Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4508
Peer-review started: February 1, 2017
First decision: March 3, 2017
Revised: May 18, 2017
Accepted: June 12, 2017
Article in press: June 12, 2017
Published online: July 7, 2017
Processing time: 164 Days and 2.5 Hours
To investigate the hypothesis that treatment with dimethyl fumarate (DMF) may ameliorate liver ischemia/reperfusion injury (I/RI).
Rats were divided into 3 groups: sham, control (CTL), and DMF. DMF (25 mg/kg, twice/d) was orally administered for 2 d before the procedure. The CTL and DMF rats were subjected to ischemia for 1 h and reperfusion for 2 h. The serum alanine aminotransferase (ALT) and malondialdehyde (MDA) levels, adenosine triphosphate (ATP), NO × metabolites, anti-oxidant enzyme expression level, anti-inflammatory effect, and anti-apoptotic effect were determined.
Histological tissue damage was significantly reduced in the DMF group (Suzuki scores: sham: 0 ± 0; CTL: 9.3 ± 0.5; DMF: 2.5 ± 1.2; sham vs CTL, P < 0.0001; CTL vs DMF, P < 0.0001). This effect was associated with significantly lower serum ALT (DMF 5026 ± 2305 U/L vs CTL 10592 ± 1152 U/L, P = 0.04) and MDA (DMF 18.2 ± 1.4 μmol/L vs CTL 26.0 ± 1.0 μmol/L, P = 0.0009). DMF effectively improved the ATP content (DMF 20.3 ± 0.4 nmol/mg vs CTL 18.3 ± 0.6 nmol/mg, P = 0.02), myeloperoxidase activity (DMF 7.8 ± 0.4 mU/mL vs CTL 6.0 ± 0.5 mU/mL, P = 0.01) and level of endothelial nitric oxide synthase expression (DMF 0.38 ± 0.05-fold vs 0.17 ± 0.06-fold, P = 0.02). The higher expression levels of anti-oxidant enzymes (catalase and glutamate-cysteine ligase modifier subunit and lower levels of key inflammatory mediators (nuclear factor-kappa B and cyclooxygenase-2 were confirmed in the DMF group.
DMF improved the liver function and the anti-oxidant and inflammation status following I/RI. Treatment with DMF could be a promising strategy in patients with liver I/RI.
Core tip: In this study, we reveal that (1) administration of dimethyl fumarate (DMF) significantly reduced tissue damage, improved liver function; and (2) DMF attenuated oxidative stress and inflammation, and raised anti-oxidant status in rats with hepatic ischemia/reperfusion injury (I/RI). These findings suggest that DMF treatment could be a promising strategy to improve clinical outcome in patients with liver I/RI.
- Citation: Takasu C, Vaziri ND, Li S, Robles L, Vo K, Takasu M, Pham C, Farzaneh SH, Shimada M, Stamos MJ, Ichii H. Treatment with dimethyl fumarate ameliorates liver ischemia/reperfusion injury. World J Gastroenterol 2017; 23(25): 4508-4516
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4508
Liver ischemia/reperfusion injury (I/RI) is a common pathologic process caused by many clinical settings, such as liver resection, liver transplantation, hypovolemic shock, and trauma[1]. Temporary clamping of the portal triad, which is a common strategy during liver surgery, produces liver I/RI[2] that may result in postoperative liver dysfunction[3]. I/R generates reactive oxygen species (ROS), which can damage lipids, proteins and tissues and lead to local inflammatory responses, endothelial and Kupffer cell activation, cytokine/chemokine release, and cell apoptosis[4]. The I/R-induced oxidative stress and tissue damage involve multiple cell signaling pathways that result in liver failure[5].
BG-12 has been approved by the Food and Drug Administration for the treatment of patients with multiple sclerosis, in whom it reduces disease activity and the progression of relapsing-remitting multiple sclerosis[6]. Dimethyl fumarate (DMF), which has a mild side effect profile, has been available for medical use for over twenty years, despite an unclear mechanism of action. Methyl fumarates were first investigated for their anti-proliferative and anti-oxidant effects; however, they quickly became repurposed for use as potent anti-psoriatic drugs in Europe[7]. We have investigated the usefulness of DMF and reported its effects on acute[8] and chronic pancreatitis[9]. However, to our knowledge, the effects of DMF on liver I/RI have not been investigated. The aim of the present study was to test the hypothesis that treatment with the potent anti-oxidant DMF may attenuate the severity of liver I/RI in experimental animals by upregulating cellular anti-oxidant and anti-inflammatory machinery in liver tissue.
Sprague-Dawley male rats weighing 230-250 g were purchased from Charles River (Wilmington, MA, United States). The rats were free of all pathogens and housed under standard conditions (room temperature: 22 °C: humidity: 50% ± 5%, 12:12 h light/dark cycle). The study was approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
The rats were randomly divided into 3 experimental groups: (1) the sham group (n = 6) was subjected to exposure of the hepatic artery, portal vein, and bile duct region but no I/RI; (2) the control (CTL) group (n = 7) was subjected to 1 hour of ischemia followed by 2 h of reperfusion. Finally; and (3) the DMF group (n = 6) received DMF (25 mg/kg, twice/d) by oral gavage 2 d prior to the operation.
Stock solutions of DMF (Sigma, St. Louis, MO, United States) were prepared in dimethyl sulfoxide (DMSO) (Hybri-MAX, St. Louis, MO, United States). Experimental rats were given oral DMF (25 mg/kg, twice/day) dissolved in methyl cellulose (Sigma, St. Louis, MO, United States). Intragastric gavage administration was conducted in conscious animals using straight gavage needles appropriate for the animal size.
Under general anesthesia using isoflurane, a midline incision was made. The left and median portal triads were occluded by a microvascular clamp to achieve 70% liver ischemia. After 1 h of clamping, the clamp was removed, and restored hepatic blood flow was confirmed visually prior to wound closure. During the surgery, the body temperature was maintained at approximately 37.5 °C with a heating blanket. Two hours post clamp removal (reperfusion time), blood samples and ischemic liver lobes were collected under deep general anesthesia for analysis, and the animals were sacrificed[10].
Histopathological analysis was performed as previously described[11]. The severity of I/RI was blindly graded using the modified Suzuki criteria[12].
Apoptosis was quantified by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) technique (Millipore, Bedford, MA, United States). For each section, 20 random fields were examined by confocal microscopy (20 × objective). An apoptotic index (i.e., the number of nuclei labeled by the TUNEL method/the total number of nuclei) was calculated[13].
Serum alanine aminotransferase (ALT) levels were determined to assess the liver function using commercial kits (BioVision, Milpitas, CA, United States) according to the manufacturer’s instructions. The serum malondialdehyde (MDA) formation assay was performed using a thiobarbituric acid reactive substances (TBARS) assay kit (Cayman, Ann Arbor, MI, United States) according to the manufacturer’s instructions.
The adenosine triphosphate (ATP) levels of liver tissue were determined using a colorimetric/fluorometric assay kit (Bio Vision, Milpitas, CA, United States) according to the manufacturer’s instructions. We used 20 mg of liver tissue for the assay and calculated the ATP content. Protein concentration was performed with a BioRad assay kit (Bio-Rad, Richmond, CA, United States) based on the Lowry method[14].
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver[15] and was determined using an MPO colorimetric assay kit (BioVision, Milpitas, CA, United States) according to the manufacturer’s instructions.
Western blots were performed as previously described[11]. The following reagents were used: rabbit polyclonal antibody to cyclooxygenase-2 (COX-2) (AbcamInc, Cambridge, MA, United States), glutamate-cysteine ligase modifier subunit (GCLM) (AbcamInc, Cambridge, MA, United States), inducible nitric oxide (NO) synthase (iNOS) (Santa Cruz Biotechnology, Santa Cruz, CA, United States), endothelial NO synthase (eNOS) (Santa Cruz Biotechnology, Santa Cruz, CA, United States), glutamate-cysteine ligase catalytic subunit (GCLC) (AbcamInc, Cambridge, MA), glutathione (GSH) peroxidase (GPx) (AbcamInc, Cambridge, MA, United States), heme oxygenase-1 (HO-1) (AbcamInc, Cambridge, MA, United States), superoxide dismutase (SOD) (Santa Cruz Biotechnology, Santa Cruz, CA, United States), glyceraldehyde-3-phosphate dehydrogenase (GADPH) (Cell Signaling, Danvers, MA, United States), rabbit monoclonal antibody to NF-κB (Cell Signaling, Danvers, MA, United States), catalase (CAT) (Rockland Immunochemicals, Limerick, PA, United States), and mouse monoclonal antibody to nicotinamide adenine dinucleotide phosphate (NAD(P)H) quinone oxidoreductase-1 (NQO-1) (AbcamInc, Cambridge, MA, United States) followed by secondary anti-rabbit or mouse immunoglobulin G (Cell Signaling, Danvers, MA, United States).
The levels of serum inflammatory mediators were determined using a standard rat cytokine kit (Ray Biotech, Norcross, GA, United States) according to the manufacturer’s instructions. Fifteen rat cytokines/chemokines were analyzed.
All the results are presented as the mean ± SD. Comparisons between two groups were performed with Student’s t-test or Mann-Whitney’s U test, as appropriate, using Stat View-J 5.0 software (SAS, Cary, NC, United States). Statistical significance was defined as a P value less than 0.05.
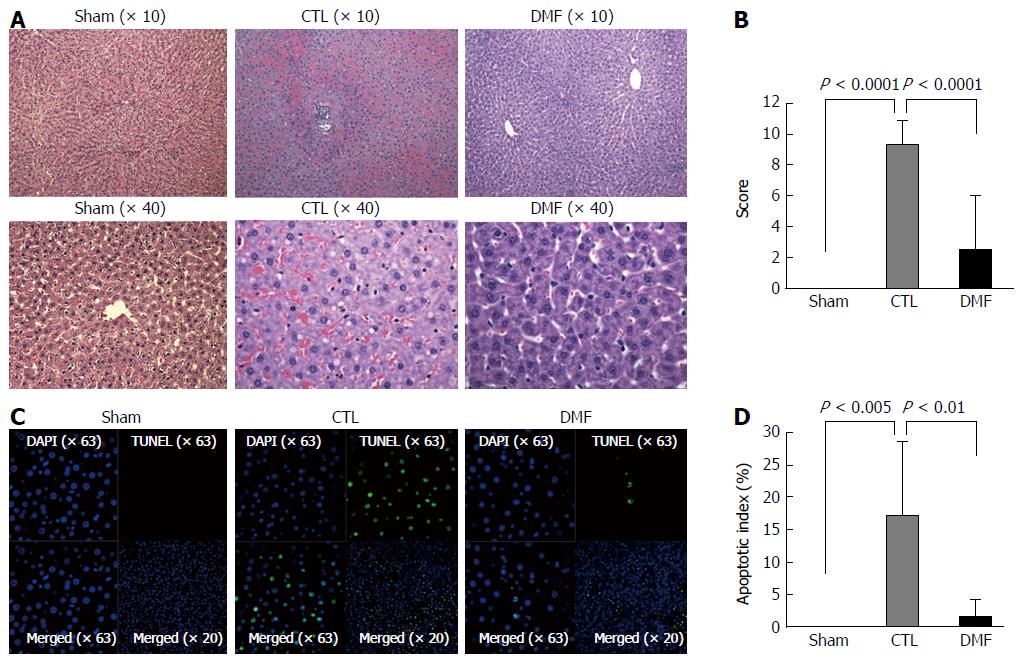
The histopathological findings are given in Figure 1A and B. The liver tissue was histologically normal in the sham group (Figure 1A). In contrast, substantial intracellular vacuolization, sinusoidal dilatation, congestion, and focal necrosis of the liver parenchyma were observed in the CTL group (Figure 1A). These changes were notably reduced in the DMF treatment group (Figure 1A). The Suzuki scores of the groups differed significantly (CTL 9.3 ± 0.5 vs DMF 2.5 ± 1.2, P < 0.0001; Figure 1B), indicating that pretreatment with DMF ameliorated the I/RI-induced histological changes.
Representative confocal microscopy images of hepatocyte-labeling TUNEL (green fluorescence) are shown in Figure 1C and D. No TUNEL-positive apoptotic cells were found in the sham group. Although the number of TUNEL-positive apoptotic cells increased dramatically in the CTL group, only a few positive cells were detected in the DMF group. The apoptotic index in the DMF group (1.3 ± 0.9) was significantly lower than that of the CTL group (17.2 ± 3.6) (Figure 1D; P = 0.002). These results suggest that DMF protected the liver from hepatocellular apoptosis.
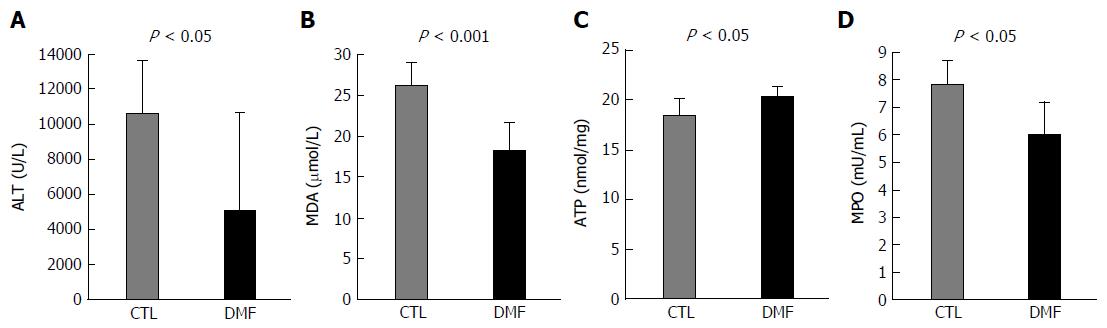
To further confirm the protective effect of DMF on hepatic I/R injury, the level of ALT, a hepatocyte damage marker, was measured. The ALT levels were significantly lower in the DMF group than in the CTL group (5026 ± 2305 U/L vs 10592 ± 1152 U/L, respectively, P = 0.04; Figure 2A). To determine the cellular damage under oxidative stress after I/RI, the serum MDA level, which is a valid biochemical marker of lipid peroxidation, was measured. The serum MDA levels were significantly lower in the DMF group (DMF 18.2 ± 1.4 μmol/L vs CTL 26.0 ± 1.0 μmol/L, P = 0.0009; Figure 2B).
To assess the ability of hepatocytes, we studied the cellular ATP levels. DMF-treated livers demonstrated significantly higher ATP contents than those in the CTL group (20.3 ± 0.4 nmol/mg vs 18.3 ± 0.6 nmol/mg, respectively, P = 0.02; Figure 2C).
Neutrophil infiltration in the DMF-treated group, as analyzed by MPO activity, was significantly lower (7.8 ± 0.4 mU/mL) than that in the CTL group (6.0 ± 0.5 mU/mL) (Figure 2D, P = 0.01), indicating that DMF treatment decreased neutrophil migration into the hepatocytes.
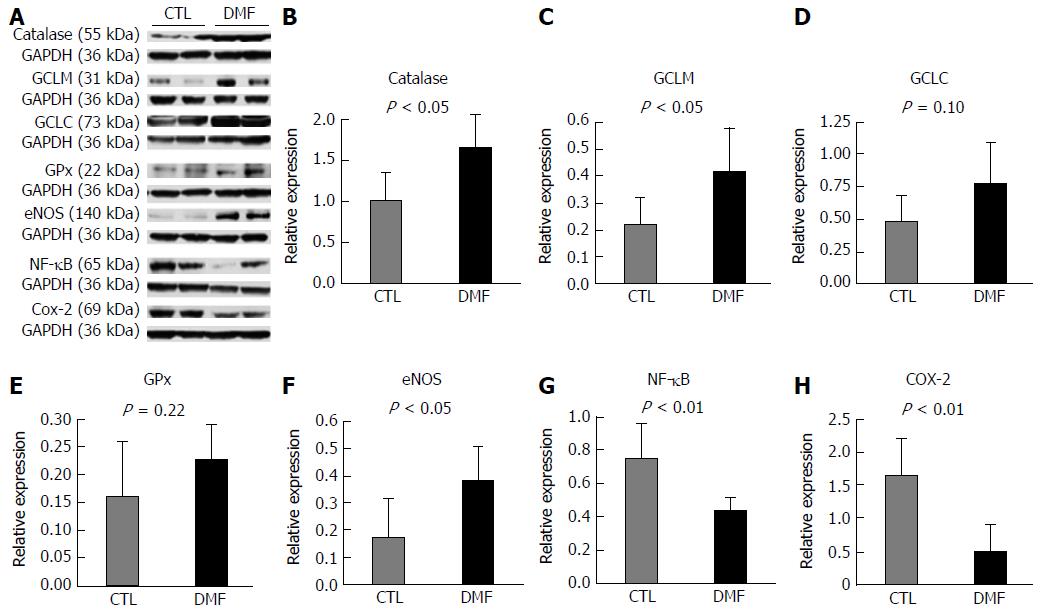
Figure 3B-D shows the protein expression of nuclear factor erythroid 2-related factor 1 (Nrf2) pathway target anti-oxidant enzymes. CAT and GCLM expression levels in the liver were significantly enhanced by DMF treatment compared with those in the CTL group (CAT: 1.50 ± 0.18-fold vs 0.93 ± 0.13-fold, respectively, P = 0.03, Figure 3B; GCLM: 0.42 ± 0.07-fold vs 0.22 ± 0.05-fold, respectively, P = 0.04, Figure 3C). Additionally, GCLC protein expression was higher in the DMF group than in the CTL group (CTL: 0.48 ± 0.08-fold vs DMF 0.77 ± 0.13-fold, P = 0.10; Figure 3D). The protein expression levels of GPx, HO-1, SOD and NQO-1 did not differ significantly between the two groups (GPx: P = 0.22, HO-1: P = 0.39, SOD: P = 0.32 and NQO-1: P = 0.95).
To determine whether DMF exerts its protective role through NO-mediated production, we detected the levels of NO synthases (eNOS and iNOS). The protein expression of eNOS was enhanced by DMF administration relative to that in the CTL group (0.38 ± 0.05-fold vs 0.17 ± 0.06-fold, respectively, P = 0.02; Figure 3F). However, no significant difference in iNOS expression was found between the two groups (CTL: 0.027 ± 0.12-fold vs DMF 0.027 ± 0.009-fold, P = 0.97).
To elucidate the effect of DMF on inflammation, western blot analysis of NF-κB and COX-2 proteins were performed. Both the expression levels of both NF-κB and COX-2 decreased significantly in DMF-treated animals’ liver tissues (NF-κB: CTL: 0.75 ± 0.08-fold vs DMF 0.44 ± 0.04-fold, P = 0.01, Figure 3G; COX-2: CTL: 1.64 ± 0.26-fold vs DMF 0.48 ± 0.19-fold, P = 0.007; Figure 3H) compared to those of the CTL group.
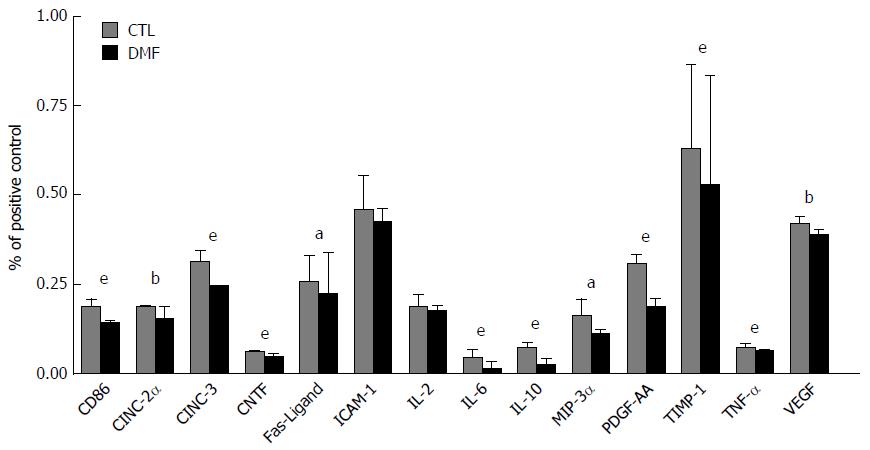
We analyzed direct changes in serum cytokine and chemokine production in response to I/RI. DMF administration significantly decreased the serum levels of many inflammatory mediators relative to those in the CTL group (Figure 4). Significant reductions of the following were observed in the DMF-treated group: cluster of differentiation 86 (CD86), cytokine-induced neutrophil chemoattractant-2α (CINC-2α), CINC-3, ciliary neurotrophic factor (CNTF), Fas ligand, interleukin-6 (IL-6), IL-10, macrophage inflammatory protein-3α (MIP-3α), platelet-derived growth factor-AA (PDGF-AA), tissue inhibitor of metalloproteinase 1 (TIMP-1), tumor necrosis factor α (TNF-α) and vascular endothelial growth factor (VEGF).
In this study, we evaluated the effects of DMF, a potent anti-oxidant, on a rodent hepatic I/RI model. Our data demonstrated that the pharmacological supplementation of DMF resulted in a significant increase of anti-oxidant enzymes and a substantial reduction of inflammatory mediators, leading to significant amelioration of the liver damage caused by I/R. Many studies have shown that oxidative stress plays a crucial role in the pathogenesis of I/RI[16]. MDA is an indicator of lipid peroxidation and cellular damage under oxidative stress, and the elevation of MDA is associated with liver I/RI[17]. We observed that the MDA levels were significantly decreased in the DMF-treated rats, suggesting lower I/RI-induced oxidative stress in the DMF-treated liver. ROS can be contained by endogenous anti-oxidant enzymes, such as SOD, CAT and GPx[1]. ROS-induced lipid peroxidation can be blocked by these anti-oxidative enzymes. CAT is an anti-oxidant enzyme that converts H2O2 to water and thereby prevents the transformation of H2O2 into highly toxic hydroxyl radicals. Previous reports showed that CAT has beneficial effects toward the end of the ischemic period and that its activities are decreased after I/RI[18]. GSH protects cells from oxidative injury by scavenging radicals and reducing lipid peroxidation products. GSH is synthesized by GSH synthetase and glutamate-cysteine ligase (GCL), which is a heterodimer composed of GCLM and GCLC subunits. Both GCLM and GCLC are upregulated for GSH synthesis by oxidants, providing a protective mechanism against oxidative stress[19]. We observed that DMF increased CAT, GCLM and GCLC expression, which may explain the observed decrease in ROS-related liver damage.
The accumulation of neutrophils, as confirmed by measuring the MPO activity, was significantly reduced by the administration of DMF. Neutrophils are believed to mediate hepatic damage caused by the production of ROS and reactive halogen species. These toxic products can directly damage hepatocytes and endothelial cells and induce other inflammatory mediators[20]. The initial phase of injury, which occurs within 2 h after reperfusion, is characterized by Kupffer cell-induced oxidant stress. Activated Kupffer cells produce and secrete pro-inflammatory cytokines, including TNF-α, IL-6, COX-2, and iNOS[3]. In this study, we observed significantly decreased TNF-α, IL-6 and COX-2 protein expression, indicating that DMF may inhibit the activation of Kupffer cells by enhancing anti-oxidant effects. We also found a significant decrease in the expression level of NF-κB 2 h after reperfusion in the DMF-pretreated rats. A previous report showed that DMF inhibited pro-inflammatory cytokine production and NF-κB signaling by inhibiting nuclear translocation[21]. Previous reports also demonstrated that DMF has a cardioprotective effect during I/RI through the Nrf-2 and NF-κB pathway[22], strongly supporting our results. Moreover, recent studies have demonstrated that NF-κB is involved in the regulation of COX-2 and iNOS expression[3]. Our data showed that DMF can inhibit NF-κB expression and suppress COX-2 expression but does not affect iNOS expression.
We found that twelve plasma cytokines and chemokines were decreased by DMF administration. CINC and MIP are members of the CXC chemokine family, are potent chemotactic factors for neutrophils, and contribute to neutrophil recruitment in inflammation[23]. MIP-3α is expressed mainly in the liver and is produced by dendritic cells and macrophages after an inflammatory response, resulting in the recruitment of activated T cells into the liver[24]. CD86 was also reported to be involved as the costimulatory signaling molecule for antigen-presenting cells (APCs) in T-cell activation[25]. The up-regulation of CD86 in sinusoidal endothelial cells contributes to liver injury after warm I/R[26]. VEGF is a well-established angiogenesis factor and has been recently found to have potent pro-inflammatory properties in the early period after transplant[27]. PDGF-AA is an important mediator of connective tissue expansion during liver fibrosis[28]. TIMP-1 is significantly increased in patients with fulminant hepatitis, reflecting severe hepatic inflammation[29]. Fas ligand is expressed on infiltrating immune cells and induces apoptosis of hepatocytes, leading to hepatocyte injury[30]. CNTF, a member of the IL-6 superfamily, is involved in fever induction and a hepatic acute-phase protein response[31]. Taken together, DMF suppressed plasma cytokines and chemokines related to apoptosis, inflammation and fibrosis. Further studies will be conducted to explore this Nrf2 anti-inflammatory effect.
The histological investigation suggested that I/RI caused severe pathological alterations in the liver. Pretreatment with DMF ameliorated I/RI-induced histological changes, and this effect was attributed to its anti-oxidant efficacy. The observed endothelial dysfunction could be a consequence of decreased synthesis or bioavailability of NO[32]. A previous report showed that the observed endothelial dysfunction is characterized by a marked reduction in NO availability and decreased expression and phosphorylation of eNOS[17]. Our results showed that DMF enhanced eNOS protein expression, suggesting that DMF pretreatment may protect endothelial function from I/RI. Furthermore, cellular apoptosis consumes large amounts of nicotinamide adenine dinucleotide (NAD+), and the process to resynthesize NAD+ decreases the level of cellular ATP[33]. Our data showed that the ATP content was increased in the DMF group compared to that in the CTL group, suggesting that DMF has an anti-apoptotic effect. In fact, the apoptotic index in the DMF group was significantly lower than that in the CTL group. A previous report showed that liver I/RI mainly occurred in the form of apoptosis[13]. Excessive oxygen free radicals could directly react with unsaturated fatty acids on the surfaces of mitochondrial membranes, leading to the destruction of their structure and function. Large amounts of cytochrome C and other apoptosis-promoting substances were released, triggering cell apoptosis. In this study, we showed that DMF may have inhibited the apoptotic pathway mediated by mitochondria.
In conclusion, our biochemical and histological findings indicated that DMF pretreatment was very effective in preventing tissue damage in liver I/RI. First, the burden of oxidative stress, which increases during I/RI, was alleviated by DMF, which increased the expression of anti-oxidant enzymes and decreased the level of NF-κB. Second, DMF improved the recovery of endothelial dysfunction and the production of ATP in hepatocytes. Other protective effects against I/RI on hepatocytes were clearly demonstrated by DMF administration, including improvements in the ALT level and the histopathological features. Future studies are needed to explore the efficacy of DMF in the management of patients with liver interventions.
Liver ischemia/reperfusion injury (I/RI). generates reactive oxygen species, which can damage lipids, proteins and tissues and lead to local inflammatory responses, endothelial and Kupffer cell activation, cytokine/chemokine release, and cell apoptosis. The I/R-induced oxidative stress and tissue damage involve multiple cell signaling pathways that result in liver failure.
To knowledge, the effects of dimethyl fumarate (DMF) on liver I/RI have not been investigated.
DMF treatment could be a promising strategy to improve clinical outcome in patients with liver I/RI.
First, the burden of oxidative stress, which increases during I/RI, was alleviated by DMF, which increased the expression of anti-oxidant enzymes and decreased the level of NF-κB. Second, DMF improved the recovery of endothelial dysfunction and the production of adenosine triphosphate in hepatocytes.
The manuscript by Takasu et al describes effects of DMF on the I/RI. The authors found that treatment of rats with DMF significantly reduces tissue damage mediated by I/RI. This reduction of liver damage correlated with corrections of many blood parameters and correction of liver functions. The authors’ conclusion that DMF improves liver functions is supported by convincing results.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Timchenko N S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3970] [Cited by in RCA: 3779] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 2. | Schauer RJ, Gerbes AL, Vonier D, Meissner H, Michl P, Leiderer R, Schildberg FW, Messmer K, Bilzer M. Glutathione protects the rat liver against reperfusion injury after prolonged warm ischemia. Ann Surg. 2004;239:220-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Dogan S, Aslan M. Hepatic ischemia-reperfusion injury and therapeutic strategies to alleviate cellular damage. Hepatol Res. 2011;41:103-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 5. | Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15-G26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 634] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 6. | Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 362] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Held KD, Epp ER, Clark EP, Biaglow JE. Effect of dimethyl fumarate on the radiation sensitivity of mammalian cells in vitro. Radiat Res. 1988;115:495-502. [PubMed] |
| 8. | Robles L, Vaziri ND, Li S, Takasu C, Masuda Y, Vo K, Farzaneh SH, Stamos MJ, Ichii H. Dimethyl fumarate ameliorates acute pancreatitis in rodent. Pancreas. 2015;44:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Robles L, Vaziri ND, Li S, Masuda Y, Takasu C, Takasu M, Vo K, Farzaneh SH, Stamos MJ, Ichii H. Dimethyl fumarate protects pancreatic islet cells and non-endocrine tissue in L-arginine-induced chronic pancreatitis. PLoS One. 2014;9:e107111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Eum HA, Cha YN, Lee SM. Necrosis and apoptosis: sequence of liver damage following reperfusion after 60 min ischemia in rats. Biochem Biophys Res Commun. 2007;358:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Masuda Y, Vaziri ND, Takasu C, Li S, Robles L, Pham C, Le A, Vo K, Farzaneh SH, Stamos MJ. Salutary effect of pre-treatment with an Nrf2 inducer on ischemia reperfusion injury in the rat liver. Gastroenterol Hepatol (Que). 2014;1:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Suzuki S, Nakamura S, Koizumi T, Sakaguchi S, Baba S, Muro H, Fujise Y. The beneficial effect of a prostaglandin I2 analog on ischemic rat liver. Transplantation. 1991;52:979-983. [PubMed] |
| 13. | Sun K, Liu ZS, Sun Q. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic postconditioning. World J Gastroenterol. 2004;10:1934-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 15. | Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157-167. [PubMed] |
| 16. | Kaplan N, Yagmurdur H, Kilinc K, Baltaci B, Tezel S. The protective effects of intravenous anesthetics and verapamil in gut ischemia/reperfusion-induced liver injury. Anesth Analg. 2007;105:1371-1378, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kireev R, Bitoun S, Cuesta S, Tejerina A, Ibarrola C, Moreno E, Vara E, Tresguerres JA. Melatonin treatment protects liver of Zucker rats after ischemia/reperfusion by diminishing oxidative stress and apoptosis. Eur J Pharmacol. 2013;701:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Fouad AA, El-Rehany MA, Maghraby HK. The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rats. Eur J Pharmacol. 2007;572:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kobayashi T, Watanabe Y, Saito Y, Fujioka D, Nakamura T, Obata JE, Kitta Y, Yano T, Kawabata K, Watanabe K. Mice lacking the glutamate-cysteine ligase modifier subunit are susceptible to myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2010;85:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Ward PA, Varani J. Mechanisms of neutrophil-mediated killing of endothelial cells. J Leukoc Biol. 1990;48:97-102. [PubMed] |
| 21. | Hafez T, Moussa M, Nesim I, Baligh N, Davidson B, Abdul-Hadi A. The effect of intraportal prostaglandin E1 on adhesion molecule expression, inflammatory modulator function, and histology in canine hepatic ischemia/reperfusion injury. J Surg Res. 2007;138:88-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Ashrafian H, Czibik G, Bellahcene M, Aksentijević D, Smith AC, Mitchell SJ, Dodd MS, Kirwan J, Byrne JJ, Ludwig C. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Takano K, Nakagawa H. Contribution of cytokine-induced neutrophil chemoattractant CINC-2 and CINC-3 to neutrophil recruitment in lipopolysaccharide-induced inflammation in rats. Inflamm Res. 2001;50:503-508. [PubMed] |
| 24. | Shimizu Y, Murata H, Kashii Y, Hirano K, Kunitani H, Higuchi K, Watanabe A. CC-chemokine receptor 6 and its ligand macrophage inflammatory protein 3alpha might be involved in the amplification of local necroinflammatory response in the liver. Hepatology. 2001;34:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Satoh S, Suzuki A, Asari Y, Sato M, Kojima N, Sato T, Tsuchiya N, Sato K, Senoo H, Kato T. Glomerular endothelium exhibits enhanced expression of costimulatory adhesion molecules, CD80 and CD86, by warm ischemia/reperfusion injury in rats. Lab Invest. 2002;82:1209-1217. [PubMed] |
| 26. | Kojima N, Sato M, Suzuki A, Sato T, Satoh S, Kato T, Senoo H. Enhanced expression of B7-1, B7-2, and intercellular adhesion molecule 1 in sinusoidal endothelial cells by warm ischemia/reperfusion injury in rat liver. Hepatology. 2001;34:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, Kupiec-Weglinski JW. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol. 2006;168:695-705. [PubMed] |
| 28. | Geremias AT, Carvalho MA, Borojevic R, Monteiro AN. TGF beta1 and PDGF AA override collagen type I inhibition of proliferation in human liver connective tissue cells. BMC Gastroenterol. 2004;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Nakamura T, Ushiyama C, Suzuki S, Shimada N, Ebihara I, Suzaki M, Takahashi T, Koide H. Effect of plasma exchange on serum tissue inhibitor of metalloproteinase 1 and cytokine concentrations in patients with fulminant hepatitis. Blood Purif. 2000;18:50-54. [PubMed] |
| 30. | Nakajima H, Mizuta N, Fujiwara I, Sakaguchi K, Ogata H, Magae J, Yagita H, Koji T. Blockade of the Fas/Fas ligand interaction suppresses hepatocyte apoptosis in ischemia-reperfusion rat liver. Apoptosis. 2008;13:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Espat NJ, Auffenberg T, Rosenberg JJ, Rogy M, Martin D, Fang CH, Hasselgren PO, Copeland EM, Moldawer LL. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am J Physiol. 1996;271:R185-R190. [PubMed] |
| 32. | He X, Zhao M, Bi XY, Yu XJ, Zang WJ. Delayed preconditioning prevents ischemia/reperfusion-induced endothelial injury in rats: role of ROS and eNOS. Lab Invest. 2013;93:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Iida A, Yoshidome H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Morita Y, Takeuchi D, Miyazaki M. Does prolonged biliary obstructive jaundice sensitize the liver to endotoxemia? Shock. 2009;31:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |