Published online May 14, 2017. doi: 10.3748/wjg.v23.i18.3367
Peer-review started: January 22, 2017
First decision: February 10, 2017
Revised: February 19, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: May 14, 2017
Processing time: 113 Days and 6.5 Hours
To evaluate the efficacy of antimicrobial susceptibility-guided therapy before first-line treatment for infection in patients with dual or triple antibiotic resistance.
A total of 1034 patients infected by Helicobacter pylori (H. pylori) during 2013-2014 were tested for antimicrobial susceptibility. 157 of 1034 (15%) patients showed resistance to two (127/1034; 12%) and to three (30/1034; 3%) antibiotics. Sixty-eight patients with dual H. pylori-resistance (clarithromycin, metronidazole or levofloxacin) were treated for 10 d with triple therapies: OAL (omeprazole 20 mg b.i.d., amoxicillin 1 g b.i.d., and levofloxacin 500 mg b.i.d.) 43 cases, OAM (omeprazole 20 mg b.i.d., amoxicillin 1 g b.i.d., and metronidazole 500 mg b.i.d.) 12 cases and OAC (omeprazole 20 mg b.id., amoxicillin 1 g b.i.d., and clarithromycin 500 mg b.i.d.) 13 cases based on the antimicrobial susceptibility testing. Twelve patients showed triple H. pylori-resistance (clarithromycin, metronidazole and levofloxacin) and received for 10 d triple therapy with OAR (omeprazole 20 mg b.id., amoxicillin 1 g b.i.d., and rifabutin 150 mg b.i.d.). Eradication was confirmed by 13C-urea breath test. Adverse effects and compliance were assessed by a questionnaire.
Intention-to-treat eradication rates were: OAL (97.6%), OAM (91.6%), OAC (92.3%) and OAR (58.3%). Cure rate was significantly higher in naïve patients treated with OAR-10 compared to patients who had two or three previous treatment failures (83% vs 33%). Adverse events rates for OAL, OAM, OAC and OAR were 22%, 25%, 23% and 17%, respectively, all of them mild-moderate.
Antimicrobial susceptibility-guided triple therapies during 10 d for first-line treatment leads to an eradication rate superior to 90% in patients with dual antibiotic H. pylori resistance.
Core tip: This study evaluated the efficacy of antimicrobial susceptibility-guided therapy: proton-pump inhibitor and two or three antibiotics for ten days before first-line treatment in patients with dual or triple Helicobacter pylori resistance to clarithromycin, metronidazole and/or levofloxacin. Intention to treat analysis demonstrates that eradication rates in patients with dual Helicobacter pylori resistance are high (around 95%). Cure rate was significantly higher in naive patients with dual resistance compared to those with triple resistance (95% vs 83%).
- Citation: Cosme A, Montes M, Ibarra B, Tamayo E, Alonso H, Mendarte U, Lizasoan J, Herreros-Villanueva M, Bujanda L. Antimicrobial susceptibility testing before first-line treatment for Helicobacter pylori infection in patients with dual or triple antibiotic resistance. World J Gastroenterol 2017; 23(18): 3367-3373
- URL: https://www.wjgnet.com/1007-9327/full/v23/i18/3367.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i18.3367
Helicobacter pylori (H. pylori) eradication therapy success is compromised mainly due to antibiotic resistance. Resistance prevalence varies by country and within same region by periods of time. Among possible causes of failure are the increased use of different antibiotics in the general population- adult and children for dental, respiratory, gynecological and parasitic infectious diseases.
Prevalence of H. pylori antibiotic resistance has been increasing remarkably worldwide and concordantly, eradication rate has been declining globally[1]. Since multiresistance is frequently developed against most commonly implemented antibiotics and this phenomena leads to an increasing inadequate success rates, new therapeutic strategies are urgently needed.
In Gipuzkoa, a region in Northern Spain, during 2006-2012 the prevalence of clarithromycin-resistant H. pylori varied from 15.7% to 21.6% and from 32% to 44.3% for metronidazole. Between 2000 and 2012, the resistance to two or three antibiotics (clarithromycin, metronidazole, or/and levofloxacin) was observed in 14.8% of 5998 H. pylori isolates tested for antimicrobial susceptibility[2].
The objective of our study was to evaluate the efficacy of antimicrobial susceptibility-guided therapy: proton-pump inhibitor (PPI) and two antibiotics for ten days before first-line treatment in patients with dual H. pylori-resistance (clarithromycin, metronidazole or levofloxacin) or triple H. pylori-resistance (clarithromycin, metronidazole and levofloxacin).
This was a prospective observational study including all patients infected by H. pylori during 2013 and 2014 in our region. Susceptibility testing was performed in all strains isolates from gastric biopsies. Exclusion criteria included severe concomitant disease (insulin-dependent type I diabetes mellitus and neoplastic diseases), previous gastric surgery, pregnancy or lactation, and allergy to any of the drugs used in the study.
This study was conducted in accordance with the principles of the Declaration of Helsinki, ICH Guidelines for Good Clinical Practice and full conformity with relevant regulations.
For each patient, two antral and two corpus biopsies were taken for the bacterial culture. Susceptibility testing was assessed by E-test (BioMerieux) in 1034 patients. Primary and relapse isolates were included. E-test strips were placed on Brucella agar plates with 5% hemolized horse blood, supplemented with 1% Vitox and were incubated at 37 °C for 48-72 h under microaerophilic conditions with 80% humidity[3].
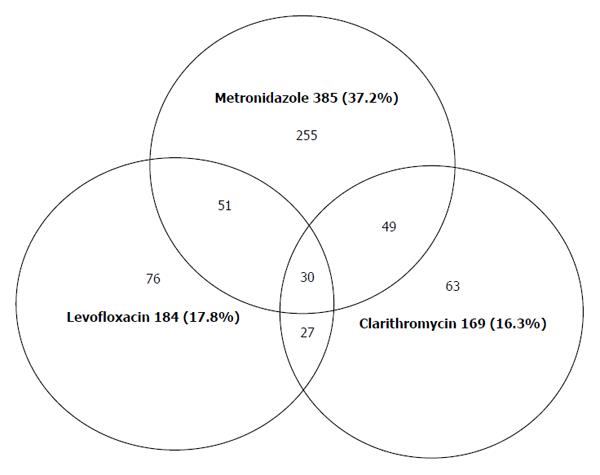
It was observed that 53.3% (551/1034) of isolates were resistant to at least one antibiotic, 12.3% of them to two antibiotics and 2.9% to three antibiotics (Figure 1). In Gipuzkoa the rate of H. pylori amoxicillin resistance was 0%. It is well known that resistance to clarithromycin and fluoroquinolone are mainly codified in the 23SrRNA and gyrA genes, respectively.
Patients with dual H. pylori-resistance were treated based on the antimicrobial susceptibility results: (1) levofloxacin-based triple therapy: omeprazole 20 mg b.i.d., amoxicillin 1 g b.i.d., and levofloxacin 500 mg b.i.d. (OAL), if the H. pylori-isolates were resistant to clarithromycin and metronidazole but sensitive to levofloxacin; (2) metronidazole-based triple therapy: omeprazole 20 mg b.i.d., amoxicillin 1 g b.i.d., and metronidazole 500 mg b.i.d. (OAM), if the H. pylori-isolates were resistant to both clarithromycin and levofloxacin but sensitive to metronidazole; and (3) clarithromycin-based triple therapy: omeprazole 20 mg b.i.d., amoxicillin 1 g b.i.d., and clarithromycin 500 mg b.i.d. (OAC), if the H. pylori-isolates were resistant to metronidazole and levofloxacin but sensitive to clarithromycin, in all cases twice a day for 10 d.
Patients with triple H. pylori-resistance to clarithromycin, metronidazole and levofloxacin received rifabutin-based triple therapy: omeprazole 20 mg b.i.d., amoxicillin 1 g b.i.d., and rifabutin 150 mg b.i.d. (OAR) for 10 d. No patients had previously received rifampicin and 6 of them referred two or three H. pylori eradication failures (3 with OAC and bismuth quadruple therapy, 2 with OAC, bismuth quadruple therapy and OAL, and 1 with OAC and OAL, in all cases for 10 d).
Treatment was clearly explained to all patients. Drugs were self-administered orally after meals. Drugs used in all groups were generic branding.
Primary end point: confirmed H. pylori eradication by intention-to-treat (ITT) in each group of at least 6 wk after treatment completion using the urea breath test (UBT) with 100 mg of urea, in accordance with a previously described protocol[3].
Secondary end point: adherence to treatment regimen and adverse events associated with treatment. Adherence to treatment was defined as in take of -at least- 90% of the medication prescribed assessed by using a questionnaire and counting empty medication sachets returned. Side effects were evaluated with a specific postreatment questionnaire completed. Depending of the intensity, adverse effects were classified by physicians as mild (symptoms appear but do not interfere with daily life), moderate (symptoms interfere with daily life) or severe (symptoms prevent daily life or requires discontinuation of the drug).
A total of 1034 H. pylori-positive consecutive adult patients with antimicrobial-susceptibility testing were enrolled from January 2013 to December 2014 in Gipuzkoa (Basque Country), a region in Northern Spain of around 700000 residents. Considering the total of patients, dual H. pylori-resistance to two antimicrobials (clarithromycin, metronidazole or levofloxacin) was observed in 127 (12.3%) and triple H. pylori-resistance to three antimicrobials in 30 (2.9%) of them. Patients with dual H. pylori-resistance were treated with OAL-10, OAM-10 or OAC-10 d, and patients with triple H. pylori-resistance with OAR-10 d. A subgroup of 80 patients (68 with dual H. pylori-resistance, and 12 with triple H. pylori-resistance) attending to Donostia Hospital (San Sebastián, Spain) were only included. The population represented in this area of San Sebastián is around 393000 residents (Figure 2).
Baseline characteristics of each group of patients, efficacy of therapy on H. pylori-eradication, compliance and adverse events are shown in Table 1. ITT analysis demonstrated that eradication rates in patients with dual H. pylori-resistance treated with OAL-10, OAM-10, and OAC-10 were 97.6% (42/43), 91.6% (11/12) and 92.3% (12/13), respectively, and with triple H. pylori-resistance treated with OAR-10, 58.3% (7/12). The eradication rate in naïve patients with OAR-10 was 83% (5/6) and 33% (2/6) in patients after previous regimen failures (OAC and bismuth quadruple therapy, and OAC, bismuth quadruple therapy and OAL, respectively).
| Dual H. pylori - resistance | Triple H. pylori- resistance | |||
| C + M | C + L | M + L | C + M + L | |
| OAL-10 | OAM-10 | OAC-10 | OAR-10 | |
| (43) | (12) | (13) | (12) | |
| Gender (females) | 70% | 67% | 69% | 67% |
| Age (yr) | ||||
| Mean | 51.6 | 52.8 | 50 | 53.3 |
| Median (range) | 51 (18-76) | 52 (24-76) | 50 (31-75) | 53 (42-77) |
| Indication | ||||
| Dyspepsia | 95% | 83% | 84% | 75% |
| Ulcer | 5% | 17% | 16% | 25% |
| ITT eradication | 42/43 (97.6%) | 11/12 (91.6%) | 12/13 (92.3%) | 7/12 (58.3%) |
| Compliance | 93% | 93% | 93% | 94% |
| Adverse events | 22% | 25% | 23% | 17% |
Seventy-five out of 80 patients with dual or triple H. pylori-resistance showed 100% compliance to prescribed medications. No significant differences were observed in compliance between patients with dual and triple H. pylori-resistance (93% vs 94% respectively).
Adverse events rate for OAL, OAM, OAC, and OAR were 22% (abdominal pain in 3 patients, nausea in 3 patients, asthenia in 1 patient, diarrhea in 1 patient and metallic taste in 1 patient), 25% (myalgia in 1 patient and metallic taste in 2 patients), 23% (diarrhea in 1 patient, nausea in 1 patient and metallic taste in 1 patient) and 17% (headache in 1 patient, and increased transaminases in 1 patient) respectively, all of them classified as mild to moderate.
In this study we demonstrated that in patients with dual clarithromycin, levofloxacin or metronidazole resistance, antimicrobial susceptibility-guided therapy including a combination of PPI and two antibiotics for 10 d leads to an eradication rate (evaluated by ITT) of 95.5%.
In our reference center-Donostia Hospital, cultures are carried out as “routine practice” since 1994[4]. During 1994-1998 dual H. pylori-resistance to two antimicrobials (clarithromycin, metronidazole or levofloxacin) was 10.5%[4,5]. In Gipuzkoa (Basque country), from 2000-2012 the dual resistance was observed in 12.4% of isolates of H. pylori[2], being 12.3% considering the period 2013-2014. By contrast, multiple H. pylori-resistance to three antimicrobials increased from 2.4% during the period 2000-2012 to 2.9% for the period 2013-2014.
Patients with dual or triple resistance represents a new scenario to evaluate efficacy of current antibiotics regimens. According to the Maastrich V/Florence Consensus Conference on managing H. pylori, bismuth quadruple therapy (BQT) for first-line treatment or non-bismuth quadruple concomitant therapy (non-BQT) as alternative were recommended when clarithromycin resistance of H. pylori is > 15%, metronidazole H. pylori-resistance is less than 40% and dual clarithromycin and metronidazole H. pylori-resistance is < 15%[6].
In patients with dual clarithromycin and metronidazole H. pylori resistance, Georgopoulos et al[7], reported 78% (18 out 23) of eradication rates when non-bismuth quadruple concomitant therapy (PPI regime plus amoxicillin, clarithromycin and metronidazole for 10 d) was prescribed and 33% (9 out 27) with quadruple sequential therapy administration (5 d PPI plus amoxicillin, followed by a further 5 d with clarithromycin and metronidazole). Recently, in the European H. pylory Registry (31 countries and 18270 pacients with complete treatment, 12% of them with culture and antibiogram), eradication rates with quadruple concomitant and sequential therapy were 80% (4 of 5) and 62.5% (5 of 8) respectively[8].
On the other hand, the eradication rate with bismuth quadruple therapy for 10 d was lower in pretreated patients with metronidazole resistance[9,10]. Furthermore, different studies analyzing bismuth quadruple therapy concluded that in vitro resistant strains to metronidazole could be effectively heated by using higher doses of metronidazole administrated for 14 d[11,12]. However, this classic bismuth quadruple therapy requires a complex scheme of administration, increases side effects and it is not worldwide available due to distribution restrictions.
A number of strategies have been recommended to potentially overcome dual H. pylori-resistance (clarithromycin and metronidazole) including longer duration of quadruple therapies (concomitant-14 d, bismuth -14 d), higher dose of new-generations - PPIs (esomeprazole or rabeprazole), hybrid therapy (a 7 d of PPI plus amoxicillin, followed by 7 d of PPI, amoxicillin, clarithromycin and metronidazole) or quadruple therapy with levofloxacin and bismuth - 14 d (PPI regime plus amoxicillin, bismuth and levofloxacin)[13-16]. Quadruple sequential therapy (14 d), hybrid therapy and non-bismuth quadruple concomitant therapy (14 d) are expected to fail if the rate of dual clarithromycin and metronidazole resistant strains are > 5%, > 9%, and > 15%, respectively[7]. Our results showed that antimicrobial susceptibility-guided triple therapies for 10 d in a population with clarithromycin - H. pylori resistance > 16% and dual H. pylori-resistance (clarithromycin, metronidazole or levofloxacin) > 12% was more effective than quadruple therapies for first-line (BQT or non-BQT) for first-line treatment.
In this study eradication rate of OAR for 10 d in naïve patients with triple H. pylori-resistance (clarithromycin, metronidazole and levofloxacin) was 83% (5 of 6) vs 33% (2 of 6) in patients after two or three previous H. pylori treatment failures. Several studies have confirmed that most of rifabutin-resistant isolates of H. pylori were obtained from patients after treatment failures, suggesting that previous unsuccessful attempts of eradication seem to be an important risk factor for the development of resistance to rifabutin and/or multiple resistance[17,18]. Rifabutin rescue regimen is effective after multiple previous H. pylori eradication failures[19,20]. A recent study, involving nine Spanish hospitals and 100 consecutive patients, supports that the use of rifabutin-based rescue therapy in patients with three H. pylori eradication failures (OAC, BQT, and OAL, in all cases for 10 d) may be effective in approximately 50% of the cases[21].
Different virulence factors play an important role in the pathogenesis of H. pylori and treatment resistance. For instance, a significant association has been found between dupA1 genotype and A214G clarithromycin resistance mutation by Hussein et al[22]. Further molecular studies are needed in order to clarify biomarkers that could causes of H. pylori resistance.
Some of the limitations of this study were: first, the number of patients is too limited to draw definitive conclusions on the efficacy of these therapies. Second, rifabutin resistance evaluation was not assessed in patients treated with rifabutin-based triple therapy either before or after administration.
Dual or triple H. pylori-resistance to various families of antimicrobials may be addressed by the adequate use of antibiotics, in a cost-effective manner and shortening the regimens as much as possible.
In summary, triple therapies including PPI and two antibiotics for 10 d, based in the knowledge of pretreatment antimicrobial susceptibility showed good eradication rates of H. pylori infection in our region with an increased prevalence of dual or triple drugs H. pylori-resistance (clarithromycin, metronidazole and/or levofloxacin).
Frequency of dual or triple antimicrobial resistance has been rising during the last decade and Helicobacter pylori (H. pylori) infections are an important challenge for physicians. To tailor recommendations for optimal treatments, efficacy evaluations must be performed in different geographical areas.
H. pylori eradication therapy success is compromised mainly due to antibiotic resistance and numerous researchers are trying to overcome multiresistance. Resistance prevalence varies by country and within same region, by periods of time. The aim of this study was to evaluate the efficacy of antimicrobial susceptibility-guided therapy before first-line treatment for infection in patients with dual or triple antibiotic resistance.
In this study the authors demonstrated that in patients with dual clarithromycin, levofloxacin or metronidazole resistance, antimicrobial susceptibility-guided therapy including a combination of PPI and two antibiotics for 10 d leads to an eradication rate of 95.5%. Compared to different strategies previously purposed, our results shows that antimicrobial susceptibility-guided triple therapies for 10 d in a population with clarithromycin - H. pylori resistance > 16% and dual H. pylori-resistance (clarithromycin, metronidazole or levofloxacin) > 12% was more effective than quadruple therapies for first-line treatment of infections.
Dual or triple H. pylori resistance to various families of antimicrobials may be addressed by the adequate use of antibiotics in a cost-effective manner that allows shorter regimens.
Common therapies for H. pylori treatment. Triple therapies: OAL (omeprazole 20 mg b.id., amoxicillin 1 g b.i.d., and levofloxacin 500 mg b.i.d.) 43 cases, OAM (omeprazole 20 mg b.id., amoxicillin 1 g b.i.d., and metronidazole 500 mg b.i.d.) 12 cases and OAC (omeprazole 20 mg b.id., amoxicillin 1 g b.i.d., and clarithromycin 500 mg b.i.d.) 13 cases based on the antimicrobial susceptibility testing. Twelve patients showed triple H. pylori-resistance and received for 10 d triple therapy with OAR (omeprazole 20 mg b.id., amoxicillin 1 g b.i.d., and rifabutin 150 mg b.i.d.).
In the present paper, Cosme et al evaluated the impact of antimicrobial resistance in H. pylori in the first line eradication therapy. Starting from a sample size of 1034 patients undergoing culture, they selected cases with double or triple resistance and assigned a tailored triple therapy (omeprazole + amoxicillin + clarithromycin or metronidazole or levofloxacin or rifabutin). Therefore, they demonstrated a success rate > 90% in all regimens, excluding when rifabutin was adopted (58.3%) in triple resistant strains.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Emara MH, Hussein NR, Ierardi E, Yamaoka Y S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 552] [Article Influence: 61.3] [Reference Citation Analysis (2)] |
| 2. | Fernandez-Reyes M, Montes M, Lizasoain J, Piñeiro L, Bujanda L, Perez-Trallero E. Increased trend in Helicobacter pylori antimicrobial resistance during a 13 year period (2000-2012, Gipuzkoa, Basque Country, Spain). Helicobacer. 2013;18:103. |
| 3. | Martos M, Bujanda L, Salicio Y, Sarasqueta C, Ibarra B, Mendarte U, Fernández-Reyes M, Cosme A. Clarithromycin for first-line treatment of Helicobacter pylori infection after culture in high-resistance regions. Eur J Gastroenterol Hepatol. 2014;26:1380-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Perez-Trallero E, Montes M, Larrañaga M, Arenas JI. How long for the routine Helicobacter pylori antimicrobial susceptibility testing? The usefulness of the string test to obtain Helicobacter for culture. Am J Gastroenterol. 1999;94:3075-3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Cosme A, Montes M, Martos M, Gil I, Mendarte U, Salicio Y, Piñeiro L, Recasens MT, Ibarra B, Sarasqueta C. Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clin Microbiol Infect. 2013;19:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1973] [Article Influence: 246.6] [Reference Citation Analysis (1)] |
| 7. | Georgopoulos SD, Xirouchakis E, Mentis A. Is there a nonbismuth quadruple therapy that can reliably overcome bacterial resistance? Gastroenterology. 2013;145:1496-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | McNicholl AG, Tepes B, Gasbarrini A, Pérez-Aisa A, Vaira D, Bordin DS, Lerang F, Castro M, Bujanda L. Registro Europeo del manejo de H. pylori (HP-Eureg): Análisis intermedio resistencia antibiótica. Gastroenterol Hepatol. 2016;39:144. |
| 9. | Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Graham DY, Lee SY. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol Clin North Am. 2015;44:537-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Mégraud F. The challenge of Helicobacter pylori resistance to antibiotics: the comeback of bismuth-based quadruple therapy. Therap Adv Gastroenterol. 2012;5:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;145:121-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Molina-Infante J, Gisbert JP. Optimizing clarithromycin-containing therapy for Helicobacter pylori in the era of antibiotic resistance. World J Gastroenterol. 2014;20:10338-10347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Mc Nicholl AG, Molina-Infantae J, Bermejo F, Yarb H, Ferrer-Barcelo L, Modolell I, Anton R, Alcedo J, Angeles Perez-Aisa M, Barenys M. Non bismuth quadruple “concomitant”therapies in the eradication of Helicobacter pylori. Standard vs optimized (14 days, high-dose PPI) regimes in clinical practice. United Eur Gastroenterol J. 2015;3:A65. |
| 16. | Gisbert JP, Molina-Infante J, Amador J, Bermejo F, Bujanda L, Calvet X, Castro-Fernández M, Cuadrado-Lavín A, Elizalde JI, Gene E. IV Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol Hepatol. 2016;39:697-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Heep M, Lehn N, Brandstätter B, Rieger U, Senzenberger S, Wehrl W. Detection of rifabutin resistance and association of rpoB mutations with resistance to four rifamycin derivatives in Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2002;21:143-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Glocker E, Bogdan C, Kist M. Characterization of rifampicin-resistant clinical Helicobacter pylori isolates from Germany. J Antimicrob Chemother. 2007;59:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Van der Poorten D, Katelaris PH. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment Pharmacol Ther. 2007;26:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Gisbert JP, Calvet X. Review article: rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2012;35:209-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Hussein NR, Tunjel I, Majed HS, Yousif ST, Aswad SI, Assafi MS. Duodenal ulcer promoting gene 1 (dupA1) is associated with A2147G clarithromycin-resistance mutation but not interleukin-8 secretion from gastric mucosa in Iraqi patients. New Microbes New Infect. 2015;6:5-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |