Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4559
Peer-review started: March 15, 2017
First decision: March 30, 2017
Revised: April 4, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: July 7, 2017
Processing time: 117 Days and 23 Hours
To evaluate the anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium (DSS)-induced colitis model.
An acute colitis mouse model was induced by oral administration of 5% DSS in the drinking water for 7 d. In the treated group, rosuvastatin (0.3 mg/kg per day) was administered orally before and after DSS administration for 21 d. On day 21, mice were sacrificed and the colons were removed for macroscopic examination, histology, and Western blot analysis. In the in vitro study, IEC-6 cells were stimulated with 50 ng/mL tumor necrosis factor (TNF)-α and then treated with or without rosuvastatin (2 μmol/L). The levels of reactive oxygen species (ROS), inflammatory mediators, and apoptotic markers were measured.
In DSS-induced colitis mice, rosuvastatin treatment significantly reduced the disease activity index and histological damage score compared to untreated mice (P < 0.05). Rosuvastatin also attenuated the DSS-induced increase of 8-hydroxy-2’-deoxyguanosine and NADPH oxidase-1 expression in colon tissue. Multiplex ELISA analysis revealed that rosuvastatin treatment reduced the DSS-induced increase of serum IL-2, IL-4, IL-5, IL-6, IL-12 and IL-17, and G-CSF levels. The increased levels of cleaved caspase-3, caspase-7, and poly (ADP-ribose) polymerase in the DSS group were attenuated by rosuvastatin treatment. In vitro, rosuvastatin significantly reduced the production of ROS, inflammatory mediators and apoptotic markers in TNF-α-treated IEC-6 cells (P < 0.05).
Rosuvastatin had the antioxidant, anti-inflammatory and anti-apoptotic effects in DSS-induced colitis model. Therefore, it might be a candidate anti-inflammatory drug in patients with inflammatory bowel disease.
Core tip: Oxidative stress in the intestinal tract is considered a major factor that contributes to the pathogenesis and progression of inflammatory bowel disease (IBD). We report that rosuvastatin has the antioxidant, anti-inflammatory and anti-apoptotic effects in dextran sulfate sodium (DSS)-induced colitis mice. We assume the possibility of anti-inflammatory effects of rosuvastatin through the regulation of oxidative stress, and first describe the anti-apoptotic effects of rosuvastatin in a DSS-induced colitis model. Therefore, rosuvastatin might be a candidate anti-inflammatory drug in patients with IBD.
- Citation: Shin SK, Cho JH, Kim EJ, Kim EK, Park DK, Kwon KA, Chung JW, Kim KO, Kim YJ. Anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium-induced colitis model. World J Gastroenterol 2017; 23(25): 4559-4568
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4559
Inflammatory bowel disease (IBD) encompasses a range of intestinal diseases, including two major disorders: ulcerative colitis (UC) and Crohn’s disease (CD). IBD is characterized by a chronic or relapsing inflammatory condition within the gastrointestinal tract. In recent years, several studies have focused on colonic inflammation and oxidative stress, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) as one of the major mechanisms involved in the pathophysiology of IBD[1,2]. Inhibition of both inflammatory mediators and ROS production could be an important protective and therapeutic treatment for IBD. Many drugs have been used to prevent inflammation and mutagenesis in patients with IBD[3]. However, until now, only a couple of drugs were commercially available to control IBD. In animal studies, antioxidants such as S-adenosylmethionine, green tea polyphenols, and 2(R,S)-n-propylthiazolidine-4(R)-carboxylic acid attenuated dextran sulfate sodium (DSS)-induced colitis, and exogenous 8-hydroxy-2′-deoxyguanosine paradoxically blocked Rac1 activation and subsequent nitrogen oxide (NO) inactivation in DSS-induced colitis and inflammation-associated carcinogenesis models[4-6]. In our previous studies, pantoprazole significantly reduced oxidative stress and the degree of colon inflammation through suppression of tumor necrosis factor-alpha (TNF-α) and NO in a DSS-induced colitis mouse model, and infliximab was suggested as a preventive drug in a colitis-associated carcinogenesis model[7,8]. Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in the mevalonate pathway, and have long been used to lower cholesterol levels through inhibition of this pathway. Recently, their anti-inflammatory and endothelial cell protective actions, independent of their anti-hyperlipidemic effects were suggested[9]. In addition, a previous report demonstrated that rosuvastatin may be effective for preventing the development of DSS-induced colitis in mice via inhibition of mucosal inflammatory responses associated with the preservation of endothelial NO synthase transcription[10].
However, the precise mechanism of anti-inflammatory effects by rosuvastatin in DSS-induced colitis is still unclear, and few studies have described the anti-apoptotic effects of rosuvastatin in DSS-induced colitis. We describe the antioxidant, anti-inflammatory and anti-apoptotic effects of rosuvastatin in a DSS-induced colitis model.
Rosuvastatin was obtained from Sigma-Aldrich (St. Louis, MO, United States) and 2’,7’-dichlorofluorescein diacetate (DCFH-DA) was purchased from Molecular Probes (Eugene, OR, United States). Antibodies to cyclooxygenase (COX)-2 (#12282), cleaved caspase-3 (#9664), caspase-7 (#8438) and poly (ADP-ribose) polymerase (PARP) (#5625) were purchased from Cell Signaling Technology (Beverly, MA, United States). β-actin (LF-PA0207) antibodies were obtained from Ab Frontier (Seoul, South Korea).
Seven-week-old SPF male C57BL/6 mice (Orient Bio, Sungnam, Korea) were used for the experiments. A total of 15 mice were divided into three groups, with five mice per group. In the untreated control group, mice did not receive DSS or rosuvastatin. In the DSS (molecular weight of 35-50 kDa; MP Biomedicals) group, mice were given drinking water containing 5% DSS for one week. In the rosuvastatin treatment group, rosuvastatin (0.3 mg/kg per day) was administered orally for 21 d (before 7 d and after 7 d of DSS administration). On day 21, all of the mice were sacrificed and their colons were removed, measured, opened longitudinally, and rinsed with ice-cold phosphate-buffered saline (PBS). For histological examination, the isolated tissues were stained with hematoxylin and eosin (H&E), and for immunofluorescence, tissues were subjected to immunohistochemistry staining. Tissue samples were also processed for immunoblotting.
We evaluated colitis severity using a histologic damage score and a disease activity index (DAI) score. The histologic damage score was assessed in tissues by H&E staining and was obtained by evaluating crypt architecture, inflammatory cell infiltration, goblet cell depletion, and crypt abscess. The histologic damage score consists of four grades of crypt architecture (0, normal; 1, distortion up to 1/3; 2, distortion up to 2/3; and 3, complete loss), four grades of inflammatory cell infiltration (0, normal; 1, mild; 2, moderated; and 3, dense), two grades of goblet cell depletion (0, absent; and 1, present), and two grades of crypt abscess (0, absent; and 1, present). These grades are combined to calculate the histologic damage score[11]. The DAI consists of scores for weight change, gross bleeding, and stool consistency. There are five grades of weight loss (0, no loss or weight gain; 1, 1%-10% loss; 2, 10%-15% loss; 3, 15%-20% loss; and 4, > 20% loss), two grades of stool consistency (0, normal; and 4, diarrhea), and two grades of gross bleeding (0, normal; and 4, gross bleeding). The combined scores were used to calculate the DAI after 1, 2, and 3 wk of DSS administration. After determining the DAI, the mice were sacrificed by cervical dislocation on day 21, and the colons were resected between the ileocecal junction and the proximal rectum, close to its passage under the pelvisternum. The colons were placed on a nonabsorbent surface and measured with a ruler. The entire colon was divided into three segments (proximal, middle, and distal), and a part of each segment was fixed in 10% neutral buffered formalin. After fixation, the specimens were embedded in paraffin, divided into 7 μm sections, and stained with H&E.
Sections (3-10 μm) were deparaffinized in xylene and rehydrated through a graded ethanol series. Antigen retrieval was achieved by immersing the sections in 10 mmol/L sodium citrate buffer (pH 6.0), heating to 95 °C for 10 min, and cooling in cold water for 30 min. Slides were treated with 3% hydrogen peroxidase for 10 min to block endogenous peroxidase, rinsed in PBS for 5 min, and incubated with a mouse monoclonal antibody to 8-hydroxydeoxyguanosine (8-OHdG) (ab48508; Abcam, Cambridge, MA, United States) at 4 °C overnight. Immunohistochemical assays were performed using a Dako REAL EnVision Detection system (Peroxidase/DAB+) (K500711, DaKo, Denmark). Nuclei were counterstained with hematoxylin.
Immunofluorescence assays were performed using anti-NADPH oxidase (NOX)-1 (NBP1-31546; Novus Biologicals, Canada) and anti-NOX2 (ab80508; abcam, Cambridge, MA, United States) antibodies. The nuclei were stained with propidium iodide (Invitrogen, Carlsbad, CA, United States) and images were acquired using a Zeiss LSM 700 confocal microscope.
The concentrations of serum cytokines (IL-2, IL-3, IL-4, IL-5, IL-6, IL-12 and IL-17, MCP-1 and GM-CSF) were quantified using a Mouse Cytokine Array Kit (110951MS, Quansys Biosciences, UT, United States). Serum samples were diluted 1:2 with the supplied diluents and run in duplicate according to the manufacturer’s protocol.
The normal rat intestinal epithelial cell line IEC-6 was purchased from the Korean Cell Lines Bank (KCLB, Seoul, South Korea) and cultured in DMEM medium (Hyclon, NY, United States) supplemented with 10% fetal bovine serum (GIBCO, Grand Island, NY, United States) and 1% antibiotic-antimycotic in a humidified 5% CO2 atmosphere.
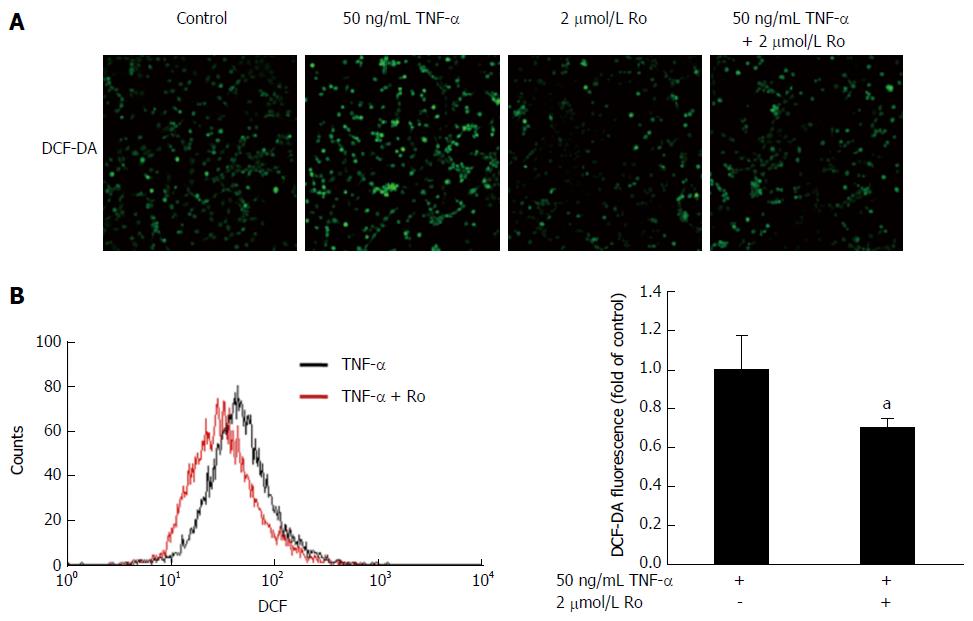
Levels of ROS were detected using DCF-DA, which measures the production of hydrogen peroxide and superoxide anion. IEC-6 cells were stimulated with 50 ng/mL TNF-α or 2 μmol/L rosuvastatin for 20 min. Cells were incubated with 25 μmol/L DCF-DA in the dark for 40 min. DCF fluorescence was detected by confocal microscopy and the fluorescence intensity was measured using a FACSCalibur apparatus (Beckton Dickinson, San Jose, CA, United States).
RNA was isolated using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). To remove genomic DNA, the extracted total RNA was treated with DNase I (New England BioLabs, Ipswich, MA, United States). A total of 1 μg purified total RNA was reverse transcribed by one-step RT-PCR using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States). For relative mRNA quantification, the reactions were tested using TaKaRa SYBR Premix Ex taq II (TaKaRa, Japan), and the PCR reactions were run on an iCycler (Bio-Rad, Hercules, CA, United States). The following primer pairs were used: mIL-1beta, 5’-TACCTATGTCTTGCCCGTGGAG-3’ and 5’-ATCATCCCACGAGTCACAGAGG-3’; mIL-8, 5’-CATTAATATTTAACGATGTGGATGCGTTTCA-3’ and 5’-GCCTACCATCTTTAAACTGCACAAT-3’; COX-2, 5’-TGATCGAAGACTACGTGCAACA-3’ and 5’-AAAAGCAGCTCTGGGTCGAA-3’; and GAPDH, 5’-CTCCCATTCTTCCACCTTTG-3’ and 5’-ATGTAGGCCATGAGGTCCAC-3’. GAPDH was amplified as a reference.
Harvested cells and mice tissue were lysed in cold RIPA lysis buffer (0.5 mol/L Tris-HCl, pH 7.4, 1.5 mol/L NaCl, 2.5% deoxycholic acid, 10% NP-40, and 10 mmol/L EDTA) containing protease and phosphatase inhibitors (GenDEPOT, San Jose, CA, United States). Cell lysates were subjected to SDS-PAGE and then transferred to a nitrocellulose membrane. After blocking in 5% bovine serum albumin, the membrane was probed with anti-COX-2, anti-poly ADP-ribose polymerase (PARP), anti-cleaved caspase-7, and anti-cleaved caspase-3 antibodies, followed by incubation with a secondary antibody conjugated to horseradish peroxidase. Then the membranes then stripped and reprobed with an anti-β-actin antibody.
Animals were handled in an accredited animal facility in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines under the Center of Animal Care and Use facility of the Gachon University Lee Gil Ya Cancer and Diabetes Institute (LCDI-2015-0044).
The data are presented as mean ± SD. Statistical significance of the difference between experimental groups was assessed using the two-tailed Student’s t-test, with a P < 0.05 considered statistically significant.
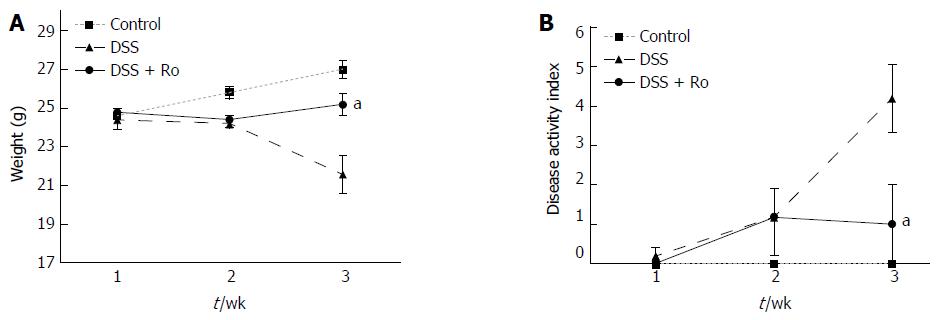
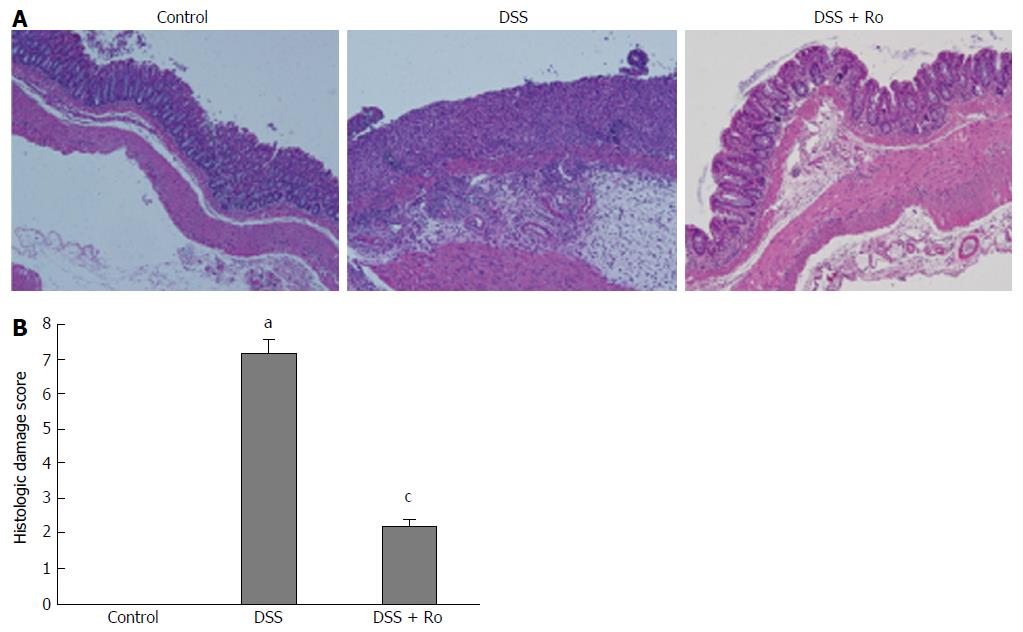
The mean colon length (89 ± 4.2 mm vs 100.4 ± 17.8 mm) was lower in the DSS-induced colitis group than in the control group. Although the decrease in colon length after DSS administration (89.0 ± 4.2 mm vs 97.0 ± 9.1 mm, P = 0.127) was reversed by rosuvastatin, the difference was not statistically significant. Significant weight loss was observed in the DSS-induced colitis group compared to the control group, and the rosuvastatin group showed less weight loss than the DSS-induced colitis group (21.6 ± 2.2 g vs 25.2 ± 1.3 g, P = 0.018) (Figure 1A). On day 21, the DAI score in the DSS-induced colitis group was higher than that in the control group and was significantly lower in the rosuvastatin-treated group (4.2 ± 1.9 vs 1.0 ± 2.2, P = 0.041) (Figure 1B). H&E staining showed that DSS administration distorted glandular formation and led to the recruitment of inflammatory cells into the submucosal layer, leading to mucosal destruction. On the other hand, rosuvastatin treatment significantly attenuated these pathologic changes (Figure 2A). In the DSS-induced colitis group, the histologic damage score on day 21 was higher than that in the control group and was significantly decreased in the rosuvastatin-treated group (7.2 ± 0.4 vs 2.2 ± 0.2, P < 0.001) (Figure 2B).
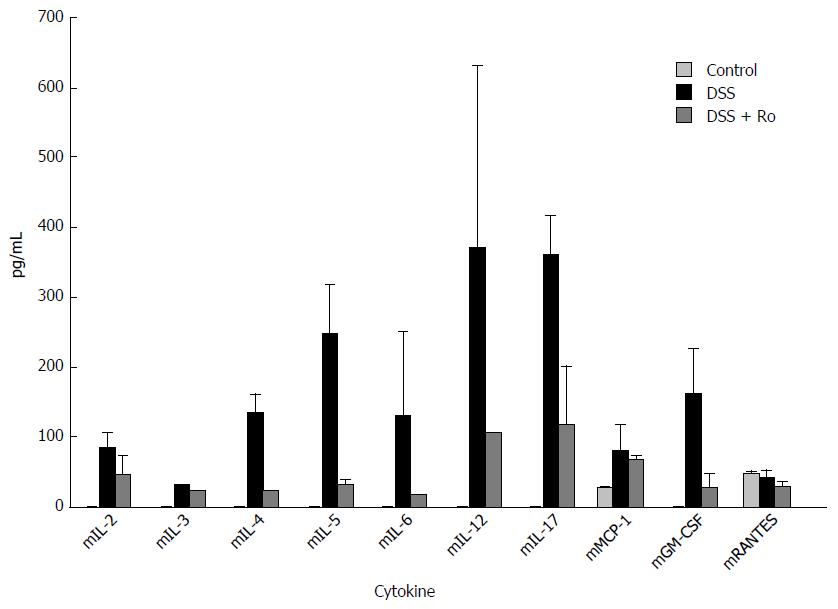
After 7 d of 5% DSS ingestion, serum levels of IL-2, IL-4, IL-5, IL-6, IL-12 and IL-17, and G-CSF were markedly decreased in the rosuvastatin-treated group. However, serum levels of IL-3, mMCP-1, and mRANTES levels were not markedly different (Figure 3).
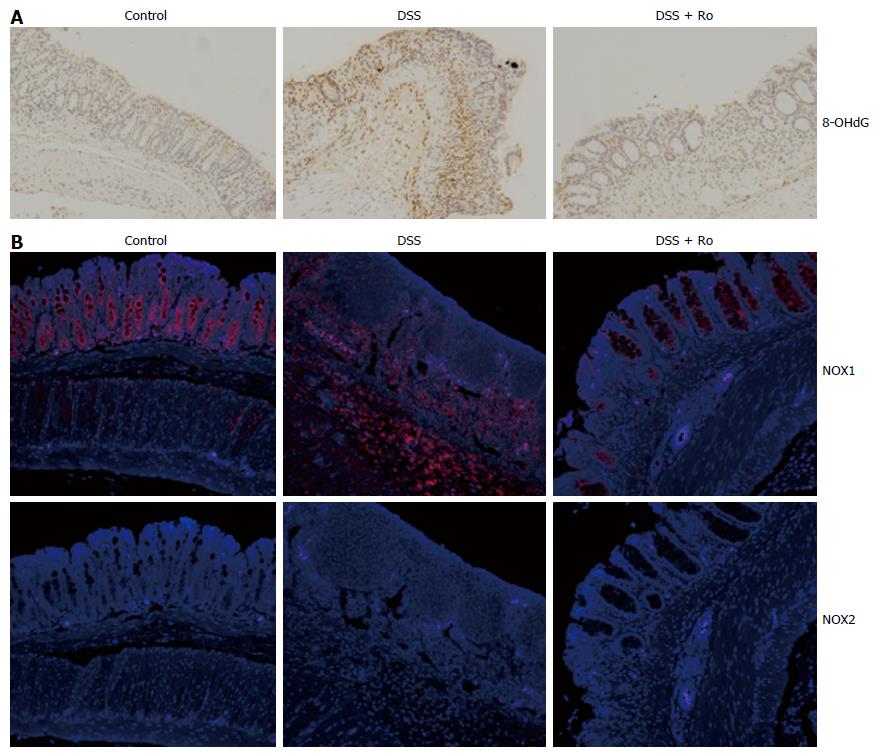
It is well known that oxidative stress leads to the accumulation of 8-OHdG in colon tissue. To evaluate the antioxidant effects of rosuvastatin in a DSS-induced colitis model, we analyzed the expression of 8-OHdG by immunohistochemistry. In the DSS-induced colitis group, 8-OHdG expression was increased compared to the control group and rosuvastatin treatment attenuated the 8-OHdG expression in colon tissue (Figure 4A). To assess ROS production via NOX, we measured NOX1 expression in colon tissue from the DSS-induced colitis group and found that NOX1 expression was increased compared to the control group. In the rosuvastatin-treated group, NOX1 expression in colon tissue was attenuated. However, NOX2 expression in the DSS-induced colitis group was not different compared to the control group (Figure 4B). To evaluate the antioxidant effects of rosuvastatin, ROS production was measured by confocal microscopy and flow cytometry. As shown in Figure 5A, DCF fluorescence intensity was higher in TNF-α-treated IEC-6 cells than in control cells. TNF-α induced an increase in DCF fluorescence intensity that was suppressed in IEC-6 cells stimulated with 2 μmol/L rosuvastatin for 20 min. In TNF-α-treated IEC-6 cells, rosuvastatin significantly decreased ROS levels, as measured by DCF fluorescence using fluorescence-activated cell sorting (FACS) analysis (Figure 5B).
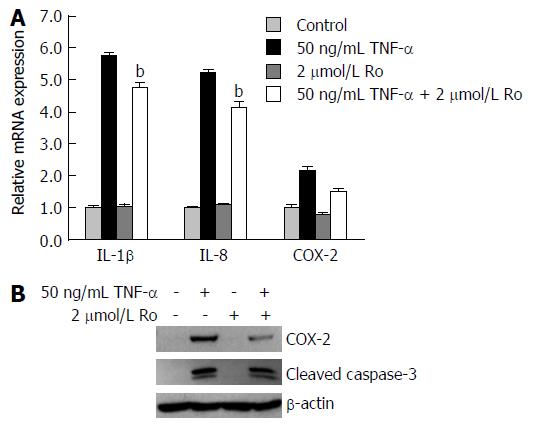
To explore whether rosuvastatin can modulate the inflammatory process, real-time quantitative RT-PCR (qRT-PCR) was used to measure mRNA levels of IL-1β, IL-8, and COX-2 in IEC-6 cells. Western blotting was also used to measure cleaved caspase-3 and COX-2 protein levels. The mRNA levels of IL-1β, IL-8, and COX-2 were all increased compared to the control, and were suppressed by rosuvastatin treatment (Figure 6A). The protein levels of COX-2 were increased in TNF-α-treated IEC-6 cells and were suppressed in rosuvastatin-treated cells (Figure 6B).
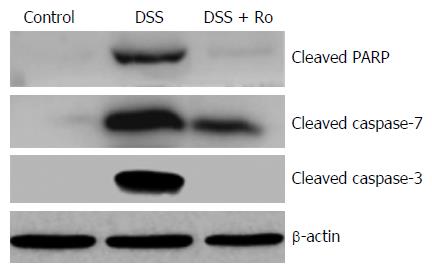
To evaluate the effects of rosuvastatin on apoptosis, protein levels of cleaved caspase-3, caspase-7, and PARP were evaluated by Western blotting in DSS-induced colitis group and in IEC-6 cells treated with 50 ng/mL TNF-α. Increased levels of cleaved caspase-3, caspase-7, and PARP in the DSS-induced colitis group were attenuated by rosuvastatin treatment (Figure 7). An increase in cleaved caspase-3 in TNF-α-treated IEC-6 cells was attenuated by rosuvastatin treatment (Figure 6B).
DSS is a chemical colitogen with anticoagulant properties that induces damage in the epithelial monolayer lining in the intestine, leading to the dissemination of pro-inflammatory intestinal contents including ROS[12]. A previous report showed that a mouse model of DSS-induced colitis had neutrophil accumulation and increased expression of TNF-α in the colon[10]. DSS-induced colitis models are commonly used to study for IBD. DSS induces bloody diarrhea, weight loss, shortening of the colon, and mucosal ulceration in mice[12]. Previously, Naito et al[10] found that in DSS-treated mice, histological findings and DAI scores, as determined by weight loss, stool consistency, and blood in the stool, were improved in rosuvastatin-treated mice. In our study, rosuvastatin treatment also ameliorated DSS-induced colonic injury as assessed by the DAI and the histologic damage score.
Oxidative stress in the intestinal tract is considered a major factor that contributes to the pathogenesis and progression of IBD, and is supported by recent data that suggests a positive correlation between upregulated NOX expression and gastrointestinal inflammation[13]. NOX has a dedicated function of generating reactive oxygen. Previous studies have shown that the epithelial NOX homologs, NOX1 and DUOX2, generate a higher level of superoxide in the colon compared to phagocyte NOX2[14]. In particular, a previous report showed that NOX1 was highly expressed in human colon epithelial cells, and lymphocytes within lesions of CD and UC had high levels of NOX1 expression[15]. Another report showed that NOX1 expression was increased in the colon of mice treated by TNF-α, a pro-inflammatory cytokine that is increased in the mucosa of IBD patients and mouse models of DSS-induced colitis[16,17]. In addition, it is well known that oxidative stress leads to the accumulation of products that cause oxidative damage such as 8-OHdG in colon tissue. In this study, expression levels of NOX1 and 8-OHdG in colon tissue from DSS-induced colitis mice were higher than those in control mice. Furthermore, ROS production was increased in TNF-α-treated IEC-6 cells.
Previous reports have demonstrated the anti-inflammatory effects of statins in colitis models. Maheshwari et al[18] reported that simvastatin or rosuvastatin led to a significant reduction in oxidative stress levels by virtue of increasing levels of superoxide dismutase (SOD) and glutathione (GSH), and decreasing levels of malondialdehyde (MDA) in a trinitrobenzene sulfonic acid-induced colitis rat model. Naito et al[10] suggested that rosuvastatin-induced TNF-α inhibition and eNOS transcription upregulation was accompanied by significant suppression of intestinal inflammation. However, few studies have investigated whether statins can suppress NOX-dependent ROS production in DSS-induced colitis models. Recently, it was suggested that statins reduced NOX-derived ROS in the vascular wall that was mediated by mevalonate-reversible inhibition of isoprenoid formation and membrane translocation of the small GTP protein Rac1[19]. In this study, we confirmed that in DSS-treated mice, the expression levels of NOX1 and 8-OHdG in colon tissue, and the production of TNF-α-induced ROS, were suppressed by rosuvastatin treatment.
The pro-inflammatory enzyme, COX-2, is overexpressed in the large intestinal epithelium in active human IBD, and the expression of COX-2 can be induced by the cytokines such as IL-1β and IL-8[20,21]. In our in vitro study, rosuvastatin treatment reduced the expression of pro-inflammatory cytokines such as IL-1β, IL-8, and COX-2, and attenuated serum levels of pro-inflammatory cytokines such as IL-2, IL-4, IL-5, IL-6, IL-12 and IL-17, and G-CSF in DSS-treated mice.
High levels of apoptosis are observed in the intestinal epithelium of IBD patients, and in UC, ROS are thought to play an important role in inducing epithelial cell apoptosis[22]. Cell death mechanisms have been associated with the development of IBD in humans and mice. The activation of extrinsic (mediated by caspase-8) and intrinsic (mediated by caspase-9) pathways leads to the activation of caspase-3, caspase-6, and caspase-7, which leads to the cleavage of other proteins[23]. One of the essential substrates cleaved by both caspase-3 and caspase-7 is PARP, an abundant DNA-binding enzyme that detects and signals DNA strand breaks[24]. PARP has been implicated in intestinal barrier dysfunction in the development of IBD[25]. In our study, rosuvastatin inhibited the apoptotic pathway by activating caspase-3 in TNF-α-treated IEC-6 cells and activating caspase-3, caspase-7, and PARP in DSS-treated mice.
In conclusion, we assume the possibility of anti-inflammatory effects of rosuvastatin through the regulation of oxidative stress, and first describe the anti-apoptotic effects of rosuvastatin in a DSS-induced colitis model. These results show that rosuvastatin may be a potential anti-inflammatory drug for the treatment of IBD.
Oxidative stress in the intestinal tract is considered a major factor that contributes to the pathogenesis and progression of inflammatory bowel disease (IBD). Recently, anti-inflammatory actions of statins, independent of their anti-hyperlipidemic effects were suggested. The authors investigated the anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium (DSS)-induced colitis model.
The precise mechanism of anti-inflammatory effects by rosuvastatin in DSS-induced colitis is still unclear, and few studies have described the anti-apoptotic effects of rosuvastatin in DSS-induced colitis.
This study demonstrates the possibility of anti-inflammatory effects of rosuvastatin through the regulation of oxidative stress, and is the first to describe the anti-apoptotic effects of rosuvastatin in a DSS-induced colitis model.
Rosuvastatin might be a candidate anti-inflammatory drug in patients with IBD.
Oxidative stress in the intestinal tract is considered a major factor that contributes to the pathogenesis and progression of IBD, and is supported by recent data that suggests a positive correlation between upregulated NADPH oxidase (NOX) expression and gastrointestinal inflammation.
Authors tested a hypothesis of presence of protective effect of rosuvastatin in animal DSS-induced colitis model and also performed a IEC6 cell line model of another in vitro study. The study showed promising results about protective effects of rosuvastatin by reduction of oxidative stress, reduced apoptosis and decreased inflammation. The study is interesting and could possibly to further investigations for a possible treatment modality.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Harmanci O S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Wendland BE, Aghdassi E, Tam C, Carrrier J, Steinhart AH, Wolman SL, Baron D, Allard JP. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr. 2001;74:259-264. [PubMed] |
| 2. | Damiani CR, Benetton CA, Stoffel C, Bardini KC, Cardoso VH, Di Giunta G, Pinho RA, Dal-Pizzol F, Streck EL. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol. 2007;22:1846-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Park DI. Current status of biosimilars in the treatment of inflammatory bowel diseases. Intest Res. 2016;14:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Oz HS, Chen TS, McClain CJ, de Villiers WJ. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Myoung DS, Park YL, Joo SY, Myung E, Chung CY, Park HC, Kim JS, Cho SB, Lee WS, Kim HS. Epigallocatechin-3-gallate Inhibits the expression of adhesion molecules by blocking nuclear factor kappa B signaling in intestinal epithelial cells. Intest Res. 2013;11:261-267. |
| 6. | Ock CY, Kim EH, Hong H, Hong KS, Han YM, Choi KS, Hahm KB, Chung MH. Prevention of colitis-associated colorectal cancer with 8-hydroxydeoxyguanosine. Cancer Prev Res (Phila). 2011;4:1507-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention of colitis-induced colorectal carcinogenesis beyond acid suppression. Cancer Prev Res (Phila). 2010;3:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 2010;3:1314-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Oda H, Keane WF. Recent advances in statins and the kidney. Kidney Int Suppl. 1999;71:S2-S5. [PubMed] |
| 10. | Naito Y, Katada K, Takagi T, Tsuboi H, Isozaki Y, Handa O, Kokura S, Yoshida N, Ichikawa H, Yoshikawa T. Rosuvastatin, a new HMG-CoA reductase inhibitor, reduces the colonic inflammatory response in dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:997-1004. [PubMed] |
| 11. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 12. | Oh SY, Cho KA, Kang JL, Kim KH, Woo SY. Comparison of experimental mouse models of inflammatory bowel disease. Int J Mol Med. 2014;33:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Lam G, Apostolopoulos V, Zulli A, Nurgali K. NADPH oxidases and inflammatory bowel disease. Curr Med Chem. 2015;22:2100-2109. [PubMed] |
| 14. | Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245-313. [PubMed] |
| 15. | Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705-1709. [PubMed] |
| 17. | Mouzaoui S, Djerdjouri B, Makhezer N, Kroviarski Y, El-Benna J, Dang PM. Tumor necrosis factor-α-induced colitis increases NADPH oxidase 1 expression, oxidative stress, and neutrophil recruitment in the colon: preventive effect of apocynin. Mediators Inflamm. 2014;2014:312484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Maheshwari RA, Balaraman R, Sailor GU, Sen DB. Protective effect of simvastatin and rosuvastatin on trinitrobenzene sulfonic acid-induced colitis in rats. Indian J Pharmacol. 2015;47:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Antoniades C, Bakogiannis C, Tousoulis D, Reilly S, Zhang MH, Paschalis A, Antonopoulos AS, Demosthenous M, Miliou A, Psarros C. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation. 2010;122:S66-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297-306. [PubMed] |
| 21. | Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3011] [Cited by in RCA: 3171] [Article Influence: 132.1] [Reference Citation Analysis (0)] |
| 22. | Souza HS, Tortori CJ, Castelo-Branco MT, Carvalho AT, Margallo VS, Delgado CF, Dines I, Elia CC. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways. Int J Colorectal Dis. 2005;20:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Nunes T, Bernardazzi C, de Souza HS. Cell death and inflammatory bowel diseases: apoptosis, necrosis, and autophagy in the intestinal epithelium. Biomed Res Int. 2014;2014:218493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002;3:275-283. [PubMed] |
| 25. | Zingarelli B, Hake PW, Burroughs TJ, Piraino G, O’connor M, Denenberg A. Activator protein-1 signalling pathway and apoptosis are modulated by poly(ADP-ribose) polymerase-1 in experimental colitis. Immunology. 2004;113:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (1)] |