Published online Mar 28, 2017. doi: 10.3748/wjg.v23.i12.2251
Peer-review started: November 26, 2016
First decision: January 10, 2017
Revised: January 24, 2017
Accepted: March 2, 2017
Article in press: March 2, 2017
Published online: March 28, 2017
Processing time: 123 Days and 18.6 Hours
Breast cancer with stomach metastasis rare with an incidence of 1% or less among metastatic breast cancer patients. We experienced a case of breast cancer metastasizing to the stomach in 65-year-old female patient. She experienced dyspepsia and poor oral intake before visiting the clinic. Diffuse infiltration with nodular mucosal thickening of the stomach wall was observed, suggesting advanced gastric cancer based on gross endoscopic finding. Spread of poorly cohesive tumor cells in the gastric mucosa observed upon hematoxylin and eosin stain resembled signet ring cell carcinoma, but diffuse positive staining for GATA3 in immunohistochemical stain allowed for a conclusive diagnosis of breast cancer metastasizing to the stomach. Based on the final diagnosis, systemic chemotherapy was administered instead of primary surgical resection. After 2 cycles of docetaxel administration, she showed a partial response based on abdominal computed tomography scan. This case is an unusual presentation of breast cancer metastasizing to the gastrointestinal tract.
Core tip: This case report describes a patient who was clinically diagnosed as advanced gastric cancer, but final pathological confirm diagnosis was to be breast cancer with gastric metastasis. Patient received systemic chemotherapy and is currently on partial response state at present.
- Citation: Yim K, Ro SM, Lee J. Breast cancer metastasizing to the stomach mimicking primary gastric cancer: A case report. World J Gastroenterol 2017; 23(12): 2251-2257
- URL: https://www.wjgnet.com/1007-9327/full/v23/i12/2251.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i12.2251
Breast cancer commonly metastasizes to bone, lung, liver, and brain, but metastasis to the gastrointestinal tract is rare[1,2]. In Korea, fewer than 10 cases of breast cancer metastasizing to the gastrointestinal tract have been reported[3]. Breast cancer with gastrointestinal metastasis requires systemic chemotherapy. However, if breast cancer with gastrointestinal metastasis is misdiagnosed as a primary gastrointestinal cancer, unnecessary surgical resection may take over place. Herein, the authors present a case of breast cancer metastasizing to the stomach, initially suspected to be primary gastric cancer. This patient was successfully treated with systemic chemotherapy.
A 65-year-old female patient was referred to the oncology department for evaluation of indigestion and epigastric discomfort. She had been previously diagnosed with breast cancer, treated with modified radical mastectomy (invasive lobular carcinoma, pT2N3M0), adjuvant chemotherapy (cyclophosphamide, methotrexate, 5-FU) and adjuvant radiation. Two years after surgery, she experienced cancer recurrence with bone metastasis and received an aromatase inhibitor (letrozole) as treatment for another 2 years. At the time she visited the oncology department, she was currently on aromatase inhibitor (letrozole). Other than breast cancer, she had no other medical history. Her last endoscopy was performed 2 years ago, with no specific findings.
Initial white blood cell (WBC) counts, hemoglobin level and hematocrit were 4790 cell/mm3 (neutrophil count 82%, lymphocytes count 25.8%), 13.1 g/dL (normal range 13.0-18.0 g/dL), and 369000/mm3 (normal range 150000-450000/mm3). Other laboratory findings including those of blood chemistry and urine analysis were in the normal range. Serum carcinoembryonic antigen level was increased up to 23.25 ng/dL.
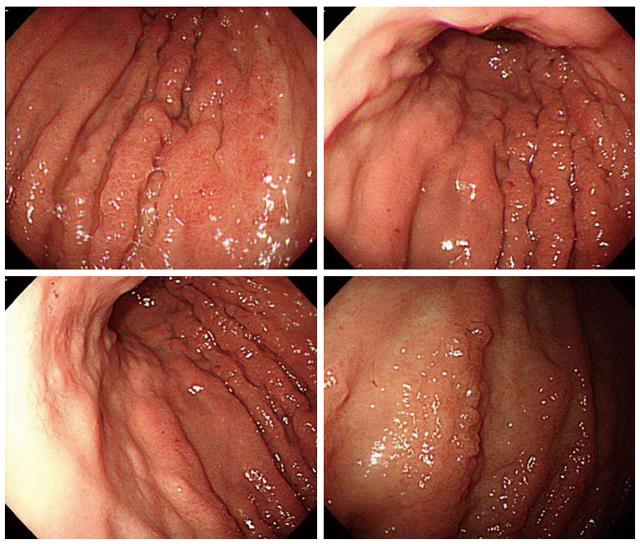
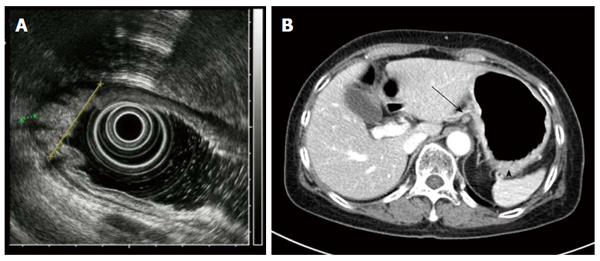
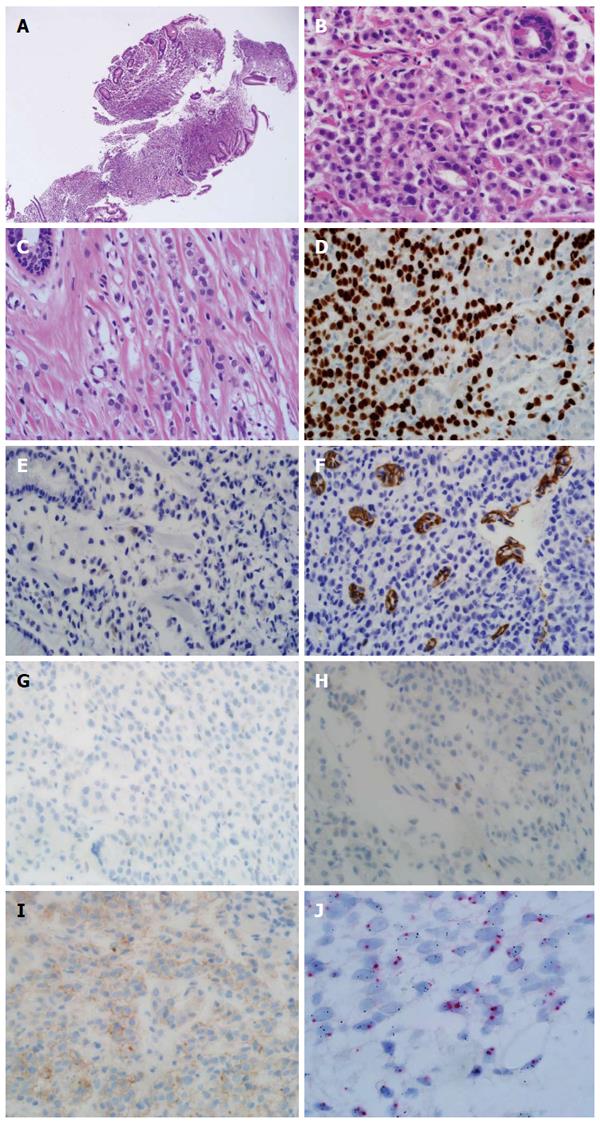
Endoscopy revealed diffuse infiltration with nodular mucosal thickening of the stomach wall, involving the lower two-thirds of the stomach body (Figure 1). Based on endoscopy, endoscopic ultrasound (Figure 2A) and abdominal CT scan (Figure 2B), advanced gastric cancer (cT3N1M0) was suspected. Hematoxylin and eosin (H&E) staining of the endoscopic biopsy revealed poorly cohesive tumor cells spreading into the gastric mucosa, suggesting signet ring cell carcinoma. However, no intracytoplasmic mucin was found in the tumor cells, with scant to moderate pinkish cytoplasm. Normal stomach glandular tissue was found in the biopsy specimen, with no cancer cells connected to the glandular structure (Figure 3A and B). These findings were not consistent with typical gastric signet ring cell carcinoma. Because the patient was diagnosed with invasive lobular carcinoma, archival breast tumor tissue was re-evaluated for comparison.
Breast tissue pathology showed a similar appearance to the endoscopic biopsy specimen, such as a de-cohesive pattern with cells arranged in an Indian file pattern, and a centrally located enlarged nucleus (Figure 3C). In the immunohistochemical (IHC) test, the tumor cells showed diffuse strong nuclear staining for GATA3 binding protein (GATA3) (Figure 3D). IHC results of gross cystic disease fluid protein-15 (GCDFP-15) (Figure 3E), E-cadherin (Figure 3F), estrogen receptor (ER, Figure 3G) and progesterone receptor (PR, Figure 3H) were negative. HER-2 IHC staining showed weak membranous staining consistent with equivocal (+2) positivity (Figure 3I). Silver in situ hybridization (SISH) for HER-2 gene was performed, and the dual-probe HER2/Chr17 ratio was 3.2 (161/51), consistent with HER-2 amplification (Figure 3J).
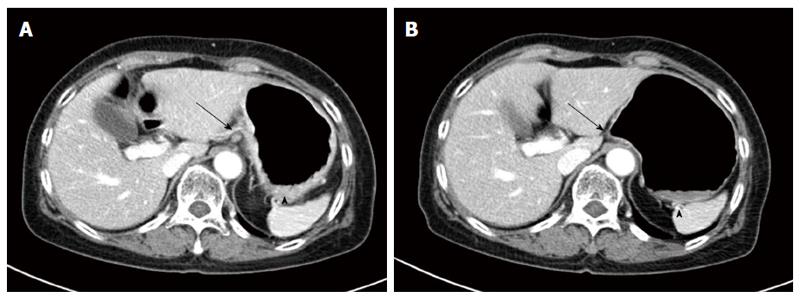
Based on the pathologic findings, breast cancer metastasizing to the stomach was diagnosed. The stomach metastasis developed 4 years after surgery and 2 years after the initiation of an aromatase inhibitor use. As systemic treatment, docetaxel combined with trastuzumab was considered but trastuzumab was not available due to insurance guidelines. Docetaxel (75 mg/m2 intravenously [I.V.], day 1) was administered every 3 wk. After 2 cycles of systemic chemotherapy, follow up abdominal CT scans showed decreased stomach wall thickness, and perigastric lymph nodes showed a partial response (PR) based on the Response Evaluation Criteria in Solid Tumors (Figure 4). During 2 cycles of systemic chemotherapy, the patient’s symptoms of indigestion and epigastric discomfort regressed. Currently, the patient is in persistent PR state and 6 cycles of docetaxel have been administered.
Cancer metastasizing to the gastrointestinal (GI) tract is reported to be rare, but breast cancer is the second most common cancer metastasizing to the GI tract after lung cancer[2,4]. However, the incidence of breast cancer with GI tract metastasis is reported to be 1% or lower[5,6]. Invasive lobular carcinoma tends to metastasize to the GI tract more frequently compared to invasive ductal carcinoma[7]. The most common metastatic sites in the GI tract are the colon and rectum, stomach, small intestine and esophagus, in that order[4]. In Korea, 7 cases of breast cancers metastasizing to the GI tract have been reported, with 5 cases of breast cancer with gastric metastases and 2 cases of synchronous stomach and colorectal metastases[3]. The clinical characteristics of the previous cited cases are summarized in Table 1[2,3,8-19].
| Ref. | Age | Duration after initial diagnosis | Clinical presentation | Endoscopy | Pathology | IHC | Surgery | Treatment | Other metastases site | Overall survival | ||
| ER | PR | C-erbB2 | ||||||||||
| Our case | 65 | 4 | Epigastric Discomfort | Diffuse infiltrative mucosal lesion | ILC | neg | neg | pos | No | Bone | - | |
| Indigestion | Extensive nodular thickening | |||||||||||
| Pera et al[18] | 45 | 7 | Epigastric pain | Erosion of gastric wall | ILC | pos | pos | - | Subtotal gastrectomy | H | - | - |
| heart burn | ||||||||||||
| Jones et al[2] | 51 | 3 | No symptom | Polyp at antrum wall | ILC | neg | neg | neg | Total gastrectomy | Palliative | Bone | - |
| 61 | 6.9 | Dysphagia | Fungating mass | ILC | pos | pos | neg | No | C,R | Brain, bone, pleura | - | |
| weight loss | ||||||||||||
| Eo et al[11] | 48 | 9 | Nausea | Elevated mucosal lesion | IDC | pos | pos | neg | No | C | Liver, bone, pleura | - |
| anorexia | ||||||||||||
| Arrangoiz et al[8] | 70 | 1 | Diarrhea | Mucosal thickening | ILC | pos | neg | neg | No | H | Lung, rectum | - |
| constipation | ||||||||||||
| Koike et al[16] | 42 | 5 | Epigastric pain | Mucosal erosion | ILC | pos | pos | neg | No | C | - | - |
| 54 | 6 | Epigastric pain | Mucosal erosion | ILC | pos | pos | neg | No | C, H | Liver, bone, peritoneum | 5 | |
| 54 | 3 | Epigastric pain | Submucosal tumor | IDC | pos | pos | pos | No | C | Bone | 2.3 | |
| vomiting | ||||||||||||
| Geredeli et al[12] | 47 | 3 | Increased serum CEA, CA15-3 | ILC | neg | neg | neg | Subtotal gastrectomy | C | Bone | - | |
| Buka et al[9] | 58 | 1.2 | Abdominal pain | Polypoid infiltration | ILC | pos | pos | neg | Total gastrectomy | C, R | Colon, pleura | 7.2 |
| weight loss | ||||||||||||
| Lee et al[17] | 48 | 5.7 | Melena | Mucosal erosion | - | - | - | - | - | C | Bone, liver | - |
| Yim et al[19] | 48 | Initial diagnosis | Epigastric discomfort | Mucosal erosion | ILC | neg | neg | - | No | C | Bone | - |
| Jeon et al[14] | 49 | 5 | Melena | Volcano shaped ulcers | IDC | pos | neg | - | No | C | Bone | |
| Kim et al[15] | 53 | 10 | Dyspepsia | Mucosal erosion | IDC | neg | neg | - | No | C, H | Kidney, ovary, colon, bone, peritoneal LN | 2.4 |
| lower abdominal pain | ||||||||||||
| small caliper of stool | ||||||||||||
| Hwang et al[13] | 66 | 17 | Back pain | Flat mucosal lesion | ILC | neg | pos | endoscopic mucosal resection | C | Bone | - | |
| Cheoi et al[10] | 56 | 4 | Upper abdominal discomfort | Mucosal erosion | IDC | neg | pos | pos | - | C, H | - | 1.3 |
| Yu et al[3] | 63 | 10 | Melena | Linitis plastica | ILC | pos | pos | pos | No | C, H | Colon, bone marrow | - |
| small caliper of stool | flat ulcer | |||||||||||
Most breast cancer patients with gastric metastasis present with GI symptoms[3,16], similar to primary gastric cancer. In our case, the patient complained of indigestion, early satiety, and weight loss. Endoscopy with sufficient mucosal biopsy is mandatory for the diagnosis. Diffuse infiltration of the gastric wall with linitis plastica formation may be found[2], but approximately 50% of patients may have shallow mucosal lesion indistinguishable from benign gastric mucosal lesions[20]. Our patient showed extensive nodular mucosal thickening with a thickened gastric fold, with a primary suspicion of advanced gastric cancer.
Pathologic findings of breast cancer metastasizing to the stomach are morphologically similar to poorly cohesive gastric carcinoma, especially in invasive lobular carcinoma[21,22]. However, some morphological differences are present. In metastatic mammary carcinoma, sialomucin is present in the intracytoplasmic lumina with a central location of the nucleus. In contrast, primary gastric signet ring cell carcinoma contains clear intracytoplasmic acid mucin that pushes the nucleus to the periphery[21]. In the present case, the nuclei of the tumor cells were located in the center, and there were no clear intracytoplasmic inclusions.
Also, IHC study is helpful for differential diagnosis. GCDFP-15 staining was traditionally used for differential diagnosis of mammary origin carcinoma. However, it shows relatively low sensitivity (55%-76%) for detecting a breast origin cancer[23]. Recently, GATA3 is widely known as a mammary cancer and urothelial cancer marker. GATA3 expression shows 100% positivity in involving breast lobular carcinoma and 96% positivity in breast ductal carcinoma. However, only 5% of tumors are positive for GATA3 in gastric adenocarcinoma[24]. In our case, although GCDFP-15 staining was negative, GATA3 showed diffuse strong nuclear positivity, consistent with a mammary origin of the carcinoma.
Metastatic breast cancer involving the stomach is treated with systemic agents such as cytotoxic chemotherapeutic agents or hormonal agents. Surgical resection of the stomach has a limited role in treatment, and does not affect the survival outcomes of patients presenting with gastric metastasis[4]. However, surgical treatment may have a role in palliative treatment such as relieving obstructive symptoms.
Breast cancer patients have a superior survival outcome compared to other cancers, raising the possibility of a double primary cancer during the clinical course. However, metastasis of primary breast cancer must also be considered. In a breast cancer patient who complains of gastrointestinal symptoms, prompt endoscopy and biopsy are necessary for an accurate diagnosis. Sufficient pathologic review of gastric biopsy and previous breast specimens, with immunohistochemical examination is warranted. When metastasis of breast cancer to the stomach is suspected, appropriate systemic treatment is necessary for further treatment.
A 65-year-old female patient who was diagnosed as metastatic breast cancer visited the hospital for evaluation of epigastric discomfort.
Epigastric discomfort and indigestion.
Gastric ulcer, primary gastric cancer showed be differentiated by endoscopic biopsy.
Serum carcinoembryonic antigen was increased up to 23.25 ng/dL.
Endoscopy showed diffuse infiltration with nodular mucosal thickening of stomach wall.
Metastatic invasive lobular carcinoma to stomach was diagnosed by immunohistochemical stain.
Docetaxel 150 mg/m2 intravenous, every 3 wk.
Breast cancer rarely metastasize to gastrointestinal tract and should be diagnosed by careful review of the pathologic specimen. If patient have underlying breast cancer, metastatic breast cancer should be considered other than primary gastric cancer during the diagnosis.
GATA3 refers to GATA3 binding protein used for differential marker for diagnosis of breast cancer. Partial response (PR) means more than 30% decrease in the sum of the longest diameters of target lesions during response evaluation.
Early differential diagnosis of metastatic breast cancer to stomach is important for appropriate systemic chemotherapy and avoidance of unnecessary surgery.
This is generally an interesting and useful paper.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Thota PN, Serban ED S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Menuck LS, Amberg JR. Metastatic disease involving the stomach. Am J Dig Dis. 1975;20:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Jones GE, Strauss DC, Forshaw MJ, Deere H, Mahedeva U, Mason RC. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol. 2007;5:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Yu HA, Kim EY, Seo MJ, Chung E, Cho MJ, Oh HJ, Jang JH, Park JC, Lee JU, Park SY. Stomach and Colon Metastasis from Breast Cancer. Ewha Med J. 2014;37:98-104. [DOI] [Full Text] |
| 4. | McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, Donohue JH. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Khadim MI. The effects of Pan and its ingredients on oral mucosa. J Pak Med Assoc. 1977;27:353-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Matsuda I, Matsubara N, Aoyama N, Hamanaka M, Yamagishi D, Kuno T, Tsukamoto K, Yamano T, Noda M, Ikeuchi H. Metastatic lobular carcinoma of the breast masquerading as a primary rectal cancer. World J Surg Oncol. 2012;10:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Harris M, Howell A, Chrissohou M, Swindell RI, Hudson M, Sellwood RA. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer. 1984;50:23-30. [PubMed] |
| 8. | Arrangoiz R, Papavasiliou P, Dushkin H, Farma JM. Case report and literature review: Metastatic lobular carcinoma of the breast an unusual presentation. Int J Surg Case Rep. 2011;2:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Buka D, Dvořák J, Richter I, Hadzi ND, Cyrany J. Gastric and Colorectal Metastases of Lobular Breast Carcinoma: A Case Report. Acta Medica (Hradec Kralove). 2016;59:18-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Cheoi KS, Eum YO, Kim HS, Lee OJ, Lee KH. A case of stomach metastasis from breast cancer. Korean J Med. 2006;71:567-572. |
| 11. | Eo WK. Breast cancer metastasis to the stomach resembling early gastric cancer. Cancer Res Treat. 2008;40:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Geredeli C, Dogru O, Omeroglu E, Yilmaz F, Cicekci F. Gastric Metastasis of Triple Negative Invasive Lobular Carcinoma. Rare Tumors. 2015;7:5764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Hwang SY, Ryu DY, Park JH, Lee DW, Lee DH, Kim TO, Kim GH, Heo J, Kang DH, Song GA. [A case of gastric metastasis of breast carcinoma resembling early gastric cancer]. Korean J Gastroenterol. 2005;46:481-484. [PubMed] |
| 14. | Jeon SH, Kwon TK, Kim SH, Kwon DY, Park KS. A case of gastric metastasis from breast carcinoma manifested by upper gastrointestinal bleeding. Korean J Gastrointest Endosc. 2002;24:220-224. |
| 15. | Kim DY, Yun T, Kim TY, Heo DS, Bang YJ. Renal, gastric, and multiple intestinal metastases of invasive ductal carcinoma of breast. Korean J Med. 2003;65:S836-S840. |
| 16. | Koike K, Kitahara K, Higaki M, Urata M, Yamazaki F, Noshiro H. Clinicopathological features of gastric metastasis from breast cancer in three cases. Breast Cancer. 2014;21:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Lee SI, Moon YM, Kang JK, Park IS, Choi HJ, Kim BS, Kim TS. A case of gastric metastasis from breast cancer. Korean J Gastroenterol. 1983;15:157-162. |
| 18. | Pera M, Riera E, Lopez R, Viñolas N, Romagosa C, Miquel R. Metastatic carcinoma of the breast resembling early gastric carcinoma. Mayo Clin Proc. 2001;76:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Yim H, Jin YM, Shim C, Park HB. Gastric metastasis of mammary signet ring cell carcinoma--a differential diagnosis with primary gastric signet ring cell carcinoma. J Korean Med Sci. 1997;12:256-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Lorimier G, Binelli C, Burtin P, Maillart P, Bertrand G, Verriele V, Fondrinier E. Metastatic gastric cancer arising from breast carcinoma: endoscopic ultrasonographic aspects. Endoscopy. 1998;30:800-804. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol. 2004;121:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89:2214-2221. [PubMed] |
| 23. | Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, Matsuura M, Ito Y, Akiyama F, Sakamoto G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727-3734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 24. | Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, Langfort R, Waloszczyk P, Biernat W, Lasota J. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 495] [Article Influence: 45.0] [Reference Citation Analysis (0)] |