Published online May 14, 2017. doi: 10.3748/wjg.v23.i18.3269
Peer-review started: January 20, 2017
First decision: February 9, 2017
Revised: April 25, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: May 14, 2017
Processing time: 114 Days and 14.4 Hours
To investigate the effects of sleeve gastrectomy plus trunk vagotomy (SGTV) compared with sleeve gastrectomy (SG) in a diabetic rat model.
SGTV, SG, TV and Sham operations were performed on rats with diabetes induced by high-fat diet and streptozotocin. Body weight, food intake, oral glucose tolerance test, homeostasis model assessment of insulin resistance (HOMA-IR), hepatic insulin signaling (IR, IRS1, IRS2, PI3K and AKT), oral glucose stimulated insulin secretion, GLP-1 and ghrelin were compared at various postoperative times.
Both SG and SGTV resulted in better glucose tolerance, lower HOMA-IR, up-regulated hepatic insulin signaling, higher levels of oral glucose-stimulated insulin secretion, higher postprandial GLP-1 and lower fasting ghrelin levels than the TV and Sham groups. No significant differences were observed between the SG and SGTV groups. In addition, no significant differences were found between the TV and Sham groups in terms of glucose tolerance, HOMA-IR, hepatic insulin signaling, oral glucose-stimulated insulin secretion, postprandial GLP-1 and fasting ghrelin levels. No differences in body weight and food intake were noted between the four groups.
SGTV is feasible for diabetes control and is independent of weight loss. However, SGTV did not result in a better improvement in diabetes than SG alone.
Core tip: To investigate the effects of sleeve gastrectomy plus trunk vagotomy (SGTV) compared with sleeve gastrectomy (SG) in a diabetic rat model, SGTV, SG, TV and Sham operations were performed on diabetic rats. The result showed that SG and SGTV resulted in better glucose regulation, but SGTV did not result in a better improvement in diabetes than SG alone.
- Citation: Liu T, Zhong MW, Liu Y, Huang X, Cheng YG, Wang KX, Liu SZ, Hu SY. Effects of sleeve gastrectomy plus trunk vagotomy compared with sleeve gastrectomy on glucose metabolism in diabetic rats. World J Gastroenterol 2017; 23(18): 3269-3278
- URL: https://www.wjgnet.com/1007-9327/full/v23/i18/3269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i18.3269
Bariatric surgery has evolved since the 1950s and has become the most effective treatment for morbid obesity, and results in a marked improvement in weight loss and serious obesity-related comorbidities, especially type 2 diabetes mellitus (T2DM). Recently, bariatric surgery has been included in the treatment algorithm of T2DM and is accepted worldwide by medical and scientific organizations.
In addition to bariatric surgery, gastrointestinal metabolic surgery has been widely accepted by most major bariatric surgery societies. According to a global survey, Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are the most frequently performed procedures worldwide. The number of SGs has markedly increased since 2003 and has exceeded RYGB as the most popular procedure in North America, the Asia/Pacific region and Europe. With accumulating evidence that SG induces weight loss and diabetes remission, the more easily performed SG compared with RYGB has been recognized as a stand-alone bariatric operation. Given that records of follow-up after SG are few, further long-term surveillance is necessary. Furthermore, the anti-diabetic effect of SG seems to be inferior to RYGB[1,2]. Therefore, RYGB, rather than SG, is still accepted as the gold-standard procedure for diabetes control.
Research on the mechanisms of improvement in diabetes after bariatric surgery[3] has shown that the small intestine plays a key role in this mechanism, which involves the roles of gut hormones, bile acid metabolism, nutrient sensing, incretins, and the gut microbiome which are induced by bypass of the proximal intestinal and rapid distal gut nutrient delivery. Moreover, novel bariatric techniques involving only the small intestine, such as duodenal-jejunal bypass and ileal transposition, performed on rodents or patients, proved to have marked effects on glucose metabolism[4].
Based on these findings, and with the purpose of enhancing the effect of SG on diabetes control, pioneer surgeons developed novel procedures and combined various procedures with SG, involving bypass or transposition of different parts of the small intestine. These novel procedures include SG plus (SG+) single-anastomosis duodeno-ileal bypass (SADI-S)[5], SG plus duodenal-jejunal bypass (SG-DJB), SG plus jejunal-jejunal bypass (SG-JJB), SG plus jejunal-ileal bypass (SG-JIB)[6], SG plus side-to-side jejunoileal anastomosis (SG-JIA), and SG plus ileal transposition (SG-IT) DA. Although all these SG+ procedures were proved to be feasible and effective for T2DM in rats and patients, randomized trials comparing the effects of SG+ and SG alone in patients are expected. In addition, all the SG+ procedures mentioned above involved operations on the small intestine and at least one extra anastomosis, which resulted in similar surgical risks to RYGB. Therefore, simpler SG+ procedures have yet to be developed.
The vagus nerve has multiple physiologic functions related to food intake, energy metabolism and glycemic control[7]. Preclinical and clinical studies have suggested that vagal interruption has effects on insulin secretion and hepatic glucose metabolism[7,8], and truncal vagotomy (TV) results in early satiety and weight loss[9-11]. Furthermore, electrical vagal nerve blockade is associated with significant excess weight loss and sustained improvements in HbA1c[12,13]. Therefore, we developed SG plus TV (SGTV), a less invasive procedure than other SG+ procedures requiring additional anastomoses, and performed SGTV on a rat model of diabetes. In this study, we compared SGTV with SG alone to evaluate the effect of vagotomy on glucose metabolism and compared TV with SGTV and SG to evaluate its effect on diabetes improvement, with an aim to determine whether SGTV is a feasible and safe procedure for inducing diabetes remission.
All experiments were approved by the Animal Care and Utilization Committee of Shandong University, Jinan, China. The animals were housed in separate independently ventilated cages, at a constant temperature of 24-26 °C, humidity of 50%-60%, a 12 h light/dark cycle, and had free access to tap water and food at the Laboratory Animal Center of Shandong University. Male Wistar rats (age, 8 wk, body weight, 160-180 g) were fed with a high-fat diet (HFD) (40% of calories as fat) rodent chow for 8 wk, and then injected with streptozotocin (STZ) intraperitoneally (35 mg/kg). Two weeks later, the rats were fasted overnight and received a three-hour long oral glucose tolerance test (OGTT, 1 g/kg glucose by gavage). Rats with a peak blood glucose of ≥ 11.1 mmol/L and ≤ 16.0 mmol/L were considered diabetic and selected for further studies.
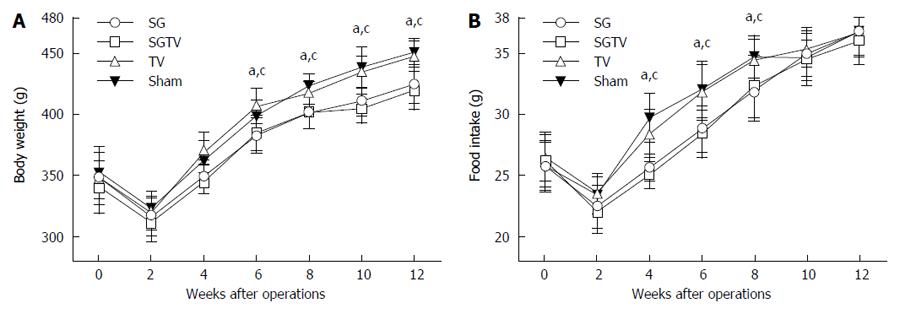
Forty diabetic rats were included in the study and randomly assigned to the SG (n = 10), SGTV (n = 10), TV (n = 10) and Sham (n = 10) groups. After surgery, all rats in the three treatment groups were given a common rodent chow for 12 wk. Body weight and food intake were monitored biweekly during the entire study.
Before surgery, all rats were fed 10% Ensure (Abbott Laboratories, UNI) for 2 d, and then fasted overnight. Rats were anesthetized with 10% chloral hydrate (3 mL/kg, Qilu Hospital, China) before surgery, and had access to water 2 h after surgery. Subsequently, the rats were fed 10% Ensure for 3 d, followed by common rodent chow until the end of the study.
SG: SG surgery involved (1) a 4-cm midline epigastric incision; (2) dissection of the gastric omentum to expose the cardium; (3) ligation of all vessels around the greater curvature using 7-0 silk suture (Ningbo Medical Needle, Co. Ltd., Ningbo, China); (4) removal of the fundus and most of the gastric body; and (5) closure of the remnant stomach using 5-0 silk suture (Ningbo Medical Needle, Co. Ltd.).
TV: TV surgery involved (1) a 4-cm midline abdominal incision; (2) dissociation of the perigastric ligaments and gentle manipulation of the stomach to reveal the esophagus and the trunks of the vagus; and (3) a 5 mm section from both the dorsal and ventral nerve trunks above the point of bifurcation into the celiac and gastric or hepatic and accessory celiac branches, respectively. Care was taken not to damage the esophagus and the left gastric artery.
SGTV: SGTV surgery involved the same 4-cm midline abdominal incision. TV was performed first followed by SG.
Sham operation: Rats in the Sham group underwent laparotomy to expose the stomach, esophagus, and vagus trunk around the esophagus. The operative time was prolonged to generate a comparable degree of anesthetic stress to that in the SGTV group. No other procedures were carried out.
OGTT: OGTT was performed at baseline, 4 and 12 wk postoperatively, and areas under the curves for OGTT (AUCOGTT) were calculated to evaluate the effect of diabetes control in each group. For the OGTT, rats were fasted overnight and administered 1 g/kg of glucose by oral gavage, and blood samples were obtained from the tail vein at baseline and 10, 30, 60, 120 and 180 min after gavage. Blood glucose was measured after gavage (1 g/kg) using a glucometer (Roche One Touch® Ultra; Lifescan, Johnson and Johnson, Milpitas, CA, United States).
Homeostasis model assessment of insulin resistance (HOMA-IR) was adopted as a surrogate of insulin sensitivity and calculated at baseline, 4 and 12 wk postoperatively according to the following formula: HOMA-IR = fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5.
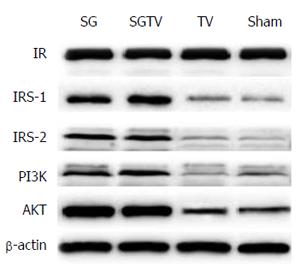
All rats were sacrificed at 12 wk postoperatively. Livers were sampled and immediately frozen in liquid nitrogen and stored at -80 °C until analysis. Alterations in the insulin signaling pathway, as indicated by the protein expression of insulin receptor (IR), insulin receptor substrate 1 (IRS-1), insulin receptor substrate 2 (IRS-2), PI3K, and AKT were determined by Western blotting. Samples were mechanically dissociated and lysed in radioimmunoprecipitation assay 37 (RIPA) buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L Na2-EDTA, 1% NP-40, 0.25% Na-deoxycholate) containing protease and phosphatase inhibitor cocktails (Roche, United States). Following brief sonication and heating, the supernatants were subjected to SDS-PAGE and transferred to PVDF membranes. Blots were incubated overnight at 4 °C with primary antibodies (anti-insulin receptor antibody; anti-IRS1 antibody; anti-IRS2 antibody; anti-PI3K P85 alpha antibody; anti-pan-AKT antibody; anti-beta-actin antibody, all from Abcam) and were then incubated with secondary antibodies (Abcam). Blots were visualized with an enhanced chemiluminescence reagent (Millipore) and quantified with Image Lab (Bio-Rad).
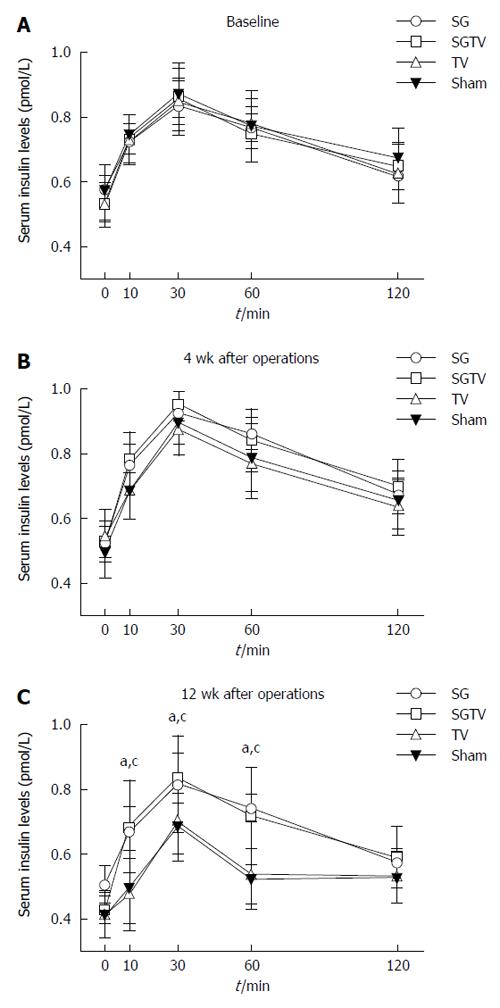
Oral glucose stimulated insulin secretion was measured using the serum samples as a surrogate index of β-cell function at baseline, and at 4 and 12 wk postoperatively. Rats were deprived of food overnight and then administered 1 g/kg of glucose by oral gavage. Blood was collected from the retrobulbar venous plexus at baseline and 10, 30, 60, and 120 min after gavage into tubes containing ethylenediaminetetraacetic acid (EDTA) and a dipeptidyl peptidase IV inhibitor. After centrifugation at 3000 rpm at 4 °C for 15 min, the separated serum was immediately removed to EP tubes and stored at -80 °C until analyzed. Insulin was measured with rat/mouse insulin ELISA kits (Merck Millipore, United States).
Total GLP-1 levels after glucose gavage and fasting serum ghrelin were measured in the serum collected at baseline, and at 4 and 12 wk postoperatively. GLP-1 was measured with multi-species GLP-1 total ELISA kits (Merck Millipore, United States). Ghrelin was measured with Rat/Mouse Ghrelin (total) ELISA (Merck Millipore, United States).
Quantitative data were expressed as mean ± SD. Data that were not normally distributed or did not satisfy homogeneity of variance were logarithmically transformed before analysis. Areas under curves for the OGTT (AUCOGTT) were calculated by trapezoidal integration. All statistical analyses were performed using SPSS Version 19.0. AUCOGTT, HOMA-IR, and ghrelin data at each time point, and band intensity of Western blots were compared by one-way analysis of variance (ANOVA). Body weight, food intake, postprandial insulin, and GLP-1 data were compared by two-factor repeated measures (RM) ANOVA. Post hoc analysis and adjustment using Bonferroni’s correction, were performed when necessary. Differences were considered significant when P value was < 0.05.
Ten rats were included in each experimental group preoperatively. When the study ended 12 wk after surgery, the numbers of rats alive in the SG, SGTV, TV and Sham groups were 8, 7, 7 and 7, respectively. The causes of death were diabetes complications (n = 5), anastomotic leakage (n = 2), and intestinal obstruction (n = 4).
As shown in Figure 1, there were no significant differences in body weight and food intake between the SG and SGTV group, or between the TV and Sham group preoperatively. Due to perioperative food restriction and surgical and anesthetic stress, body weight in all groups was minimal 1 wk postoperatively and was restored to preoperative values 2 wk after surgery.
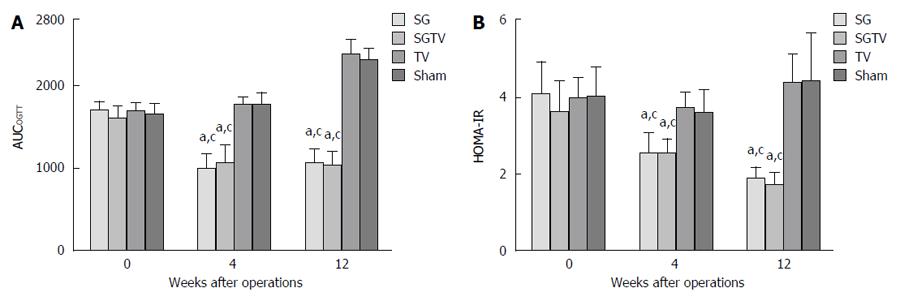
There were no significant differences in AUCOGTT between the groups preoperatively. Compared with the Sham group, the SG and SGTV groups both exhibited lower AUCOGTT 4 and 12 wk postoperatively (all P < 0.001). However, the TV group showed a similar AUCOGTT to the Sham group. Notably, the AUCOGTT in the SGTV groups were not statistically lower than that in the SG group after surgery, and there were significant differences in AUCOGTT at 4 and 12 wk postoperatively (all P < 0.001). There were no significant differences in AUCOGTT between the TV group and Sham group at 4 and 12 wk postoperatively (Figure 2A).
The HOMA-IR also showed no differences preoperatively between the groups. The SG group showed a significantly lower postoperative HOMA-IR than the TV and Sham groups. In addition, postoperative HOMA-IR in the TV group was statistically similar to that in the Sham group. However, the SGTV group showed no significant difference compared with the SG group in postoperative HOMA-IR, but it was still lower than that in the TV and Sham groups (Figure 2B).
As shown in Figure 3, the expression of hepatic IRS-1, IRS-2, PI3K and AKT increased in the SG and SGTV groups compared with the Sham group, indicating that the insulin signaling pathway was up-regulated in the liver. However, the expression of IR was not different between the groups. There was no statistical difference in the expression of IRS-1, IRS-2, PI3K and AKT between the SG and SGTV groups. The TV group showed similar expression of IRS-1, IRS-2, PI3K and AKT to the Sham group.
In order to verify the role of insulin secretion in improved glucose tolerance, we measured serum insulin concentration after an oral glucose load preoperatively and at 4 and 12 wk postoperatively. There were no significant differences in serum insulin levels between the four groups at 4 wk postoperatively, and the insulin levels in the SG and SGTV groups were higher than those in the TV and Sham groups at 12 wk postoperatively. Furthermore, there was no difference between the SG and SGTV groups, or the TV and Sham groups (Figure 4).
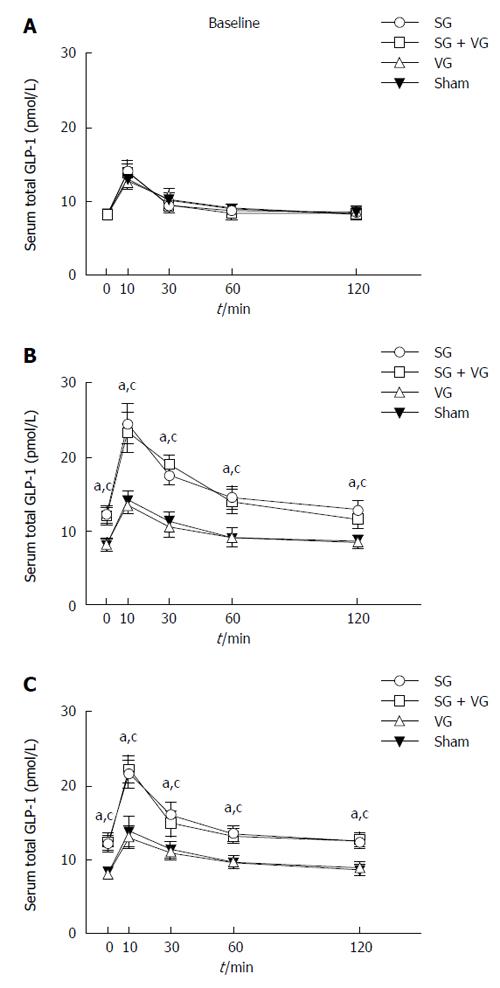
At 4 and 12 wk postoperatively, the SG and SGTV groups displayed both higher total and peak GLP-1 levels than the TV and Sham groups. No difference in GLP-1 levels was detected between the SG and SGTV groups, and between the TV and Sham groups at 4 or 12 wk postoperatively (Figure 5).
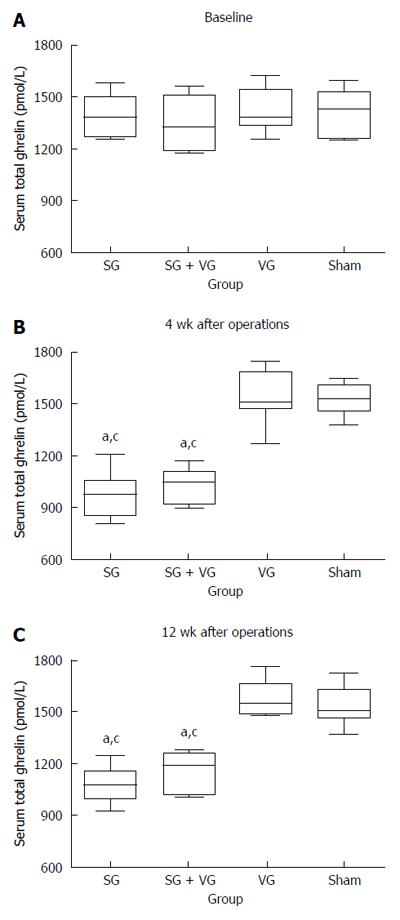
At 4 and 12 wk postoperatively, the SGTV group displayed significantly lower fasting serum ghrelin to that in the TV and Sham groups, but a similar level to that in the SG group. The TV group did not exhibit a significantly different fasting serum ghrelin level to that in the Sham group (Figure 6).
The vagus nerve provides innervation to the stomach, small intestine, liver and pancreas, and has an effect on gastrointestinal motility, hormone secretion and glucose metabolism[14]. TV has been extensively adopted to treat patients with refractory peptic ulcers[9]. Recently, TV was performed for the treatment of marginal ulcers after RYGB for morbid obesity[15]. Following vagotomy, loss of appetite, food and liquid intake and weight loss or failure to regain weight were observed, and it was proposed that TV useful for the treatment of severe obesity[16,17]. TV alone as a treatment for severe obesity did not gain further attention, possibly due to slower weight loss, than gastrointestinal bypass and vertical banded gastroplasty (VBG), the predominant procedure in the 1980s and 1990s. TV was then combined with VGB which provided enhanced excess weight loss at 5 years compared with VGB alone[18]. TV has also been reported in combination with adjustable gastric banding (AGB) and RYGB[19-21]. Angrisani et al[19] and Martin et al[20] added TV to AGB and compared it with AGB alone in prospective studies, and found that TV reduced band adjustment, but did not enhance weight loss. RYGB with TV resulted in a similar outcome to AGB, with no augmentation of excess weight loss compared with RYGB alone[21]. Selective hepatic branch vagotomy has also been reported in some studies. Shin et al[22] reported that there were no significant differences in the regulation of energy metabolism between RYGB and RYGB with hepatic branch vagotomy. Few studies have reported the effects of these procedures on diabetes control. Qiu et al[23] also compared selective hepatic branch vagotomy following RYGB with RYGB alone in rats, and observed similar diabetes control. However, they found that RYGB alone resulted in more weight loss, which was the opposite of the results reported above.
The present study provides an initial report on SGTV, and its effect on weight loss and glucose metabolism compared with SG alone. The results demonstrated that SGTV achieved rapid and sustained improvement in diabetes, with a significant improvement in glucose tolerance and insulin sensitivity. The effect of this novel procedure on diabetes control was independent of weight loss. Unexpectedly, SGTV did not exhibit superiority over SG alone in terms of weight reduction or diabetes control. In addition, TV alone in this study resulted in no food intake, weight loss or anti-diabetic effects. Based on these findings, we conclude that TV has no effect on weight reduction or glucose metabolism, and the vagus nerve is dispensable for the effect of SG on glucose homeostasis.
Delayed gastric emptying, loss of appetite, decreased food and liquid intake, and changes in some gastrointestinal hormones are considered to be possible factors contributing to weight reduction after TV[7]. Clinical and preclinical investigations, including the present study, on the effect of TV or selective vagotomy, either alone or combined with bariatric procedures, have shown inconsistent results. Enhanced, non-enhanced and even decreased weight loss were all observed in different reports. These diverse results suggested that the effect of the vagus nerve on energy metabolism involved other unknown factors and further investigations are necessary. Hao et al[11] provided another explanation for these diverse results. Although vagal innervation of the intestine contributed to weight loss after RYGB, the effect was small and only existed during the early post-operative period. Therefore, the effect of TV was possibly concealed and could not be observed during mid- or long-term follow-up.
With the exception of TV, electrical vagal nerve blockade is another important method of interrupting the vagus. Electrical vagal nerve blockade has been employed in the treatment of refractory epilepsy. Accompanied weight reduction was reported[24,25], although not in all cases[26]. Vagal blocking therapy (VBLOC) was developed to induce intermittent intra-abdominal vagal blockade for the treatment of morbid obesity using high-frequency electrical currents. After confirmation of the reversible inhibition of pancreatic exocrine secretion and gastric contractions in preclinical studies[27,28], VBLOC therapy was used in patients and led to significant weight loss and an improvement in diabetes and cardiovascular risk factors control[12,13,29]. Although the EMPOWER study found no difference in weight loss between the VBLOC and control groups[30], a modified randomized controlled trial known as the ReCharge study reported better and sustained weight loss in the vagal block group compared with the sham device group[31,32]. Unlike TV, VBLOC therapy provided reversible inhibition of the propagation of the vagus, and was more acceptable to patients. SG with VBLOC may provide another feasible and effective alternative to SG+ procedures. Insulin sensitivity was estimated by HOMA-IR in this study, and the hepatic insulin signaling pathway, including IR, IRS1, IRS2, PI3K and AKT, was also examined. The SGTV group exhibited fast and significant improvement in insulin sensitivity, and the hepatic insulin signaling pathway was upregulated at 12 wk postoperatively. However, there were no differences observed between the SGTV and SG groups in HOMA-IR or the expression of proteins in the insulin signaling pathway. Moreover, TV alone did not result in changes in HOMA-IR or the insulin signaling pathway. These results suggest that TV had no effect on the regulation of insulin sensitivity, at least in the diabetic rat model used in this study. Furthermore, the vagus innervations were possibly unrelated to the improvement in insulin sensitivity, especially hepatic insulin sensitivity. Shin et al[22] reported similar results to ours in that the integrity of vagal nerve innervations was not necessary for the effect of RYGB in patients. Wang et al[33] and Yue et al[34] drew the contrasting conclusion that the integrity of vagal nerve innervations was necessary for the gut-brain-liver axis to regulate hepatic insulin sensitivity, and subdiaphragmatic vagotomy or gut vagal deafferentation interrupted the transmission of neural signals between the small intestine and the brain, and impaired the ability of the DJB in the regulation of hepatic glucose production[35]. These diverse results indicated that the effect of vagal interruption on glucose metabolism requires further investigation.
The function of beta-cells evaluated by oral glucose stimulated insulin secretion showed higher curves of serum insulin level in the SGTV and TV alone groups than in the TV and Sham groups at 12 wk postoperatively. Given that there is no evidence to support islet hyperplasia or beta-cell turnover after bariatric surgery until now, we speculate that SG had a protective effect on the insulin secretion function of beta-cells, but could not improve their function. In addition, the vagus was not necessary for the effect of SG on insulin secretion. As expected, serum ghrelin levels decreased and GLP-1 levels increased after SG. No difference was observed between the SG and SGTV groups.
We suggest that vagus innervations of the stomach, proximal small intestine, liver and pancreas are not essential for the effect of SG on weight loss, improvement in insulin sensitivity and beta-cell function, and hormone changes. However, the vagus nerve plays a key role in the gut-brain-gut axis in the autonomic neurohumoral pathway integrating these elements of energy homeostasis. Why did SGTV result in a similar improvement in diabetes, insulin sensitivity, insulin secretion to oral glucose stimulation and changes in gastrointestinal hormones as SG without the vagus? We speculate that this was possibly due to collateral innervations formed after TV, and counterbalancing metabolic or neural pathways were upregulated through visceral spinal afferents and sympathetic efferents. Therefore, the role of the vagus nerve in regulation of glucose homeostasis after SG requires further research.
In conclusion, SGTV for diabetes control is feasible and independent of weight loss, but did not result in better diabetes control. The integrity of vagal innervations was not necessary for the effect of SG on the improvement in hepatic insulin sensitivity or beta-cell function. Possible involvement of the vagus nerve in the beneficial effects of SG on glucose homeostasis remains to be determined.
The vagus nerve has an effect on gastrointestinal motility, hormone secretion and glucose metabolism. Trunk vagotomy (TV) was performed for severe obesity recently. With the purpose of enhancing the effect of sleeve gastrectomy (SG) on diabetes control, SG plus TV (SGTV) may provide a better glucose regulationg.
SG is currently the most frequently performed bariatric procedures worldwide and included in diabetes treatment algorithms. SG+ procedures are now researched for a better postoperative glucose regulation. SGTV has less trauma to the patients and has the potential to be a new procedure.
In this study, the authors created a surgery model of SG plus TV. They detect body weight, food intake, OGTT, HOMA-IR, hepatic insulin signaling (IR, IRS1, IRS2, PI3K and AKT), oral glucose stimulated insulin secretion, GLP-1 and ghrelin to investigate the effects of SGTV compared with SG.
The authors established a surgery model of SGTV and compared it with SG in the ability to improve glucose metabolism. Although the result showed that SGTV has no advantage with SG, the authors have developed the method to find new surgery procedure, and have interest in SG plus VBLOC.
SG is a popular bariatric procedures performed worldwide, which has a similar effect to RYGB, and less complications than RYGB. TV is a new procedure to treat morbid obesity, and the effect is not clear.
Very interesting about the SGTV was developed using a diabetic rat model and compared with SG and TV on the effect of diabetes control.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ciezki JP, Takamatsu S S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, Pothier CE, Brethauer S, Nissen S, Gupta M. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 1590] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 3. | Batterham RL, Cummings DE. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care. 2016;39:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Balibrea JM, Vilallonga R, Hidalgo M, Ciudin A, González Ó, Caubet E, Sánchez-Pernaute A, Fort JM, Armengol-Carrasco M. Mid-Term Results and Responsiveness Predictors After Two-Step Single-Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy. Obes Surg. 2017;27:1302-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Hassn A, Luhmann A, Rahmani S, Morris-Stiff G. Medium-Term Results of Combined Laparoscopic Sleeve Gastrectomy and Modified Jejuno-Ileal Bypass in Bariatric Surgery. Obes Surg. 2016;26:2316-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Nishi S, Seino Y, Ishida H, Seno M, Taminato T, Sakurai H, Imura H. Vagal regulation of insulin, glucagon, and somatostatin secretion in vitro in the rat. J Clin Invest. 1987;79:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Cardin S, Walmsley K, Neal DW, Williams PE, Cherrington AD. Involvement of the vagus nerves in the regulation of basal hepatic glucose production in conscious dogs. Am J Physiol Endocrinol Metab. 2002;283:E958-E964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Goligher JC, Pulvertaft CN, Irvin TT, Johnston D, Walder B, Hall RA, Willson-Pepper J, Matheson TS. Five- to eight-year results of truncal vagotomy and pyloroplasty for duodenal ulcer. Br Med J. 1972;1:7-13. [PubMed] |
| 10. | Smith DK, Sarfeh J, Howard L. Truncal vagotomy in hypothalamic obesity. Lancet. 1983;1:1330-1331. [PubMed] |
| 11. | Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes Surg. 2014;24:2145-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Camilleri M, Toouli J, Herrera MF, Kulseng B, Kow L, Pantoja JP, Marvik R, Johnsen G, Billington CJ, Moody FG. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Shikora S, Toouli J, Herrera MF, Kulseng B, Zulewski H, Brancatisano R, Kow L, Pantoja JP, Johnsen G, Brancatisano A. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes. 2013;2013:245683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20 Suppl 1:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Steinemann DC, Bueter M, Schiesser M, Amygdalos I, Clavien PA, Nocito A. Management of anastomotic ulcers after Roux-en-Y gastric bypass: results of an international survey. Obes Surg. 2014;24:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Boss TJ, Peters J, Patti MG, Lustig RH, Kral JG. Laparoscopic truncal vagotomy for severe obesity: Six month experience in 10 patients from a prospective, two-center study. Surg Obes Relat Dis. 2007;3:292. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kral JG, Görtz L, Hermansson G, Wallin GS. Gastroplasty for obesity: long-term weight loss improved by vagotomy. World J Surg. 1993;17:75-78; discussion 79. [PubMed] |
| 19. | Angrisani L, Cutolo PP, Ciciriello MB, Vitolo G, Persico F, Lorenzo M, Scarano P. Laparoscopic adjustable gastric banding with truncal vagotomy versus laparoscopic adjustable gastric banding alone: interim results of a prospective randomized trial. Surg Obes Relat Dis. 2009;5:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Martin MB, Earle KR. Laparoscopic adjustable gastric banding with truncal vagotomy: any increased weight loss? Surg Endosc. 2011;25:2522-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Okafor PN, Lien C, Bairdain S, Simonson DC, Halperin F, Vernon AH, Linden BC, Lautz DB. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes Res Clin Pract. 2015;9:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg. 2012;255:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Qiu NC, Zhang Q, Song X, Liu ME, Li XK, Shan CX, Qiu M. Impact of the hepatic branch of the vagus and Roux-en-Y gastric bypass on the hypoglycemic effect and glucagon-like peptide-1 in rats with type 2 diabetes mellitus. J Surg Res. 2014;191:123-129. |
| 24. | Burneo JG, Faught E, Knowlton R, Morawetz R, Kuzniecky R. Weight loss associated with vagus nerve stimulation. Neurology. 2002;59:463-464. [PubMed] |
| 25. | Abubakr A, Wambacq I. Long-term outcome of vagus nerve stimulation therapy in patients with refractory epilepsy. J Clin Neurosci. 2008;15:127-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Koren MS, Holmes MD. Vagus nerve stimulation does not lead to significant changes in body weight in patients with epilepsy. Epilepsy Behav. 2006;8:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Tweden KS, Anvari M, Bierk MD, Billington CJ, Camilleri M, Honda CN, Knudson MB, Larson DE, Wilson RR, Freston JW. Vagal Blocking for Obesity Control (VBLOC): Concordance of Effects of Very High Frequency Vagal Blocking Currents at the Neural and Organ Levels Using Two Pre-clinical Models. Gastroenterology. 2006;130:A-148. [DOI] [Full Text] |
| 28. | Tweden KS, Sarr MG, Camilleri M, Kendrick ML, Moody FG, Bierk MD, Knudson MB, Wilson RR, Anvari M. Vagal Blocking for Obesity Control (VBLOC): Studies of pancreatic and gastric function and safety in a porcine model. Surg Obes Relat Dis. 2006;2:301-302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Shikora SA, Toouli J, Herrera MF, Kulseng B, Brancatisano R, Kow L, Pantoja JP, Johnsen G, Brancatisano A, Tweden KS. Intermittent Vagal Nerve Block for Improvements in Obesity, Cardiovascular Risk Factors, and Glycemic Control in Patients with Type 2 Diabetes Mellitus: 2-Year Results of the VBLOC DM2 Study. Obes Surg. 2016;26:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Sarr MG, Billington CJ, Brancatisano R, Brancatisano A, Toouli J, Kow L, Nguyen NT, Blackstone R, Maher JW, Shikora S. The EMPOWER study: randomized, prospective, double-blind, multicenter trial of vagal blockade to induce weight loss in morbid obesity. Obes Surg. 2012;22:1771-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Ikramuddin S, Blackstone RP, Brancatisano A, Toouli J, Shah SN, Wolfe BM, Fujioka K, Maher JW, Swain J, Que FG. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 32. | Shikora SA, Wolfe BM, Apovian CM, Anvari M, Sarwer DB, Gibbons RD, Ikramuddin S, Miller CJ, Knudson MB, Tweden KS. Sustained Weight Loss with Vagal Nerve Blockade but Not with Sham: 18-Month Results of the ReCharge Trial. J Obes. 2015;2015:365604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Yue JT, Lam TK. Lipid sensing and insulin resistance in the brain. Cell Metab. 2012;15:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Breen DM, Rasmussen BA, Kokorovic A, Wang R, Cheung GW, Lam TK. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |