Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4644
Peer-review started: January 10, 2017
First decision: February 23, 2017
Revised: March 8, 2017
Accepted: April 12, 2017
Article in press: April 12, 2017
Published online: July 7, 2017
Processing time: 179 Days and 1.4 Hours
To evaluate the anti-apoptotic effect of banhasasim-tang (BHSST) on chronic acid reflux esophagitis (CARE) using a rat model.
A surgically-induced CARE model was established in Sprague-Dawley rats. The modeled rats were divided into a treatment group or untreated group, and given BHSST (1 g/kg body weight per day) or water, respectively, for 15 consecutive days (n = 7 each group). Changes in expression of proteins related to nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and apoptosis were assessed by western blotting. Changes in esophageal pathology were analyzed by gross and histological examinations.
The CARE exposure modeled rats showed increased levels of the NADPH oxidase subunit, NOX4 and p47phox in the esophagus. The BHSST treatment completely resolved these CARE-related increases. The CARE rats also showed markers of cytokine stress, including elevated levels of TNF-α and reactive oxygen species as well as of the consequent increase in JNK activation, and subsequent decrease in pro-survival gene expression, such as of Bcl-2. BHSST treatment resolved the CARE-related changes. BHSST also exerted an anti-apoptotic effect, as evidenced by altered expression of the apoptosis-related genes for bax, cytochrome c, and caspase 3. Finally, the BHSST treatment markedly ameliorated the CARE-related esophageal mucosal ulcerations.
In the rat model of CARE, BHSST can suppress development of esophageal mucosal ulceration via regulation of reactive oxygen species-dependent apoptosis.
Core tip: Banhasasim-tang (BHSST) has been used widely as an herbal prescription in East Asia for its therapeutic effects on symptoms associated with gastroesophageal reflux disease. In this study, BHSST is shown to play a protective role against chronic acid reflux esophagitis-induced esophageal mucosal ulcer in a rat model, and that this effect involves regulation of reactive oxygen species-dependent apoptosis.
- Citation: Shin MR, An HJ, Seo BI, Roh SS. Anti-apoptotic effect of banhasasim-tang on chronic acid reflux esophagitis. World J Gastroenterol 2017; 23(25): 4644-4653
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4644.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4644
Gastroesophageal reflux disease (GERD) is one of the most frequently encountered gastrointestinal disorders worldwide, occurring in children as well as adults and showing a trend towards increasing risk in the past few decades[1]. Various etiologies have been demonstrated as underlying the disease pathogenesis, ranging from diet, the increased social life stress of modern-day life, and a diverse array of medical conditions[2]. As such, the symptoms of GERD are equally diverse, ranging from heartburn and regurgitation to severe erosive esophagitis and its associated complications. Patients with GERD can also suffer sleep disturbances, chest pains or respiratory symptoms[3].
Economic analyses have indicated that the increased, incidence of GERD is accompanied by increased direct and indirect costs related to its diagnosis, treatment and supervision, as well as of costs related to its complications. The currently prescribed medications for GERD are pharmaceutical drugs-primarily H2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs)[4]. However, these drugs are associated with multiple adverse effects, such as headaches, diarrhea, nausea (H2RAs and PPIs) and constipation (PPIs)[5,6]. Herbal medicines have been proposed as a useful alternative, and the field of GERD research has begun to search for an herbal therapeutic useful for the treatment and/or prevention of GERD and to define the molecular mechanisms underlying any such beneficial activities.
Recently, Lee et al[7] uncovered a close relationship between chronic acid reflux esophagitis (CARE) and an altered profile of markers of oxidative stress (OS). An exaggerated or unregulated prolonged inflammatory process, in response to various exogenous and endogenous stimuli, can induce tissue damage and has been implicated in the development and progression of many chronic diseases, including CARE[8]. Continuous reflux of gastric contents causes ulceration and destruction of the normal squamous epithelium of the esophagus. Over time, the esophagus adapts to the continuous reflux stimuli with metaplastic conversion to columnar epithelium[9,10]. These series of processes led to Barrett’s esophagus, a common premalignant lesion of esophageal adenocarcinoma. Accordingly, management of Barrett’s esophagus (via suppression of the gastroesophageal reflux) is recommended, at the earliest stage possible, to prevent the development of gastroesophageal carcinoma in an early stage[11].
Banhasasim-tang (BHSST) is a classic herbal formulation in Traditional Chinese Medicine (TCM). Its first recorded description is in the Shang-Han Lun (transl. Treatise on Cold Damage and Miscellaneous Diseases) written by the Chinese physician Zhang Zhong-Jing (A.D. 150-219). He suggested the treatment of disease and a proper mixture of medicines in Shang-Han Lun after having synthesized clinical experiences and therapeutic principles based on the existing medical books[12,13]. It continues to be in use throughout Asia, including in South Korea, for treating symptoms associated with GERD[14-16].
However, the molecular mechanisms underlying its observed protective effects against GERD remain unknown. The research study described herein was designed to begin to investigate the molecular mechanisms of BHSST protection against esophageal mucosal ulcer by using a rat model of CARE.
Protease inhibitor mixture cocktail, 4,6-dihydroxy-2-mercaptopyrimidine (2-thiobarbituric acid; TBA), and ethylenediaminetetraacetic acid (EDTA) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 2′,7′-Dichlorofluorescein diacetate (DCF-DA) was obtained from Molecular Probes (Eugene, OR, United States). Phenylmethylsulfonyl fluoride (PMSF) was purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, United States). Pierce’s bicinchoninic acid protein assay kit was obtained from Thermo Fisher Scientific (Waltham, MA, United States). Enhanced chemiluminescence (ECL) western blotting detection reagents and nitrocellulose membranes were supplied by GE Healthcare (Chicago, IL, United States). Rabbit polyclonal antibodies against bax (1:1000; SC-7480), p47phox (1:1000; SC-14015) and cytochrome c (1:1000; SC-13156); goat polyclonal antibodies against tumor necrosis factor-α (TNF-α) (1:1000; SC-1351); mouse monoclonal antibodies against phosphor-c-Jun NH2-terminal kinase (p-JNK) (1:1000; SC-6254), histone (1:1000; SC-8030) and β-actin (1:1000; SC-4778) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, United States). Mouse monoclonal antibody against activator protein-1 (AP-1) subunit c-Jun (1:1000; #2315) was obtained from Cell Signaling Technology, Inc. (Danvers, MA, United States). Mouse monoclonal anti-caspase-3 (1:1000; 3004-100) was purchased from BioVision Inc. (Mountain View, CA, United States). Rabbit polyclonal anti-reduced nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) was purchased from LifeSpan BioSciences (Seattle, WA, United States). Rabbit anti-goat (1:3000; SC-2774), goat anti-rabbit (1:3000; SC-2004) and goat anti-mouse (1:3000; SC-2005) immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated secondary antibodies were acquired from Santa Cruz Biotechnology, Inc. All other chemicals and reagents used were of the analytical grade purchased from Sigma-Aldrich Co.
Light brown granules of BHSST were purchased from Hankook Shinyak Corp (Nonsan-si, Chungcheongnam-do, South Korea) and had been produced according to the Korean Good Manufacturing Practice under permit granted by regulation of the Korean Food & Drug Administration (Seoul, South Korea). For experimentation, 1.5 g BHSST was dissolved in distilled water. The specific composition of the BHSST formulation is presented in Table 1.
| Herb | Amount in g |
| Pinelliae rhizoma | 1.67 |
| Scutellariae radix | 1.00 |
| Zingiberis rhizoma siccus | 0.83 |
| Ginseng radix | 1.00 |
| Glycyrrhizae radix | 1.00 |
| Jujubae fructus | 1.00 |
| Coptidis rhizoma | 0.33 |
All procedures involving animals were carried out in full accordance with the “Guidelines for Animal Experimentation” with pre-approval given by the Ethics Committee of the Daegu Haany University. Male Sprague-Dawley rats (Nara Biotec Co, Pyeongtaek, South Korea), weighing 160-170 g, were housed in cages with constant temperature of 24 ± 2 °C, relative humidity of 60%, automated 12:12 h light/dark cycle (light on at 7:00 AM), and free access to food and water.
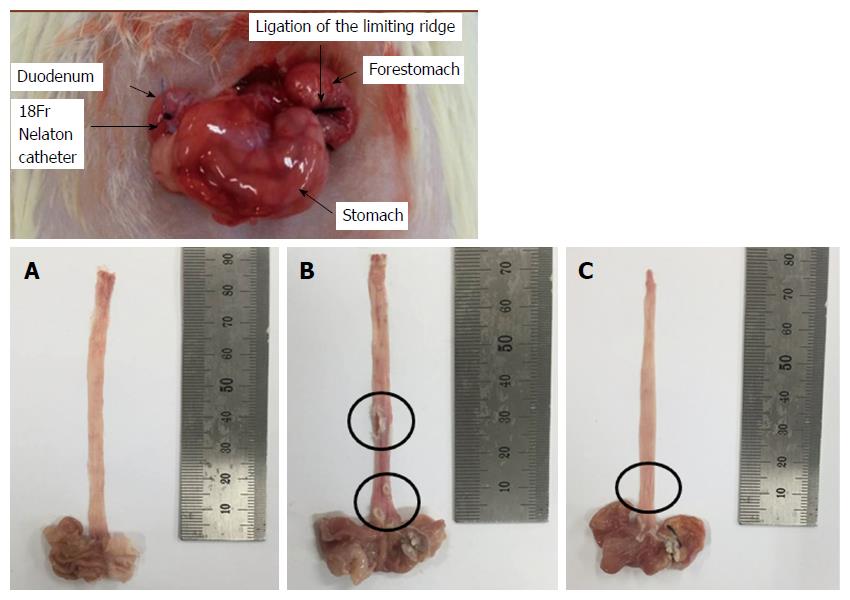
CARE was surgically induced as described by Omura et al[17]. Briefly, a midline laparotomy was performed to expose the stomach and transitional region (i.e., limiting ridge) between the fundus, and the glandular portion of the stomach was ligated with 2-0 silk thread in order to restrict the compliance of the stomach, which led to the reflux of gastric contents into the esophagus. Additionally, a latex ring (2 mm in thickness; ID, 4 mm, made from 18-Fr Nelaton catheter) was placed around the pyloric sphincter so as to restrict the emptying of gastric contents. Rats were then injected with gentamicin sulfate (antibiotic, subcutaneous injection) and dexamethasone (anti-inflammatory agent, subcutaneous injection) to prevent infection.
After surgery, the rats were fasted for a further 48 h but water was provided at 24 h after surgery. All animals had an operational adjustment for 7 d post-surgery. Body weight was recorded every day over a course of 22 d from the surgery day (during the “operational adjustment period” of the first 7 d post-surgery and then during the “BHSST treatment period” of the next 15 d. Food intake was recorded every day during the 15-d BHSST treatment period. For the 15-d treatment course, the CARE modeled rats were divided into two groups (n = 7 each) and given an oral administration (via stomach tube) of either water (CARE group) or BHSST at 1 g/kg body weight (BHSST-treated CARE group). Blood samples were collected by vena cava puncture from anesthetized rats. At day 22 post-surgery, all rats were sacrificed and the entire esophagus was removed, examined rapidly for gross mucosal injury and frozen in liquid nitrogen. Both the esophageal tissues and serum samples were kept at -80 °C until analysis.
Serum reactive oxygen species (ROS) level was measured as described by Ali et al[18]. Briefly, 25 mmol/L DCF-DA was added to the serum sample and allowed to incubate for 30 min. The DCF-DA-induced change in fluorescence value was measured at an excitation wavelength of 486 nm and emission wavelength of 530 nm. TBA-reactive substance (TBARS) level was estimated according to the method of Mihara and Uchiyama[19].
Protein extraction was performed according to the method described by Komatsu[20], with minor modifications. Briefly, esophageal tissue was homogenized in ice-cold lysis buffer A (250 mL) containing 10 mmol/L HEPES (pH 7.8), 10 mmol/L KCl, 2 mmol/L MgCl2, 1 mmol/L DTT, 0.1 mmol/L EDTA, 0.1 mmol/L PMSF, and 1250 μL protease inhibitor mixture cocktail. The homogenate was then incubated at 4 °C for 20 min, after which 10% NP-40 was added and mixed well. After centrifugation at 13400 × g for 2 min at 4 °C (5415R centrifuge; Eppendorf, Hamburg, Germany), the supernatant liquid (cytosolic fraction) was removed to a new tube. The leftover pellet was then washed twice with buffer A, centrifuged and the supernatant discarded. The leftover pellet was suspended in lysis buffer C (20 mL) containing 50 mmol/L HEPES (pH 7.8), 50 mmol/L KCl, 300 mmol/L NaCl, 1 mmol/L DTT, 0.1 mmol/L EDTA, 0.1 mmol/L PMSF, 1% (v/v) glycerol, and 100 μL protease inhibitor mixture cocktail and incubated at 4 °C for 30 min. After centrifugation at 13400 × g for 10 min at 4 °C, the nuclear fraction was obtained. Both cytosolic and nuclear fractions were kept at -80 °C until use in analysis.
For estimation of c-Jun and histone, 13.6 μg of protein from each nuclear fraction was resolved by electrophoresis through a 10% sodium dodecylsulfate polyacrylamide gel (SDS-PAGE). The separated proteins were transferred to a nitrocellulose membrane, blocked by incubating with 5% (w/v) skim milk solution for 1 h, and then incubated with primary antibodies (c-Jun and histone) for overnight at 4 °C. After the blots were washed, they were incubated with anti-rabbit or anti-mouse IgG HRP-conjugated secondary antibody for 1 h at room temperature. In addition, 10-16 μg protein of each cytosolic fraction was electrophoresed by 10%-14% SDS-PAGE for immunodetection of TNF-α, p-JNK, NOX4, p47phox, bax, cytochrome c, caspase 3, and β-actin. Each antigen-antibody complex was first processed with the ECL regents and then detected by the Sensi-Q 2000 Chemidoc instrument (Lugen Sci Co., Ltd., Gyeonggi-do, South Korea). Band densities were measured using ATTO Densitograph Software (ATTO Corporation, Tokyo, Japan) and quantified as the ratio to histone or β-actin. Protein levels of the groups are expressed as relative to those of normal rat (set to 1.0).
The data are expressed as mean ± SE. Statistical analysis was performed using SPSS version 22.0 software (IBM SPSS Inc, Armonk, NY, United States). P values less than 0.05 were considered to indicate statistical significance.
CARE modeled-rats had significantly lower body weight and food intake during the BHSST treatment period than the of normal rats (Table 2). However, the BHSST-treated CARE modeled rats had significantly higher food intake than the untreated CARE rats. The BHSST treatment appeared to have no significant effect on the body weight of the CARE modeled rats.
| Group | Body weight change in g, following surgery | Food intake in g/d | ||||
| 0 d | 4 d | 7 d | 14 d | 21 d | ||
| Drug treatment | - | - | 1 d | 8 d | 15 d | |
| Normal rats | 169 ± 2.4 | 170 ± 2.4 | 219 ± 2.6 | 278 ± 3.9 | 329 ± 5.6 | 27.32 ± 0.47 |
| CARE modeled rats | ||||||
| Untreated | 166 ± 2.2 | 137 ± 2.0b | 142 ± 2.7b | 150 ± 7.5b | 178 ± 12.8b | 12.27 ± 0.63b |
| BHSST-treated | 167 ± 1.8 | 135 ± 1.8 | 146 ± 3.8 | 169 ± 10.1 | 194 ± 19.1 | 14.99 ± 0.58d |
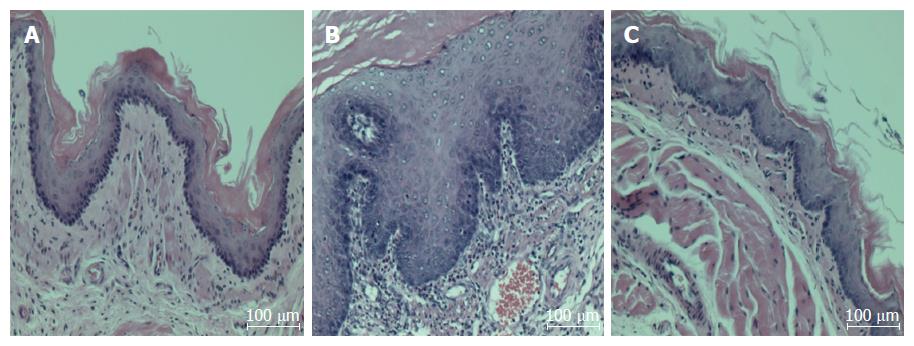
Normal rats had no detectable esophageal mucosa lesions, whereas esophageal ulcers were readily apparent in the middle or distal esophagus of the untreated CARE modeled rats (Figure 1). As compared to the normal rats, the untreated CARE modeled rats exhibited basal layer thickening and inflammatory cell infiltration; the BHSST-treated CARE modeled rats showed remarkably less extensive damage (Figure 2).
As shown in Table 3, the levels of the OS-related biomarkers, ROS and TBARS, in untreated CARE modeled rats were markedly higher than those of normal rats (P < 0.01). The BHSST-treated CARE modeled rats showed a decrease in these CARE-induced elevations, to levels even lower than those in normal rats. In addition, the BHSST treatment led to significant decreases in serum ROS and TBARS, but only the ROS decrease was statistically significant (P < 0.05).
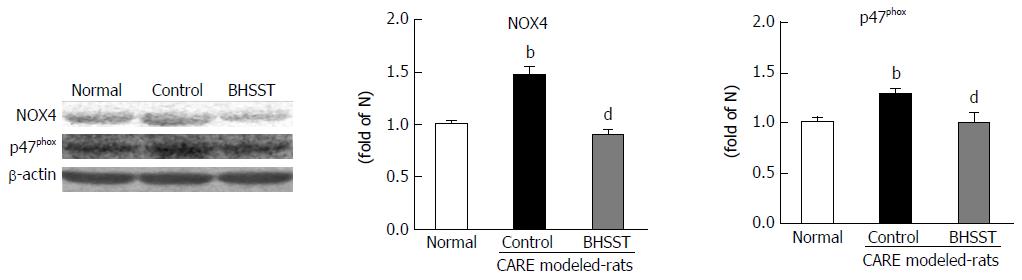
The expressions of both p47phox and NOX4 proteins [the markers of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in the esophageal tissues] were augmented in the CARE modeled rats (vs normal rats, P < 0.01). The BHSST-treated CARE modeled rats, however, had significantly down-regulated NADPH oxidase (vs untreated CARE modeled rats, P < 0.01) (Figure 3).
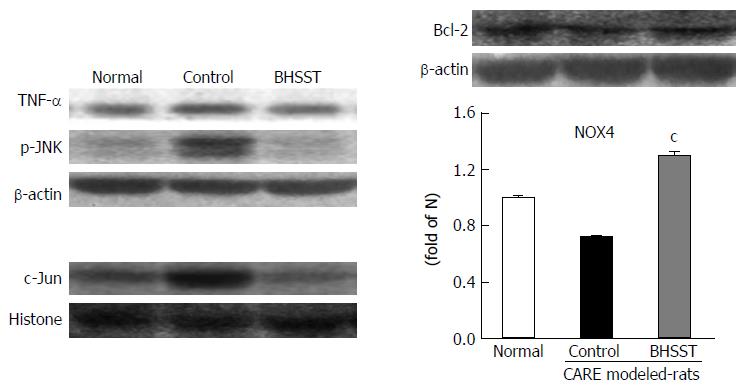
The untreated CARE modeled rats had increased protein expression for TNF-α and p-JNK in the cytosolic fraction, and for c-Jun in the nuclear fraction. The up-regulation was reversed by the BHSST treatment (Figure 4).
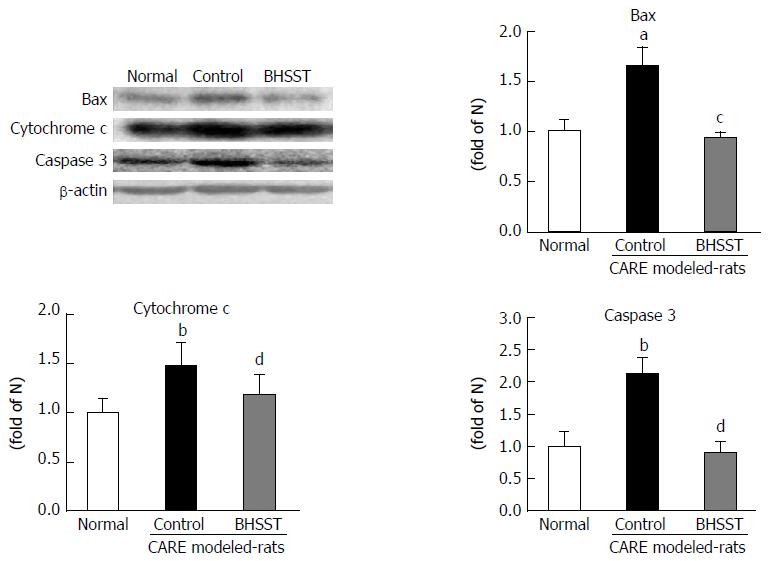
As shown in Figure 5, the apoptosis-related factors bax, cytochrome c and caspase 3 were examined in the cytosolic fraction. All three of these proteins were significantly higher in the untreated CARE modeled rats (vs normal rats, P < 0.05, < 0.001 and < 0.01, respectively). These elevated expressions were substantially enhanced by the BHSST treatment, with bax and caspase 3 expression being decreased to a level near that seen in the normal (P < 0.05 and < 0.01, respectively).
Several herbals from TCM have been shown to exert therapeutic effect through the inhibition of apoptosis, particularly in the treatment of GERD[21]. However, the role of BHSST still lacks evidential data of its underlying molecular mechanism against CARE. The present study provided evidence that supplementation of BHSST significantly ameliorated CARE-induced esophageal ulcer.
Traditional herbal formulas have been generally used throughout East Asia for prevention and treatment of various inflammatory disorders[22]. BHSST is one such herbal medicine, and its formulation consists of the following seven herbs (in 5:3:3:3:3:3:1 proportions): Pinelliae rhizoma, Scutellariae radix, Zingiberis rhizoma siccus, Ginseng radix alba, Glycyrrhizae radix, Zizyphi fructus, and Coptidis rhizoma. Studies of the various constituents have elucidated the antiemetic effect of Pinelliae rhizoma[23], the anti-inflammatory and antitumor effects of Scutellariae radix[24] and Ginseng radix alba[25], and the antioxidant effect of Glycyrrhizae radix[26], Zingiberis rhizoma siccus[27] and Coptidis Rhizoma[28]; Ziziphus jujuba[29] has been widely used to ameliorate the symptoms of gastrointestinal disorders in Eastern Asia. The 13 major bioactive components of BHSST are homogentisic acid, 3,4-dihydroxybenzaldehyde (from Pinelliae rhizoma), spinosin (Zizyphi fructus), liquiritin, liguiritigenin and glycyrrhizin (Glycyrrhizae radix), baicalin, baicalein, wogonoside and wogonin (Scutellariae radix), ginsenoside Rg1 and ginsenoside Rb1 (Ginseng radix alba), and 6-gingerol (Zingiberis rhizoma siccus)[30]. Baicalin represents the most abundant content. BHSST formulations including these bioactive components have been shown to exert gastroprotective effects and to do so through inhibition of inflammatory proteins[31,32].
Based on the previous studies of BHSST, we predicted that a formulation containing these bioactive herbs would allow for improvement of esophageal ulcer induced by CARE in a rat model. For this reason, the present study was conducted using the same CARE model system frequently used in the previous experimental studies[17]. Firstly, body weight gain during the experimental period and food intake during the BHSST treatment periods were confirmed. As shown in Table 2, although the normal rats and CARE modeled rats started out at similar body weights, the CARE modeled rats experienced weight loss until recovery from the surgery (4 d), during which time the normal rats experienced a gradual increase and this was due to low food intake. However, the significant increase in food intake upon BHSST administration led to some weight gain (but without statistical significance). Thus, the CARE-inducing surgery appears to have brought about the changes in food intake and body weight[33].
Next, gross morphological changes such as mucosal thickening and esophageal ulcer, both of which are associated with the metaplastic process of mucosal epithelial cells were observed in the CARE modeled rats; this is in contrast to the features of hemorrhage, hyperemia and multiple erosions in acute reflux esophagitis[34]. The CARE-related tissue injuries were located in the lower part of esophagus, in particular. However, esophageal ulceration was completely absent in the normal rats. The normal esophagus exhibited a thin epithelial layer with squamous cells and few inflammatory cells in the submucosal layer, while the CARE esophagus exhibited the characteristic basal layer thickening and inflammatory cell infiltration[35]. Importantly, the CARE modeled rats that were treated with BHSST showed remarkably less damage than their untreated CARE control counterparts.
Apoptosis, or programmed cell death, serves to remove superfluous, damaged, infected, or transformed cells, and is a key cellular process for maintenance of tissue homeostasis. Two main pathways lead to apoptosis: the extrinsic (or death receptor) pathway, and the intrinsic (or mitochondrial) pathway[36,37]. The extrinsic pathway requires activation of membrane “death” receptors, such as those for TNF. The intrinsic pathway, which functions independently of the death receptors, involves the mitochondria and is mediated through stimulation by stress molecules (i.e., ROS)[38]. An important cellular source of ROS is the NADPH oxidase family. Previous studies have reported that pharmacological suppression of NADPH oxidase activity prevents tissue apoptosis[39]. These findings suggest that NADPH oxidases may contribute to apoptosis of esophageal tissue, a feature of GERD in humans and CARE in rats[40]. In the present study, CARE modeled rats showed up-regulation of both NADPH oxidase and TNF-α in esophageal ulcer, these CARE-related changes were reversed by BHSST administration.
In the absence of OS, JNK can bind to glutathione S-transferase, resulting in inhibition of JNK activity. Under OS conditions, which are characterized by ROS overproduction, glutathione S-transferase is dissociated from JNK, resulting in its activation[41]. Increased levels of ROS, which exceed the capacity of cellular antioxidant defense systems, lead to OS and have been implicated in the pathology of several chronic diseases[42]. Numerous studies have shown that the JNK/AP-1 pathway can stimulate expression of pro-apoptotic genes, such as TNF. JNK is also known to decrease the expression of pro-survival genes, as has been shown for Bcl-2. Besides, the pro-apoptotic protein bax may promote cytochrome c release and the subsequent activation of effector caspases. In a healthy cell, Bcl-2 is expressed on the external surface of the mitochondrial membrane, where it is available for binding to Apaf-1, which is thereby kept inactivated. Any alteration in the internal equilibrium of the cell, such as ROS accumulation, then causes mitochondrial release of cytochrome c. In turn, Bcl-2 liberates Apaf-1, that then binds to the released cytochrome c[43]. Furthermore, the release of cytochrome c from mitochondria into the cytoplasm is an important regulatory step in caspase 3 activation.
In the current study, JNK activation was moderately enhanced in CARE modeled rats, as compared to normal rats. BHSST treatment facilitated a substantial down-regulation of the CARE-related enhancement of JNK activation. Moreover, BHSST was found to increase transcription of the gene encoding the anti-apoptotic Bcl-2-like protein, which would serve to inhibit the pro-apoptotic factor bax and to attenuate the transcription of genes encoding for pro-apoptotic proteins, such as bax. Taken together, regulating expression of genes encoding Bcl-2 during CARE could be relevant to explain the beneficial effects of BHSST in treatment of CARE. Nevertheless, the mechanism of action of BHSST remains to be unambiguously defined and further research is required.
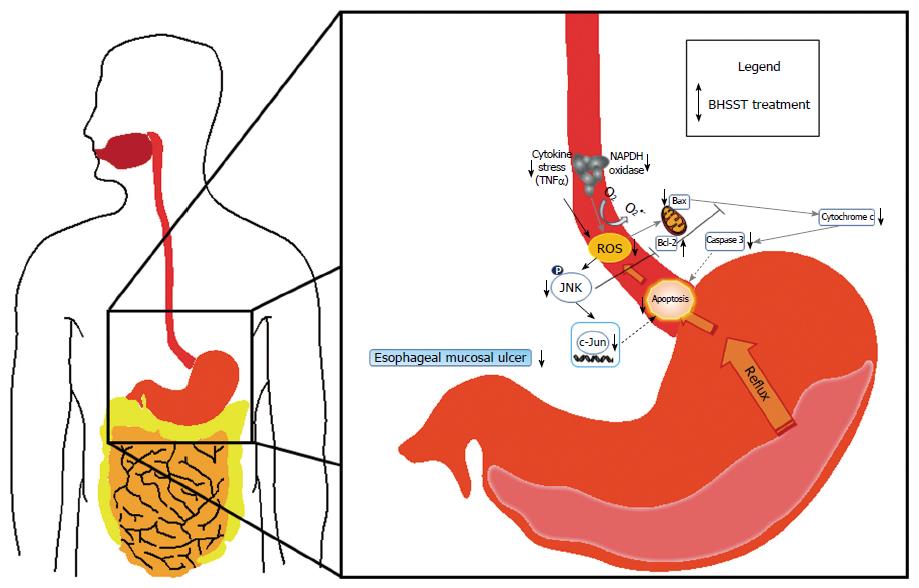
In conclusion, data from the present study indicates that the down-regulation of TNF-α or the suppression of NADPH oxidase activity affects the esophageal apoptotic response in CARE. Meanwhile, BHSST treatment was shown to exert beneficial anti-apoptotic effects in CARE, as shown in Figure 6. Accordingly, BHSST may be a promising herbal formula for the prevention or treatment of GERD. However, the relationship between the prevalence of esophageal mucosal ulcerations and other risk or etiological factors, such as gastrointestinal motility, gastric acid and pepsin secretion, or esophageal sensitivity is unknown, and further study is necessary in order to adequately understand this relationship.
Gastroesophageal reflux disease (GERD), including reflux esophagitis, is mainly caused by excessive or continuous exposure of the esophageal mucosa to the gastric contents. The existing therapeutic strategy for GERD is primarily acid suppression, and commonly involves the use of pharmacological antacids, H2-receptor antagonists, and proton pump inhibitors. Despite their well-known efficacies, recurrence among the treated patients is considerably high. Recent studies have reported that oxidative stress plays an important role in the pathogenesis of reflux esophagitis. Hence, the authors sought to determine whether banhasasim-tang (BHSST) treatment exerts a protective effect under oxidative stress status of chronic acid reflux esophagitis (CARE) using a rat model.
GERD, which causes symptoms of heartburn and acid regurgitation, is a common disease affecting modern life, despite the great achievements that have increased our understanding of the pathophysiology and treatment of the disease. Incidence of GERD has grown worldwide in recent decades, and has detrimental effects on the quality of life of sufferers. The findings from this research may help health care professionals in dealing with GERD. Consequently, BHSST, one of more safe and effective herbal formulations, may prove useful as a therapeutic to increase the quality of life of GERD patients.
This novel study demonstrated that BHSST plays a protective role against esophageal mucosal ulcer, possibly through modulation of apoptotic proteins via suppression of oxidative stress.
This study may provide a future strategy for therapeutic intervention for esophageal mucosal ulcer that is induced in CARE model rats.
The esophageal mucosal ulcer is involved in CARE. CARE leads to oxidative stress. But suppression of oxidative stress can affect apoptosis in the esophagus. As the result, BHSST administration exerts a protective effect through the inhibition of reactive oxygen species.
This is a well written and planned study demonstrating the protective effects of BHSST on a rat CARE model system. The protective effects of BHSST seem to arise from regulation of reactive oxygen species-dependent apoptosis. The results of this study are very interesting and good.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Dong H, Guo XZ, Teramoto-Matsubara OT S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst Rev. 2014;CD008550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Darvishmoghadam S, Zahedi MJ, Hayatbakhsh Abbasi MM, Haghdoost AA, Khalilyzade M, Karimi Goughari E. Review of Clinical Spectrum of Gastroesophageal Reflux Disease in a General Population; A Study from South-East Iran. Middle East J Dig Dis. 2016;8:310-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Flook N, Jones R, Vakil N. Approach to gastroesophageal reflux disease in primary care: Putting the Montreal definition into practice. Can Fam Physician. 2008;54:701-705. [PubMed] |
| 4. | Watson TJ, Peters JH. Lower esophageal sphincter injections for the treatment of gastroesophageal reflux disease. Thorac Surg Clin. 2005;15:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 6. | Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol. 2015;80:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Kim HJ, Hahm KB. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett’s esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001;480-481:189-200. [PubMed] |
| 8. | Biswas SK. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid Med Cell Longev. 2016;2016:5698931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 753] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 9. | Ribeiro AR, Diniz PB, Pinheiro MS, Albuquerque-Júnior RL, Thomazzi SM. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem Biol Interact. 2016;244:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Kim A, Park WY, Shin N, Lee HJ, Kim YK, Lee SJ, Hwang CS, Park DY, Kim GH, Lee BE. Cardiac mucosa at the gastroesophageal junction: An Eastern perspective. World J Gastroenterol. 2015;21:9126-9133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Maes S, Sharma P, Bisschops R. Review: Surveillance of patients with Barrett oesophagus. Best Pract Res Clin Gastroenterol. 2016;30:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Bang JK. A study on the relationship between the symptom of Shanghanlon and the defensive Gi. J Korean Med Classics. 2016;29:151-163. |
| 13. | Park JW, Ryu B, Yeo I, Jerng UM, Han G, Oh S, Lee J, Kim J. Banha-sasim-tang as an herbal formula for the treatment of functional dyspepsia: a randomized, double-blind, placebo-controlled, two-center trial. Trials. 2010;11:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Chen FP, Chen FJ, Jong MS, Tsai HL, Wang JR, Hwang SJ. Modern use of Chinese herbal formulae from Shang-Han Lun. Chin Med J (Engl). 2009;122:1889-1894. [PubMed] |
| 15. | Lee KG, Cui X, Lim JP. Effect of the concurrent administration of Banhasasim-tang with cimetidine on gastric ulcer in rats. Korean J Orient Physiol Pathol. 2002;16:572-576. |
| 16. | Lee JS, Yoon SHm Kim JS, Ryu BH. Effect of Banhasasimtang granule on gastric emptying in rats. Korean J Orient Int Med. 2006;27:471-479. |
| 17. | Omura N, Kashiwagi H, Chen G, Suzuki Y, Yano F, Aoki T. Establishment of surgically induced chronic acid reflux esophagitis in rats. Scand J Gastroenterol. 1999;34:948-953. [PubMed] |
| 18. | Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637-648. [PubMed] |
| 19. | Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271-278. [PubMed] |
| 20. | Komatsu S. Extraction of nuclear proteins. Methods Mol Biol. 2007;355:73-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Zikri NN, Riedl KM, Wang LS, Lechner J, Schwartz SJ, Stoner GD. Black raspberry components inhibit proliferation, induce apoptosis, and modulate gene expression in rat esophageal epithelial cells. Nutr Cancer. 2009;61:816-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Ling W, Huang Y, Xu JH, Li Y, Huang YM, Ling HB, Sui Y, Zhao HL. Consistent Efficacy of Wendan Decoction for the Treatment of Digestive Reflux Disorders. Am J Chin Med. 2015;43:893-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Cai Y, Wang L, Liu H, Wang X. Optimization of processing technology of Rhizoma Pinelliae Praeparatum and its anti-tumor effect. Afr Health Sci. 2015;15:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Chen JJ, Huang CC, Chang HY, Li PY, Liang YC, Deng JS, Huang SS, Huang GJ. Scutellaria baicalensis Ameliorates Acute Lung Injury by Suppressing Inflammation In Vitro and In Vivo. Am J Chin Med. 2017;45:137-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Li C, Tian ZN, Cai JP, Chen KX, Zhang B, Feng MY, Shi QT, Li R, Qin Y, Geng JS. Panax ginseng polysaccharide induces apoptosis by targeting Twist/AKR1C2/NF-1 pathway in human gastric cancer. Carbohydr Polym. 2014;102:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Zhang Y, Luo H, Liu K, Jia H, Chen Y, Wang Z. Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Sci. 2015;105:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Danwilai K, Konmun J, Sripanidkulchai B, Subongkot S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: a pilot study. Cancer Manag Res. 2017;9:11-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Choo BK, Roh SS. Berberine protects against esophageal mucosal damage in reflux esophagitis by suppressing proinflammatory cytokines. Exp Ther Med. 2013;6:663-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Anantachoke N, Lomarat P, Praserttirachai W, Khammanit R, Mangmool S. Thai Fruits Exhibit Antioxidant Activity and Induction of Antioxidant Enzymes in HEK-293 Cells. Evid Based Complement Alternat Med. 2016;2016:6083136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Seo CS, Shin HK. Quantitative Determination of the Thirteen Marker Components in Banhasasim-Tang Decoction Using an Ultra-Performance Liquid Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. Korean J Pharmacogn. 2016;47:62-72. |
| 31. | Lee KG, Cui X. Lim JP. Effect of the concurrent administration of Banhasasim-tang with cimetidine on gastric ulcer in rats. Korean J Orient Physiol Pathol. 2002;16:572-576. |
| 32. | Jin SE, Lim HS, Kim Y, Seo CS, Yoo SR, Shin HK, Jeong SJ. Traditional Herbal Formula Banhasasim-tang Exerts Anti-Inflammatory Effects in RAW 264.7 Macrophages and HaCaT Keratinocytes. Evid Based Complement Alternat Med. 2015;2015:728380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Wei JJ, Tang DP, Xie JJ, Yang LY, Zhuang ZH. Decreased n-6/n-3 polyunsaturated fatty acid ratio reduces chronic reflux esophagitis in rats. Prostaglandins Leukot Essent Fatty Acids. 2016;112:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Kwon OJ, Kim MY, Shin SH, Lee AR, Lee JY, Seo BI, Shin MR, Choi HG, Kim JA, Min BS. Antioxidant and Anti-Inflammatory Effects of Rhei Rhizoma and Coptidis Rhizoma Mixture on Reflux Esophagitis in Rats. Evid Based Complement Alternat Med. 2016;2016:2052180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Liu G, Han X, Liu J, Li GX, Zou DW, Li ZS. Inhibition of p38 MAPK activation attenuates esophageal mucosal damage in a chronic model of reflux esophagitis. Neurogastroenterol Motil. 2015;27:1648-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305-1308. [PubMed] |
| 37. | Serrano-García N, Pedraza-Chaverri J, Mares-Sámano JJ, Orozco-Ibarra M, Cruz-Salgado A, Jiménez-Anguiano A, Sotelo J, Trejo-Solís C. Antiapoptotic Effects of EGb 761. Evid Based Complement Alternat Med. 2013;2013:495703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Zimmermann KC, Bonzon C, Green DR. The machinery of programmed cell death. Pharmacol Ther. 2001;92:57-70. [PubMed] |
| 39. | Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4609] [Cited by in RCA: 5127] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 40. | Si J, Fu X, Behar J, Wands J, Beer DG, Souza RF, Spechler SJ, Lambeth D, Cao W. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett’s esophageal adenocarcinoma cells. J Biol Chem. 2007;282:16244-16255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Wang T, Arifoglu P, Ronai Z, Tew KD. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J Biol Chem. 2001;276:20999-21003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 238] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Magenta A, Greco S, Gaetano C, Martelli F. Oxidative stress and microRNAs in vascular diseases. Int J Mol Sci. 2013;14:17319-17346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2713] [Article Influence: 77.5] [Reference Citation Analysis (1)] |