADAMTS13:Ag and inflammatory markers. However, ADAMTS13act was inversely correlated with fibrinogen (r = -0.36, P = 0.0286) and CRP (r = -0.45, P = 0.0048).
This is the first report of the effects of novel parameters associated with the structure and function of VWF on haemostasis in IBD. On the one hand, ADAMTS13:Ag was lower in IBD patients than in controls, particularly in subjects with UC; this resulted in an increased number of circulating multimers of VWF and thus an elevated thrombotic risk. The prothrombotic effects are enhanced by markedly elevated VWF:Ag. On the other hand, the incidence of AVWS, which leads to increased risk for bleeding, was higher in IBD patients. These findings provide insight into the elevated risk for thromboembolic events and bleeding observed in UC and less frequently in CD. The dysregulation of the balance between VWF function and ADAMTS13 reported herein might contribute to the complex haemostatic abnormalities observed in IBD.
Data on VWF and ADAMTS13 in IBD are scarce. Feys et al[29] reported no differences in VWF:Ag, ADAMTS13:Ag, and ADAMTS13act between UC and CD patients compared to healthy controls; the ADAMTS13:Ag was lower only in those with a CRP > 10 mg/L. However, that study included only 27 patients with CD and 17 with UC. Moreover, the ADAMTS13act/ADAMTS13:Ag ratio exhibited no intergroup differences. Similarly, we did not find any differences in the ADAMTS13act/ADAMTS13:Ag coefficient between our IBD and control groups. Lack of these differences may point to the quantitative nature of the defect[30].
A congenital or acquired deficit in ADAMTS13 has been reported in numerous diseases accompanied by TMA (e.g., TTP, ischaemic stroke). For example, Klonizakis et al[31] reported that ADAMTS13 is a prognostic marker of microthrombotic manifestations in systemic lupus erythematosus. Other authors have demonstrated that the levels of ADAMTS13:Ag and ADAMTS13act in disseminated intravascular coagulation are associated with disease severity and mortality[32]. Enooku et al[33] showed in a Japanese population that the ADAMTS13 concentration was negatively correlated with the levels of inflammatory markers, arterial pressure, and pulse pressure. ADAMTS13 plays a role in inflammatory processes, oxidative stress, and atherosclerosis[33,34]. A reduction in ADAMTS13act and increases in VWF:Ag may increase risk for ischaemic stroke, cerebral infarction, and myocardial infarction[35,36]. In patients with infectious and noninfectious systemic inflammatory response syndrome, ADAMTS13 levels are negatively correlated with platelet count[12]. Such an association in UC patients is our novel finding.
It is possible that during inflammation, there is an increase in the number of large VWF multimers and a decrease in the level and activity of ADAMTS13:Ag, while the degree of disturbance in the balance of these markers is related to the severity of inflammation and the degree of organ failure[16,21]. An acquired decrease in ADAMTS13 level can be caused by several mechanisms[33]. These include excessive consumption of ADAMTS13 due to increased HMWM release from activated endothelium. ADAMTS13 becomes inactive after cleaving its substrate[37]. In addition, inhibition of ADAMTS13 synthesis by proinflammatory cytokines (interleukin 6 or TNF-alpha), or inactivation of ADAMTS13 by proteases released from neutrophils during inflammation occur in inflammatory diseases[38,39]. Interleukin 6 also has the ability to degrade ADAMTS13[21]. ADAMTS13 activity may be regulated by coagulation proteinases; thrombin, plasmin, and factor Xa activity may lead to inactivation of ADAMTS13[38,39].
Based on a mouse model, Chauchan et al[40] suggested that by cleaving UL-VWF multimers, ADAMTS13 downregulates inflammation, while its deficit leads to increased leukocyte adhesion in vessels with inflammatory lesions, as well as increased extravasation of leukocytes. The diversity of mechanisms affecting the level and activity of ADAMTS13 confirms the interdependence of inflammation and coagulation.
A decrease in ADAMTS13act in association with an increase in the VWF:Ag/ADAMTS13act coefficient may be a risk factor for thrombosis and coagulopathy[41]. Claus et al[21] reported that significant disturbances in the balance between VWF:Ag and ADAMTS13act can result in the formation of UL-VWF multimers. The authors proposed that the VWF:Ag/ADAMTS13act ratio should be defined as the TMA index. High TMA index values in patients with inflammation and sepsis suggest that the index may be employed to diagnose highly prothrombotic states, and that its diagnostic value may be higher than that of VWF multimers alone[21]. In vitro studies have demonstrated a correlation between the presence of UL-VWF multimers and VWF:RCo[42].
Administration of recombinant ADAMTS13 in a mouse model of acquired TTP prevents development of symptoms; in contrast, in a mouse model of cerebral ischaemia it decreases the extent of stroke and improves the functional state[43,44]. Straat et al[45] showed that administration of frozen fresh plasma improved endothelial damage and inflammatory parameters, which was related to an increase in ADAMTS13 levels. Therefore, ADAMTS13 may be a new, alternate therapeutic agent for diseases involving disturbances of the balance between VWF and ADAMTS13.
We showed that the VWF:Ag/ADAMTS13act ratio was higher in patients with UC and CD compared to controls. These results confirm that hypercoagulability occurs in IBD, to which VWF is an important contributor. Notably, in approximately 50% of IBD patients, the VWF:Ag value was greater than 150%, compared to 8% in the controls. The inflammatory nature of IBD is associated with increased levels of VWF:Ag, which our findings support.
In contrast, the VWF:CB level in the present study was lower in the UC group than the control and CD groups. This suggests reduced collagen binding by VWF, leading to impaired platelet adhesion to damaged endothelium, as collagen itself has limited platelet-binding ability[13]. Clinically, this may manifest as gastrointestinal bleeding, which is more common in UC patients.
The VWF:CB/VWF:Ag ratio was lower in the UC and CD groups than the control group, and the OR of a VWF:CB/VWF:Ag ratio lower than 0.7 was 12- and 13-fold higher in the UC and CD groups, respectively. The VWF:RCo/VWF:Ag ratio did not differ among the CD, UC, and control groups; however, the IBD group included patients with a VWF:RCo/VWF:Ag coefficient below 0.7, while the control group did not.
VWF:RCo/VWF:Ag and VWF:CB/VWF:Ag coefficients of less than 0.7 are seen in patients with type 2A VWD, indicating substantial depletion of HMWM[45]. Riddell et al[18] reported that VWF:CB assessment is a more sensitive method for diagnostic management of type 2A VWD. Thus, the findings of our study suggest that the risk for AVWS in IBD patients is almost 20-fold higher than that in healthy individuals. This is of practical importance, for example, in patients undergoing antithrombotic prophylaxis during periods of IBD exacerbation, as it enables the identification of patients at increased risk for gastrointestinal bleeding.
This study had several limitations. First, the groups contained relatively few patients. Second, the presence of large VWF multimers in plasma was not analysed. Associations do not necessarily indicate a causal relationship; therefore, in vitro and animal model studies are necessary to elucidate the molecular mechanisms underlying our findings. Finally, this was a case-control study and patients were not followed in terms of thromboembolic events or the duration and severity of bleeding.
To our knowledge, the present study is the first to demonstrate a decrease in ADAMTS13act levels in CD patients, and in ADAMTS13:Ag and ADAMTS13act in UC patients, in whom these parameters were negatively correlated with disease activity and the levels of inflammatory markers. ADAMTS13:Ag represents a link between blood coagulation and inflammation in IBD. ADAMTS13:Ag deficit in IBD is quantitative by nature, possibly due to increased proteolysis of large VWF multimers. The elevated TMA index in IBD patients indicates increased risk for thromboembolic complications. In this group, the use of anticoagulation prophylaxis might be considered during disease flares not only in patients being hospitalised.
We also report for the first time the presence of abnormalities typical of type 2A AVWS in IBD patients. Determination of the VWF:Ag concentration and VWF activity may facilitate the identification of IBD patients at risk for bleeding complications and the management of patients with exacerbated disease, particularly when anticoagulation prophylaxis is recommended. In this group, a specific for AVWS treatment might be implemented during invasive procedures, especially surgery. Further studies involving larger patient populations and long-term follow-up are needed to validate our finding of a role for VWF in IBD.
Inflammation and blood coagulation are closely related. A tightly regulated balance between Von Willebrand factor (VWF) and ADAMTS13 is important for haemostasis, and its dysregulation might predispose to either thrombotic events or bleeding. Impaired cleavage of large VWF multimers due to inadequate ADAMTS13 levels and/or impaired ADAMTS13 activity leads to thrombotic microangiopathies. In severe inflammatory states, in which the VWF level is markedly elevated, a normal or slightly decreased ADAMTS13 level may be insufficient to control ultra-large VWF multimers.
Inflammatory bowel disease (IBD) patients are at threefold higher risk for thromboembolic complications than the general population. This risk is especially high during hospitalizations, surgery or active disease and results from various hereditary and acquired factors.
This study demonstrated a decrease in ADAMTS13 activity levels in Crohn’s disease patients and in ADAMTS13 antigen and ADAMTS13 activity in ulcerative colitis patients, in whom these parameters were negatively correlated with disease activity and inflammatory markers. On the other hand, the study showed for the first time the presence of abnormalities typical of type A2 acquired von Willebrand syndrome in IBD patients.
If further studies confirm the clinical relevance of these results, they may facilitate the individualization of antithrombotic therapy in patients with IBD. The elevated VWF antigen/ADAMTS13 activity ratio indicates an increased risk for thromboembolic complications. In this group, the use of anticoagulation prophylaxis might be considered not only in patients undergoing surgery or hospitalizations due to disease flare-ups but also in outpatients with disease exacerbation. On the other hand, determination of the VWF antigen concentration and VWF activity may facilitate the identification of IBD patients at higher risk for bleeding complications and the management of patients with exacerbated disease, particularly when anticoagulation prophylaxis is recommended. In this group, a specific for acquired von Willebrand syndrome treatment might be implemented during invasive procedures, especially surgery.
VWF is an acute-phase protein and a marker of endothelial damage. Its function is crucial for platelet adhesion and aggregation. ADAMTS13 is a glycoprotein that cleaves large VWF multimers to smaller, less-active multimers. Type 2A acquired von Willebrand syndrome is characterised by an acquired qualitative VWF deficit associated with high-molecular-weight multimers depletion, which increases the risk of mucocutaneous bleeding.
The manuscript Levels and activities of von Willebrand factor and metalloproteinase with thrombospondin type-1 motif, number 13 in IBD is an interesting original research with clear objectives.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Krawiec P S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review. Am J Gastroenterol. 2011;106:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Novacek G, Weltermann A, Sobala A, Tilg H, Petritsch W, Reinisch W, Mayer A, Haas T, Kaser A, Feichtenschlager T. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779-787, 787.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Dhillon AP, Anthony A, Sim R, Wakefield AJ, Sankey EA, Hudson M, Allison MC, Pounder RE. Mucosal capillary thrombi in rectal biopsies. Histopathology. 1992;21:127-133. [PubMed] |
| 4. | Owczarek D, Cibor D, Głowacki MK, Rodacki T, Mach T. Inflammatory bowel disease: epidemiology, pathology and risk factors for hypercoagulability. World J Gastroenterol. 2014;20:53-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Thompson NP, Wakefield AJ, Pounder RE. Inherited disorders of coagulation appear to protect against inflammatory bowel disease. Gastroenterology. 1995;108:1011-1015. [PubMed] |
| 6. | Owczarek D, Cibor D, Sałapa K, Głowacki MK, Mach T, Undas A. Reduced plasma fibrin clot permeability and susceptibility to lysis in patients with inflammatory bowel disease: a novel prothrombotic mechanism. Inflamm Bowel Dis. 2013;19:2616-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Bernhard H, Deutschmann A, Leschnik B, Schweintzger S, Novak M, Hauer A, Muntean W. Thrombin generation in pediatric patients with Crohn’s disease. Inflamm Bowel Dis. 2011;17:2333-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Undas A, Kaczmarek P, Sladek K, Stepien E, Skucha W, Rzeszutko M, Gorkiewicz-Kot I, Tracz W. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease. Beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Kwasny-Krochin B, Gluszko P, Undas A. Unfavorably altered fibrin clot properties in patients with active rheumatoid arthritis. Thromb Res. 2010;126:e11-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Cibor D, Domagala-Rodacka R, Rodacki T, Jurczyszyn A, Mach T, Owczarek D. Endothelial dysfunction in inflammatory bowel diseases: Pathogenesis, assessment and implications. World J Gastroenterol. 2016;22:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:507-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 12. | Claus RA, Bockmeyer CL, Sossdorf M, Lösche W. The balance between von-Willebrand factor and its cleaving protease ADAMTS13: biomarker in systemic inflammation and development of organ failure? Curr Mol Med. 2010;10:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Yoshida H, Yilmaz CE, Granger DN. Role of tumor necrosis factor-α in the extraintestinal thrombosis associated with colonic inflammation. Inflamm Bowel Dis. 2011;17:2217-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: The clot thickens. Am J Gastroenterol. 2007;102:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 283] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Owczarek D, Cibor D, Sałapa K, Cieśla A, Głowacki MK, Pocztar H, Mach TH. Anti-inflammatory and anticoagulant properties of the protein C system in inflammatory bowel disease. Pol Arch Med Wewn. 2012;122:209-216. [PubMed] |
| 16. | Owczarek D, Cibor D, Mach T. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA), arginine, and 8-iso-prostaglandin F2alpha (8-iso-PGF2alpha) level in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Reininger AJ. The function of ultra-large von Willebrand factor multimers in high shear flow controlled by ADAMTS13. Hamostaseologie. 2015;35:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Riddell AF, Jenkins PV, Nitu-Whalley IC, McCraw AH, Lee CA, Brown SA. Use of the collagen-binding assay for von Willebrand factor in the analysis of type 2M von Willebrand disease: a comparison with the ristocetin cofactor assay. Br J Haematol. 2002;116:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Natorska J, Mazur P, Undas A. Increased bleeding risk in patients with aortic valvular stenosis: From new mechanisms to new therapies. Thromb Res. 2016;139:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis. 2014;25:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Claus RA, Bockmeyer CL, Budde U, Kentouche K, Sossdorf M, Hilberg T, Schneppenheim R, Reinhart K, Bauer M, Brunkhorst FM. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009;101:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Reiter RA, Varadi K, Turecek PL, Jilma B, Knöbl P. Changes in ADAMTS13 (von-Willebrand-factor-cleaving protease) activity after induced release of von Willebrand factor during acute systemic inflammation. Thromb Haemost. 2005;93:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Mazur P, Plicner D, Zdziarska J, Sadowski J, Undas A. Decreased von Willebrand factor ristocetin cofactor activity and increased ADAMTS13 antigen increase postoperative drainage after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2014;45:e26-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Stenson WF. Inflammatory bowel diseases. Textbook of Gastroenterology, Vol. 2, 2nd ed. Philadelphia: JB Lippincott; 1995; 1761-1772. |
| 25. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2354] [Article Influence: 123.9] [Reference Citation Analysis (2)] |
| 26. | Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 27. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2252] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 28. | Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26400] [Cited by in RCA: 34972] [Article Influence: 1942.9] [Reference Citation Analysis (0)] |
| 29. | Feys HB, Canciani MT, Peyvandi F, Deckmyn H, Vanhoorelbeke K, Mannucci PM. ADAMTS13 activity to antigen ratio in physiological and pathological conditions associated with an increased risk of thrombosis. Br J Haematol. 2007;138:534-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Stepanian A, Cohen-Moatti M, Sanglier T, Legendre P, Ameziane N, Tsatsaris V, Mandelbrot L, de Prost D, Veyradier A. Von Willebrand factor and ADAMTS13: a candidate couple for preeclampsia pathophysiology. Arterioscler Thromb Vasc Biol. 2011;31:1703-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Klonizakis P, Tselios K, Sarantopoulos A, Gougourellas I, Rouka E, Onufriadou Z, Kapali P, Kyriakou D, Boura P. ADAMTS-13 metalloprotease abnormalities in systemic lupus erythematosus: is there a correlation with disease status? Lupus. 2013;22:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Ohshiro M, Kuroda J, Kobayashi Y, Akaogi T, Kawata E, Uoshima N, Kamitsuji Y, Kaneko H, Shimura K, Shimazaki C. ADAMTS-13 activity can predict the outcome of disseminated intravascular coagulation in hematologic malignancies treated with recombinant human soluble thrombomodulin. Am J Hematol. 2012;87:116-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Enooku K, Kato R, Ikeda H, Kurano M, Kume Y, Yoshida H, Ono T, Aizawa K, Suzuki T, Yamazaki T. Inverse correlations between serum ADAMTS13 levels and systolic blood pressure, pulse pressure, and serum C-reactive protein levels observed at a general health examination in a Japanese population: a cross-sectional study. Clin Chim Acta. 2013;421:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Schwameis M, Schörgenhofer C, Assinger A, Steiner MM, Jilma B. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb Haemost. 2015;113:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 35. | Andersson HM, Siegerink B, Luken BM, Crawley JT, Algra A, Lane DA, Rosendaal FR. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Qu L, Jiang M, Qiu W, Lu S, Zhao Y, Xia L, Ruan C, Zhao Y. Assessment of the Diagnostic Value of Plasma Levels, Activities, and Their Ratios of von Willebrand Factor and ADAMTS13 in Patients with Cerebral Infarction. Clin Appl Thromb Hemost. 2016;22:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Wang Y, Chen J, Ling M, López JA, Chung DW, Fu X. Hypochlorous acid generated by neutrophils inactivates ADAMTS13: an oxidative mechanism for regulating ADAMTS13 proteolytic activity during inflammation. J Biol Chem. 2015;290:1422-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Mannucci PM, Capoferri C, Canciani MT. Plasma levels of von Willebrand factor regulate ADAMTS-13, its major cleaving protease. Br J Haematol. 2004;126:213-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Crawley JT, Lam JK, Rance JB, Mollica LR, O’Donnell JS, Lane DA. Proteolytic inactivation of ADAMTS13 by thrombin and plasmin. Blood. 2005;105:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Chauhan AK, Kisucka J, Brill A, Walsh MT, Scheiflinger F, Wagner DD. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008;205:2065-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Soares RP, Bydlowski SP, Jatene MB, Hironaka JF, Lopes AA. Decreased plasma ADAMTS-13 activity as a predictor of postoperative bleeding in cyanotic congenital heart disease. Clinics (Sao Paulo). 2013;68:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Budde U, Metzner HJ, Müller HG. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost. 2006;32:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Tersteeg C, Schiviz A, De Meyer SF, Plaimauer B, Scheiflinger F, Rottensteiner H, Vanhoorelbeke K. Potential for Recombinant ADAMTS13 as an Effective Therapy for Acquired Thrombotic Thrombocytopenic Purpura. Arterioscler Thromb Vasc Biol. 2015;35:2336-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 45. | Straat M, Müller MC, Meijers JC, Arbous MS, Spoelstra-de Man AM, Beurskens CJ, Vroom MB, Juffermans NP. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care. 2015;19:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |