Published online May 28, 2017. doi: 10.3748/wjg.v23.i20.3690
Peer-review started: January 19, 2017
First decision: February 9, 2017
Revised: March 7, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: May 28, 2017
Processing time: 128 Days and 23.2 Hours
To investigate the prognostic value of the radiological response after transarterial chemoembolization (TACE) and inflammatory markers in patients affected by hepatocellular carcinoma (HCC) awaiting liver transplantation (LT).
We retrospectively evaluated the preoperative predictors of HCC recurrence in 70 patients treated with conventional (n = 16) or doxorubicin-eluting bead TACE (n = 54) before LT. The patient and tumour characteristics, including the static and dynamic alpha-fetoprotein, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio (PLR) measurements, were recorded. Treatment response was classified according to the modified Response Evaluation Criteria in Solid Tumours (mRECIST) and the European Association for the Study of the Liver (EASL) criteria as complete response (CR), partial response (PR), stable disease or progressive disease. After examination of the explanted livers, histological necrosis was classified as complete (100% of the cumulative tumour area), partial (50%-99%) or minimal (< 50%) and was correlated with the preoperative radiological findings.
According to the pre-TACE radiological evaluation, 22/70 (31.4%) and 12/70 (17.1%) patients were beyond Milan and University of San Francisco (UCSF) criteria, respectively. After TACE procedures, the objective response (CR + PR) rates were 71.4% and 70.0% according to mRECIST and EASL criteria, respectively. The agreement between the two guidelines in defining the radiological response was rated as very good both for the overall and target lesion response (weighted k-value: 0.98 and 0.93, respectively). Complete and partial histological necrosis were achieved in 14/70 (20.0%) and 28/70 (40.0%) patients, respectively. Using histopathology as the reference standard, mRECIST criteria correctly classified necrosis in 72.9% (51/70) of patients and EASL criteria in 68.6% (48/70) of cases. The mRECIST non-response to TACE [Exp(b) = 9.2, p = 0.012], exceeding UCSF criteria before TACE [Exp(b) = 4.7, p = 0.033] and a preoperative PLR > 150 [Exp(b) = 5.9, p = 0.046] were independent predictors of tumour recurrence.
The radiological response and inflammatory markers are predictive of tumour recurrence and allow the proper selection of TACE-treated candidates for LT.
Core tip: The response to loco-regional therapy and biological markers appear to stratify the prognosis of hepatocellular carcinoma patients awaiting liver transplantation (LT) better than morphological criteria; however, their role in the selection scheme still needs validation. We analysed a homogeneous cohort of 70 patients treated exclusively by transarterial chemoembolization (TACE) prior to LT; the radiological response was assessed by two different enhancing methods (mRECIST and EASL criteria) that provided an accurate preoperative estimation of histological necrosis. We also demonstrated that a lack of response to TACE and a high platelet-to-lymphocyte ratio before surgery are strongly predictive of tumour recurrence, independently of the Milan criteria status at referral.
- Citation: Nicolini D, Agostini A, Montalti R, Mocchegiani F, Mincarelli C, Mandolesi A, Robertson NL, Candelari R, Giovagnoni A, Vivarelli M. Radiological response and inflammation scores predict tumour recurrence in patients treated with transarterial chemoembolization before liver transplantation. World J Gastroenterol 2017; 23(20): 3690-3701
- URL: https://www.wjgnet.com/1007-9327/full/v23/i20/3690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i20.3690
Liver transplantation (LT) is the best chance of a cure for patients with hepatocellular carcinoma (HCC)[1], removing both the tumour and underlying cirrhosis, known to be a premalignant condition. Over the past two decades, several dimensional criteria, based on tumour size and number, have been validated to identify patients with a lower risk of recurrence after LT. The Milan Criteria (MC)[2] are currently considered the gold-standard for selecting patients for LT at most transplant centres worldwide[3]. Tumour features including microvascular invasion (mVI), poor histopathological differentiation and gene expression profiles are universally recognized as strong prognostic indicators[4,5]; however, these features are rarely assessable at the pre-operative diagnostic work-up.
Locoregional treatments (LRTs) are used in patients awaiting LT to help prevent progression while on the waiting list (bridging therapy) and as neoadjuvant treatment to downstage HCC according to the commonly accepted MC or University of San Francisco criteria (UCSF)[6].
It has been shown that applying the MC to baseline imaging may incorrectly stage a patient with HCC in over 30% of cases compared with assessing the explanted histopathological specimen[7,8]. Furthermore, two prospective studies have demonstrated favourable outcomes in patients with advanced HCC treated with neoadjuvant therapy with successful down-staging prior to LT[9,10]. These findings suggest that reliable selection criteria for LT candidates require more than static dimensional criteria based on preoperative liver imaging.
Transarterial chemoembolization (TACE) is the most extensively studied and widely used neoadjuvant therapy in current clinical practice[11,12]. To define the treatment response, the modified Response Evaluation Criteria In Solid Tumours (mRECIST)[13] and European Association for the Study of the Liver (EASL)[14] criteria are enhancement methods used in the transplant[15-17] and non-transplant settings[18,19]. Although the post-LT prognosis can be predicted using mRECIST response dynamics following TACE[15,20], the accuracy of this criterion remains controversial compared with the histopathological standard of reference[16,21,22].
Regarding serum tumour markers, alpha-fetoprotein (AFP) levels[23-25] and their fluctuation during the waiting list (WL) period[26-28] have been shown to predict tumour recurrence in many clinical experiences[23-25]. However, there is no universally agreed upon cut-off value that would make a patient ineligible for LT[26-28]. Besides AFP, a growing number of recent studies have emphasized the association between tumour biological aggressiveness and the “so-called” inflammatory markers, namely the neutrophil-to-lymphocyte ratio (NLR)[29-31] and platelet-to-lymphocyte ratio (PLR)[32]. These laboratory markers are inexpensive and readily available, but their prognostic role in HCC recurrence and WL dropout remains widely debated[33].
We evaluated the radiological response and preoperative inflammation scores as prognostic variables to predict post-transplant HCC recurrence, through comprehensive multivariate analysis of preoperative risk factors in 70 consecutive patients treated with TACE at our institution. The accuracy of mRECIST and EASL criteria for the prediction of histological necrosis was also evaluated.
The study population was retrieved from the institutional, prospectively entered database of the Hepatobiliary and Transplant Unit of Polytechnic University of Marche, Ancona. Between August 2005 and December 2014, 114 liver transplants were performed in patients with histologically confirmed HCC in the background of liver cirrhosis. Patients who did not receive neoadjuvant treatment (n = 24), who received a type of neoadjuvant therapy other than TACE (n = 15) or who were transplanted before the scheduled post-TACE imaging evaluation (n = 5) were excluded from the analysis. The final study population included 70 patients who exclusively underwent one or more TACE sessions prior to LT.
The preoperative diagnosis of HCC was based on EASL guidelines[1]. Thoracic and abdominal contrast-enhanced computed tomography (CT) or multiphasic contrast-enhanced MRI imaging was performed before TACE to exclude intra-abdominal or pulmonary tumour spread, lymphatic metastasis or macrovascular invasion of the portal branches. In line with our institution’s policy, TACE was performed in patients with a greater tumour burden than described by the MC at initial imaging, or in patients fulfilling MC with an expected waiting list time of longer than 2 mo. The patient eligibility for LT was discussed during the weekly local multidisciplinary liver transplant committee meeting, considering all aspects of the pre-operative work-up and staging test results.
The patient demographics, aetiology of cirrhosis, Child-Pugh and Model for End-Stage Liver Disease (MELD) scores, and imaging and pathological records were collected for each patient. Laboratory factors related to tumour biology such as serum AFP and inflammation-based scores were recorded at two different well-defined time points: the day of admission to perform the first TACE and immediately before surgery. The NLR is expressed as the ratio between the absolute blood count of neutrophils and lymphocytes. The PLR is the ratio between the absolute blood count of platelets and lymphocytes. The prognostic value of both static and dynamic (difference between initial and final values divided by the time lapse between the two referral points) AFP, NLR and PLR values were evaluated.
The transplant procedures were performed using deceased donor allografts. The immunosuppressive schedule included mammalian target of rapamycin inhibitors (Everolimus) in association with low-dose calcineurin inhibitor (Tacrolimus) in most patients. Steroids were gradually tapered and discontinued after 3 mo in all patients.
Screening for tumour recurrence involved serial AFP measurements, 3 monthly abdominal ultrasounds and annual contrast-enhanced CT chest/abdomen imaging.
All patients underwent baseline celiac and superior mesenteric arteriography using femoral artery puncture. Conventional TACE was performed by administering 50 mg of epirubicin in an emulsion with Lipiodol followed by embolization with gelatin sponge particles. Doxorubicin-eluting bead TACE (DEB-TACE) was performed using DC beads impregnated with 75 mg of doxorubicin in each vial. When the radiologic findings demonstrated residual viable tumour in the treated nodules or new lesions, patients with a low risk of decompensation underwent further TACE therapy.
Two radiologists (Agostini A and Giovagnoni A, with 5 and 25 years of experience, respectively, in liver imaging) evaluated the baseline contrast-enhanced imaging (dynamic MRI or CT) and last available imaging before LT to define the tumour response according to mRECIST and EASL guidelines. Both mRECIST and EASL guidelines define viable tumour as the area of enhancement during the arterial phase. For each measurable lesion, the largest diameter and largest perpendicular diameter were recorded; the first was used to assess the target lesion response according to mRECIST (based on the sum of unidimensional measurements), and the product of both measurements was used to assess the response of measurable lesions according to EASL. Both criteria define a complete response (CR) as the absence of arterial enhancement within a lesion. mRECIST defines a partial response as at least a 30% decrease in the sum of the longest diameter of target lesions, taking as the reference the baseline sum longest diameter; progressive disease (PD) is defined as an increase of at least 20% of the sum of the longest diameter of the target lesions. EASL defines a PR as a decrease of at least 50% of the sum of cross products of the enhancing diameters, while PD is defined as an increase of at least 25%. Stable disease (SD) occurs when neither PR nor PD is assigned with both criteria. New lesions denote PD for mRECIST and EASL. Overall, the patient response is a result of the combined assessment of target lesions, non-target lesions, and new lesions. Patients obtaining a CR or PR after TACE cycles are defined as objective responders (OR).
A dedicated liver pathologist performed the analysis of all explanted livers that were serially cut into sections of approximately 0.5-cm thick. Tumour grade according to the Edmonson and Steiner classification was assessed except when complete necrosis of the tumour was achieved. The presence of satellite lesions and mVI were also reported. Nodule necrosis was expressed as the percentage of necrotic tissue within the whole area of the nodule. In patients with multiple lesions, the necrosis of the cumulative tumour area (% of necrosis on CTA) was calculated, including that of non-treated tumours, as a mean of necrosis rates weighted on nodular areas. To explore the accuracy of radiological criteria in predicting the histological outcome, we assumed that the CR corresponds to 100% of necrosis on CTA (no viable cells detected), whereas PR corresponds to 50%-99% of necrosis on CTA and SD or PD correspond to < 50% necrosis on CTA. For the radio-histological correlation of target lesions, the percentage of necrosis on CTA was re-calculated considering only nodules ≥ 1 cm in diameter at pathology. Adherence to MC or UCSF criteria was re-assessed at pathological examination using only the viable portion of each nodule.
Categorical variables are reported as numbers and percentages and were compared with Fisher’s exact test. Continuous variables are reported as medians and interquartile ranges (IQRs); the Mann-Whitney U test was applied to compare continuous variables in different subgroups of patients, whereas any difference in laboratory values before and after TACE therapy was investigated using the Wilcoxon test. Continuous variables such as AFP serum levels, AFP slope, NLR, and PLR were dichotomized according to previously reported threshold values (400 ng/mL for the static AFP value and 15 ng/mL per month for the AFP slope; 4 for NLR and 150 for PLR)[23,31-37]. Third-quartile values, corresponding to 0.24 and 3.04/mo, were used for the NLR and PLR slopes, respectively. The rank correlation test for nonparametric continuous variables (Kendall’s tau) was applied to investigate the relationship between the amount of necrosis and different categories of the radiological response. The agreement in defining the radiological response between mRECIST and EASL criteria was explored by the k-cohen test. The impact of each individual variable in determining HCC recurrence-free survival (RFS) was assessed by the Kaplan-Meier method and was compared by the log-rank test. A multivariate Cox proportional regression model (stepwise method) was designed to investigate risk factors independently related to HCC recurrence, considering only preoperative variables that proved to be significant (p value < 0.05) after univariate analysis. Statistical significance was set at a p value < 0.05.
The demographics and clinical characteristics of the 70 patients included in the study are summarized in Table 1. The median post-LT follow-up was 38.4 (IQR: 24.8-68.6) mo. At the initial radiological evaluation, multifocal HCC was detected in 30 (42.9%) patients, and the median diameter of the largest nodule was 2.6 (IQR: 2.0-3.4) cm. Twenty-two (31.4%) patients were beyond MC, and 12 (17.1%) were also outside the UCSF criteria.
| Variable | All treated patients (n = 70) |
| Demographics and indications | |
| Age at LT (yr) [median (IQRs)] | 57 (51-62) |
| Male gender | 62 (88.6) |
| Biochemical MELD Score [median (IQRs)] | 11 (7-15) |
| Child-Pugh class A/B/C | 29 (41.4)/27 (38.6)/14 (20.0) |
| Virus B-related cirrhosis | 15 (21.4) |
| Virus C-related cirrhosis | 41 (58.6) |
| Pre-TACE radiological evaluation | |
| Type of imaging technique (CT/MR) | 49 (70.0)/21 (30) |
| Exceeding Milan criteria | 22 (31.4) |
| Exceeding UCSF criteria | 12 (17.1) |
| Number of nodules [median (IQRs)] | 1 (1-2) |
| Single/multiple | 40 (57.1)/30 (42.9) |
| Sum of nodule diameters (cm) [median (IQRs)] | 3.35 (2.1-5.4) |
| Sum of nodule diameters > 5 cm | 22 (31.4) |
| Diameter of the largest nodule (cm) [median (IQRs)] | 2.6 (2.0-3.4) |
| Diameter of the largest nodule > 5 cm | 7 (10.0) |
| Pre-TACE laboratory evaluation | |
| 1AFP (ng/mL) [median (IQRs)] | 12.5 (5.8-52.0) |
| AFP > 400 ng/mL | 5 (8.1) |
| 2NLR [median (IQRs)] | 2.0 (1.4-3.1) |
| NLR > 4 | 13 (20.3) |
| 3PLR [median (IQRs)] | 67.2 (44.6-84.0) |
| PLR > 150 | 2 (3.1) |
| AST/ALT (U/L) [median (IQRs)] | 69 (43.7-108.7)/56 (34.0-88.2) |
| WBC (× 103/mmc) [median (IQRs)] | 4.7 (3.7-5.8) |
| Characteristics of TACE and time-intervals between procedures | |
| Number of treatments [median (IQRs)] | 2 (1-2) |
| Repeated TACE | 37 (52.9) |
| Type of TACE (DEB/conventional) | 54 (77.1)/16 (22.9) |
| Interval of last imaging-LT (mo) [median (IQRs)] | 1.4 (0.7-2.7) |
| Interval of last TACE-LT (mo) [median (IQRs)] | 3.9 (2.1-7.4) |
| Interval of first TACE-LT (mo) [median (IQRs)] | 6.9 (3.7-11.0) |
Globally, 124 TACE procedures were performed in 70 LT candidates; 37 (52.8%) patients underwent two or more TACE sessions. The number of treatments did not significantly differ between the patients classified within or beyond MC according to the initial imaging (p = 0.1546). The median time interval between the first TACE and LT was 6.9 (IQR: 3.7-11.0) mo. DEB-TACE was employed more frequently (77.1% of patients) than c-TACE (22.9%).
According to mRECIST criteria, the OR rate was 71.4%, with 24 (34.3%) patients achieving CR and 26 (37.1%) patients achieving PR (Table 2). When EASL criteria were applied to define the response to TACE, a CR was seen in 24 (34.3%) patients, a PR in 25 (35.7%), SD in 11 (15.7%) and PD in 10 (14.3%). The agreement between the two guidelines in defining the radiological response was rated as very good both for the overall and target lesion response (weighted k-value 0.98 and 0.93, respectively).
| Variable | All treated patients (n = 70) |
| Pre-LT radiological evaluation | |
| mRECIST overall response | |
| Complete/partial response | 24 (34.3)/26 (37.1) |
| Stable/progressive disease | 10 (14.3)/10 (14.3) |
| EASL overall response | |
| Complete/partial response | 24 (34.3/25 (35.7) |
| Stable/progressive disease | 11 (15.7)/10 (14.3) |
| Number of enhancing nodules [median (IQRs)] | 1 (0.0-2.0) |
| None/single/multiple | 24 (34.3)/22 (31.4)/24 (34.3) |
| Sum of enhancing diameters (cm) [median (IQRs)] | 1.4 (0.0-3.3) |
| Sum of enhancing diameters > 5 cm | 8 (11.4) |
| Diameter of the largest enhancing nodule (cm) [median (IQRs)] | 1.3 (0.0-2.1) |
| Diameter of the largest enhancing nodule > 5 cm | 1 (1.4) |
| Pre-LT laboratory evaluation | |
| 1AFP (ng/mL) [median (IQRs)] | 13.5 (5.3-65.0) |
| AFP > 400 ng/mL | 6 (9.1) |
| 2AFP increase > 15 ng/mL per month | 6 (10.2) |
| NLR [median (IQRs)] | 2.6 (1.8-3.8) |
| NLR > 4 | 15 (21.4) |
| 3NLR increase > 0.24 | 16 (29.6) |
| PLR [median (IQRs)] | 62.9 (49.7-85.9) |
| PLR > 150 | 5 (7.1) |
| 3PLR increase > 3.04 | 16 (29.6) |
| AST/ALT (UI/L) [median (IQRs)] | 68 (43-100)/49 (32-76) |
| WBC (× 103/mmc) [median (IQRs)] | 4.7 (3.7-5.8) |
| Tumour histopathological characteristics | |
| Number of viable nodules [median(IQRs)] | 1 (1-3) |
| Number of viable nodules > 3 | 11 (15.7) |
| Tumour differentiation (4Gx/G1-G2/G3-G4) | 14 (20.0)/48 (68.6)/8 (11.4) |
| Microvascular invasion | 8 (11.4) |
| Exceeding Milan criteria | 12 (17.1) |
| Exceeding UCSF criteria | 11 (15.7) |
| % of necrosis on cumulative tumour area (100/99-50/< 50) | 14 (20.0)/28 (40.0)/28 (40.0) |
Considering only the viable portion of the nodules, 12 (17.1%) patients were beyond MC at pathological examination. The median percentage of necrosis on CTA was 62.5% (IQR: 23.3-95.4); 14 (20.0%) patients exhibited complete necrosis. Histological necrosis significantly differed across the different response categories as defined by mRECIST and EASL. Using mRECIST, the patients classified as CR, PR or SD/PD showed a percentage of necrosis on CTA of 86.6%, 64.1% and 16.4%, respectively (Kendall’s Tau: 0.66, p < 0.0001). Similarly, patients assigned to CR, PR or SD/PD as defined by EASL showed a % of necrosis on CTA of 86.6%, 60.5% and 22.9%, respectively (Kendall’s Tau: 0.61, p < 0.0001). No correlation was found between the amount of histological necrosis and time interval between the last radiological assessment and LT or the interval between the last TACE and LT.
The accuracy of the mRECIST overall response was 72.9% (51 patients). In 17 (24.3%) cases, mRECIST overestimated histopathological necrosis, and in 2 (2.9%) cases, mRECIST underestimated tumour necrosis as seen on imaging before LT. When EASL criteria were applied, the accuracy was slightly lower (48 patients; 68.6%), with 18 (25.7%) cases of overestimation and 4 (5.7%) cases of underestimation (Table 3). Overall, the accuracy in discriminating responders from non-responders was 85.7% and 81.4% for mRECIST and EASL criteria, respectively.
| Overall response | Target lesion response | |||||||
| Patients (n) | % of necrosis on CTA | Patients (n) | % of necrosis on CTA1 | |||||
| 100 | 50-99 | < 50 | 100 | 50-99 | < 50 | |||
| mRECIST | ||||||||
| Complete response | 24 | 13 (54.2) | 8 (33.3) | 3 (12.5) | 29 | 16 (55.2) | 8 (27.6) | 5 (17.2) |
| Partial response | 26 | 1 (3.8) | 19 (73.1) | 6 (23.1) | 25 | 2 (8.0) | 16 (64.0) | 7 (28.0) |
| Stable/progressive disease | 20 | 0 (0.0) | 1 (5.0) | 19 (95.0) | 16 | 0 (0.0) | 0 (0.0) | 16 (100.0) |
| EASL | ||||||||
| Complete response | 24 | 13 (54.2) | 8 (33.3) | 3 (12.5) | 29 | 16 (55.2) | 8 (27.6) | 5 (17.2) |
| Partial response | 25 | 1 (4.0) | 17 (68.0) | 7 (28.0) | 25 | 1 (4.0) | 15 (60.0) | 9 (36.0) |
| Stable/progressive disease | 21 | 0 (0.0) | 3 (14.3) | 18 (85.7) | 16 | 1 (6.3) | 1 (6.3) | 14 (87.5) |
Before TACE, 5 (8.1%) patients had an AFP value higher than 400 ng/mL, and 13 (20.3%) patients had an NLR > 4. The median PLR initial value was 67.2 (IQR: 44.6-84.0) (Table 1). Considering the initial radiological evaluation, no differences were found in terms of AFP, NLR or PLR between patients fulfilling or exceeding MC (p = 0.5148, p = 0.2672 and p = 0.3780, respectively). The pre-LT values of biological markers and their fluctuation during the observation period are displayed in Table 2. An increase in AFP of more than 15 ng/mL per month was seen in 6 (10.2%) patients. At the time of LT, patients who did not respond to TACE (SD or PD) did not have significantly higher levels of AFP, NLR or PLR compared with those who achieved an OR (CR or PR). With respect to their pre-TACE values, a significant increase in the median NLR was seen in patients who experienced an OR after LRT (1.9 vs 2.9, p = 0.0025); this increment was less evident in patients who did not respond to TACE (2.0 vs 2.4, p = 0.0539).
Eight (11.4%) patients in the entire cohort developed HCC recurrence after a median period of 16.9 mo (IQR: 14.2-23.4, range: 9.7-38.3). The three-year patient and recurrence-free survival rates were 79.9% and 86.4%, respectively. Five patients were found to have recurrence in the liver, one patient had spinal cord recurrence in addition to liver lesions, a further patient had an adrenal gland deposit, and the final patient was found to have recurrent pulmonary disease. Two of eight patients were alive with recurrence at the time of data extraction.
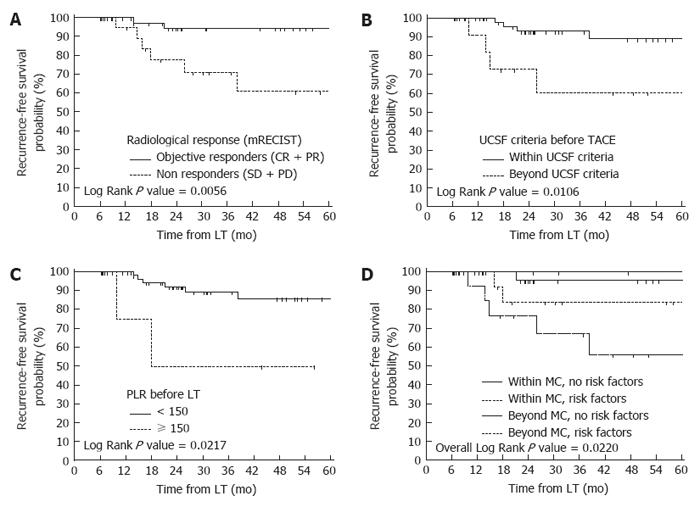
On multivariate Cox regression analysis, mRECIST non-response to TACE at the last imaging before LT [Exp(b) = 9.2, CI: 1.6-51.3, p = 0.0119] was the strongest predictor of HCC recurrence. The lack of fulfilment of UCSF criteria before TACE [Exp(b) = 4.7, CI: 1.1-19.3, p = 0.0331] and an increased (> 150) PLR before LT [Exp(b) = 5.9, CI: 1.0-33.9, p = 0.0458] were also independently associated with tumour recurrence (Table 4).
| Risk factors | Univariate analysis | Multivariate analysis | |||
| 3-yr RFS rate (%) | HR (95%CI) | Log-rank P value | Exp(b) (95%CI) | P value | |
| Pre-TACE radiological and laboratory evaluation | |||||
| Exceeding Milan criteria | 77.7 vs 91.2 | 3.41 (0.78-14.92) | 0.074 | ||
| Exceeding UCSF criteria | 60.6 vs 92.8 | 5.06 (0.78-32.74) | 0.011 | 4.69 (1.14-19.30) | 0.033 |
| Multiple nodules | 77.2 vs 93.4 | 4.33 (1.06-17.72) | 0.049 | 1.77 (0.27-11.48) | 0.550 |
| AFP > 400 ng/mL | 80.0 vs 85.3 | 1.96 (0.13-30.81) | 0.520 | ||
| NLR > 4 | 100 vs 80.4 | NA | 0.185 | ||
| PLR > 150 | 50.0 vs 88.6 | 5.98 (0.06-573.48) | 0.059 | ||
| Pre-LT radiological and laboratory evaluation | |||||
| mRECIST non response | 71.2 vs 94.3 | 6.96 (1.54-31.50) | 0.006 | 9.19 (1.65-51.30) | 0.012 |
| EASL non response | 76.8 vs 91.6 | 3.67 (0.82-16.34) | 0.056 | ||
| AFP > 400 ng/mL | 83.3 vs 89.0 | 4.74 (0.29-77.77) | 0.034 | 1.43 (0.23-9.10) | 0.703 |
| AFP increase > 15 ng/mL per month | 41.7 vs 87.7 | 3.89 (0.30-50.72) | 0.072 | ||
| NLR > 4 | 80.8 vs 87.9 | 1.54 (0.25-9.41) | 0.594 | ||
| NLR increase > 0.24/mo | 83.3 vs 100 | NA | 0.114 | ||
| PLR > 150 | 50.0 vs 89.1 | 5.32 (0.28-101.01) | 0.022 | 5.95 (1.04-33.95) | 0.046 |
| PLR increase > 3.04 | 80.8 vs 88.7 | 1.48 (0.24-9.04) | 0.636 | ||
The presence of multiple nodules at initial imaging and an AFP level greater than 400 ng/mL prior to performing TACE were significantly associated with HCC recurrence after univariate analysis but were not confirmed as independent prognostic factors after multivariate analysis. Notably, the MC status at initial radiological evaluation was not significantly associated with HCC recurrence in our cohort (p = 0.0736).
Stratifying the entire cohort according to the risk factors that proved statistically significant after multivariate analysis (pre-LT PLR > 150, mRECIST non response and exceeding UCSF criteria before TACE), patients beyond MC at the initial radiological evaluation with at least one risk factor (15 patients) experienced the worst outcome in terms of recurrence (3-year RFS = 67.3%) (Figure 1). Interestingly, patients initially classified as MC-OUT without risk factors (7 patients) achieved excellent 3-year RFS rates (100%), similar to MC-IN patients without risk factors (95.5%) and even slightly better than patients fulfilling MC with risk factors (83.9%).
Dimensional selection criteria, in particular MC, are widely used to define eligibility for LT in HCC patients in many transplant centres. There is increasing evidence to show that additional variables may play an important role in patient prognosis because it is recognized that some patients falling within the MC have a poor outcome following LT, whereas some patients falling outside of the criteria demonstrate a good outcome. There are several factors that affect a patient’s prognosis; in particular, a significant association has been demonstrated between the presence of mVI, poorly differentiated tumour grading and HCC recurrence[4,5]. Unfortunately, the usefulness of these variables for selecting patients for LT is limited because they usually cannot be assessed in the pre-operative setting[38,39]. Moreover, the radiological staging for HCC at referral (‘‘inside’’ or ‘‘outside’’ MC) may be unreliable, under-staging or over-staging up to 25% of cases compared with surgical histopathology[7,8]. In the context of LRT, this discrepancy is confirmed when considering the last imaging available before LT, with incorrect radiological staging in up to 26% of patients[40]. This suggests that further variables, in addition to lesion size and number, should be considered when selecting patients for LT.
TACE is the most commonly used technique[41] for bridging or down-staging patients with HCC prior to LT, and the treatment response has been suggested as a surrogate marker of tumour biology and predictor of post-LT outcome[9,12,42-45]. Recently, Lai et al[20] conducted a European multicentre study looking at the radiological response to LRT in 422 patients with HCC. The authors demonstrated that an AFP slope of more than 15 ng/mL per month and radiological PD according to mRECIST were unique independent risk factors for tumour recurrence and death, both in patients classified within or beyond MC before LRT. Similarly, Kim and colleagues identified that nonresponse to TACE (SD or PD) and a tumour size greater than 3 cm were preoperative predictors of recurrence in 173 patients treated with TACE before LT[15].
However, the reproducibility of these study findings is affected by treatment heterogeneity, the centre-specific criteria for LT and the reported outcome measures (post-LT recurrence or survival, achievement of tumour necrosis and classification of radiological response). Thus, some authors stratified the radiological response according to traditional RECIST criteria, or “self-established” radiological criteria, which probably lack reproducibility in other patient cohorts. For example, Otto and colleagues defined “any progression” as any increase in the sum of the diameter of target lesions (even if less than 20%), in contradiction to mRECIST or EASL guidelines[40]. Furthermore, the team from UCLA, who have reported on the largest single-institution series of HCC patients undergoing LRT and LT, defined the radiological response by the arterial enhancement of treated lesions [absent, possible or definite viable tumour (or new tumours)]. This seems to be an oversimplification of the commonly accepted mRECIST criteria.
The use of validated and well-defined parameters such as mRECIST or EASL guidelines has been shown to be the most accurate method of evaluating treatment response; in contrast to the RECIST criteria, they consider intratumoural necrosis when estimating a decrease in the tumour burden and not only the reduction in the overall tumour size. In our analysis, we applied these two criteria to explore their prognostic performance, inter-method agreement and accuracy in predicting different subcategories of necrosis. The agreement between the two guidelines was rated as very good for both the overall and target lesion responses; a non-response according to mRECIST criteria, namely SD or PD, was seen to be an independent prognostic factor for recurrence. This important point should be highlighted because there is currently no consensus regarding the radiological response and association with disease recurrence or patient drop-out from WL. In particular, some authors have previously suggested that only radiological tumour progression is predictive of post-LT tumour recurrence[20,40,46]; in keeping with these findings, patients classified as SD following LRT, with no evidence of progression, may be prioritized for LT. However, in the absence of a radiological response, these patients with SD could be at a higher risk of recurrence.
Accurate preoperative radiological estimation of tumour necrosis is essential, particularly in light of recent evidence. In two large series of patients, complete or nearly complete histological response to LRT was shown to improve long-term survival after LT[47,48]. At the per-patient level, the accuracy of the mRECIST overall response was 72.9%, whereas EASL criteria correctly defined histological necrosis in 68.6% of our patients. Other studies reporting on the reliability of radiological criteria via a pathological-radiological correlation have reported accuracies ranging from 57% to 74.3% for EASL criteria[21,49,50] and from 67.4% to 76.3% for mRECIST criteria[16,51]. Noticeably, the unique prospective study in this field reported that mRECIST has a low accuracy (56.2%) 1 mo following radioembolization[52]. The authors concluded that neither EASL nor mRECIST criteria correctly predict the pathological necrosis.
Recently, several reports have demonstrated that increased systemic inflammation is related to the poor prognosis of various types of cancers, promoting angiogenesis and tumour invasion through the upregulation of cytokines[53-56]. The prognostic performance of NLR and PLR, two simple and easily accessible serum parameters of systemic inflammation, were tested in the clinical scenario of LT, leading to controversial results[29-31,36,37,57-59]. An intention-to-treat study by Lai et al[32] demonstrated that NLR is a good predictor for dropout from the waiting list, while PLR is a good predictor of post-LT recurrence. Parisi et al[33] did not confirm these findings in a study involving 150 patients fulfilling the MC. To the best of our knowledge, our study is the first of its kind to look at the combination of inflammatory markers and the tumour radiological response as competitive risk factors using multivariate analysis. As reported by other authors[32,60], a high pre-LT PLR value was an independent risk factor for tumour recurrence in our cohort.
The definition of a well-established “upper limit” when considering patients with HCC for a down-staging protocol remains controversial. Our results suggest that applying radiological UCSF criteria before beginning TACE can provide significant prognostic information; when patients exceed the criteria, the risk of recurrence is unacceptably high. This was shown to be independent from the other static and dynamic variables included in the regression model.
This study has the following limitations: first, despite the data extraction being prospective, the analysis was retrospective. Second, the relatively small number of patients and events (i.e., recurrences) may affect the power of the study; in particular, some relevant preoperative variables (AFP and AFP slope) failed to reach statistical significance after univariate analysis. Third, as an intention-to-treat analysis of the entire WL population was not performed, the prognostic ability of the radiological and biological parameters in relation to the risk of drop-out was not tested.
However, as advised by Lai et al[20], we chose to analyse a homogeneous cohort of patients treated with only one type of LRT (i.e., TACE) at a single centre to eliminate bias derived from the different treatments’ efficacy, timing and modality. The radiological response was assessed rigidly following two enhancing criteria, namely mRECIST and EASL guidelines, to make the results reproducible, and an accurate radiological-pathological analysis of explanted livers was conducted to explore the reliability of these criteria in predicting histological necrosis. Our regression analysis of risk factors for post-LT recurrence was performed considering radiological (morphological and response to therapy) and biological variables (AFP, NLR and PLR, including their slopes) at two well-defined time-points (before starting TACE therapy and immediately before LT).
In conclusion, our data suggest that patients who experience an OR to TACE according to mRECIST criteria or not exceeding a pre-LT PLR value of 150 can achieve optimal results in terms of tumour-free survival, independent from their MC status at the initial evaluation. Further studies involving larger cohorts of patients are required to validate these new parameters as selection criteria in TACE-treated candidates for LT.
Liver transplantation (LT) is the best chance of cure for patients with hepatocellular carcinoma (HCC). Transarterial chemoembolization (TACE) is widely used in HCC patients awaiting LT, to prevent tumor progression whilst on the waiting list or to downstage the tumor within the commonly accepted Milan or University of San Francisco criteria. Recently, the radiological response to TACE and others biological markers, as alfa-fetoprotein (AFP) and inflammatory markers, have been demonstrated to predict the risk of post-LT tumor recurrence better than morphological criteria; however, the use of these parameters as selection criteria for LT is still to validate.
The authors analyzed a single-center homogeneous cohort of 70 patients treated exclusively by TACE prior to LT; noticeably, 31.4% of them were beyond Milan criteria at the initial radiological evaluation. Radiological response to TACE was assessed rigidly following two enhancing criteria, namely modified Response Evaluation Criteria in Solid Tumors (mRECIST) and the European Association for the Study of the Liver (EASL) criteria, to make the results reproducible. An accurate radiological-pathological analysis of explanted livers was conducted to explore the reliability of these criteria in predicting histological necrosis. Multivariate analysis of competitive risk factors for post-LT recurrence was performed taking in account radiological (morphological and response to therapy) and biological variables at two well-defined time-points (before starting TACE therapy and immediately before LT).
In this manuscript, we demonstrated that a lack of response to TACE and a high platelet-to-lymphocyte ratio before surgery are strongly predictive of tumor recurrence, independently from the Milan criteria status at referral. The overall diagnostic accuracy in predicting histological necrosis was 72.9% and 68.6% for mRECIST and EASL criteria, respectively.
This study highlights the prognostic role of “biological” and “dynamic” tumor parameters in HCC recurrence after LT. These preoperative factors should be integrated in the selection algorithm to increase the number of transplantable patients and to improve the recurrence-free survival rates in TACE-treated candidates for LT.
TACE is an image-guided, endovascular procedure that is used to treat malignant lesions in the liver, by injecting selectively small embolic particles into an artery directly supplying the tumor. These particles both block the blood supply and induce cytotoxicity by releasing chemotherapeutic drugs. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have been used as markers to evaluate the systemic inflammatory responses; the theoretical background for their possible predictive value in cancer-related prognosis lies in the close association of chronic inflammation and carcinogenesis.
The retrospective study was the first of its kind to look at the combination of inflammatory markers and tumor radiological response as competitive risk factors using multivariate analysis. The regression analysis of risk factors for post-LT recurrence was performed taking in account radiological (morphological and response to therapy) and biological variables (AFP, NLR and PLR, including their slopes) at two well-defined time-points (before starting TACE therapy and immediately before LT). The results are reliable and convincing, and better reference value to predict the tumor recurrence and allow a proper selection of TACE-treated candidates for LT.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW, Qin JM, Zhang X S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 2. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5305] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 3. | Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 438] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 4. | Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, Lipshutz G, Yersiz H, Lu DS, Lassman C. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502-509; discussion 509-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 6. | Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 7. | Sotiropoulos GC, Malagó M, Molmenti E, Paul A, Nadalin S, Brokalaki E, Kühl H, Dirsch O, Lang H, Broelsch CE. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumor classification before transplantation realistic? Transplantation. 2005;79:483-487. [PubMed] |
| 8. | Shah SA, Tan JC, McGilvray ID, Cattral MS, Cleary SP, Levy GA, Greig PD, Grant DR. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation. 2006;81:1633-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 10. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 11. | Bargellini I, Vignali C, Cioni R, Petruzzi P, Cicorelli A, Campani D, De Simone P, Filipponi F, Bartolozzi C. Hepatocellular carcinoma: CT for tumor response after transarterial chemoembolization in patients exceeding Milan criteria--selection parameter for liver transplantation. Radiology. 2010;255:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3293] [Article Influence: 219.5] [Reference Citation Analysis (36)] |
| 14. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 15. | Kim DJ, Clark PJ, Heimbach J, Rosen C, Sanchez W, Watt K, Charlton MR. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant. 2014;14:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Bargellini I, Bozzi E, Campani D, Carrai P, De Simone P, Pollina L, Cioni R, Filipponi F, Bartolozzi C. Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: CT-pathologic correlation in 178 liver explants. Eur J Radiol. 2013;82:e212-e218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Kim HD, Shim JH, Kim GA, Shin YM, Yu E, Lee SG, Lee D, Kim KM, Lim YS, Lee HC. Optimal methods for measuring eligibility for liver transplant in hepatocellular carcinoma patients undergoing transarterial chemoembolization. J Hepatol. 2015;62:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, Suh DJ. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 19. | Jung ES, Kim JH, Yoon EL, Lee HJ, Lee SJ, Suh SJ, Lee BJ, Seo YS, Yim HJ, Seo TS. Comparison of the methods for tumor response assessment in patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Hepatol. 2013;58:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 20. | Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, Goffette P, Vogel W, Pitton MB, Lerut J. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | El-Gazzaz G, Sourianarayanane A, Menon KVN, Sanabria J, Hashimoto K, Quintini C, Kelly D, Eghtesad B, Miller C, Fung J. Radiologic-histological correlation of hepatocellular carcinoma treated via pre-liver transplant locoregional therapies. Hepatobiliary & Pancreatic Diseases International. 2013;12:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Odisio BC, Galastri F, Avritscher R, Afonso BB, Segatelli V, Felga GE, Salvalaggio PR, Ensor J, Wallace MJ, Nasser F. Hepatocellular carcinomas within the Milan criteria: predictors of histologic necrosis after drug-eluting beads transarterial chemoembolization. Cardiovasc Intervent Radiol. 2014;37:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 24. | Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, Kim IH, Yi NJ, Lee KU. A revised scoring system utilizing serum alphafetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery. 2007;141:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 379] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Dumitra TC, Dumitra S, Metrakos PP, Barkun JS, Chaudhury P, Deschênes M, Paraskevas S, Hassanain M, Tchervenkov JI. Pretransplantation α-fetoprotein slope and milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation. 2013;95:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-994.e3; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 724] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 28. | Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Bertuzzo VR, Cescon M, Ravaioli M, Grazi GL, Ercolani G, Del Gaudio M, Cucchetti A, D’Errico-Grigioni A, Golfieri R, Pinna AD. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 31. | Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013;96:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Parisi I, Tsochatzis E, Wijewantha H, Rodríguez-Perálvarez M, De Luca L, Manousou P, Fatourou E, Pieri G, Papastergiou V, Davies N. Inflammation-based scores do not predict post-transplant recurrence of hepatocellular carcinoma in patients within Milan criteria. Liver Transpl. 2014;20:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Ciccarelli O, Lai Q, Goffette P, Finet P, De Reyck C, Roggen F, Sempoux C, Doffagne E, Reding R, Lerut J. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int. 2012;25:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Lai Q, Avolio AW, Manzia TM, Sorge R, Agnes S, Tisone G, Berloco PB, Rossi M. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant. 2012;26:E125-E131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 37. | Yoshizumi T, Ikegami T, Yoshiya S, Motomura T, Mano Y, Muto J, Ikeda T, Soejima Y, Shirabe K, Maehara Y. Impact of tumor size, number of tumors and neutrophil-to-lymphocyte ratio in liver transplantation for recurrent hepatocellular carcinoma. Hepatol Res. 2013;43:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 785] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 39. | Takada Y, Tohyama T, Watanabe J. Biological markers of hepatocellular carcinoma for use as selection criteria in liver transplantation. J Hepatobiliary Pancreat Sci. 2015;22:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Otto G, Schuchmann M, Hoppe-Lotichius M, Heise M, Weinmann A, Hansen T, Pitton MP. How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol. 2013;59:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Rahimi RS, Trotter JF. Liver transplantation for hepatocellular carcinoma: outcomes and treatment options for recurrence. Ann Gastroenterol. 2015;28:323-330. [PubMed] |
| 42. | De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 43. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688-701; discussion 701-703. [PubMed] |
| 45. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 46. | De Carlis L, Di Sandro S, Giacomoni A, Slim A, Lauterio A, Mangoni I, Mihaylov P, Pirotta V, Aseni P, Rampoldi A. Beyond the Milan criteria: what risks for patients with hepatocellular carcinoma progression before liver transplantation? J Clin Gastroenterol. 2012;46:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Agopian VG, Morshedi MM, McWilliams J, Harlander-Locke MP, Markovic D, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Hiatt JR. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg. 2015;262:536-545; discussion 543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 48. | Allard MA, Sebagh M, Ruiz A, Guettier C, Paule B, Vibert E, Cunha AS, Cherqui D, Samuel D, Bismuth H. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 49. | Riaz A, Lewandowski RJ, Kulik L, Ryu RK, Mulcahy MF, Baker T, Gates V, Nayar R, Wang E, Miller FH. Radiologic-pathologic correlation of hepatocellular carcinoma treated with chemoembolization. Cardiovasc Intervent Radiol. 2010;33:1143-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Riaz A, Memon K, Miller FH, Nikolaidis P, Kulik LM, Lewandowski RJ, Ryu RK, Sato KT, Gates VL, Mulcahy MF. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic-pathologic correlation. J Hepatol. 2011;54:695-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Shim JH, Han S, Shin YM, Yu E, Park W, Kim KM, Lim YS, Lee HC. Optimal measurement modality and method for evaluation of responses to transarterial chemoembolization of hepatocellular carcinoma based on enhancement criteria. J Vasc Interv Radiol. 2013;24:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Vouche M, Kulik L, Atassi R, Memon K, Hickey R, Ganger D, Miller FH, Yaghmai V, Abecassis M, Baker T. Radiological-pathological analysis of WHO, RECIST, EASL, mRECIST and DWI: Imaging analysis from a prospective randomized trial of Y90 ± sorafenib. Hepatology. 2013;58:1655-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, Foulis AK, Horgan PG, McMillan DC. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633-2641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 606] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 54. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 55. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8170] [Article Influence: 544.7] [Reference Citation Analysis (0)] |
| 56. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11271] [Article Influence: 490.0] [Reference Citation Analysis (2)] |
| 57. | Limaye AR, Clark V, Soldevila-Pico C, Morelli G, Suman A, Firpi R, Nelson DR, Cabrera R. Neutrophil-lymphocyte ratio predicts overall and recurrence-free survival after liver transplantation for hepatocellular carcinoma. Hepatol Res. 2013;43:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 58. | Na GH, Kim DG, Han JH, Kim EY, Lee SH, Hong TH, You YK. Inflammatory markers as selection criteria of hepatocellular carcinoma in living-donor liver transplantation. World J Gastroenterol. 2014;20:6594-6601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, Zhu HB, Zhang Q, Chen GH. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6:e25295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Xia W, Ke Q, Wang Y, Wang W, Zhang M, Shen Y, Wu J, Xu X, Zheng S. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J Surg Oncol. 2015;13:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |