Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2705
Peer-review started: December 6, 2016
First decision: February 9, 2017
Revised: February 22, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: April 21, 2017
Processing time: 138 Days and 3.8 Hours
To investigate the effects of Hwangryunhaedok-tang (HHT) on gastrointestinal (GI) motility in mice.
The effects of a boiling water extract of HHT (HHTE) on GI motility were investigated by calculating percent intestinal transit rates (ITR%) and gastric emptying (GE) values using Evans Blue and phenol red, respectively, in normal mice and in mice with experimentally induced GI motility dysfunction (GMD). In addition, the effects of the four components of HHT, that is, Gardeniae Fructus (GF), Scutellariae Radix (SR), Coptidis Rhizoma (CR), and Phellodendri Cortex (PC), on GI motility were also investigated.
In normal ICR mice, ITR% and GE values were significantly and dose-dependently increased by the intragastric administration of HHTE (0.1-1 g/kg). The ITR% values of GMD mice were significantly lower than those of normal mice, and these reductions were significantly and dose-dependently inhibited by HHTE (0.1-1 g/kg). Additionally, GF, CR, and PC dose-dependently increased ITR% and GE values in normal and GMD mice.
These results suggest that HHT is a novel candidate for the development of a gastroprokinetic agent for the GI tract.
Core tip: Hwangryunhaedok-tang, a traditional herbal medicine, has been widely used in Korea for many years to ameliorate gastrointestinal (GI) disorders. Our data suggest that Hwangryunhaedok-tang may be a novel candidate for the development of a gastroprokinetic agent and for the treatment of GI motility dysfunction.
- Citation: Kim H, Kim I, Lee MC, Kim HJ, Lee GS, Kim H, Kim BJ. Effects of Hwangryunhaedok-tang on gastrointestinal motility function in mice. World J Gastroenterol 2017; 23(15): 2705-2715
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2705
Hwangryunhaedok-tang (HHT; Huang-Lian-Jie-Tang in Chinese or Oren-gedoku-to in Japanese) is prepared using Coptidis Rhizoma (Coptis chinensis Franch., Ranunculaceae), Scutellariae Radix (Scutellaria baicalensis Georgi, Labiatae), Phellodendri Cortex (Phellodendron amurense Rupr., Rutaceae), and Gardeniae Fructus (Gardenia jasminoides Ellis, Rubiaceae)[1]. HHT has been used to treat various symptoms, including those of inflammatory diseases[2,3], cardiovascular diseases[4], diabetes mellitus[5], liver diseases[6], brain injury[7] and obesity[8]. It is also used to prevent or ameliorate gastrointestinal (GI) diseases[9-12]. However, no study has previously addressed the effect of HHT on GI motility.
Prokinetic agents can enhance coordinated GI motility and the transit of GI tract contents[13]. The recent importance use of cohabitation prokinetic agents hashas increased quality of life in patients with GI motility disorders[14], and there is an increasing need to develop safer and more effective gastroprokinetic agents. However, despite the widespread use of HHT to treat GI disorders, little is known of its regulatory effects on GI motility. Therefore, we undertook this study to investigate the effects of HHT on the mouse GI tract in vivo.
HHT was purchased from I-WORLD Pharmaceuticals (Incheon, South Korea). It is composed of Coptidis Rhizoma, Scutellariae Radix, Phellodendri Cortex, and Gardeniae Fructus (Table 1). The materials were authenticated by Professor Hyungwoo Kim (Division of Pharmacology, Pusan National University, School of Korean Medicine, Yangsan, South Korea). The extract of HHT (HHTE) was prepared by boiling HHT in water for three hours. The standard adult dose of HHTE is 10-15 g (based on HHT) per day. More information about HHT can be found at the I-WORLD Pharmaceuticals Homepage (http://i-pharm.koreasme.com). The HHT was dissolved in distilled water at a concentration of 0.5 g/mL and stored in a refrigerator until required.
| Herbal name | Amount (g) |
| Coptidis Rhizoma | 0.67 |
| Scutellariae Radix | 1.00 |
| Phellodendri Cortex | 1.00 |
| Gardeniae Fructus | 1.00 |
| Total | 3.67 |
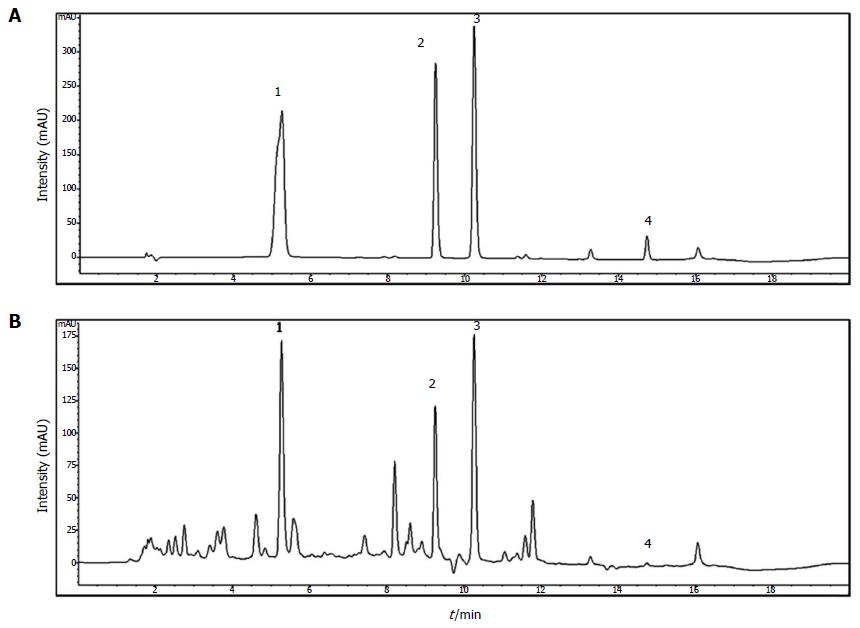
Geniposide, berberine chloride, baicalin, and wogonin were purchased from Wako (Japan). HPLC-grade water and methanol were obtained from JT Baker Inc. (Phillipsburg, NJ, United States). Trifluoroacetic acid was purchased from Sigma-Aldrich (St. Louis, MO, United States). These standard compounds were accurately weighed and dissolved in methanol at 1000 μg/mL; these solutions were diluted 10-fold before injection. An Agilent 1200 (Agilent Technologies, Santa Clara, CA, United States) equipped with a quaternary pump, autosampler, column oven, and diode-array detector was used. The acquired data were processed using ChemStation software (ver.B.03.02). Chromatographic separation was performed on an XDB C18 column (4.6 × 150 mm, 5 μm; Agilent, United States) at 35 °C. The mobile phase consisted of water containing 0.1% trifluoroacetic acid (A) and methanol (B). The program used was as follows: 30% (B) for 1 min, 30%-80% (B) over 1-13 min, and held at 80% B for 1 min, followed by a re-equilibration with 30% (B). The flow rate was set at 0.8 mL/min and the injection volume at 10 μL. The detection wavelengths used were 240 nm for geniposide, 260 nm for berberine chloride, and 275 nm for baicalin and wogonin. Standard compounds were detected in the chromatogram of HHTE at the following retention times: 5.2 min for geniposide, 9.1 min for berberine chloride, 10.1 min for baicalin, and 14.7 min for wogonin (Figure 1).
Animal care and experiments were conducted in accordance with the guidelines issued by the Institutional Animal Care and Use Committee at Pusan National University (Busan, South Korea; Approval no. PNU-2015-1036) and those issued by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Male ICR mice (Samtako BioKorea Co., Ltd., Osan, Republic of Korea) weighing 20-25 g were used to investigate the in vivo effects of HHTE on GI motility. The animals were maintained under controlled conditions (20 ± 4 °C, relative humidity 51% ± 5%, lights on 6 a.m.-6 p.m.). The animals were allowed free access to a commercial diet and tap water but were deprived of food for 24 h before the experiments. All experiments were conducted between 10 a.m. and 3 p.m.
To determine the intestinal transit rates (ITR%) associated with HHTE in vivo, we used Evans Blue solution (5% w/v in distilled water (DW)). Evans Blue solution was administered (0.1 mL/kg of body weight, i.g.) through an orogastric tube 30 min after HHTE was administered i.g. to normal ICR mice. The animals were sacrificed 30 min after Evans Blue administration, and intestinal transit distances were determined by measuring the distance the Evans Blue had migrated in the intestine (from the pylorus to its most distal point). Intestinal transit was quantified using ITR% values, which were calculated by expressing the distance traveled by Evans Blue in 30 min as a percentage of total small intestine length (from the pylorus to the ileal terminus).
Two experimental GI motility dysfunction models, that is, an acetic acid (AA)-induced peritoneal irritation mouse model and an STZ-induced diabetic mouse model, were used. For the AA model, peritoneal irritation was induced by administering AA to ICR mice 30 min after the i.g. administration of HHTE (or DW as a vehicle control). AA (0.6% w/v in saline) was injected intraperitoneally (i.p.) at 10 mL/kg. After the injection of AA, the mice were placed in individual cages and allowed to recover for 30 min. For the STZ-induced diabetic mouse model, male ICR mice aged 4-5 wk and weighing 20-25 g were used to investigate the in vivo effects of HHTE on GI motility. The mice were randomly allocated to two groups: a control group and a diabetic group. To produce diabetes, the mice were fasted overnight, and on the following day, STZ (Sigma-Aldrich, St. Louis, MO, United States) solution was administered i.p. Fresh STZ solution was prepared in 0.1 mol/L ice-cold citrate buffer (pH 4.0) and administered at 200 mg/kg body weight[13]. Control mice received the same volume of 0.1 mol/L citrate buffer i.p. Two months after STZ injection, blood was withdrawn from a tail vein after an 8 h fast, and blood glucose concentrations were measured using a ONE-TOUCH Select Simple kit (Johnson & Johnson Medical Company). Diabetes was defined as a blood glucose level of > 16 mmol/L. No mortality occurred during the study period, and no mouse recovered from STZ-induced diabetes.
GE was assessed by administering a 0.05% (w/v) phenol red solution (0.5 mL/mouse) 30 min after administering HHTE. The mice were sacrificed 20 min later, and the stomachs were immediately removed, cut into several pieces in 5 mL of 0.01 N NaOH, and homogenized. The homogenates were treated with 0.2 mL of 20% trichloroacetic acid per mL of homogenate. The mixtures were centrifuged for 10 min at 1050 g, and the supernatants (0.05 mL) so obtained were added to 0.5 N NaOH (0.2 mL). The absorbances of these mixtures were measured using a spectrometer at 560 nm. Gastric emptying (GE) (%) was calculated using 100-(A/B) × 100, where A is test stomach absorbance (560 nm) and B is control stomach absorbance (560 nm) immediately after phenol red administration.
All drugs were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, United States). In addition, an aqueous extract of the dried immature fruit of Poncirus trifoliata Raf. (PF) was prepared, as previously described[15,16], and its prokinetic activities were compared with those of HHTE. PF is one of the most popular traditional folk medicines in Korea and is obtained from the fruits of Rutaceae. Furthermore, PF has been shown to possess unique, potent prokinetic activities in normal rodents and in rodents with GI motility dysfunction (GMD)[15].
The results are expressed as the mean ± SE. Statistical analysis was performed using Student’s t-test or by ANOVA followed by Bonferroni’s post hoc test, as appropriate. Statistical significance was accepted for P values < 0.05.
The presence of geniposide, berberine chloride, baicalin, and wogonin in HHTE was confirmed by HPLC, and their levels were quantified using calibration curves obtained using purchased standards (Table 2 and Figure 1). Validation of the method used confirmed its reliability and stability.
| Compound | Content (%) |
| Geniposide | 2.684 ± 0.014 |
| Berberine chloride | 0.993 ± 0.006 |
| Baicalin | 5.081 ± 0.027 |
| Wogonin | 0.040 ± 0.003 |
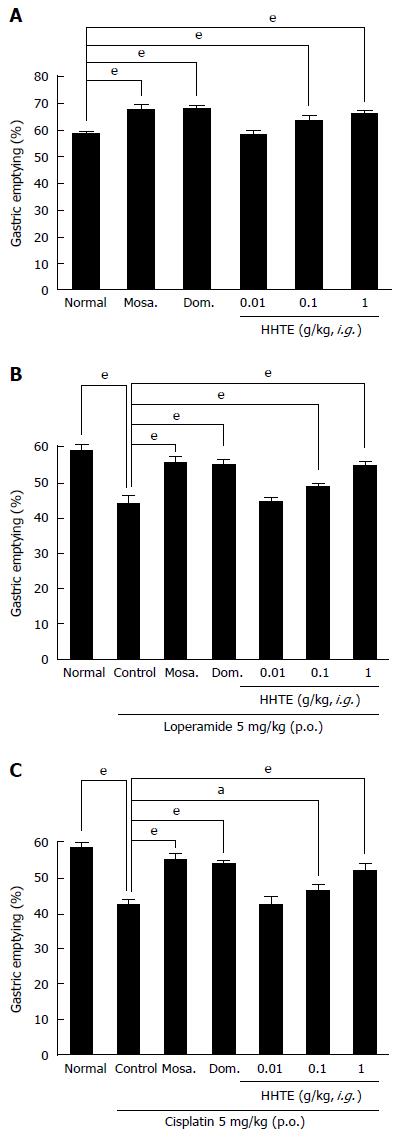
In normal mice, HHTE (0.01, 0.1 and 1 g/kg)-treated groups showed significantly higher GE (%) values than non-treated controls [GE values for HHTE at 0.01, 0.1 and 1 g/kg were 58.7% ± 1.2%, 63.4% ± 1.9% (P < 0.001) and 66.2% ± 0.9% (P < 0.001), respectively; Figure 2A]. Furthermore, its effects were dose dependent in the dosage range 0.01 to 1 g/kg, and at 1 g/kg HHTE had effects similar to those of mosapride at 5 mg/kg [67.4% ± 1.8% (P < 0.001)] and domperidone at 5 mg/kg [67.8% ± 1.3% (P < 0.001)] (Figure 2A). Next, we examined loperamide-induced and cisplatin-induced models of GE delay to determine whether HHTE could increase GE in these abnormally depressed GE models. In the loperamide-induced model of GE delay, the mean GE was lower than normal [44.2% ± 2.2% (P < 0.001); Figure 2B], and this decrease was inhibited by HHTE at doses from 0.01 to 1 g/kg [GE values for HHTE at 0.01, 0.1 and 1 g/kg were 44.6% ± 0.9%, 48.7% ± 1.3% (P < 0.001) and 54.9% ± 1.1% (P < 0.001), respectively; Figure 2B]. The highest efficacy was obtained at 1 g/kg HHTE, and this effect was comparable to that of 5 mg/kg mosapride [55.6% ± 1.3% (P < 0.001)] or 5 mg/kg domperidone [55.2% ± 1.5% (P < 0.001)] (Figure 2B). In addition, in the cisplatin-induced model of GE delay, decreased GE was also inhibited by HHTE (0.01, 0.1 or 1 g/kg) [GE values at HHTE 0.01, 0.1 and 1 g/kg were 42.4% ± 2.3%, 46.0 ± 1.9% (P < 0.05) and 51.8% ± 2.0% (P < 0.001), respectively; Figure 2C]. The highest efficacy was obtained at an HHTE dose of 1 g/kg, and this effect was comparable to that of 5 mg/kg mosapride [54.9% ± 1.6% (P < 0.001)] or 5 mg/kg domperidone [53.6% ± 0.9% (P < 0.001)] (Figure 2C).
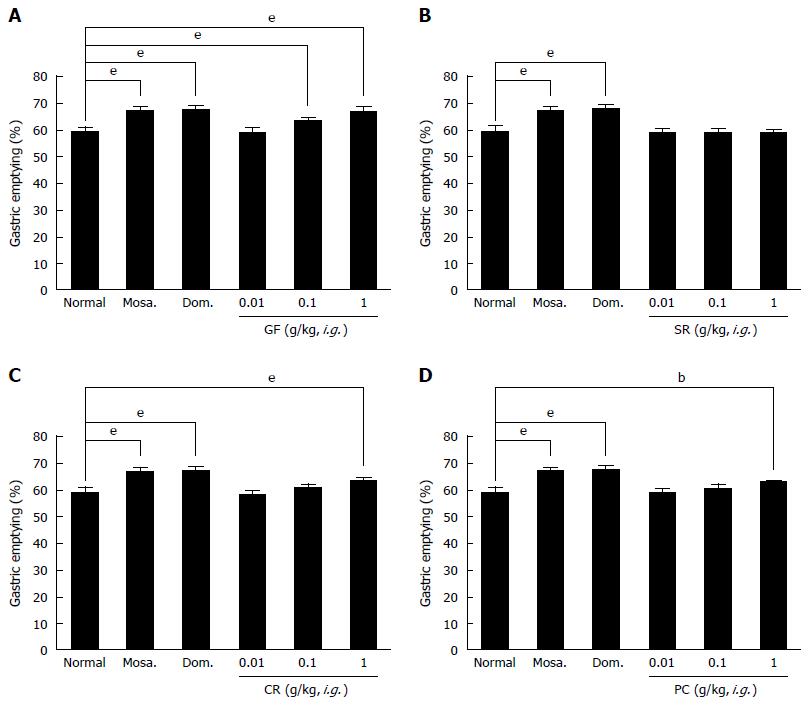
HHT is composed of Gardeniae Fructus (Gardenia jasminoides Ellis), Scutellariae Radix (Scutellaria baicalensis Georgi), Coptidis Rhizoma (Coptis chinesis Franch.), and Phellodendri Cortex (Phellodendron amurense Rupr.)[1]. Therefore, we investigated the effects of these components on GE in normal mice. Gardeniae Fructus (GF) (0.1 or 1 g/kg)-treated groups showed significantly higher GE (%) values than normal controls [GE values at HHTE 0.01, 0.1 and 1 g/kg were 58.9% ± 1.6%, 63.5% ± 0.9% (P < 0.001) and 66.5% ± 1.8% (P < 0.001), respectively; Figure 3A], and its effects were dose dependent in the dosage range 0.01 to 1 g/kg. HHTE at 1 g/kg had effects similar to those of mosapride at 5 mg/kg [67.1% ± 1.4% (P < 0.001)] and domperidone at 5 mg/kg [67.6% ± 1.5% (P < 0.001)] (Figure 3A). However, Scutellariae Radix (SR) (0.01, 0.1 and 1 g/kg) had no effect on GE (%) values [GE values at HHTE 0.01, 0.1 and 1 g/kg were 58.9% ± 1.3%, 59.0% ± 1.4% and 58.7% ± 0.9%, respectively; Figure 3B]. On the other hand, Coptidis Rhizoma (CR) (1 g/kg) significantly enhanced GE (%) values [GE values at HHTE 0.01, 0.1, and 1 g/kg were 58.4% ± 1.6%, 61.2% ± 1.3% and 64.0% ± 0.9% (P < 0.001), respectively; Figure 3C], and its effects were dose dependent in the dosage range 0.01 to 1 g/kg. Furthermore, CR at 1 g/kg had effects similar to those of mosapride at 5 mg/kg and domperidone at 5 mg/kg (Figure 3C). Similarly, Phellodendri Cortex (PC) (1 g/kg) significantly enhanced GE (%) values compared with normal controls [GE values at HHTE 0.01, 0.1, and 1 g/kg were 58.9% ± 1.2%, 60.8% ± 1.5% and 62.9% ± 0.8% (P < 0.01), respectively; Figure 3D]. Its effects were dose dependent in the dosage range 0.01 to 1 g/kg, and at 1 g/kg PC had effects similar to those of mosapride at 5 mg/kg and domperidone at 5 mg/kg (Figure 3D). These results indicate that GF, CR, and PC enhanced GE in mice.
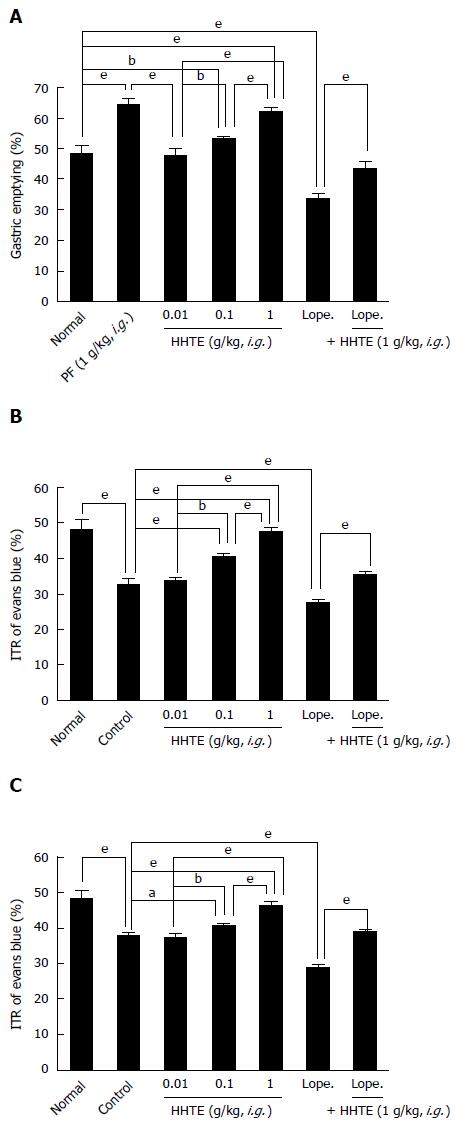
The mean ITR% for Evans Blue after 30 min in normal mice was 48.4% ± 2.4% (Figure 4A). PF (1 g/kg), which has been demonstrated to have prokinetic activity in the GI tract[15], significantly increased ITR% [64.4% ± 1.8% (P < 0.001)]. HHTE increased ITR% dose-dependently [ITR% values at 0.01, 0.1 and 1 g/kg were 47.8% ± 1.9%, 53.2% ± 0.8% (P < 0.01) and 62.2% ± 1.1% (P < 0.001), respectively; Figure 4A]. Loperamide decreased ITR%, which is consistent with previous reports[16], and HHTE inhibited this loperamide-induced ITR% decrease [ITR% for loperamide 33.8% ± 1.3%, and ITR% for loperamide with HHTE was 43.6% ± 2.1% (P < 0.001); Figure 4A].
To examine the effect of HHTE on GI motility, we used AA and STZ-induced diabetic mouse models of experimental GMD. The AA mouse model showed a significant ITR% decrease (to 32.8% ± 1.3% vs the 48.4 ± 2.4% of normal controls; P < 0.001; Figure 4B). However, intragastric treatment with HHTE at 0.01, 0.1, or 1 g/kg significantly inhibited this reduction [to 34.1% ± 0.7%, 40.6% ± 0.9% (P < 0.001) and 47.6% ± 1.1% (P < 0.001), respectively; Figure 4B]. No abnormal clinical signs or changes in AA mice were observed after administration of HHTE. In addition, loperamide decreased ITR% in AA mice [to 27.6% ± 0.9% (P < 0.001)], and HHTE reduced this decrease [to 35.4% ± 0.9% (P < 0.001); Figure 4B]. Furthermore, STZ-induced diabetic mice also showed significant ITR% reduction (to 38.1% ± 0.7%; Figure 4C), and this was also significantly inhibited by HHTE at 0.01, 0.1 or 1 g/kg [to 37.4% ± 0.9%, 40.6% ± 0.9% (P < 0.05) and 46.4% ± 1.1% (P < 0.001), respectively; Figure 4C]. No abnormal clinical signs or changes were observed in STZ-induced diabetic mice after administration of HHTE. In addition, loperamide decreased ITR% in STZ-induced diabetic mice [to 29.2% ± 0.8% (P < 0.001)], and HHTE reduced this decrease [to 39.1% ± 0.7% (P < 0.001); Figure 4C]. These results indicate that HHTE increased ITR% in mice with GMD.
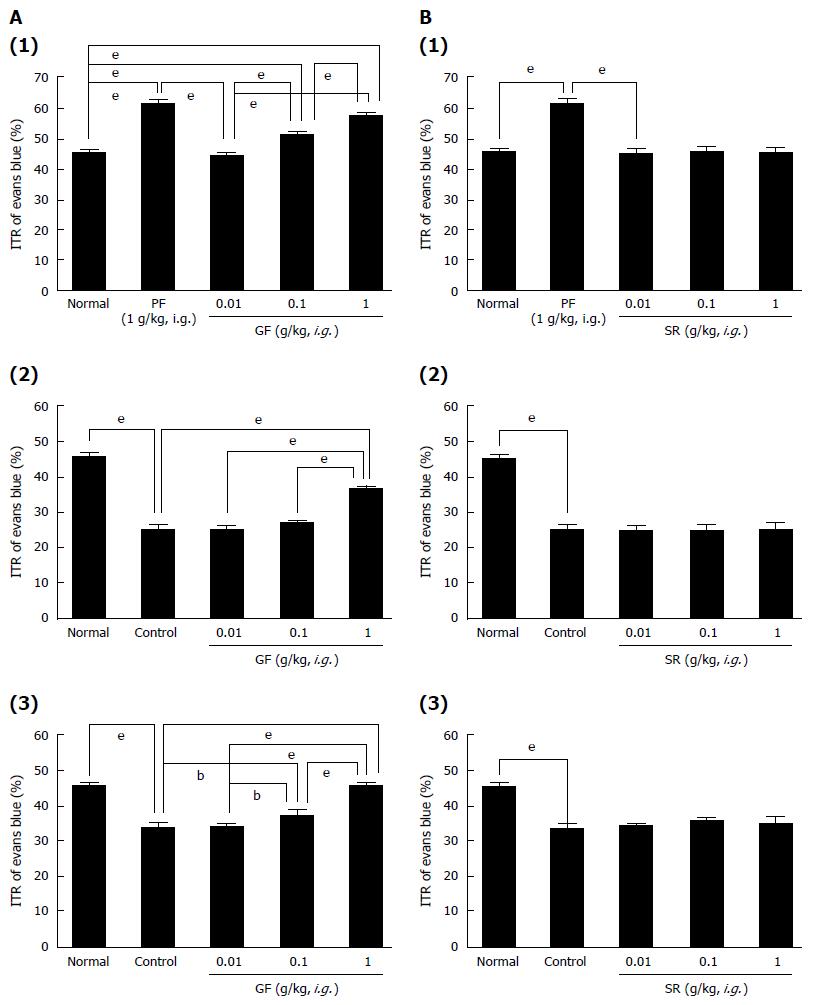
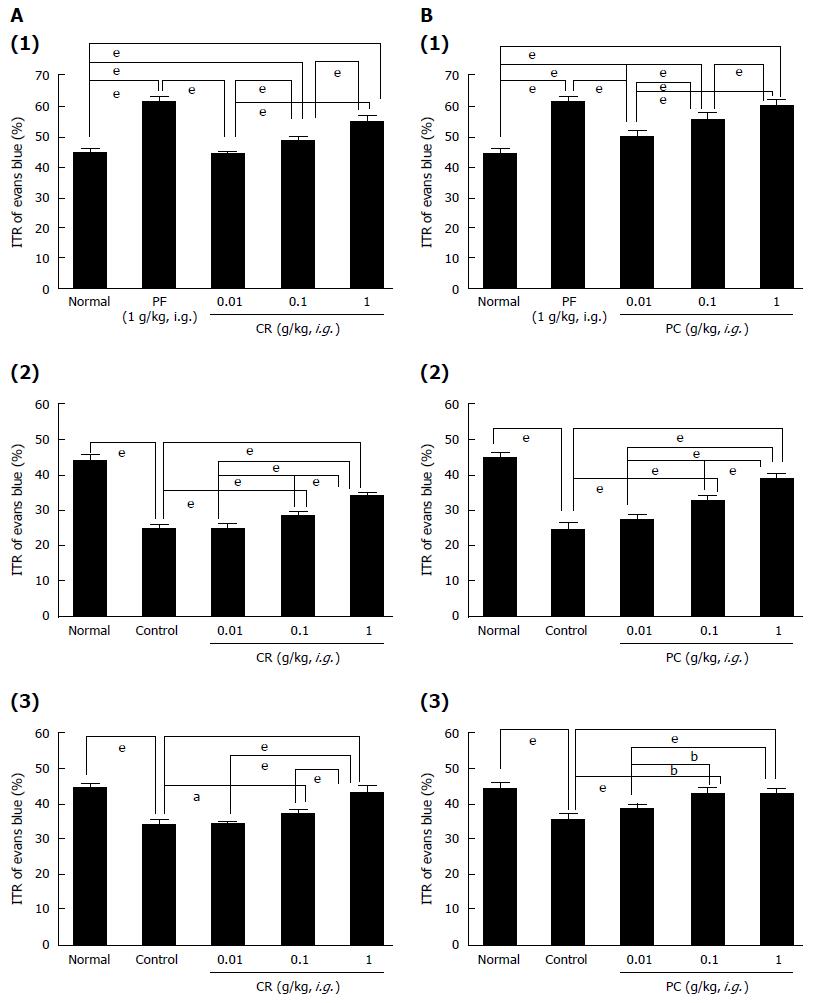
GF increased ITR% dose-dependently [ITR% values at 0.01, 0.1 and 1 g/kg were 44.4% ± 1.1%, 51.6% ± 0.9% (P < 0.001) and 57.8% ± 0.8% (P < 0.001), respectively, vs the 48.4% ± 2.4% of normal controls; (Figure 5A1)]. As mentioned above, the AA mouse model showed significant ITR% reduction [to 25.2% ± 1.2% (P < 0.001 vs normal controls); (Figure 5A2)]. However, significant inhibition of this reduction was observed when GF was administered at 0.01, 0.1, or 1 g/kg intragastrically [to 25.1% ± 1.3%, 27.3% ± 0.7% and 36.4% ± 0.9% (P < 0.001), respectively; (Figure 5A2)], but not when SR was administered at these levels (Figure 5B2). In addition, STZ-induced diabetic mice showed significant ITR% reduction (to 33.8% ± 1.3%; Figure 5A3), and this was also significantly inhibited by GF at 0.01, 0.1 or 1 g/kg [to 34.2% ± 0.8%, 37.2% ± 1.5% (P < 0.01) and 45.8% ± 1.0% (P < 0.001), respectively; (Figure 5A3)] but not by SR (0.01, 0.1 and 1 g/kg) (Figure 5B1). CR increased ITR% dose-dependently when administered to normal controls [ITR% values at 0.01, 0.1 and 1 g/kg were 44.2% ± 0.8%, 48.6% ± 1.1% (P < 0.001) and 55.2% ± 1.6% (P < 0.001), respectively; (Figure 6A1)]. The AA mouse model showed significantly lower ITR% values [25.1% ± 1.3% (P < 0.001 vs normal controls); (Figure 6A2)]. However, significant inhibition of this reduction was observed when CR was administered at 0.01, 0.1, or 1 g/kg intragastrically [25.1% ± 1.1%, 28.8% ± 1.2% (P < 0.001) and 34.2% ± 0.8% (P < 0.001), respectively; (Figure 6A2)]. In addition, STZ-induced diabetic mice showed significant ITR% reduction [to 34.1% ± 1.6%; (Figure 6A3)], and this was also significantly inhibited by CR at 0.01, 0.1 or 1 g/kg [34.1% ± 0.9%, 37.1% ± 1.4% (P < 0.05) and 43.2% ± 1.9% (P < 0.001), respectively; (Figure 6A3)]. Furthermore, PC increased ITR% dose-dependently [ITR% values at 0.01, 0.1 and 1 g/kg were 50.1% ± 2.1% (P < 0.001), 55.6% ± 2.3% (P < 0.001) and 60.2% ± 1.8% (P < 0.001), respectively; (Figure 6B1)]. The AA mouse model showed significant reduction in ITR% [to 24.4% ± 1.8% (P < 0.001 vs normal controls); (Figure 6B2)]. However, PC at 0.01, 0.1, or 1 g/kg significantly inhibited this reduction [to 27.5% ± 1.5%, 32.6% ± 1.4% (P < 0.001) and 38.8% ± 1.5% (P < 0.001), respectively; (Figure 6B2)]. STZ-induced diabetic mice showed significant ITR% reduction (to 35.8% ± 1.3%; Figure 6B3) vs normal controls, and this was also significantly inhibited by PC at 0.01, 0.1 or 1 g/kg [to 38.4% ± 1.6%, 42.8% ± 1.4% (P < 0.001) and 42.8% ± 1.5% (P < 0.001), respectively; (Figure 6B3)]. No abnormal clinical signs or changes were observed in AA mice or STZ-induced diabetic mice after administration of GF, SR, CR, or PC. These results indicate that GF, CR, and PC increased ITR% in mice with GMD.
In this study, HHTE-treated mice had significantly greater GE values than normal mice, and HHTE at 1 g/kg was found to have effects similar to those of mosapride and domperidone (Figure 2A). Furthermore, in abnormally depressed (loperamide- and cisplatin-induced) GE models, HHTE increased GE (Figure 2B and C). Additionally, GF, CR and PC, that is, three of the four components of HHTE, also enhanced GE in mice (Figure 3). In addition, HHTE significantly and dose-dependently enhanced ITR% (Figure 4A). In experimental GMD (AA mouse and STZ-induced diabetic mouse) models, HHTE significantly inhibited GMD-induced reductions in ITR% (Figure 4B and C). Additionally, GF, CR, and PC increased ITR% in normal and GMD mice (Figures 5 and 6). Gastroprokinetic agents are considered a first-line option for treating GI motility disorders, especially delayed gastric emptying[17]. These drugs stimulate peristalsis and may specifically improve GI motility function by influencing GI contractions and rhythms[17,18] and include 5-hydroxytryptamine (5-HT)4 receptor agonists (e.g., cisapride, tegaserod, and prucalopride), dopamine D2 receptor antagonists (e.g., metoclopramide and domperidone), and motilin receptor agonists such as erythromycin[17-20]. Among the three common types of gastroprokinetic agents, 5-HT4 receptor agonists are probably the most attractive because they effectively regulate gastrointestinal motility and sensation[21,22]. 5-HT4 receptor agonists stimulate motility and secretion by increasing the release of acetylcholine from excitatory motor neurons[21,22]. In addition, they improve gastric accommodation and ameliorate impaired visceral sensation[21,22]. However, they have been withdrawn from the market due to their side effects on cardiac rhythmicity (related to prolongation of QT intervals)[23].
Traditional herbal medicines are widely used for the initial treatment of GI motility disorders[24]. However, unfortunately, clinical trials have shown that most are ineffective[25]. Thus, new pharmacological therapeutics are needed to achieve relief from GI-related diseases and normalize GI motility[25]. Based on the results of this study, we believe HHT might mimic the major excitatory neurotransmitters of the GI tract and act as a gastroprokinetic agent. Additionally, herbal products offer an attractive alternative based on perceptions of “natural” origins and low risks of side effects[26].
The interstitial cells of Cajal (ICCs) contribute to normal GI function by generating electrical slow waves and mediating neuromuscular signaling[27,28]. Damage to ICCs or reductions in ICC numbers has been described in many GI motility disorders[29,30], and thus it is of considerable importance that studies be conducted to elucidate the mechanisms underlying ICC pacemaker activity.
In summary, in normal ICR mice, HHTE and three of its four components dose-dependently increased ITR% and GE values. Furthermore, the ITR% reductions in GMD models were significantly and dose dependently reduced by treatment with HHTE or its active components. In addition, HHTE prevented observed GE delays in our loperamide- and cisplatin-induced models. Taken together, our results suggest that HHT is a good candidate starting point for the development of a gastroprokinetic agent.
Hwangryunhaedok-tang (HHT) [composed of Coptidis Rhizoma (Coptis chinensis Franch), Scutellariae Radix (Scutellaria baicalensis Georgi), Phellodendri Cortex (Phellodendron amurense Rupr.), and Gardeniae Fructus (Gardenia jasminoides Ellis)] is a traditional Chinese medicinal formula, and has been widely used in East Asia for many years to ameliorate the symptoms of gastrointestinal (GI) disorders. However, despite the considerable use of HHT in tradition medicine to treat GI dysfunction, little was known of its in vivo regulatory effects on GI motility.
HHT is a good candidate material for the development of a gastroprokinetic agent.
In normal ICR mice, HHT and three of its components dose-dependently increased ITR% and GE values. Furthermore, ITR% reductions observed in GMD models were significantly and dose-dependently inhibited by treatment with HHT or its active components. In addition, HHT prevented observed GE delays in our loperamide- and cisplatin-induced models of GE delay.
HHT should be considered a novel candidate prokinetic agent for the pharmacological treatment of GI motility disorders.
GI motility: movements of the digestive system, and the transit of contents within it; ITR%: Rate of passage of food (sometimes in the form of a test meal) through the GI tract; Gastric emptying: the emptying of stomach contents.
The manuscript is well designed and suggests Hwangryunhaedok-tang may serve as a novel candidate of a gastroprokinetic agent for the treatment of GI motility dysfunction. It is excellent that both experimental GI motility dysfunction models and pharmacological methods were applied in the manuscript, which would make the data convincing.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liu MJ S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Lu T, Song J, Huang F, Deng Y, Xie L, Wang G, Liu X. Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol. 2007;110:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Lu J, Wang JS, Kong LY. Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical compounds. J Ethnopharmacol. 2011;134:911-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Yue R, Zhao L, Hu Y, Jiang P, Wang S, Xiang L, Liu W, Zhang W, Liu R. Rapid-resolution liquid chromatography TOF-MS for urine metabolomic analysis of collagen-induced arthritis in rats and its applications. J Ethnopharmacol. 2013;145:465-475. [PubMed] [DOI] [Full Text] |
| 4. | Seo CS, Kim OS, Kim JH, Shin HK. Simultaneous quantification and antiatherosclerosis effect of the traditional Korean medicine, Hwangryunhaedok-tang. BMC Complement Altern Med. 2015;15:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Yu YL, Lu SS, Yu S, Liu YC, Wang P, Xie L, Wang GJ, Liu XD. Huang-lian-jie-du-decoction modulates glucagon-like peptide-1 secretion in diabetic rats. J Ethnopharmacol. 2009;124:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Hsu YL, Kuo PL, Tzeng TF, Sung SC, Yen MH, Lin LT, Lin CC. Huang-lian-jie-du-tang, a traditional Chinese medicine prescription, induces cell-cycle arrest and apoptosis in human liver cancer cells in vitro and in vivo. J Gastroenterol Hepatol. 2008;23:e290-e299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Zhang Q, Ye YL, Yan YX, Zhang WP, Chu LS, Wei EQ, Yu YP. Protective effects of Huanglian-Jiedu-Tang on chronic brain injury after focal cerebral ischemia in mice. Zhejiang Daxue Xuebao Yixueban. 2009;38:75-80. [PubMed] |
| 8. | Yamakawa J, Ishigaki Y, Takano F, Takahashi T, Yoshida J, Moriya J, Takata T, Tatsuno T, Sasaki K, Ohta T. The Kampo medicines Orengedokuto, Bofutsushosan and Boiogito have different activities to regulate gene expressions in differentiated rat white adipocytes: comprehensive analysis of genetic profiles. Biol Pharm Bull. 2008;31:2083-2089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Ohta Y, Kobayashi T, Nishida K, Sasaki E, Ishiguro I. Preventive effect of Oren-gedoku-to (Huanglian-Jie-Du-Tang) extract on the development of stress-induced acute gastric mucosal lesions in rats. J Ethnopharmacol. 1999;67:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Zhou H, Mineshita S. The effect of Oren-gedoku-to on experimental colitis in rats. J Pharm Pharmacol. 1999;51:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Hong T, Jin G, Cyong J. Effect of components of Oren-gedoku-to (Huang-Lian-Jie-Du-Tang) on murine colitis induced by dextran sulfate sodium. J Trad Med. 2000;17:173-179. |
| 12. | Watanabe-Fukuda Y, Yamamoto M, Miura N, Fukutake M, Ishige A, Yamaguchi R, Nagasaki M, Saito A, Imoto S, Miyano S. Orengedokuto and berberine improve indomethacin-induced small intestinal injury via adenosine. J Gastroenterol. 2009;44:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Hwang MW, Kim JN, Song HJ, Lim B, Kwon YK, Kim BJ. Effects of Lizhong Tang on cultured mouse small intestine interstitial cells of Cajal. World J Gastroenterol. 2013;19:2249-2255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1075] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 15. | Kim BJ, Kim HW, Lee GS, Choi S, Jun JY, So I, Kim SJ. Poncirus trifoliate fruit modulates pacemaker activity in interstitial cells of Cajal from the murine small intestine. J Ethnopharmacol. 2013;149:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci. 2003;20:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther. 2008;27:724-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Yin J, Xu X, Song G, Han HS, Kim HW, Chen JD. Prokinetic effects of a new 5-HT4 agonist, YKP10811, on gastric motility in dogs. J Gastroenterol Hepatol. 2017;32:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Hasler WL. Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:619-647, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Tack J. Prokinetics and fundic relaxants in upper functional GI disorders. Curr Opin Pharmacol. 2008;8:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Tonini M, Candura SM, Onori L, Coccini T, Manzo L, Rizzi CA. 5-hydroxytryptamine4 receptor agonists facilitate cholinergic transmission in the circular muscle of guinea pig ileum: antagonism by tropisetron and DAU 6285. Life Sci. 1992;50:PL173-PL178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Prins NH, Akkermans LM, Lefebvre RA, Schuurkes JA. 5-HT(4) receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol. 2000;131:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Wang SH, Lin CY, Huang TY, Wu WS, Chen CC, Tsai SH. QT interval effects of cisapride in the clinical setting. Int J Cardiol. 2001;80:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Jia G, Meng MB, Huang ZW, Qing X, Lei W, Yang XN, Liu SS, Diao JC, Hu SY, Lin BH. Treatment of functional constipation with the Yun-chang capsule: a double-blind, randomized, placebo-controlled, dose-escalation trial. J Gastroenterol Hepatol. 2010;25:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Jiang C, Xu Q, Wen X, Sun H. Current developments in pharmacological therapeutics for chronic constipation. Acta Pharm Sin B. 2015;5:300-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Suzuki H, Inadomi JM, Hibi T. Japanese herbal medicine in functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:688-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 223] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Kim BJ, Park KJ, Kim HW, Choi S, Jun JY, Chang IY, Jeon JH, So I, Kim SJ. Identification of TRPM7 channels in human intestinal interstitial cells of Cajal. World J Gastroenterol. 2009;15:5799-5804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | He CL, Burgart L, Wang L, Pemberton J, Young-Fadok T, Szurszewski J, Farrugia G. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14-21. [PubMed] |
| 30. | Zárate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |